Abstract
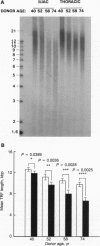
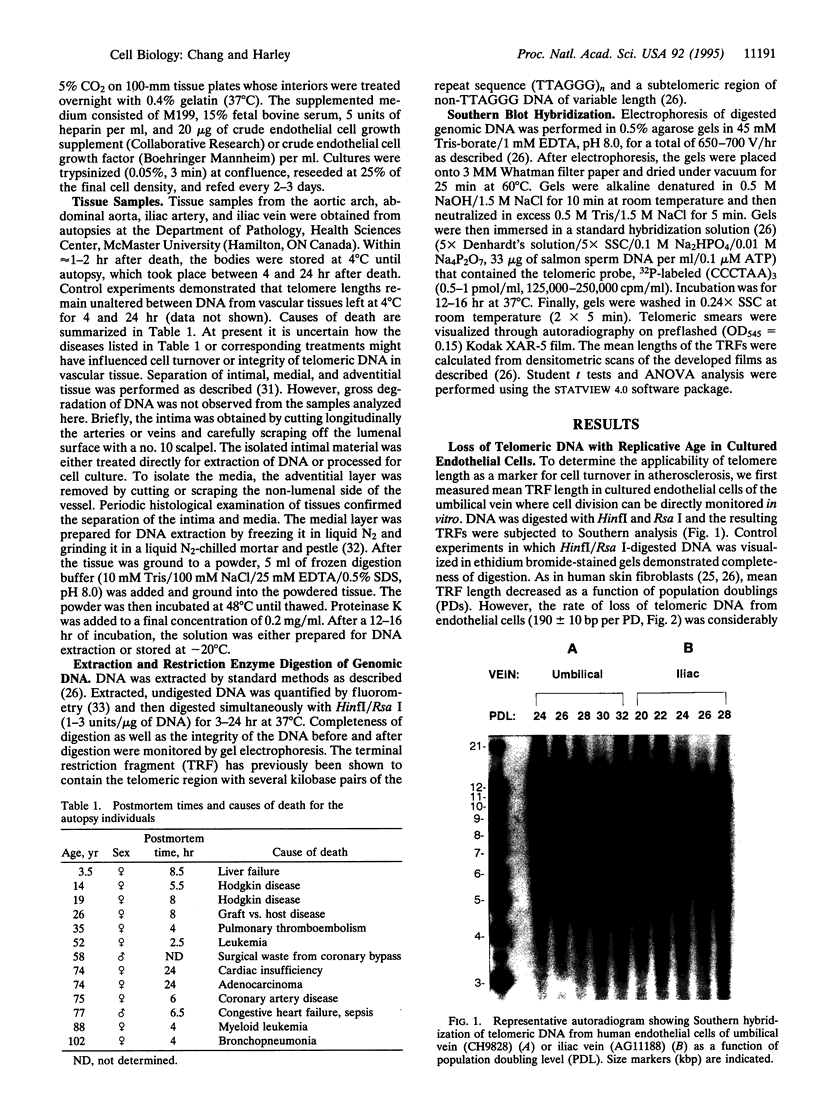
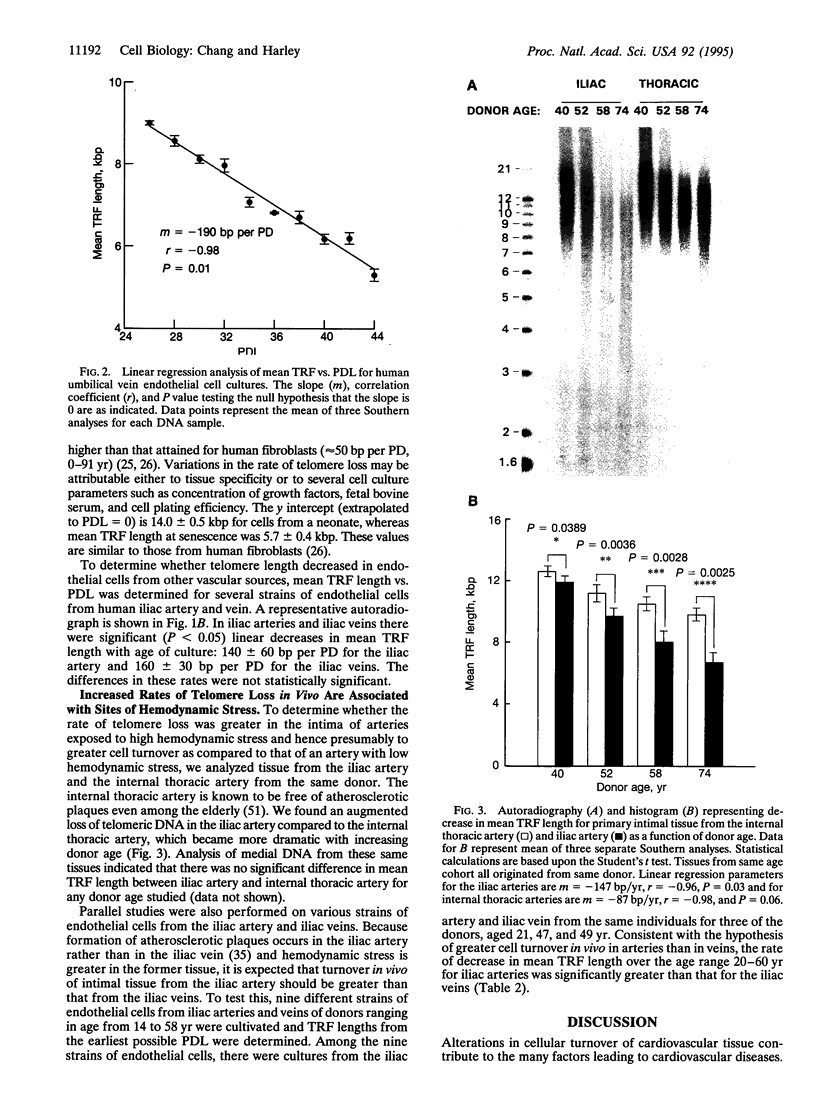
Because repeated injury of the endothelium and subsequent turnover of intimal and medial cells have been implicated in atherosclerosis, we examined telomere length, a marker of somatic cell turnover, in cells from these tissues. Telomere lengths were assessed by Southern analysis of terminal restriction fragments (TRFs) generated by HinfI/Rsa I digestion of human genomic DNA. Mean TRF length decreased as a function of population doublings in human endothelial cell cultures from umbilical veins, iliac arteries, and iliac veins. When endothelial cells were examined for mean TRF length as a function of donor age, there was a significantly greater rate of decrease for cells from iliac arteries than from iliac veins (102 bp/yr vs. 47 bp/yr, respectively, P < 0.05), consistent with higher hemodynamic stress and increased cell turnover in arteries. Moreover, the rate of telomere loss as a function of donor age was greater in the intimal DNA of iliac arteries compared to that of the internal thoracic arteries (147 bp/yr vs. 87 bp/yr, respectively, P < 0.05), a region of the arterial tree subject to less hemodynamic stress. This indicates that the effect is not tissue specific. DNA from the medial tissue of the iliac and internal thoracic arteries showed no significant difference in the rates of decrease, suggesting that chronic stress leading to cellular senescence is more pronounced in the intima than in the media. These observations extend the use of telomere size as a marker for the replicative history of cells and are consistent with a role for focal replicative senescence in cardiovascular diseases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allsopp R. C., Chang E., Kashefi-Aazam M., Rogaev E. I., Piatyszek M. A., Shay J. W., Harley C. B. Telomere shortening is associated with cell division in vitro and in vivo. Exp Cell Res. 1995 Sep;220(1):194–200. doi: 10.1006/excr.1995.1306. [DOI] [PubMed] [Google Scholar]
- Allsopp R. C., Vaziri H., Patterson C., Goldstein S., Younglai E. V., Futcher A. B., Greider C. W., Harley C. B. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker G. T., 3rd, Sprott R. L. Biomarkers of aging. Exp Gerontol. 1988;23(4-5):223–239. doi: 10.1016/0531-5565(88)90025-3. [DOI] [PubMed] [Google Scholar]
- Baron B. W., Lyon R. T., Zarins C. K., Glagov S., Baron J. M. Changes in plasma factor VIII complex and serum lipid profile during atherogenesis in cynomolgus monkeys. Arteriosclerosis. 1990 Nov-Dec;10(6):1074–1081. doi: 10.1161/01.atv.10.6.1074. [DOI] [PubMed] [Google Scholar]
- Bierman E. L. The effect of donor age on the in vitro life span of cultured human arterial smooth-muscle cells. In Vitro. 1978 Nov;14(11):951–955. doi: 10.1007/BF02616126. [DOI] [PubMed] [Google Scholar]
- Bonnet J., Garderes P. E., Aumailley M., Moreau C., Gouverneur G., Benchimol D., Crockett R., Larrue J., Bricaud H. Serum type III procollagen peptide levels in coronary artery disease (a marker of atherosclerosis). Eur J Clin Invest. 1988 Feb;18(1):18–21. doi: 10.1111/j.1365-2362.1988.tb01159.x. [DOI] [PubMed] [Google Scholar]
- Breckenridge W. C. Lipoprotein (a): genetic marker for atherosclerosis? CMAJ. 1990 Jul 15;143(2):115–115. [PMC free article] [PubMed] [Google Scholar]
- Campisi J. Gene expression in quiescent and senescent fibroblasts. Ann N Y Acad Sci. 1992 Nov 21;663:195–201. doi: 10.1111/j.1749-6632.1992.tb38663.x. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. C., Galton D. J. Genetic susceptibility to atherosclerosis. Br Med Bull. 1990 Oct;46(4):917–940. doi: 10.1093/oxfordjournals.bmb.a072446. [DOI] [PubMed] [Google Scholar]
- Conti C. R. The mysterious, internal thoracic artery. Clin Cardiol. 1991 Jan;14(1):3–4. doi: 10.1002/clc.4960140103. [DOI] [PubMed] [Google Scholar]
- Cristofalo V. J., Gerhard G. S., Pignolo R. J. Molecular biology of aging. Surg Clin North Am. 1994 Feb;74(1):1–21. doi: 10.1016/s0039-6109(16)46225-0. [DOI] [PubMed] [Google Scholar]
- Cybulsky M. I., Gimbrone M. A., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991 Feb 15;251(4995):788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- Dartsch P. C., Voisard R., Bauriedel G., Höfling B., Betz E. Growth characteristics and cytoskeletal organization of cultured smooth muscle cells from human primary stenosing and restenosing lesions. Arteriosclerosis. 1990 Jan-Feb;10(1):62–75. doi: 10.1161/01.atv.10.1.62. [DOI] [PubMed] [Google Scholar]
- Dempsey R. J., Diana A. L., Moore R. W. Thickness of carotid artery atherosclerotic plaque and ischemic risk. Neurosurgery. 1990 Sep;27(3):343–348. doi: 10.1097/00006123-199009000-00001. [DOI] [PubMed] [Google Scholar]
- Goldstein S. Replicative senescence: the human fibroblast comes of age. Science. 1990 Sep 7;249(4973):1129–1133. doi: 10.1126/science.2204114. [DOI] [PubMed] [Google Scholar]
- Gordon D., Mohai L. G., Schwartz S. M. Induction of polyploidy in cultures of neonatal rat aortic smooth muscle cells. Circ Res. 1986 Dec;59(6):633–644. doi: 10.1161/01.res.59.6.633. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Seifert P. S., Olsson G., Bondjers G. Immunohistochemical detection of macrophages and T lymphocytes in atherosclerotic lesions of cholesterol-fed rabbits. Arterioscler Thromb. 1991 May-Jun;11(3):745–750. doi: 10.1161/01.atv.11.3.745. [DOI] [PubMed] [Google Scholar]
- Hariri R. J., Hajjar D. P., Coletti D., Alonso D. R., Weksler M. E., Rabellino E. Aging and arteriosclerosis. Cell cycle kinetics of young and old arterial smooth muscle cells. Am J Pathol. 1988 Apr;131(1):132–136. [PMC free article] [PubMed] [Google Scholar]
- Harley C. B., Futcher A. B., Greider C. W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990 May 31;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Dempster M., Dunlop M. G., Thompson A. M., Green D. K., Allshire R. C. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990 Aug 30;346(6287):866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Hearing V. J. Unraveling the melanocyte. Am J Hum Genet. 1993 Jan;52(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Kalenich O. S., Tertov V. V., Novikov I. D., Vorob'eva E. G., Perova N. V., Metel'skaia V. A., Pomerantsev E. V., Liakishev A. A., Ruda M. Ia, Orekhov A. N. Kholesterin tsirkuliruiushchikh immunnykh kompleksov kak biokhimicheskii marker koronarnogo ateroskleroza. Kardiologiia. 1991 Feb;31(2):42–44. [PubMed] [Google Scholar]
- Kennedy B. P., Crim L. W., Davies P. L. Expression of histone and tubulin genes during spermatogenesis. Evidence of post-meiotic transcription. Exp Cell Res. 1985 Jun;158(2):445–460. doi: 10.1016/0014-4827(85)90468-9. [DOI] [PubMed] [Google Scholar]
- Kockx M. M., De Meyer G. R., Jacob W. A., Bult H., Herman A. G. Triphasic sequence of neointimal formation in the cuffed carotid artery of the rabbit. Arterioscler Thromb. 1992 Dec;12(12):1447–1457. doi: 10.1161/01.atv.12.12.1447. [DOI] [PubMed] [Google Scholar]
- Kumazaki T., Fujii T., Kobayashi M., Mitsui Y. Aging- and growth-dependent modulation of endothelin-1 gene expression in human vascular endothelial cells. Exp Cell Res. 1994 Mar;211(1):6–11. doi: 10.1006/excr.1994.1051. [DOI] [PubMed] [Google Scholar]
- Lerman A., Edwards B. S., Hallett J. W., Heublein D. M., Sandberg S. M., Burnett J. C., Jr Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N Engl J Med. 1991 Oct 3;325(14):997–1001. doi: 10.1056/NEJM199110033251404. [DOI] [PubMed] [Google Scholar]
- Lindsey J., McGill N. I., Lindsey L. A., Green D. K., Cooke H. J. In vivo loss of telomeric repeats with age in humans. Mutat Res. 1991 Jan;256(1):45–48. doi: 10.1016/0921-8734(91)90032-7. [DOI] [PubMed] [Google Scholar]
- Maier J. A., Statuto M., Ragnotti G. Senescence stimulates U937-endothelial cell interactions. Exp Cell Res. 1993 Sep;208(1):270–274. doi: 10.1006/excr.1993.1246. [DOI] [PubMed] [Google Scholar]
- Martin G. M., Ogburn C. E., Wight T. N. Comparative rates of decline in the primary cloning efficiencies of smooth muscle cells from the aging thoracic aorta of two murine species of contrasting maximum life span potentials. Am J Pathol. 1983 Feb;110(2):236–245. [PMC free article] [PubMed] [Google Scholar]
- Mezdour H., Parra H. J., Aguie-Aguie G., Fruchart J. C. La lipoprotéine (a). Un marqueur additionnel de l'athérosclérose. Ann Biol Clin (Paris) 1990;48(3):139–153. [PubMed] [Google Scholar]
- Morgan A. R., Lee J. S., Pulleyblank D. E., Murray N. L., Evans D. H. Review: ethidium fluorescence assays. Part 1. Physicochemical studies. Nucleic Acids Res. 1979 Oct 10;7(3):547–569. doi: 10.1093/nar/7.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss N. S., Benditt E. P. Human atherosclerotic plaque cells and leiomyoma cells. Comparison of in vitro growth characteristics. Am J Pathol. 1975 Feb;78(2):175–190. [PMC free article] [PubMed] [Google Scholar]
- Owens G. K., Rabinovitch P. S., Schwartz S. M. Smooth muscle cell hypertrophy versus hyperplasia in hypertension. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7759–7763. doi: 10.1073/pnas.78.12.7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens G. K., Schwartz S. M. Alterations in vascular smooth muscle mass in the spontaneously hypertensive rat. Role of cellular hypertrophy, hyperploidy, and hyperplasia. Circ Res. 1982 Sep;51(3):280–289. doi: 10.1161/01.res.51.3.280. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (first of two parts). N Engl J Med. 1976 Aug 12;295(7):369–377. doi: 10.1056/NEJM197608122950707. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976 Aug 19;295(8):420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Ryan U. S. Isolation and culture of pulmonary endothelial cells. Environ Health Perspect. 1984 Jun;56:103–114. doi: 10.1289/ehp.8456103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadayasu T., Nakashima Y., Yashiro A., Kawashima T., Kuroiwa A. Heparin-releasable platelet factor 4 in patients with coronary artery disease. Clin Cardiol. 1991 Sep;14(9):725–729. doi: 10.1002/clc.4960140906. [DOI] [PubMed] [Google Scholar]
- Sepehrnia B., Kamboh M. I., Ferrell R. E. Genetic studies of human apolipoproteins. III. Polymorphism of apolipoprotein C-II. Hum Hered. 1988;38(3):136–143. doi: 10.1159/000153774. [DOI] [PubMed] [Google Scholar]
- Slagboom P. E., Droog S., Boomsma D. I. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994 Nov;55(5):876–882. [PMC free article] [PubMed] [Google Scholar]
- Stringer M. D., Kakkar V. V. Markers of disease severity in peripheral atherosclerosis. Eur J Vasc Surg. 1990 Oct;4(5):513–518. doi: 10.1016/s0950-821x(05)80794-7. [DOI] [PubMed] [Google Scholar]
- Vita J. A., Treasure C. B., Nabel E. G., McLenachan J. M., Fish R. D., Yeung A. C., Vekshtein V. I., Selwyn A. P., Ganz P. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990 Feb;81(2):491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- West M. D. The cellular and molecular biology of skin aging. Arch Dermatol. 1994 Jan;130(1):87–95. [PubMed] [Google Scholar]
- Wright W. E., Shay J. W. Telomere positional effects and the regulation of cellular senescence. Trends Genet. 1992 Jun;8(6):193–197. doi: 10.1016/0168-9525(92)90232-s. [DOI] [PubMed] [Google Scholar]