Abstract
We report a novel pH-responsive gold nanoparticle-stabilized liposome system for gastric antimicrobial delivery. By adsorbing small chitosan-modified gold nanoparticles (diameter ~ 10 nm) onto the outer surface of negatively charged phospholipid liposomes (diameter ~ 75 nm), we show that at gastric pH the liposomes have excellent stability with limited fusion ability and negligible cargo releases. However when the stabilized liposomes are present in an environment with neutral pH, the gold stabilizers detach from the liposomes resulting in free liposomes that can actively fuse with bacterial membranes. Using Helicobacter pylori as a model bacterium and doxycycline as a model antibiotic, we demonstrate such pH-responsive fusion activity and drug release profile of the nanoparticle-stabilized liposomes. Particularly, at neutral pH the gold nanoparticles detach and thus the doxycycline-loaded liposomes rapidly fuse with bacteria and cause superior bactericidal efficacy as compared to the free doxycycline counterpart. Our results suggest that the reported liposome system holds a substantial potential for gastric drug delivery; it remains inactive (stable) in the stomach lumen but actively interact with bacteria once reaches the mucus layer of the stomach where the bacteria may reside.
Keywords: liposome, gold nanoparticle, pH-responsive, gastric infection, antimicrobial delivery
Introduction
Liposomes have been studied extensively as antimicrobial delivery vehicles mainly because of their unique features, including highly biocompatible lipid materials, unique bilayer structure that can fuse with bacterial membranes, high drug carrying capacity, and readily tunable formulation properties.1–6 Despite these advantageous features, the applications of liposomes, particularly those with sizes below 100 nm, are often limited by poor stability due to spontaneous fusion among liposomes, causing payload loss and undesired mixing.7–9 A widely applied approach to stabilize liposomes is to coat their surface with a stealth material such as polyethylene glycol (PEG)10–12 and zwitterionic polymers.13, 14 The PEG coating reduces the tendency of liposomes to aggregate and fuse with each other through steric stabilization. It also suppresses non-specific interactions of liposomes with blood components (opsonization) for enhanced blood residency time.15, 16 As a result, PEGylated liposomes have found great applications in systemic drug delivery. However, PEGylated liposomes are rarely used for antimicrobial delivery to treat bacterial infections. This is mainly because the polymer coating not only stabilizes liposomes against fusion with each other but also prevents them from fusing with bacterial membranes, to which the antimicrobial payloads need to be delivered.17–19 Therefore, it would be ideal to engineer advanced liposomal formulations that are stabilized against fusion prior to ‘seeing’ target bacteria, while their fusion activity resumes once they arrive at the infection sites.
An emerging strategy to stabilize liposomes for effective antimicrobial delivery is to bind tiny charged nanoparticles to liposome surfaces. The non-specific adsorption of charged nanoparticles onto phospholipid bilayers provide steric repulsion that inhibits liposomes from approaching each other and then fusing to form larger vesicles.20, 21 In addition, the nanoparticle stabilizers are found to cause lipid surface reconstruction at the points where nanoparticles adsorb. Such surface reconstruction reduces liposome surface tension and further enhances liposome stability.22 More interestingly, stabilization by small nanoparticles leaves a substantial fraction of the liposome surfaces untouched, making it possible to incorporate additional functionalities to the liposomes and allowing for controlled cargo releases. For instance, it has been shown that the uncoated liposome surfaces are highly accessible to bacterial toxins, which can punch holes on the liposomes to release encapsulated drugs at the infectious sites.8 Furthermore, the charge and charge density of both the nanoparticle stabilizers and the liposomes can be precisely tailored to enable stimulus-responsive binding and detaching of the nanoparticles, thereby allowing an on-demand control over liposome fusion activity for smart cargo delivery.18 The objective of this study is to develop a unique and robust nanoparticle-stabilized liposome system for gastric antimicrobial delivery with a particular interest in antibiotic delivery to treat Helicobacter pylori (H. pylori) infection in the stomach.
H. pylori infects about half of the people in the world and is of major public health concern. Infection with H. pylori is the main cause of chronic gastritis, peptic ulcers, and gastric malignancy.23–25 However, eradication of H. pylori is challenging regardless of the treatment regimens, partially because the bacteria locate in the stomach mucus lining, which requires drugs to tolerate the highly acidic gastric environment.26, 27 Herein, we report a novel pH-responsive gold nanoparticle-stabilized liposome system in which small gold nanoparticles (diameter: ~ 10 nm) bind to the surface of liposomes (diameter: ~ 75 nm) and thus stabilize the liposomes at acidic pH values (i.e., gastric pH). These gold stabilizers will detach from the liposomes when the environmental acidity decreases to near neutral value (i.e. pH = 7.4). The resulting free liposomes can then actively fuse with target bacterial membrane driven by reducing the high surface tension of the liposomes. This would be an ideal delivery platform for drug delivery to mucus lining in the stomach for the treatment of H. pylori infection. It has been well documented that the pH values in the mucus layer of stomach gradually increase from 1.2 at the gastric lumen side to near 7.4 at the mucus lining, where the H. pylori bacteria reside.26–29
As shown in the Figure 1, the gold nanoparticles are surface modified with chitosan (pKa ≈ 6), denoted as AuChi, which exhibit a strong positive charge at gastric pH but are deprotonated at neutral pH.30 The liposomes are comprised of hydrogenated L-aphosphatidylcholine (Egg PC) and 1,2-dioleoyl-sn-glycero-3-phosphate (sodium salt) (DOPA), a phospholipid with strong negative charge during the pH range of 1.2 to 7.4.31, 32 We demonstrate that under gastric pH AuChi tightly bind to the liposome surfaces, thereby effectively inhibiting drug release and liposome fusion with H. pylori bacteria. Once the pH level is increased to neutral pH, AuChi detach from the liposomes. The resulting free liposomes can rapidly fuse with the bacterial membranes of H. pylori, release the encapsulated antibiotics, and kill the bacteria with a superior efficacy as compared to the free antibiotic counterpart.
Figure 1.
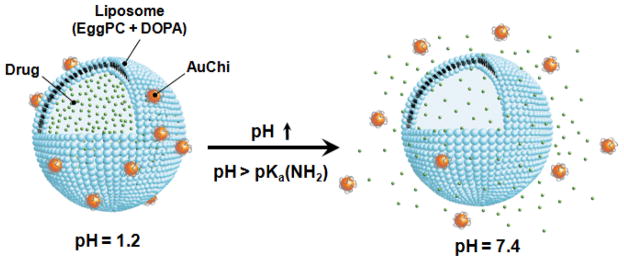
Schematic illustration of a phospholipid liposome stabilized by chitosan-modified gold nanoparticles (AuChi-liposome) for pH-responsive gastric drug delivery. At gastric pH (pH = 1.2), the liposome is stabilized by binding of protonated AuChi nanoparticles. At physiological condition (pH = 7.4), AuChi nanoparticles are deprotonated and thus detach from the liposome, resulting in bare liposome with restored fusion and drug release properties.
Results and Discussion
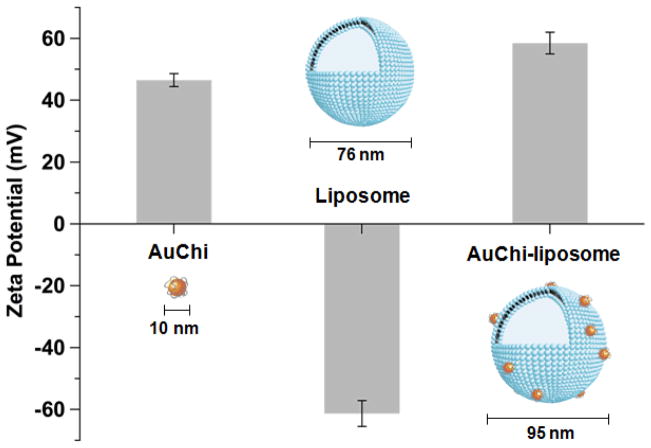
The preparation of AuChi-stabilized liposomes, denoted as AuChi-liposome, can be divided into three steps. First, AuChi nanoparticles were synthesized by following a previously established protocol, where gold hydrosol was first made by using sodium borohydride reduction of AuHCl4 and then stabilized by adding chitosan in ambient condition.8, 33 Measurements of AuChi nanoparticles with dynamic light scattering (DLS) showed a diameter of about 10 nm with a nearly uniform size distribution (Figure 2). The electrophoretic mobility measurements with DLS showed that the surface zeta potential of AuChi was 43.4 ± 1.0 mV, a strong positive charge implying the presence of cationic amine groups of chitosan on the particle surfaces. In the second step, liposomes consisting of Egg PC and DOPA (in a weight ratio of 80:20) were prepared by vesicle extrusion technique in deionized water at pH = 6.34 The subsequent DLS measurements of liposomes showed a diameter of 76.1 ± 1.0 nm (polydispersity index = 0.10 ± 0.02) and a surface zeta potential of −61.1 ± 2.3 mV. The strong negative zeta potential verifies the incorporation of DOPA into the lipid bilayers as liposomes formulated without DOPA showed a similar size but a less negative zeta potential of −7.6 ± 0.4 mV. Lastly, the resulting cationic AuChi nanoparticles and the anionic liposomes were mixed at a molar ratio of 300:1 under gentle bath sonication for 10 min. The pH value of the mixture solution was then adjusted to 1.2, simulating gastric pH. Following the preparation, DLS measurements showed that the AuChi-liposome had a diameter of 95.2 ± 1.3 nm (polydispersity index = 0.11 ± 0.01) and a surface zeta potential of 57.4 ± 0.7 mV. Compared to bare liposomes, the observed approximate 20 nm diameter increase of particle size is likely due to the adsorption of AuChi onto the liposome surfaces. The switch of zeta potential from −61.1 to 57.4 mV also confirms the binding of positively charged AuChi nanoparticles to the liposomes.
Figure 2.
The surface zeta potential and hydrodynamic size of AuChi, bare liposome (without AuChi), and AuChi-liposome with an AuChi-to-liposome molar ratio of 300:1 measured by dynamic light scattering (DLS).
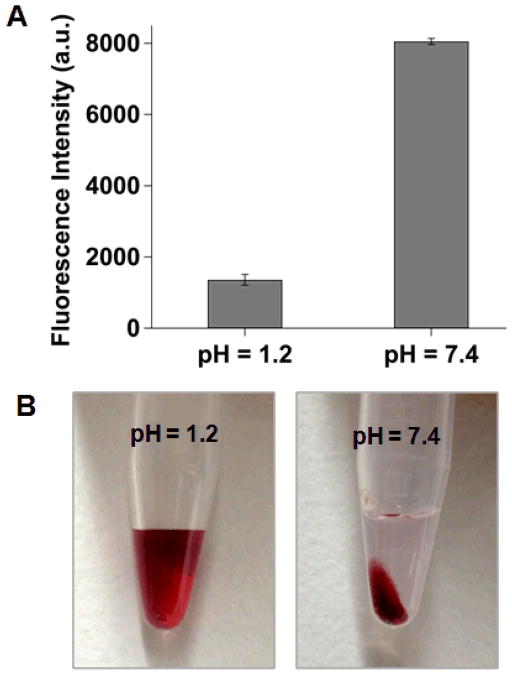
The formation of AuChi-liposome complex at gastric pH was first verified through a fluorescent assay based upon the distance-dependent fluorescence quenching phenomenon of gold nanoparticles.35, 36 To this end, a fluorescence labeled lipid molecule, 1,2-dimyristoyl-sn-glycero-3 phosphoethanolamine-N-lissamine rhodamine B sulfonyl (DMPE-RhB, excitation/emission = 550/590 nm), was incorporated into the liposome membranes prior to the preparation of AuChi-liposome. The pH value of the AuChi-liposome suspension was then set at pH = 1.2 and 7.4, simulating the gastric pH and physiological condition, respectively, and the florescence intensity of the suspensions was monitored. As shown in Figure 3A, the fluorescence intensity at pH = 1.2 was over 5-fold lower than that at pH = 7.4, indicating that much more AuChi adsorbed on the liposome surfaces at acidic pH while they detached at neutral pH. To further verify the binding of AuChi to the anionic liposomes, the AuChi-liposome solutions at different pH values were subject to an external centrifugal force (2000 ×g) for 10 min. As shown in Figure 3B, at pH = 1.2, no particle precipitate was observed and the suspension remained red, the characteristic color of gold nanoparticles. In contrast, a large amount of particle precipitates and a clear supernatant were observed at pH = 7.4. The obtained supernatant was then measured with DLS for the size and surface zeta potential, which were found to be similar to those of the corresponding bare liposomes. These results suggest that AuChi strongly bound to liposomes at acidic pH, thus inseparable from the liposomes by low centrifugal force, but they readily detached from the liposomes and precipitated from the suspension by centrifugation at neutral pH.
Figure 3.
(A) Fluorescence intensity of rhodamine B (RhB)-doped AuChi-liposome at pH = 1.2 and 7.4, respectively. 0.5 mol% of DMPE-RhB was incorporated into the liposome membranes prior to AuChi stabilization. The binding of AuChi on the liposome would quench the fluorescent probes within the membranes while detaching of AuChi would induce fluorescence recovery. (B) AuChi-liposome solutions after centrifugation to precipitate free AuChi nanoparticles. Dark red color indicates the presence of AuChi in the solution at pH = 1.2 and the sedimentation of AuChi at pH = 7.4.
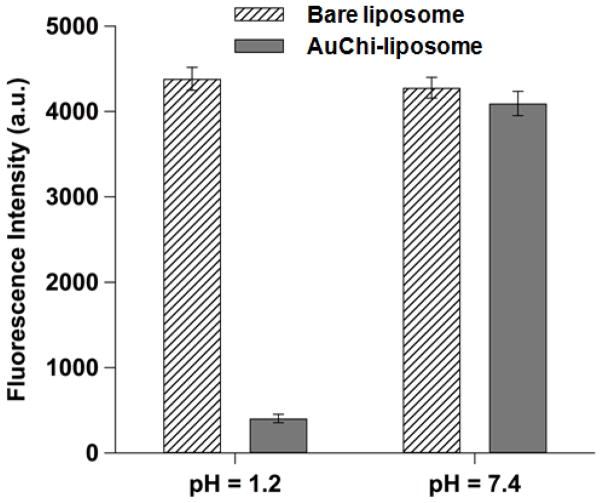
To examine the pH-responsive stability of the AuChi-liposome, we next examined their fusion ability with bacterial membranes. Fluorescence labeled AuChi-liposome (0.5 mM, containing 0.5 mol% DMPE-RhB) were prepared and mixed with H. pylori bacteria (5×108 CFU/mL) at pH = 1.2 and 7.4, respectively. The mixture suspensions were incubated at 37°C for 30 min. Then the bacteria were thoroughly washed, collected and resuspended in 1× PBS (pH = 7.4). The fluorescence intensity of the bacterial suspensions was then measured to quantify the fusion ability of the liposomes. Fluorescence labeled bare liposomes (without AuChi) were tested in parallel as a control. Figure 4 shows that H. pylori bacteria incubated with bare liposomes had comparable fluorescence intensity at pH = 1.2 and 7.4. In contrast, a much weaker fluorescence emission was obtained from the bacteria incubated with AuChi-liposome at pH = 1.2, suggesting a significantly reduced liposome fusion with the bacteria. However, when the pH was increased from 1.2 to 7.4, AuChi-liposome resumed their fusion ability to an extent comparable to that of the bare liposomes. The observed differential fusion activity of AuChi-liposome at different pH values proves the stabilization effect conferred by the binding of AuChi nanoparticles.
Figure 4.
Fusion ability of AuChi-liposome with H. pylori bacteria at pH = 1.2 and 7.4, respectively. Fluorescently labeled AuChi-liposome (0.5 mM) was incubated with H. pylori bacteria (5×108 CFU/mL) at pH = 1.2 or 7.4 for 30 min. After incubation, the bacteria pellet was collected and then measured for fluorescence intensity. The same amount of fluorescently labeled bare liposome was tested in parallel as a control. Data represent mean ± SD (n = 3).
We further evaluated the stability of the AuChi-liposome by examining the drug release kinetics from the liposomes at different pH values. Small AuChi adsorbed onto the liposome surfaces can inhibit liposome fusion, which in principle can minimize undesirable drug leakage from liposomes. Moreover, the membrane-bound AuChi may locally modulate the stiffness and morphology of the lipid bilayers, hindering diffusion of drug molecules across the liposomal membranes.22 Using doxycycline as a model antibiotic drug, we loaded it inside the AuChi-liposome with a drug concentration of 1 mM and then monitored its release profile from the liposomes. As shown in Figure 5, at pH = 1.2, AuChi-liposome only released approximately 10% of encapsulated doxycycline within 24 hr. In contrast, at pH = 7.4, over 90% of doxycycline was released within 24 hr. The significant decrease of drug release rate from the liposomes at pH = 1.2, corresponding to the strong binding of AuChi to the liposomes, confirms the stabilization of liposomes by surface adsorption of AuChi nanoparticles.
Figure 5.
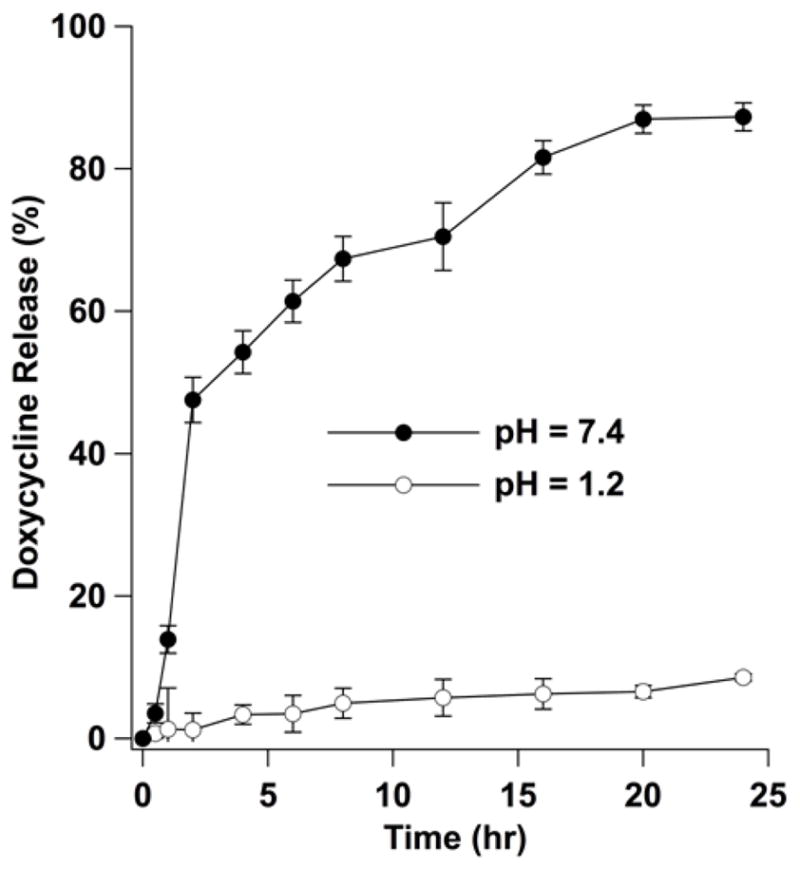
Accumulative doxycycline release profile from doxycycline-loaded AuChi-liposome at pH = 1.2 and 7.4, respectively. The released doxycycline was quantified by measuring the UV absorbance at 345 nm using a spectrophotometer and then compared to a linear standard curve to calculate the amount of doxycycline released from the AuChi-liposome.
After having verified the pH-responsive stability of the AuChi-liposome, we finally tested the antimicrobial activity of doxycycline-loaded AuChi-liposome against H. pylori bacteria in vitro. While both the fusion activity and drug release property of liposomes were effectively inhibited by the adsorption of AuChi at gastric pH, these functions would resume upon detaching of AuChi at a physiological condition. Subsequently, the drug-loaded liposomes would fuse with bacteria membranes to release therapeutic cargos. In the study, doxycycline-loaded AuChi-liposome at various doxycycline concentrations (ranging from 10 to 150 μg/mL) were mixed with H. pylori bacteria (5×106 CFU/mL) at pH = 7.4. The samples then were incubated at 37°C under microaerobic conditions for 30 min, followed by serial dilution of each sample for bacterial colony enumeration. For comparison, the same concentration of empty AuChi-liposome (without drug) and free doxycycline we tested in parallel as controls and bacteria incubating in PBS (1X) served as a negative control. As shown in Figure 6, empty AuChi-liposome at all tested concentrations did not show any inhibitory effect against H. pylori, as their incubation with the bacteria resulted in a comparable colony formation to the PBS (1X) control. Free doxycycline showed a dose-dependent antimicrobial activity, but complete killing was not observed under the experimental conditions. In contrast, doxycycline-loaded AuChi-liposome showed an enhanced therapeutic efficacy against H. pylori at all tested concentrations. Particularly, eradication of H. pylori bacteria was achieved at a doxycycline concentration of 100 μg/mL. Such significant improvement on the drug’s bactericidal efficacy is likely due to the rapid fusion between drug-loaded liposomes and bacterial membranes. Through the fusion process, all drug molecules entrapped in the liposomes are exclusively distributed into the bacteria, which may cause instant killing of the bacteria without inducing bacterial antibiotic resistance.23, 37
Figure 6.
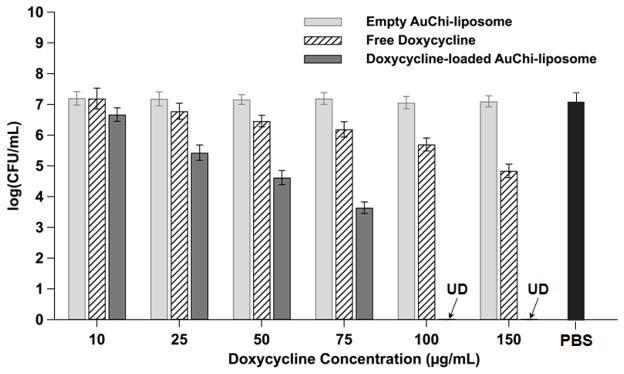
Antimicrobial activity of doxycycline-loaded AuChi-liposome against H. pylori bacteria at various doxycycline concentrations. Doxycycline-loaded AuChi-liposome were incubated with H. pylori bacteria (5×106 CFU/mL) at 37°C under microaerobic condition for 30 min, followed by serial dilution and bacterial colony enumeration on Columbia agar plates. Equivalent amounts of empty AuChi-liposome and free doxycycline were tested in parallel for comparison. PBS (1X) was used as a negative control. Data represent mean ± SD (n = 3).
Conclusions
In conclusion, by attaching chitosan-modified gold nanoparticles to the outer surface of anionic liposomes, a robust liposome-based gastric drug delivery system was developed. Such system has pH-responsive stability and fusion activity. Specifically, at gastric pH small gold nanoparticles spontaneously bound to the liposome surface, effectively inhibiting drug release and liposome fusion with H. pylori bacteria. Once the pH level was increased to neutral pH (i.e., pH value at the mucus lining of stomach), gold nanoparticles detached and resulted in free liposomes with both fusion and drug release properties restored. Using doxycycline as a model antibiotic, the gold nanoparticle-stabilized liposome formulation exhibited superior antibacterial efficacy against H. pylori bacteria when compared with the same concentrations of free doxycycline. In addition to gold nanoparticles, other solid nanoparticles, such as silica nanoparticles, have also been applied to stabilize liposomes, offering opportunities for various applications.21, 38 Our results indicate that the use of small charged nanoparticles to stabilize liposomes represents a promising strategy for developing effective antibacterial regimes, especially for the treatment of stomach bacterial infections such as H. pylori infection.
Experimental Section
Materials
Hydrogenated L-α-phosphatidylcholine (Egg PC), 1,2-dioleoyl-sn-glycero-3-phosphate (sodium salt) (DOPA) and 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-lissamine rhodamine B sulfonyl (DMPE-RhB) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). Rhodamine B and Doxycycline were purchased from Sigma Aldrich (St Louis, MO). Brain Heart Infusion (BHI) broth (1X) and Columbia agar were purchased from Becton Dickinson (Sparks, MD). Hydrogen tetrachloroaurate (HAuCl4) and sodium borohydride (NaBH4) were purchased from ACROS Organics (Geel, Belgium). Chitosan-50 was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
AuChi Preparation and Characterization
AuChi were prepared by a sodium borohydride reduction technique as previously described.8 Briefly, an aqueous solution of HAuCl4 (0.1 mM, 50 mL) was reduced by 5 mg of NaBH4 to form gold nanoparticles. To functionalize nanoparticles with chitosan, the nanoparticle suspension was incubated overnight with 0.1% w/v chitosan dissolved in 0.1 M acetic acid. Following the reaction, the AuChi nanoparticle suspension was washed three times by using an Amicon Ultra-4 centrifugal filter with a molecular weight cutoff of 10 kDa (Millipore, Billerica, MA). The hydrodynamic size, size distribution, and surface charge (zeta potential) of AuChi nanoparticles were characterized by dynamic light scattering (DLS) (Malvern Zetasizer Nano ZS, ZEN3600, Malvern Instruments, Worcestershire, UK). Clear disposable capillary cells (DTS1061) from Malvern were used for all samples. All measurements were conducted at a backscattering angle of 173° at room temperature. The average of three runs was used for each measurement.
Liposome preparation
Liposomes were prepared by following a previously described extrusion method.23 Specifically, 3 mg of lipid mixture (EggPC/DOPA=80/20 wt%) were dissolved in 1 mL chloroform and the organic solvent was evaporated by blowing nitrogen gas over the solution for 15 min to form a dried lipid film. The lipid film was rehydrated with 2 mL of deionized water containing either rhodamine B (RhB) or doxycycline at desired concentrations, followed by 1 min of vortexing and 3 min of sonication in a bath sonicator (Fisher Scientific FS30D, Pittsburgh, PA) to produce multilamellar vesicles (MLVs). Then the obtained MLVs were sonicated for 1 minute at 20 W by a titanium probe (Branson 450 sonifier, Danbury, CT) to produce unilamellar vesicles. Following the sonication, the solution was extruded through a 100 nm pore-sized polycarbonate membrane for 11 times to form narrowly distributed small unilamellar vesicles (SUVs). After the extrusion, the liposomes were purified by dialysis with a 20 kDa molecular weight cut-off to remove unencapsulated dyes or drugs.
AuChi-liposome formulation
To prepare AuChi-liposome, the pH of both AuChi and liposome solutions was adjusted to 6.5 using HCl. Then the liposomes and AuChi at desired molar ratio were mixed together, followed by 12 hr of vortexing. To quantify the adsorption of AuChi nanoparticles onto liposomes, RhB-labeled liposomes were prepared by mixing 0.5 mol% of DMPE-RhB with lipid components prior to liposome preparation. Mixing AuChi nanoparticles with the fluorescent liposomes resulted in the quenching of fluorescence intensity. It was found that the quenching effect reached the maximum at an AuChi-to-liposome molar ratio of 300:1 at pH = 1.2, indicating the saturation of AuChi nanoparticles on liposome surfaces. Hydrodynamic size, size distribution, and surface charge of AuChi-liposome were characterized with DLS. To test the quenching effect at different pH values, the solution was adjusted to desired pH levels using HCl and NaOH and measured by an Orion 3-star plus portable pH meter. The fluorescence emission spectra of RhB in the range of 550–650 nm were measured by using a fluorescent spectrophotometer (Infinite M200, TECAN, Switzerland) at an excitation wavelength of 470 nm.
AuChi-liposome fusion with H. pylori bacteria
A fluorescence method was used to study the fusion of AuChi-liposome with bacteria at pH = 1.2 and 7.4, respectively. Specifically, H. pylori Sydney strain 1 (SS1) bacteria were maintained on Columbia agar supplemented with 5% laked horse blood at 37°C under microaerobic conditions (10% CO2, 85% N2, and 5% O2). For experiments, broth cultures of H. pylori were prepared by sub-culturing fresh colonies from agar plates into BHI containing 5% fetal bovine serum (FBS) overnight at 37°C under microaerobic conditions with moderate reciprocal shaking. Then, 5×108 CFU/mL of H. pylori bacteria (determined by OD600 measurement, OD600 = 1.0 corresponding to ~ 1×108 CFU/mL) were mixed with 0.5 mM fluorescently labeled AuChi-liposome (containing 0.5 mol% of DMPE-RhB). The solutions were adjusted to desired pH values. After 30 min incubation, the bacteria pellet was collected by centrifugation at 4,000 ×g for 5 min. After removing the supernatant, the bacteria were resuspended in 1 mL PBS (1X, 10 mM Na2HPO4, 2 mM KH2PO4, 2.7 mM KCl, and 137 mM NaCl) and the fluorescence intensity of DMPE-RhB was measured. Bare liposomes containing the same amount of DMPE-RhB were used as controls. The experiment was carried out in triplicate and average value was reported.
Drug release study
Doxycycline-loaded AuChi-liposome were prepared by mixing 1 mM of doxycycline with the rehydration solution during the preparation of liposomes. The unencapsulated doxycycline molecules were then removed by dialyzing AuChi-liposome suspension for 12 hr. Then the liposome suspension was adjusted to pH = 1.2 and 7.4, respectively The samples were loaded into multiple dialysis cups with 400 μL in each and dialyzed against PBS (1X) at 37°C up to 24 hr with a frequent change of buffer solution. At each time point, three dialysis cups were collected to quantify the remaining drugs inside the liposomes. Specifcially, the liposomes remained in the cup were disrupted by adding 1% Triton X-100 and the solution was filtered with an Amicon centrifugal filter unit with a molecular weight cutoff of 100 kDa (Millipore, Billerica, MA) at 7000 rpm for 10 min. Following the centrifugation, the amount of doxycycline in filtrate was quantified by measuring absorbance at 345 nm using spectrophotometer (Infinite M200, TECAN, Switzerland). The acquired doxycycline absorbance was compared with a linear standard curve of doxycycline at different concentrations at the desired pH to calculate the amount of doxycycline released from the liposomal formulations. Doxycycline-loaded AuChi-liposome at t=0 (prior to dialysis) was used to determine the initial drug loading yield.
Antibacterial activity of AuChi-liposome
An overnight broth culture of H. pylori was centrifuged at 4000 ×g for 5 min to obtain a bacterial pellet. The pellet was washed and adjusted to an OD600 value of 1.0, corresponding to approximately 1×108 CFU/mL. Then 10 μL bacterial suspension containing 1×106 CFU bacteria was added to 190 μL of bacteria culture medium containing doxycycline-loaded AuChi-liposome at desired concentrations. The mixture was incubated at pH = 7.4 with gentle shaking at 37°C under microaerobic conditions. After 12 hr incubation, a series of 10-fold dilutions of the bacterial suspension (1:10 to 1:105) was prepared, and 5 μL from each diluted sample was inoculated onto a Columbia agar plate supplemented with 5% laked horse blood. The plates were cultured in the incubator for 4 days before colony counting. Free doxycycline served as a positive control, while empty AuChi-liposome (without doxycycline) and PBS (1X) served as negative controls. All experiments were repeated three times.
Acknowledgments
This work is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R01DK095168
References
- 1.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 2.Huang CM, Chen CH, Pornpattananangkul D, Zhang L, Chan M, Hsieh MF, Zhang L. Eradication of drug resistant Staphylococcus aureus by liposomal oleic acids. Biomaterials. 2011;32:214–221. doi: 10.1016/j.biomaterials.2010.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang D, Pornpattananangkul D, Nakatsuji T, Chan M, Carson D, Huang CM, Zhang L. The antimicrobial activity of liposomal lauric acids against Propionibacterium acnes. Biomaterials. 2009;30:6035–6040. doi: 10.1016/j.biomaterials.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, Pornpattananangkul D, Hu CM, Huang CM. Development of nanoparticles for antimicrobial drug elivery. Curr Med Chem. 2010;17:585–594. doi: 10.2174/092986710790416290. [DOI] [PubMed] [Google Scholar]
- 5.Nakatani K, Morita T, Kimura S. Vertical and directional insertion of helical peptide into lipid bilayer membrane. Langmuir. 2007;23:7170–7177. doi: 10.1021/la7002723. [DOI] [PubMed] [Google Scholar]
- 6.Li YJ, Lu JH, Liu HQ, Qu N, Peng XJ, Teng LS. Liposome formulations with strengthened stability in storage and enhanced antimicrobial activity. J Invest Med. 2013;61:S15–S16. [Google Scholar]
- 7.Marrink S, Mark AE. The mechanism of vesicle fusion as revealed by molecular dynamics simulations. J Am Chem Soc. 2003;125:11144–11145. doi: 10.1021/ja036138+. [DOI] [PubMed] [Google Scholar]
- 8.Pornpattananangkul D, Zhang L, Olson S, Aryal S, Obonyo M, Vecchio K, Huang CM, Zhang L. Bacterial toxin-triggered drug release from gold nanoparticle-stabilized liposomes for the treatment of bacterial infection. J Am Chem Soc. 2011;133:4132–4139. doi: 10.1021/ja111110e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haluska CK, Riske KA, Marchi-Artzner V, Lehn JM, Lipowsky R, Dimova R. Time scales of membrane fusion revealed by direct imaging of vesicle fusion with high temporal resolution. Proc Natl Acad Sci USA. 2006;103:15841–15846. doi: 10.1073/pnas.0602766103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Edit. 2010;49:6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 11.Stepniewski M, Pasenkiewicz-Gierula M, Rog T, Danne R, Orlowski A, Karttunen M, Urtti A, Yliperttula M, Vuorimaa E, Bunker A. Study of PEGylated Lipid Layers as a Model for PEGylated Liposome Surfaces: Molecular Dynamics Simulation and Langmuir Monolayer Studies. Langmuir. 2011;27:7788–7798. doi: 10.1021/la200003n. [DOI] [PubMed] [Google Scholar]
- 12.Yan W, Huang L. Recent advances in liposome-based nanoparticles for antigen delivery. Polymer Rev. 2007;47:329–344. [Google Scholar]
- 13.Cao ZQ, Zhang L, Jiang SY. Superhydrophilic zwitterionic polymers stabilize liposomes. Langmuir. 2012;28:11625–11632. doi: 10.1021/la302433a. [DOI] [PubMed] [Google Scholar]
- 14.Jiang SY, Cao ZQ. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv Mater. 2010;22:920–932. doi: 10.1002/adma.200901407. [DOI] [PubMed] [Google Scholar]
- 15.Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 16.Woodle MC. Controlling liposome blood clearance by surface-grafted polymers. Adv Drug Deliv Rev. 1998;32:139–152. doi: 10.1016/s0169-409x(97)00136-1. [DOI] [PubMed] [Google Scholar]
- 17.Castro GA, Ferreira LA. Novel vesicular and particulate drug delivery systems for topical treatment of acne. Expert Opin Drug Deliv. 2008;5:665–679. doi: 10.1517/17425247.5.6.665. [DOI] [PubMed] [Google Scholar]
- 18.Pornpattananangkul D, Olson S, Aryal S, Sartor M, Huang CM, Vecchio K, Zhang L. Stimuli-responsive liposome fusion mediated by gold nanoparticles. ACS Nano. 2010;4:1935–1942. doi: 10.1021/nn9018587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinico C, Fadda AM. Vesicular carriers for dermal drug delivery. Expert Opin Drug Deliv. 2009;6:813–825. doi: 10.1517/17425240903071029. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Granick S. How to stabilize phospholipid liposomes (using nanoparticles) Nano Lett. 2006;6:694–698. doi: 10.1021/nl052455y. [DOI] [PubMed] [Google Scholar]
- 21.Michel R, Plostica T, Abezgauz L, Danino D, Gradzielski M. Control of the stability and structure of liposomes by means of nanoparticles. Soft Matter. 2013;9:4167–4177. [Google Scholar]
- 22.Wang B, Zhang L, Bae SC, Granick S. Nanoparticle-induced surface reconstruction of phospholipid membranes. Proc Natl Acad Sci USA. 2008;105:18171–18175. doi: 10.1073/pnas.0807296105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obonyo M, Zhang L, Thamphiwatana S, Pornpattananangkul D, Fu V, Zhang L. Antibacterial activities of liposomal linolenic acids against antibiotic-resistant Helicobacter pylori. Mol Pharm. 2012;9:2677–2685. doi: 10.1021/mp300243w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 25.Suerbaum S, Michetti P. Helicobacter pylori infection. New Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 26.Furuta T, Graham DY. Pharmacologic aspects of eradication therapy for Helicobacter pylori Infection. Gastroenterol Clin North Am. 2010;39:465–480. doi: 10.1016/j.gtc.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–1153. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 28.Ito T, Kobayashi D, Uchida K, Takemura T, Nagaoka S, Kobayashi I, Yokoyama T, Ishige I, Ishige Y, Ishida N, Furukawa A, Muraoka H, Ikeda S, Sekine M, Ando N, Suzuki Y, Yamada T, Suzuki T, Eishi Y. Helicobacter pylori invades the gastric mucosa and translocates to the gastric lymph nodes. Lab Invest. 2008;88:664–681. doi: 10.1038/labinvest.2008.33. [DOI] [PubMed] [Google Scholar]
- 29.Necchi V, Candusso ME, Tava F, Luinetti O, Ventura U, Fiocca R, Ricci V, Solcia E. Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori. Gastroenterology. 2007;132:1009–1023. doi: 10.1053/j.gastro.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 30.Lin YH, Chang CH, Wu YS, Hsu YM, Chiou SF, Chen YJ. Development of pH-responsive chitosan/heparin nanoparticles for stomach-specific anti-Helicobacter pylori therapy. Biomaterials. 2009;30:3332–3342. doi: 10.1016/j.biomaterials.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 31.Tocanne JF, Teissie J. Ionization of phospholipids and phospholipid-supported interfacial lateral diffusion of protons in membrane model systems. Biochim Biophys Acta. 1990;1031:111–142. doi: 10.1016/0304-4157(90)90005-w. [DOI] [PubMed] [Google Scholar]
- 32.Hafez IM, Ansell S, Cullis PR. Tunable pH-sensitive liposomes composed of mixtures of cationic and anionic lipids. Biophys J. 2000;79:1438–1446. doi: 10.1016/S0006-3495(00)76395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aryal S, BKCR, Dharmaraj N, Bhattarai N, Kim CH, Kim HY. Spectroscopic identification of S-Au interaction in cysteine capped gold nanoparticles. Spectrochim Acta A. 2006;63:160–163. doi: 10.1016/j.saa.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 34.Mayer LD, Hope MJ, Cullis PR. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim Biophys Acta. 1986;858:161–168. doi: 10.1016/0005-2736(86)90302-0. [DOI] [PubMed] [Google Scholar]
- 35.Gao W, Hu CM, Fang RH, Luk BT, Su Jing, Zhang L. Surface functionalization of gold nanoparticles with red blood cell membranes. Adv Mater. 2013;25:3549–3553. doi: 10.1002/adma.201300638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raikar US, Tangod VB, Mastiholi BM, Fulari VJ. Fluorescence quenching using plasmonic gold nanoparticles. Opt Commun. 2011;284:4761–4765. [Google Scholar]
- 37.Huh AJ, Kwon YJ. Nanoantibiotics: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J Control Release. 2011;156:128–145. doi: 10.1016/j.jconrel.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Liu M, Gan L, Chen L, Xu Z, Zhu D, Hao Z, Chen L. Supramolecular core–shell nanosilica@liposome nanocapsules for drug delivery. Langmuir. 2012;28:10725–10732. doi: 10.1021/la3021645. [DOI] [PubMed] [Google Scholar]