Abstract
Treatment with therapeutic proteins is an attractive approach to targeting a number of challenging diseases. Unfortunately, the native proteins themselves are often unstable in physiological conditions, reducing bioavailability and therefore increasing the dose that is required. Conjugation with poly(ethylene glycol) (PEG) is often used to increase stability, but this has a detrimental effect on bioactivity. Here, we introduce conjugation with zwitterionic polymers such as poly(carboxybetaine). We show that poly(carboxybetaine) conjugation improves stability in a manner similar to PEGylation, but that the new conjugates retain or even improve the binding affinity as a result of enhanced protein–substrate hydrophobic interactions. This chemistry opens a new avenue for the development of protein therapeutics by avoiding the need to compromise between stability and affinity.
For decades, the attachment of poly(ethylene glycol) (PEG)—known as PEGylation—has been the benchmark for achieving protein stabilization and increased body circulation times1,2. Although greatly beneficial as a protein stabilizer, PEGylation is also known to diminish binding affinity, thereby reducing overall bioactivity3,4. Efforts have been made to control the location of PEG attachment so as to minimize this effect5, but the material problem has remained unresolved for the last 30 years. For example, the activity of marketed PEGylated interferon-α2a (Pegasys, Roche) has been seen to drop to 7% compared to its native form6. When PEG is attached to monoclonal antibodies, large reductions in activity are found7, and the effect is also observed for PEGylated enzymes8. For this reason, the current pool of available therapeutic proteins has been limited to either humanized proteins, or proteins with small-molecule substrates that are able to resist inhibition from PEGylation, such as Pegaspargase (Oncaspar) and Pegademase Bovine (Adagen)6. These are just a few of many examples.
Over the last several years, we have demonstrated how poly(zwitterions) act as super non-fouling surfaces that prevent protein adsorption from complex biological media9. We have also shown how poly(zwitterions) such as poly(carboxybetaine) (pCB) outperform PEG in terms of resistance to non-specific protein adsorption from blood serum and plasma10. This is noteworthy given that PEG has been universally used as a highly resistant non-fouling surface in many applications11,12. PEG obtains its non-fouling surface characteristics from its ability to bind large amounts of water by means of hydrogen bonding among its many ether groups. PEG therefore takes on a ballooning-type conformation that increases solution viscosity and acts as a water barrier that prevents proteins from approaching the surface13. PEG, although able to form many bonds with water, is also known to have amphiphilic character, being soluble in both polar and nonpolar organic solvents (such as toluene and dichloromethane). Zwitterions, on the other hand, are polyelectrolytes that have both positively and negatively charged groups, but have an overall neutral charge. Poly(zwitterions) obtain their non-fouling characteristics through strong ionic structuring of water, which creates a super-hydrophilic surface14. The properties through which ions such as those found with pCB affect water structure are described in what is referred to as the Hofmeister series15.
Results and discussion
In this work, pCB was conjugated to a model protein to demonstrate its stabilizing properties, and PEG was used for comparison. Ideally, PEG and pCB polymers of similar size would be chosen. However, poly(zwitterions) are known to have strong intramolecular interactions through ionic binding, and this gives pCB a higher density in solution than predicted. This is referred to as the anti-polyelectrolyte effect16. In contrast, PEG, which binds to a large number of water molecules, has an artificially larger size in solution than would be expected based on its molecular weight. For this reason, two differently sized pCB polymers were synthesized for comparison with PEG: pCB Mn (equivalent molecular weight to 5 kDa PEG, determined by NMR) and pCB Rh (equivalent hydrodynamic size to 5 kDa PEG determined by size exclusion chromatography, SEC) (Supplementary Table S1). Details of synthesis and conjugation can be found in Supplementary Fig. S1. The well studied and characterized enzyme α-chymotrypsin (CT) was chosen as the model protein. The second parameter adjusted, after polymer size, was the number of polymers per protein. This parameter was varied by adjusting the feed ratio to give three degrees of conjugation for each polymer conjugate. The degree of modification (number of polymers per CT protein) was determined by an amine sensitive assay. Conjugation was also confirmed from the SEC chromatograms (Supplementary Fig. S2).
To stress the stability of the prepared conjugates, activities were tested in 5 M urea (Fig. 1a). Values are represented as percentages relative to their activities before urea was added. As anticipated, PEG was seen to provide a stabilizing effect that correlated with increasing degree of conjugation, with the least conjugated showing no improvement over the bare enzyme. An increase in stability was also observed for the pCB conjugates, but with little dependence on polymer size or number of polymers; all conjugates had a stability that was comparable to the most stable PEG conjugate. A thermal stability test was also performed, measuring the effect of temperature on enzyme activity. It was observed that both pCB Mn and PEG conjugates had stabilizing effects at elevated temperatures, resisting a loss in activity compared to the unconjugated enzyme (Fig. 1b). Even though PEG is a known thermal stabilizing agent17, pCB Mn was seen to provide increased stability when compared to PEG conjugates with a comparable number of polymers/protein. Figure 1c shows that pCB Rh conjugates provided a significant improvement over the PEG samples. These results indicate that pCB has a strong stabilizing effect against chemical and thermal denaturation. It should be noted that, although both polymers have stabilizing properties, the proposed mechanisms by which they function are very different. PEG, as previously mentioned, acts as a protective barrier around the protein. This phenomenon results in an increased local viscosity, thereby reducing the structural dynamics of the protein, making it more difficult to unfold18. pCB, on the other hand, stabilizes globular proteins by strengthening the hydrophobic interactions holding the protein together by reducing the interaction of water with the surface19. By reducing the degree to which water can interfere with the structure of the protein, the conjugates become less likely to unfold. These different mechanisms become important when evaluating their effect on binding affinity.
Figure 1. Stability of PEG, pCB Mn (similar molecular weight) and pCB Rh (similar hydrodynamic size) conjugated with enzyme CT.
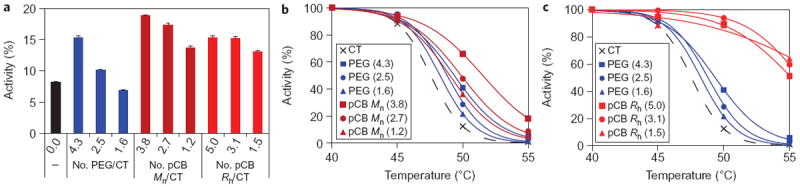
a, Activity of conjugates after 8 h incubation in 5 M urea. Values are calculated as percentages of conjugate activity in buffer without urea. Error bars represent standard deviation. For each polymer tested, a trend is observed in which conjugates with a higher degree of conjugation show increased stability. PEG and pCB polymers both showed stabilizing effects, but pCB to a high degree. b,c, Relative activity of PEG and pCB Mn conjugates (b) and PEG and pCB Rh conjugates (c) after 10 min incubation at different temperatures. The optimum operating temperature of the enzyme is known to be 40 °C. Here, a trend is also observed in which conjugates with a higher degree of conjugation show increased stability. The larger pCB polymers (pCB Rh) show significant stabilizing characteristics.
The affinities of the prepared conjugates were determined by measuring their Michaelis constants, Km. N-Succinyl-Ala-Ala-Pro-Phe p-nitroanilide was used as a substrate. This is a peptide-based substrate with an explicit binding region for cleavage by CT. This system was chosen because substrates of this size are affected by polymer inhibition8. Small-molecule substrates are known to have high affinities (small values of Km) as a result of their high diffusivities. As expected, PEG conjugates were seen to have a decreasing affinity for the peptide substrate as the number of polymers per protein increased (Fig. 2a). Lower affinities are indicated by an increase in Km, where Km is the concentration of substrate required to achieve 50% full activity (kcat). For pCB conjugates of both polymer sizes, substrate inhibition was seen to be absent. Most notably, for the larger pCB Rh conjugates, a negative correlation was observed between the amount of conjugated pCB and Km. This suggested that the addition of pCB polymer assisted in binding.
Figure 2. Affinities of prepared conjugates measured as Michaelis constants Km.

Error bars are values of standard error (s.e.m.). a, Km of prepared conjugates to the large peptide substrate. Km represents the concentration of substrate required to achieve 50% maximum activity. A decrease in Km represents an increase in binding affinity. The increase in Km with an increase in the number of PEG polymers per protein shows the inhibitory effects of PEG. In contrast, pCB Mn conjugates (similar molecular weight) showed no significant effect on binding affinity, whereas pCB Rh conjugates (similar hydrodynamic size) showed an increase in substrate affinity. b, Km values of native enzymes for the large peptide substrate in the presence of 650 Mn PEG and ammonium acetate solutions. These data support the effects observed with analogous polymers with the same chemistry. c, Km values of prepared conjugates for the small-molecule substrate. This supports the claim that Km is dependent on substrate size.
A general mechanism for molecular binding has two species with higher solubility for each other than their environment (Fig. 3a). For proteins, hydrophobic–hydrophobic interactions are significant20. Attaching polymers such as PEG to the protein surface imposes steric hindrances as well as competitive interactions with the substrate and binding site, thereby reducing the binding affinity (Fig. 3b). With PEG, amphiphilicity reduces the hydrophobic–hydrophobic driving force of the substrate–binding site interaction while also imposing a steric hindrance. In contrast, with the pCB conjugates, the binding affinity was either unaltered or improved. Owing to the super-hydrophilicity of the polymer, water is drawn away from hydrophobic regions of the protein, shifting the equilibrium to allow the substrate and binding site to interact (Fig. 3c). To support this model, the solubility of the peptide substrate was measured in solutions of PEG and pCB polymers. This was carried out by measuring the partitioning of the substrate molecule into free polymer solutions of PEG and pCB through a semi-permeable membrane (Supplementary Fig. S3a). These results showed that the substrate had higher solubility in PEG, and lower solubility in pCB compared to the buffer control (Supplementary Fig. S3b). These results support how the amphiphilic PEG polymer can interfere with substrate binding, and also how the super-hydrophilicity of the pCB polymer has low affinity for hydrophobic materials.
Figure 3. Mechanism of how PEG and pCB polymers influence binding affinity.
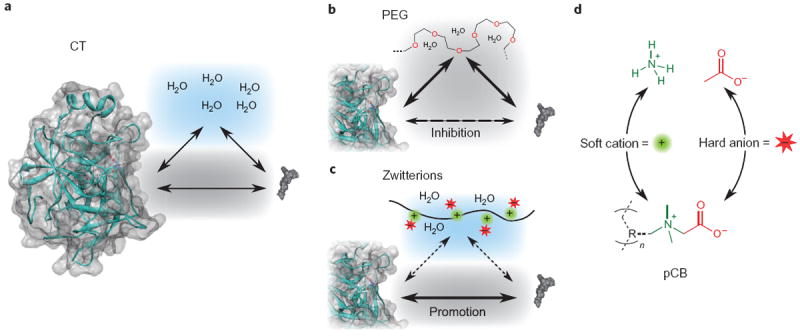
a, Relationship between enzyme and substrate without polymer. b, PEG impedes affinity by reducing enzyme–substrate hydrophobic–hydrophobic interactions as a result of its amphiphilic characteristics. c, Super-hydrophilic pCB has a strong effect on the structure of water, creating a local environment that increases enzyme–substrate hydrophobic–hydrophobic interactions, thereby increasing the substrate’s affinity for the binding pocket. d, The structural relationship between pCB and ammonium acetate, both of which contain protein-stabilizing ions found in the Hofmeister series. The R group represents a methacrylate backbone.
To confirm that the changes in affinity originated from differences in the local hydrophilicity, Km values of the bare enzyme were measured in solutions of ammonium acetate (NH4OAc) salt and 650 Mn PEG. The same improvement in binding affinity was observed with ammonium acetate as with the pCB conjugates (Fig. 2b). The effect of adding 650 Mn PEG into the solution was also seen to correlate with the observations for the conjugated PEG form—leading to significant losses in affinity. This provides further evidence that the results of enhanced affinity obtained for the pCB conjugates originated from the ionic characteristics of the polymer. Ions, and their effects on protein stability and activity, follow a principle referred to as the Hofmeister series (Supplementary Fig. S4). The trend seen in the series correlates greater enzyme performance (stability, activity and affinity) with high surface charge density (kosmotropic) anions and low surface charge density (chaotropic) cations. These are referred to as hard anions and soft cations, respectively. This effect has been shown for several enzyme and substrate pairs, demonstrating trends of increasing values of kcat and decreasing values of Km and resulting in overall greater activities21, and also for non-enzyme-based binding22. Unfortunately, this type of stabilization and increased activity, achieved by altering the environment with salts, is lost when considering applications in vivo. However, the phenomenon can be retained by covalently attaching a zwitterionic polymer such as pCB to the protein surface. The monomer unit itself is a derivative of glycine betaine, which is a natural molecule found in many living systems as a stabilizing agent. On examining the molecular structure of pCB (Fig. 3d), the betaine monomer is seen to correspond to the most stabilizing and active monovalent ions in the series, ammonium and acetate, thereby explaining the mechanism by which pCB enhances binding affinity (Fig. 3c).
To confirm that the observed effects of the polymer conjugates originated from the attached polymer, and to show the sensitivity of substrate size for polymer inhibition, a small-molecule control substrate was also evaluated (resorufin bromoacetate, Fig. 2c). It can be seen that, compared to the larger peptide-based substrate, the presence of conjugated polymer had no observable effect on affinity. The accessibility of the active site by the control substrate can be seen by noting the overall lower values of Km relative to the larger peptide-based substrate. Values of kcat were also calculated for all kinetic experiments and can be seen in Supplementary Fig. S5. For most conjugates there was no significant effect, but pCB Rh conjugates had an improved activity (kcat) at higher degrees of conjugation.
Conclusions
It has been demonstrated in this work that zwitterionic pCB polymers are able to maintain the stability of proteins without sacrificing binding affinity, a combination never achieved before. This finding represents a major improvement over the current PEGylation technique, which maintains the stability of proteins at the expense of their binding affinity. Zwitterionic pCB polymers derive their unique properties by incorporating the most stabilizing and bio-active monovalent ions (as defined in the Hofmeister series), resulting in pCB both strengthening the stability of the protein folded structure while also encouraging specific binding at the hydrophobic binding site. The mechanism is believed to mimic the action of protein stabilizing ions (kosmotropic anions and chaotropic cations). This work represents a new avenue for protein therapeutics in that it provides accessibility to a pool of potential protein therapeutics that was originally thought impossible. Other limitations of PEG have been addressed, including oxidative degradation, nonbiodegradability and the toxicity of side products23. These are issues beyond what is seen with proteins, suggesting the potential for zwitterionic pCB polymers to be used with nanoparticles24. Furthermore, the acting monomer unit of pCB is composed of the naturally occurring glycine betaine. Thus, it is expected that zwitterionic polymers can substitute the current benchmark, PEG, and take a key role in the future of protein therapeutics.
Methods
Materials
The following were used: CT from bovine pancreas type II, lyophilized powder, ≥40 units/mg protein (Sigma), copper(I) bromide (CuBr, 99.999%, Aldrich), copper(II) bromide (CuBr2, 99.999%, Aldrich), 1,1,4,7,10,10-hexamethyltriethylenetetramine (HMTETA 97%, Aldrich), N-succinyl-Ala-Ala-Pro-Phe p-nitroanilide (Sigma) and methoxy PEG succinimidyl carboxymethyl ester, Mw = 5,000 (Jenkem Technology, China).
N-Hydroxysuccinimide terminated poly(carboxybetaine methacrylate) polymer synthesis
Synthesis of the N-hydroxysuccinimide (NHS) ATRP initiator24 and poly(carboxybetaine) methacrylate with tert-butyl protected ester (CBMA-1-t-But)23 were performed as described previously. Atom transfer radical polymerization was carried out in anhydrous dimethylformamide (DMF) using a Cu(I)Br/HMTETA catalyst (Supplementary Fig. S1a). In a typical polymerization, DMF and HMTETA were separately purged of oxygen by bubbling with nitrogen. CBMA-1-t-But (1 g, 3.67 mmol) monomer and 125 mg (0.5 mmol) of NHS-initiator were added to a Schlenk tube. To a second Schlenk tube was added 71.7 mg (0.5 mmol) of Cu(I)Br. Both tubes were deoxygenated by cycling between nitrogen and vacuum three times. Deoxygenated DMF in volumes of 8 and 2 ml was added to the monomer/initiator and Cu(I)Br tubes, respectively. Deoxygenated HMTETA (136 μl, 0.5 mmol) was added to the Cu(I)Br-containing solution and was stirred for 30 min under nitrogen. The catalyst solution (Cu(I)/HMTETA) was then added to the monomer/initiator solution to start the reaction. The reaction was run to completion at room temperature while being monitored using NMR. After polymerization, the reaction was fully precipitated in ethyl ether. The precipitate was then dried under vacuum and redissolved in minimal DMF (3–5 ml). The solution was reprecipitated in anhydrous acetone to remove the soluble catalyst and trace monomer. This was repeated a total of three times to fully remove the catalyst. The remaining ester polymer was dried overnight under vacuum and analysed by NMR. To hydrolyze the tert-butal group, 500 mg NHS-pCBMA-1-t-But was dissolved in 5 ml trifluoroacetic acid (Supplementary Fig. S1b). This was allowed to sit for 2 h. The solution was then precipitated in ethyl ether, dried overnight under vacuum and subsequently analysed by NMR (Mw = 4.2 kDa) and gel permeation chromatography (Mw = 2.5 kDa, polydispersion index = 1.05).
CT polymer conjugation and purification (Supplementary Fig. S1c)
CT conjugates were prepared in 200 mM HEPES buffer (pH 8.5). The α-chymotrypsin concentration was fixed at 4 mg ml−1, and the polymer solutions were 4, 8 and 16 mg ml−1. This was done for all three polymers: PEG, pCB Mn and pCB Rh. Solutions were mixed with a stir bar for 30 min, then incubated at 4 °C for 3 h. The conjugates were purified and concentrated by ultrafiltration using a 30 kDa molecular weight cutoff membrane. The pCB Rh conjugates were further purified using a size exclusion column with a Superdex 200 10/300 column. These samples were then re-concentrated using ultrafiltration. The number of polymers per protein for each sample was determined using a trinitrobenzene sulfonate (TNBS) assay to determine the number of unreacted surface amines, together with area under the curve of SEC chromatograms.
Urea stability
Conjugates were incubated in 0.1 M Tris at pH 8.0 for 8 h at 0.5 μg ml−1, with and without 5 M urea. Measurements were performed in triplicate. Solutions were also incubated with 0.1 mg ml−1 bovine serum albumin (BSA) as a blocking agent to prevent depletion of enzyme from surface adsorption. After 8.0 h, activities were measured for all samples by adding N-succinyl-Ala-Ala-Pro-Phe p-nitroanilide at 667 μg ml−1. Activities were measured as the increase in absorbance at 412 nm with time. Values were recorded as percentage of activity with urea, relative to without urea.
Thermal stability
Conjugates were incubated in 0.1 M Tris, pH 8.0, at temperatures of 40, 45, 50 and 55 °C and a concentration of 5.0 μg ml−1. After 10 min at each temperature, each conjugate was diluted into refrigerated buffer to a concentration of 0.5 μg ml−1. Solutions were also incubated with 0.1 mg ml−1 BSA as a blocking agent. Activities were measured for all samples by adding N-succinyl-Ala-Ala-Pro-Phe p-nitroanilide to a final concentration of 667 μg ml−1. Activities were measured as the increase in absorbance at 412 nm with time. Values were recorded as percentage of each conjugate’s activity at 40 °C.
Conjugate kinetics with N-succinyl-Ala-Ala-Pro-Phe p-nitroanilide
Conjugates were incubated at 20 pM in 0.1 M Tris, pH 8.0 with 0.1 mg ml−1 BSA as a blocking agent. Low enzyme concentrations were required to achieve accurate activity measurements at low substrate concentrations. N-Succinyl-Ala-Ala-Pro-Phe p-nitroanilide was added at varying concentrations from 0 to 667 μg ml−1 (0–1,070 μM). Concentrations of each substrate were prepared in triplicate and activity was measured as an increase in absorbance with time at 412 nm.
Conjugate kinetics with resorufin bromoacetate
Conjugates were incubated at 20 pM in 0.1 M MES, pH 6.0, with 0.1 mg ml−1 BSA as a blocking agent. A lower pH was required to retain the stability of the base-labile substrate. Resorufin bromoacetate was added at varying concentrations from 0 to 50 μg ml−1 (0–150 μM). Concentrations of each substrate were prepared in triplicate and activity was measured as an increase in absorbance with time at 571 nm.
Evaluation of polymer–substrate solubility
Dialysis containers containing either buffer (control), 5 mg ml−1 PEG or 5 mg ml−1 pCB were exposed to a large bath in excess volume containing 2.5 μg ml−1 of peptide substrate (N-succinyl-Ala-Ala-Pro-Phe p-nitroanilide). A dialysis membrane separated the polymers from the substrate, which allowed the substrate to pass freely, but not to the polymers. After 3 days, the sample solutions were measured by high-performance liquid chromatography for peptide concentration.
Supplementary Material
Acknowledgments
This work was supported by the Office of Naval Research (N000140910137 and N000141010600) and the National Science Foundation (DMR-1005699). A.J.K. was partially supported through a National Cancer Institute training grant (T32CA138312). The authors thank the members of the Jiang research group, especially Zhiqiang Cao and Hong Xue, for helpful discussions.
Footnotes
Author contributions
A.J.K. performed all the experiments presented in this work. A.J.K. and S.J. designed the experiments and prepared the manuscript.
Supplementary information accompanies this paper at www.nature.com/naturechemistry.
The authors declare no competing financial interests.
References
- 1.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nature Rev Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 2.Jevsevar S, Kunstelj M, Porekar VG. PEGylation of therapeutic proteins. Biotechnol J. 2010;5:113–128. doi: 10.1002/biot.200900218. [DOI] [PubMed] [Google Scholar]
- 3.Fishburn CS. The pharmacology of PEGylation: balancing PD with PK to generate novel therapeutics. J Pharm Sci. 2008;97:4167–4183. doi: 10.1002/jps.21278. [DOI] [PubMed] [Google Scholar]
- 4.Veronese FM. Peptide and protein PEGylation: a review of problems and solutions. Biomaterials. 2001;22:405–417. doi: 10.1016/s0142-9612(00)00193-9. [DOI] [PubMed] [Google Scholar]
- 5.Shaunak S, et al. Site-specific PEGylation of native disulfide bonds in therapeutic proteins. Nature Chem Biol. 2006;2:312–313. doi: 10.1038/nchembio786. [DOI] [PubMed] [Google Scholar]
- 6.Veronese FM, Mero A. The impact of PEGylation on biological therapies. Biodrugs. 2008;22:315–329. doi: 10.2165/00063030-200822050-00004. [DOI] [PubMed] [Google Scholar]
- 7.Chapman AP. PEGylated antibodies and antibody fragments for improved therapy: a review. Adv Drug Deliv Rev. 2002;54:531–545. doi: 10.1016/s0169-409x(02)00026-1. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Martinez JA, Rivera-Rivera I, Sola RJ, Griebenow K. Enzymatic activity and thermal stability of PEG-alpha-chymotrypsin conjugates. Biotechnol Lett. 2009;31:883–887. doi: 10.1007/s10529-009-9947-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang SY, Cao ZQ. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv Mater. 2010;22:920–932. doi: 10.1002/adma.200901407. [DOI] [PubMed] [Google Scholar]
- 10.Yang W, Zhang L, Wang SL, White AD, Jiang SY. Functionalizable and ultra stable nanoparticles coated with zwitterionic poly(carboxybetaine) in undiluted blood serum. Biomaterials. 2009;30:5617–5621. doi: 10.1016/j.biomaterials.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 11.Gref R, et al. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 12.Prime KL, Whitesides GM. Self-assembled organic monolayers—model systems for studying adsorption of proteins at surfaces. Science. 1991;252:1164–1167. doi: 10.1126/science.252.5009.1164. [DOI] [PubMed] [Google Scholar]
- 13.Szleifer I. Polymers and proteins: interactions at interfaces. Curr Opin Solid State Mater Sci. 1997;2:337–344. [Google Scholar]
- 14.Kane RS, Deschatelets P, Whitesides GM. Kosmotropes form the basis of protein-resistant surfaces. Langmuir. 2003;19:2388–2391. [Google Scholar]
- 15.Cacace MG, Landau EM, Ramsden JJ. The Hofmeister series: salt and solvent effects on interfacial phenomena. Q Rev Biophys. 1997;30:241–277. doi: 10.1017/s0033583597003363. [DOI] [PubMed] [Google Scholar]
- 16.Georgiev GS, et al. Self-assembly, anti polyelectrolyte effect, and nonbiofouling properties of polyzwitterions. Biomacromolecules. 2006;7:1329–1334. doi: 10.1021/bm050938q. [DOI] [PubMed] [Google Scholar]
- 17.Treethammathurot B, Ovartlarnporn C, Wungsintaweekul J, Duncan R, Wiwattanapatapee R. Effect of PEG molecular weight and linking chemistry on the biological activity and thermal stability of PEGylated trypsin. Int J Pharm. 2008;357:252–259. doi: 10.1016/j.ijpharm.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Martinez JA, et al. Stabilization of alpha-chymotrypsin upon PEGylation correlates with reduced structural dynamics. Biotechnol Bioeng. 2008;101:1142–1149. doi: 10.1002/bit.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pegram LM, Record MT. Hofmeister salt effects on surface tension arise from partitioning of anions and cations between bulk water and the air–water interface. J Phys Chem B. 2007;111:5411–5417. doi: 10.1021/jp070245z. [DOI] [PubMed] [Google Scholar]
- 20.Lo Conte L, Chothia C, Janin J. The atomic structure of protein–protein recognition sites. J Mol Biol. 1999;285:2177–2198. doi: 10.1006/jmbi.1998.2439. [DOI] [PubMed] [Google Scholar]
- 21.Zhao H. Effect of ions and other compatible solutes on enzyme activity, and its implication for biocatalysis using ionic liquids. J Mol Catal B. 2005;37:16–25. [Google Scholar]
- 22.Ngo TT, Narinesingh D. Kosmotropes enhance the yield of antibody purified by affinity chromatography using immobilized bacterial immunoglobulin binding proteins. J Immunoassay Immunochem. 2008;29:105–115. doi: 10.1080/15321810701735203. [DOI] [PubMed] [Google Scholar]
- 23.Schubert US, Knop K, Hoogenboom R, Fischer D. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed. 2010;49:6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 24.Cao ZQ, Yu QM, Xue H, Cheng G, Jiang SY. Nanoparticles for drug delivery prepared from amphiphilic PLGA zwitterionic block copolymers with sharp contrast in polarity between two blocks. Angew Chem Int Ed. 2010;49:3771–3776. doi: 10.1002/anie.200907079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


