Figure 2.
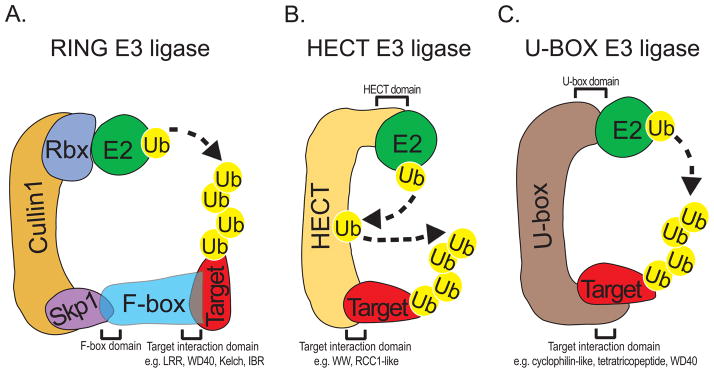
Classes of Ubiquitin E3 ligases. a. RING E3 ligases form multi-subunit protein complexes that generally include RING finger protein Rbx and a Cullin scaffold. Cullin1-based SCF ubiquitin ligase is shown. Target proteins are recruited to the complex by F-box proteins, which bind to the adaptor protein Skp1 via the conserved F-box domain. Ubiquitin-loaded E2 binds to the complex via Rbx and transfers ubiquitin to the target proteins. b. HECT E3 ligases are monomeric proteins that interact with E2 via a conserved N-terminus HECT domain and with target proteins via divergent C-terminus domains. HECT proteins have intrinsic ligase activity and act as ubiquitin acceptors from E2 before transferring Ub to target proteins. c. U-box E3 ligases bind to E2 via a conserved U-box/RING domain and to target proteins via divergent domains including WW, cyclophilin-like, and tetratricopeptide. They do not have intrinsic ligase activity and their ubiquitin transfer system is similar to RING E3 ligases.
