Abstract
Recognition of non-self molecular patterns by pattern recognition receptors is a cornerstone of innate immunity. Toll-like receptors (TLRs) exert a key role in recognizing pathogen-associated molecular patterns (PAMPs) but have also been implicated in the recognition of damage-associated molecular patterns (DAMPs). As such, TLRs regulate a wide range of biological responses including inflammatory and immune responses during carcinogenesis. The high expression of TLRs by antigen-presenting cells, including dendritic cells, and their ability to induce anti-tumor mediators such as type I interferon has led to efforts to utilize TLR agonists in tumor therapy in order to convert the often tolerant immune response towards anti-tumor responses. However, TLRs are also increasingly recognized as regulators of tumor-promoting inflammation and promoters of tumor survival signals. Here, we will review in detail the dichotomous role of TLRs in tumor biology, focusing on relevant TLR-dependent pro- and anti-tumor pathways, and discuss clinical applications of TLR-targeted therapies for tumor prevention and treatment.
Keywords: MyD88, interleukin 6, tumor necrosis factor, NF-κB, Coley’s toxin, Imiquimod
Introduction
150 years after Virchow’s postulate, the link between inflammation and cancer has been firmly established by epidemiologic investigations and animal studies 1,2. 18% of human cancers are attributable to infection, and chronic inflammation triggered by infection is an important contributor to carcinogenesis 3,4. In addition, injury may trigger local inflammation 5, and obesity can contribute to a general hyperinflammatory state 6. While many tumors arise in the setting of inflammation, it is present in virtually all established tumors. Although inflammation is controlled by numerous mediators and pathways, several systems function as key upstream regulators. Among these are pattern recognition receptors (PRR) that sense danger by detecting non-self or altered molecular patterns. Toll-like receptors (TLRs) are the best-characterized PRRs for the detection of pathogen-associated molecular patterns (PAMPs). PAMP-induced TLR activation triggers a profound activation of multiple proinflammatory and anti-viral signaling cascades to achieve pathogen elimination. However, if pathogens cannot be eliminated, they may elicit chronic inflammation, which may also in part be mediated through TLRs 7,8. The important contribution of inflammation to carcinogenesis is best exemplified by high rates of colorectal cancer in inflammatory bowel disease, high rates of gastric cancer in patients infected by H. pylori, and high rates of hepatocellular carcinoma in patients with chronic hepatitis 1. Accordingly, TLR-induced inflammation may act as a promoter of carcinogenesis, with recent studies showing strong promotion of carcinogenesis in the colon, liver, stomach and pancreas by TLRs 9–13. In addition to PAMP-induced TLR activation, inflammation and injury also induce the release of endogenous molecules termed damage-associated molecular patterns (DAMPs), some of which act as ligands for TLRs 5. Notably, signals triggered by DAMPs may promote carcinogenesis in a TLR-dependent manner 14. On the other hand, inflammatory responses may not only provide tumor-promoting signals but also contribute to anti-tumor immune responses 15,16. After decades of debate, cancer immunosurveillance has been convincingly proven, as demonstrated by increased tumor rates in RAG2−/− mice or mice that lack IFNγ signaling 17. However, we still do not know how well and in what type of tumors this system works. Based on increased immunogenicity of tumor transplants from immunosuppressed mice, it has been suggested that this system contributes to tumor elimination in early stages, but that escape mechanisms develop, resulting in long-term failure 16. One interesting aspect linking bacterial PAMPs and anti-tumor responses are historic observations, made by Busch, Bruns and Coley, that bacterial infections such as erysipelas or injection of heat-killed Streptococcus pyogenes and Serratia marcescens (“Coley’s toxin”) may lead to tumor regression and sometimes even cure 18–21. Although the mechanisms behind these historic studies remain obscure, recent advances in TLR biology and clinical studies with TLR agonists have suggested that TLR activation may indeed represent a relevant anti-tumor pathway, allowing to convert immune tolerance to anti-tumor immune responses 22–25. Here, we will briefly review TLR signaling, before discussing the dichotomous role of TLRs in tumor biology, with a particular focus on mechanisms by which TLRs may promote or inhibit cancer. Finally, we will highlight potential applications of TLR-targeting drugs for tumor prevention and therapy.
1. TLRs and TLR signaling
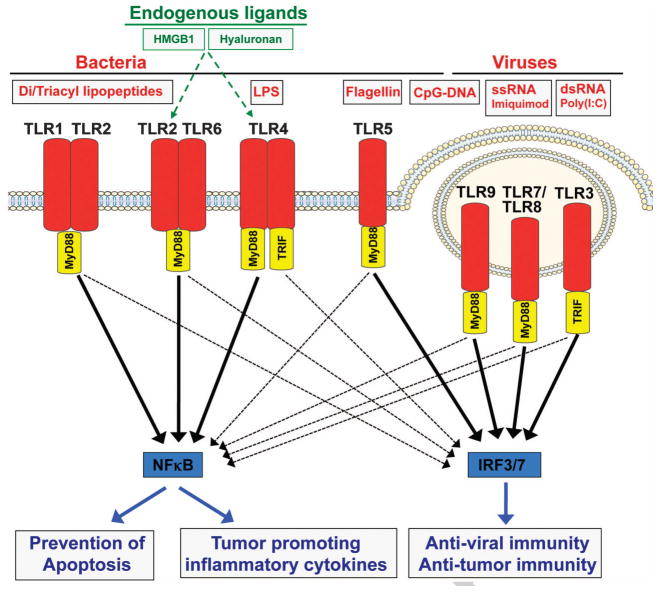
TLR signaling has been reviewed in detail elsewhere 26–28, and we will only summarize key concepts. TLRs are PRRs that recognize a wide range of bacterial, viral and fungal PAMPs, as well as endogenous DAMPs such as high mobility group box 1 (HMGB1). Although individual TLRs recognize distinct ligands, the mechanisms of TLR activation and signal transduction are highly conserved (see Fig. 1). Ligand binding occurs via leucine-rich repeats (LRRs) of extracellular TLR domains and triggers signal transduction pathways through interaction of intracellular Toll/interleukin-1 receptor (TIR) domains with conserved adaptor molecules. Most TLRs signal through the adapter molecule MyD88. Only TLR3 and TLR4 signal through a MyD88-independent pathway that relies on the adapter molecule Trif. TLR4 is the only receptor that signals through MyD88 and Trif. The MyD88-dependent and MyD88-independent pathways activate multiple proinflammatory signaling cascades including NF-κB, JNK/AP1, ERK and p38, and as well as the interferon pathway 26–28.
Figure 1. Toll-like receptor signaling.
TLRs bind bacterial and viral pathogen-associated molecular patterns (PAMPs), leading to the activation of proinflammatory and anti-viral signaling pathways including NF-κB and IRF3/IRF7, respectively. Activation of these pathways is mediated by two key adaptor molecules MyD88 and Trif. Key biological effects of NF-κB activation include prevention of apoptosis and increased inflammation. Type I interferon induces anti-viral and anti-tumor immunity.
2. Tumor promoting actions of TLRs
TLRs may promote carcinogenesis through proinflammatory, anti-apopototic, proliferative and profibrogenic signals in either the tumor microenvironment (TME) or tumor cells themselves, as described below. These effects can be either induced directly in TLR-expressing target cells, or mediated by TLR-induced cytokines.
2.1. TLR-mediated inflammation
TLRs are key regulators of inflammatory signaling, mediated by MyD88-dependent and MyD88-independent pathways. One important tumor-promoting signaling pathway induced by TLR signaling is the transcription factor NF-κB. NF-κB is a master switch of inflammation regulating the transcription of more than 100 proinflammatory genes 29, and is closely related to the avian viral oncoprotein v-Rel 30. TLR signaling upregulates well-known tumor-promoting inflammatory cytokines through NF-κB-dependent pathways, including IL-1β, TNFα and IL-6 31–33. These cytokines promote cancers in the intestine, liver, stomach and skin 34–40. TLR2-mediated inflammatory signals in macrophages, triggered by tumor-derived, TLR2-agonistic ECM protein versican, promote the secretion of TNFα and metastasis 41. Besides inflammation, NF-κB activation results in a wide range of cellular responses such as prevention of apoptosis (discussed below), proliferation and anti-oxidant defenses 42,43. Oxidative stress that typically occurs in the setting of chronic inflammation may not only contribute to the activation of tumor-promoting inflammatory signaling pathways 44 but also change molecular patterns and result in formation of DAMPs with TLR2-activating properties by lipid oxidation 45.
2.2. TLR-mediated anti-apoptotic effects
Resisting cell death is a hallmark of cancer 46,47, counteracting many of the control mechanisms that destroy malignant cells. NF-κB is considered the most relevant anti-apoptotic pathway that controls the expression of anti-apoptotic genes and also restricts the activation of proapoptotic pathways such as JNK 48,49. TLR signaling activates NF-κB both through MyD88-dependent and MyD88-independent pathways. Moreover, TLRs stimulate the release of cytokines IL-1β and TNFα that in turn promote NF-κB activation in target cells. The key role of TLR signaling in cytoprotection is highlighted by the enhanced susceptibility of MyD88-deficient mice to dextrane sulfate sodium (DSS)-induced colitis 50, which is at least in part mediated by decreased in cytoprotective pathways. Likewise, in the liver and the lung, toxic injury is suppressed in a TLR-dependent manner 9,51,52. TLR signaling may not only play a role in regulating injury responses in chronically injured precancerous organs but also in promoting survival of malignant cells. As such, TLR-induced NF-κB activation promotes tumor cell survival in colon cancer 53, liver cancer 9, stomach cancer 11 and lung cancer 54. Moreover, TLR4 on tumor cells mediates resistance of tumor cells to cell death induced by cytotoxic T lymphocytes and promotes tumor growth in vivo 55. In addition to NF-κB activation in TLR-expressing tumors, survival signals could also be provided by TLR-expressing cells in the TME such as tumor-associated macrophages (TAM) or cancer-associated fibroblasts (CAF) that release IL-1β or TNFα, which then act on tumor cells (see Fig. 2). Conditional ablation of TLRs in tumor cells and the TME will be required to differentiate between these two possibilities.
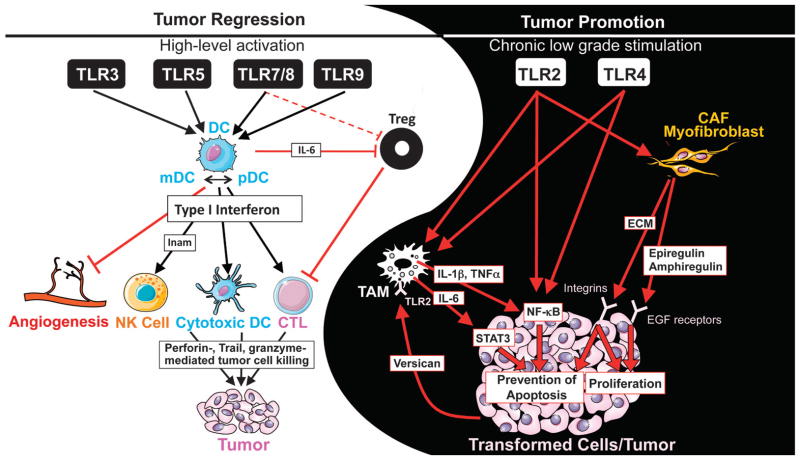
Figure 2. Yin and Yang of TLRs in tumor biology.
Potent activation of TLRs, in particular TLR3, TLR5, TLR7/8 and TLR9 may achieve anti-tumor effects by converting immune tolerance into anti-tumor immunity. High level TLR activation induces activation of dendritic cells (DC), which in turn activate key effect cells including natural killer (NK) cells and cytotoxic T lymphocytes (CTL) in a type I interferon-dependent manner. Moreover, DC themselves may also aquire cytotoxic and tumoricidal abilities. In addition, TRL-activated DC also inhibit Treg through IL-6 and angiogenesis through type I interferon. On the other hand, chronic low-grade stimulation of TLRs, in particular TLR2 and TLR4, leads to tumor-promoting inflammation and prevention of tumor apoptosis. This may be mediated through effects on tumor-associated macrophages (TAM), cancer-associated fibroblasts (CAF) or through direct effects in the tumor. NF-κB activation play as key role as it not only fuels inflammation but also prevent apoptosis. Finally, tumor-secreted factors, such as versican, may activate TLRs on cells of the tumor microevironment, further fueling tumor-promoting inflammatory signals.
2.3. TLR-mediated promotion of wound healing
The similarities between tumor stroma and wounds have led to the suggestion that cancers represent “wounds that do not heal” and a final stage of uncontrolled tissue repair processes 56. Wound healing responses may strongly promote carcinogenesis as demonstrated by studies with Rous sarcoma virus, in which injury and wound healing of infected animals amplifies carcinogenesis 57. The two events in the wound healing response that are influenced by TLR signaling include (i) epithelial regeneration and (ii) myofibroblast activation. The liver is the best-known example for the epithelial regeneration with restoration of mass after 70% partial hepatectomy within 7–10 days. Although deletion of single TLRs does not affect regeneration, deletion of the common TLR adapter MyD88 or elimination of intestinally-derived TLR ligands in germ-free mice suppresses liver regeneration 58–60. A contribution of TLR signaling to regeneration is also found in the intestine, where a TLR2/TLR4/MyD88 signaling cascade mediates epithelial regeneration after DSS-mediated injury 53,61, and in the skin, where lack of MyD88 slows the healing of excisional skin wounds 62. In the intestine and liver, TLR-MyD88 signaling regulates the expression of mitogenic EGF receptor ligands epiregulin and amphiregulin in non-hematopoietic cells. Accordingly, epiregulin-deficient mice display discreased tumor development in the liver and colon 9,63.
The accumulation of extracellular matrix constitutes a second event that may promote carcinogenesis in a TLR-dependent manner. Fibroblasts and fibroblast precursors functionally express TLR2 64,65 TLR4 66–69 and TLR9 70. Notably, TLR4 promotes fibrosis development in the liver, as demonstrated by the protective effect of TLR4-, CD14-, MD-2-, MyD88- and Trif-deficiency 71–73. Mice lacking TLR2 and TLR9 also display a reduction of hepatic fibrosis 70,74. TLRs exert only moderate profibrogenic effects in other organs, such as skin and kidney, but have no role in lung fibrosis 75.
3. TLR-mediated anti-tumor signals
Although the concept of tumor immunosurveillance is well established, the immune system is much more efficient at triggering dendritic cell (DC) activation in response to pathogens than at recognizing and eliminating tumors. In the past two decades, pharmacologic studies have established that boostering the activation of pathways, which is efficiently triggered by pathogens but not by tumors, such as TLRs and downstream mediators such as type I interferons, can be therapeutically employed to shift the balance from immunotolerance to anti-tumor effects. In particular, TLR7 agonists and interferon alpha have shown therapeutic efficacy in melanoma, basal cell cancer, renal cell cancer, and hairy cell leukemia 76.
3.1. TLR-mediated effects on dendritic cells
Because tumor cells are poor antigen-presenting cells (APC), anti-tumor immune responses typically depend on professional APCs such as DC 77. As professional APCs, that express a large number of PRR including TLRs and exert powerful effects on lymphocytes, DC are at the interface of innate and adaptive immune responses. Although DC have been in the focus of tumor immunology for their ability to launch potent anti-tumor responses, lacking activation of DC – often a result of inhibitory signals from tumor cells – may also induce immune tolerance through T cell deletion or through regulatory T cells (Treg) 77,78, thus allowing tumor progression. As type I interferon is absolutely required for immune rejection of tumors by DC 79,80, activation of the TLR-IFN signaling pathway represents a therapeutic approach to shift the balance from DC tolerance to anti-tumor response. TLR-activated DC may mediate anti-tumor responses not only through antigen presentation and effects on T cells 81,82 but also through direct cytotoxic effects on tumor cells. Accordingly, both classical and plasmacytoid DC, activated by TLR7 agonists, acquire the ability to induce anti-tumor responses and lyse tumor cells 83–85. Likewise, TLR5 activation also increases tumoricidal activity of DC 86. It is possible that DC-mediated tumor killing subsequently triggers amore efficient antigen-presentation to cytotoxic T lymphocytes, thus amplifying anti-tumor responses. Different DC subsets such as TLR9-stimulated plasmacytoid DC and myeloid DC engage in a crosstalk that promotes anti-tumor responses 87.
3.2. TLR- and IFN-mediated effects on natural killer cells
One of the earliest described immunoregulatory functions of type I IFN—its ability to regulate natural killer (NK) cell functions 88 has now been proven to be one of the major mechanisms of the regulation of tumor growth by endogenous type I IFN. Poly I:C-mediated TLR3 stimulation of myeloid DC induces NK activation and NK-mediated tumor regression in a mouse model of melanoma 89. A novel molecule termed IRF-3-dependent NK-activating molecule (INAM) provides a link between myeloid DC and NK cells89.
3.3. Inhibition of regulatory T cell functions by TLR signals
CD4+CD25+ Treg play an important role in immunity and tolerance as evidenced by development of organ-specific autoimmune diseases following their depletion 90. Activation of TLRs on DC regulates T cell activation not only through MHC class II and costimulatory molecules, but also through TLR-induced signals in DC that block the suppressive effect of Tregs in an IL-6 dependent manner 91. In addition, synthetic and natural ligands for human TLR8 can directly inhibit Treg cell function, independent of DC, and promote anti-tumor immunity 92. Altogether, these data demonstrate that TLR activation can release the brake in anti-tumor immunity via Tregs.
3.4. Additional mechanisms through which TLRs exert anti-tumor effects
Treatment with TLR3 agonist poly I:C may induce tumor regression by converting tumor-supporting macrophages to tumor suppressors that produce inflammatory cytokines and promote M1 polarization. This response is mediated by TNFα through a MyD88-independent pathway 93. TLR9 agonists can also exert anti-tumor effects through suppression of angiogenesis 94. It is likely that TLR-induced interferons play an important role as interferon alpha is well known to reduce tumor growth by blocking angiogenesis 95–97.
4. Role of specific TLRs and their ligands in experimental and human carcinogenesis
The most profound contribution of TLRs to carcinogenesis has been observed in organs that are either directly exposed to bacterial TLR ligands such as the GI tract and skin, or indirectly exposed to high amounts of intestinal TLR ligands such as the liver. Thus, tumor-promoting proinflammatory TLR activation seems to be triggered by bacterial PAMPs. Accordingly, germ-free animals display reduced carcinogenesis in the liver and the GI tract 9,98–100. However, in other organs, e.g. the pancreas, DAMPs may be the main stimulus for TLR-dependent carcinogenesis.
TLRs exert a dichotomous role in cancer. The amplitude and length of receptor activation may have a critical impact on outcome, with chronic low-grade TLR activation favoring a tumor-promoting proinflammatory state, and high-dose therapeutic TLR activation promoting anti-tumor response. Here we summarize results from the most relevant studies and classify TLRs as “largely tumor-promoting” or “largely tumor-suppressing”, with the caveat that TLRs have divergent functions in an organ-, cell-, context- and ligand-dependent manner, and that the literature contains conflicting data.
4.1. TLRs and TLR adapter molecules with largely tumor promoting functions
4.1.1. TLR4
Tumor-promoting functions of TLR4 are best established in the colon, liver, pancreas and skin. In colon, where there is constant interaction between gut microbiota and epithelial cells, TLR4 deletion strongly reduces inflammation and tumor burden in a colitis-associated neoplasia using the azoxymethane (AOM)-DSS model 101. Furthermore, transgenic mice overexpressing constitutively activated TLR4 in the intestine exhibit a higher sensitivity to colitis-associated neoplasia 102. These studies are in agreement with other studies in which either deletion of the TLR4 adapter molecule Myd88 103, or depletion of the gut microbiota, which supplies TLR4-activating PAMPs such as LPS, reduce colon cancer development 103,104. In contrast, one recent study showed that intestinal overexpression of constitutively activated TLR4 in the APCMin model of colon reduces tumor load by increasing tumor cell apoptosis 105. In the liver, several studies have demonstrated a tumor-promoting role of TLR4 9,51. Moreover, germ-free status or elimination of the gut microbiota by antibiotics decrease hepatocarcinogenesis 9. Likewise, dysbiosis induced by penicillin increases development of liver cancer whereas treatment with probiotics improves it 106. TLR4-dependent hepatocarcinogenesis is mediated by non-bone marrow-derived cells. In contrast to these numerous studies, a single study showed increased hepatocarcinogenesis in TLR4-deficient mice 107. In the skin, TLR4- and Myd88-deficient mice exhibited a strong reduction in tumor numbers induced by two-step chemical carcinogenesis 39,108. Carcinogenesis in the skin depends on TLR4 expressed by myeloid and epithelial cells, and the endogenous TLR4 ligand HMGB1. MyD88-dependent promotion of skin carcinogenesis not only depends on TLR but also on IL-1R signaling 39. The fact that TLR4 exerts a protective role in a non-inflammatory model of skin carcinogenesis suggests that the effect of TLR4 on skin cancer may be context-dependent 109. In the pancreas, TLR4 deletion decreases tumor growth, while chronic LPS treatment accelerates cancer progression 13. Tumor-promoting effects of TLR4 in pancreatic cancer are mediated by bone marrow-derived cells 13. In contrast to largely tumor-promoting role of TLR4 in the colon, liver, skin and pancreas, TLR4 in lung and breast inhibits cancer development: Mice expressing non-functional TLR4 exhibit increased tumor load in a 3-methylcholanthrene-induced lung cancer model 110. Similarly, TLR4 promotes breast cancer development, progression and metastasis in chemical carcinogenesis and syngeneic transplant models 111,112. Further studies are required to understand the organ-specific contribution TLR4. It is conceivable that TLR4-activating PAMPs act predominantly as potential tumor promoters, whereas DAMPs function as potential tumor suppressors. Accordingly, chemotherapeutic drugs or radiotherapy require TLR4-activating DAMPs to achieve therapeutic effects: HMGB1 is released following anthracycline treatment or radiotherapy resulting in HMGB1-mediated TLR4 activation on DC and tumor elimination by tumor-specific T cells. 113. Despite the large body of literature on TLR4 in experimental carcinogenesis, the functional contribution of TLR4 to human carcinogenesis is not well understood. Single nucleotide polymorphisms (SNP) that render TLR4 less responsive, such as Asp299Gly and Thr399Ile, may be associated with precancerous gastric lesions, and increased risk of gallbladder cancer 114,115. However, large-scale confirmatory studies are missing. The contribution of other TLR4 SNPs to carcinogenesis remains elusive 116. Patients that received anthracycline-based adjuvant chemotherapy for breast cancer had increased metastasis and decreased metastasis-free survival when carrying the Asp299Gly TLR4 variant, confirming the contribution of TLR4 to chemo- and radiotherapy 113.
4.1.2. TLR2
TLR2 may promote or suppress tumor growth in a context-dependent manner. A profound tumor-promoting role has been observed in gastric cancer. TLR2 is upregulated in human and murine gastric cancer samples 11. In a genetic mouse model of gastric cancer, deletion of TLR2 results in a drastically reduced tumor load 11. TLR2-mediated promotion of gastric cancer is independent of bone marrow-derived cells, and is mediated by proliferation and survival signaling pathways including PI3K/Akt, ERK1/2 and NF-κB in the epithelial compartment 11. In lung cancer, TLR2 exerts tumor- and metastasis-promoting effects. Lung cancer cells secrete extracellular matrix component versican, resulting in versican-mediated TLR2 stimulation and IL-6 and TNF production in macrophage and promotion of metastatic growth 41. In the liver, TLR2 does not affect hepatocarcinogenesis in the setting of chronic injury 9. However, in a purely genotoxic model of liver cancer, TLR2 knockout mice exhibit a higher number of tumors and decreased survival 117. Tumor cells from TLR2 knockout mice showed a lower amount of cell death and a suppressed senescence program. Similar to the liver, the role TLR2 remains controversial in colorectal cancer. In one study, there were no differences in colorectal cancer between wild-type and TLR2-deficient mice using the AOM-DSS model 118. In contrast, another study reported increased tumor development and higher IL-6, IL-17A and phospho-STAT3 levels in colorectal cancer in TLR2-deficient mice using a similar AOM-DSS model 119. TLR2 may also be the mediator of angiogenesis-promoting lipid oxidation end products such as ω-(2-carboxyethyl)pyrrole (CEP) 45. These products are not only generated during inflammation and wound healing but accumulate in highly vascularized tumors such as melanoma, and induce a VEGF-independent angiogenic response that depends on TLR2 45. The role of TLR2 in human carcinogenesis is not well understood. In gastric cancer, STAT3 pathway activation and increased TLR2 expression are associated with poor survival 11. Although TLR2 polymorphisms have been associated with gastric and cervical cancer risk 120,121, large-scale confirmatory studies are missing.
4.1.3 Adaptor Molecules and Regulators of TLR signaling
Myeloid-differentiation factor 88 (MyD88) mediates downstream signaling of the majority of TLR family members, as well as the receptors for IL-1 and IL-18 (see Fig. 1). The L265P mutation in the TIR domain of MyD88 mutations caused constitutive activation of NF-κB and JAK-STAT pathways and increased survival of malignant cells, thereby contributing to the pathogenesis of diffuse large B-cell lymphoma and Waldenström’s macroglobulinemia 122–124. Conversely, deletion of MyD88 decreases carcinogenesis in many settings, including genetic and chemical models of colon cancer 103, chemically-induced hepatocarcinogenesis 36 and chemical models of skin papilloma and fibrosarcoma 125. In contrast, deletion of MyD88 results in aggressive fibroinflammation, increased Th2 responses, and profoundly accelerated tumorigenesis in a genetic model of pancreatic cancer 13. In the latter model, carcinogenesis is driven by the MyD88-independent TRIF pathway, suggesting that MyD88 deletion might promote tumors by overactivation of the TRIF pathway 13. SIGIRR is a member of the TLR/IL-1 receptor superfamily, and acts as a negative regulator of TLR and IL-1 signaling 126. Mice deficient in SIGGIR not only display constitutive expression of inflammatory genes in the colon, that depends on the bacterial microbiota, but also increased inflammation following DSS, and increased tumorigenesis in the AOM+DSS model 127.
4.2. TLRs with largely tumor-suppressive functions
4.2.1. TLR3
TLR3 predominantly serves a tumor-suppressive function, largely mediated by TLR3-dependent stimulation of anti-tumor immunity. Accordingly, TLR3 deficiency results in development of acute lymphoblastic T cell leukemia after infection with endogenous retrovirus 128. In syngeneic and xenograft models of prostate cancer, TLR3 deficient mice display increased tumor growth rate 129. Conversely, treatment with TLR3 agonist poly I:C inhibits tumor growth in the latter model and a genetic model of prostate cancer overexpressing the TRAMP transgene 129. Systemic administration of poly I:C reduces the growth of melanoma metastasis 130. In the liver, there is a positive correlation between TLR3 expression and patient survival 131. Moreover, poly I:C treatment increased NK cell activation and reduced liver tumor growth in a syngeneic transplant model 131. Consistently in all models, TLR3 downstream effects are mediated by type I interferon and subsequent NK cell or DC activation, and NK cell- or DC-mediated death of tumor cells. In contrast to the above studies, it has also been suggested that TLR3 could promote tumor development through effects on cell proliferation and survival 132,133.
TLR5
The majority of studies deciphering the contribution of TLR5 to tumor development have revealed potent anti-tumor effects, consistent with the expression and function of TLR5 on DC and their important role in anti-tumor immune responses. These effects are best exemplified in a study where tumor cell lines expressing TLR5 agonist flagellin fused with a tumor antigen induced DC-mediated anti-tumor immune response 82. In the colon, knockdown of TLR5 or MyD88 dramatically enhanced growth and inhibited necrosis in a xenograft model 134. TLR5 is highly expressed in human breast cancer biopsies compared to non-tumor tissue 135. Implantation of TLR5-deleted breast tumor cells results in enhanced tumor growth while TLR5 agonist flagellin retarded tumor growth by increasing cell death and decreasing tumor cell proliferation 135. However, the use of immunodeficient mouse models that lack DC, is an important limitation of the above studies. Therefore, the tumor-suppressive function of TLR5 requires additional investigations in mice with a fully functional immune system.
TLR7/TLR8
TLR7 and TLR8 are structurally related receptors for single-stranded RNA, TLR7 and 8 have been the focus of research on TLRs and cancer due to the profound immunomodulatory and anti-tumor effects of small-molecule agonists 136–139. While there is considerable data on the clinical application of potent TLR7/8 agonists for anti-tumor therapy, relatively little is known about the contribution of endogenous TLR7/8 activation to carcinogenesis. A recent publication demonstrated that microRNAs, released from tumors via exosomes, bind to TLR7 and 8, and favor metastatic growth 140, suggesting that low-level activation of TLR7/8 by endogenous agonists exert tumor-promoting effects. TLR7/8 agonists are predominantly used for anti-tumor therapy of basal cell carcinoma and melanoma 136 (discussed in the next section). Anti-tumor effects are predominantly mediated by TLR7/8 activation on DC, resulting in their recruitment, activation and ability to kill tumor cells 83,85. Moreover, TLR7/8-activated DC also activate NK cells and suppress Treg 92,141,142. Despite the overwhelming literature on anti-tumor effects of TLR7/8 agonists recent studies suggest a potential tumor-promoting role TLR7/8 in some settings: In pancreatic cancer, there is not only a high expression of TLR7 in both epithelial and inflammatory cells, but also an acceleration of tumor formation following TLR7 activation 12. Conversely, pharmacologic inhibition of TLR7 decreases pancreatic cancer. TLR7 exerts its effects on pancreatic cancer in inflammatory cells as chimeric mice with absent TLR7 expression in bone marrow-derived cells displayed decreased carcinogenesis 12. In the lung, both TLR7 and TLR8 are expressed on tumor cells, and promote the survival of lung cancer cell lines through NF-κB activation and upregulation of Bcl-2 54. As discussed above, TLR7/8-agonistic microRNAs promote lung tumor growth 140. Thus, the effects of TLR7/8 on tumor may depend on the organ, tumor stage, the cell type, and the role that anti-tumor immune responses play in a particular tumor. In light of the potential clinical applications of TLR7/8 agonists for anti-tumor therapies, further pharmacologic and genetic studies are required to understand in which organs and settings TLR7/8 signaling promotes cancer.
TLR9
TLR9 is the receptor for non-methylated CpG DNA that is typically found in viral and bacterial DNA 143. Similar to TLR7/8, TLR9 has been extensively studied for its role in immunosurveillance and as potential target for anti-tumor therapy using synthetic TLR9 agonists. The contribution of TLR9 to immunosurveillance is highlighted by the fact that TLR9 deficiency results in acute lymphoblastic T cell leukemia after infection with endogenous retrovirus infection 128. These data suggest that TLR9 may be particularly relevant for virally induced tumors. Conversely, TLR9 activation by synthetic oligodeoxynucleotide agonists (CpG-ODN) has demonstrated anti-tumor activity in xenograft models of murine cervical carcinoma, neuroblastoma and colon cancer 143–147. Likewise, the combination of TLR9 agonists with Stat3 siRNA in a single molecule promoted anti-tumor responses against subcutaneously implanted melanoma cells, while at the same time suppressing the Stat3-mediated immunosuppression in the TME 148. CpG-ODNs are studied for the treatment of different tumor types as a single therapeutic agent 143, as well as in combination with radiation therapy and cytotoxic chemotherapy 149 (discussed in the next section). Mechanistically, TLR9 agonists induce type I interferon secretion in DC resulting in cytotoxic DC, activation of NK cells and cytotoxic T lymphocytes, all of which contribute an antitumor immune response 143,150–153. In addition to immune cells, TLR9 is also expressed on tumor cells from many tissues 55,154 but the function of TLR9 on tumor cells in vivo is currently not known. As it is conceivable that TLR9 stimulation may be cell-and context-dependent and possibly contribute to tumor cell survival or proliferation, additional studies in TLR9-deficient mice are needed to investigate the functional role of TLR9 in carcinogenesis in vivo 155–158.
5. TLRs as therapeutic targets for tumor prevention and therapy
Once ranked among the top 20 immunotherapy drugs for the cure of cancer, the role of TLRs in cancer biology has not only become more complex with recent studies revealing a tumor-promoting effect of several TLRs, but has also been set back by disappointing results from several phase II and phase III clinical trials 159. Altogether, these results are leading to more differentiated approaches that requires focus on (i) tumors amenable to immunotherapy, (ii) TLRs that promote anti-tumor responses most efficiently and for which a tumor-promoting role has been excluded, and (iii) combination therapies to boost immune responses to specific antigens, targeting in particular DC. The majority of clinical trials have been performed using TLR3, TLR7/8 and TLR9 agonists (Fig. 3A). On the other side of the spectrum of TLR biology, targeting specific tumor-promoting TLRs such as TLR2 and TLR4, or their ligands for tumor-prevention represents a new and evolving philosophy that could eventually be translated into clinical trials for primary or secondary tumor prevention.
Figure 3. TLR agonists for clinical anti-tumor therapies.
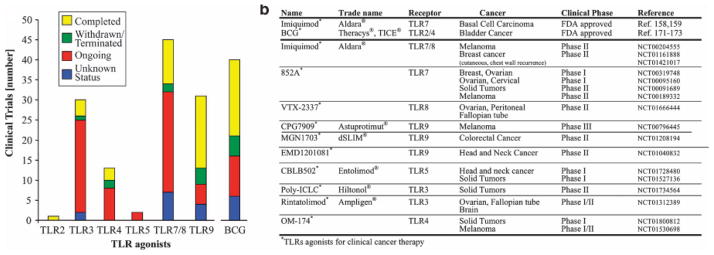
A. Summary of trials from ClinicalTrial.gov employing TLR agonists or TLR-agonistic Bacillus Calmette-Guérin (BC). B. List of FDA-approved TLR agonists for tumor therapy (upper section) and TLR agonists currently being evaluated for tumor therapy (lower section), including the National Clinical Trial (NCT) number from ClinicalTrials.gov.
5.1. TLR agonists for the induction of anti-tumor responses
5.1.1. TLR7/8 agonists
The most successful translation of immunomodulatory and anti-cancer effects of all TLR agonists has been achieved for TLR7/8. Accordingly, the TLR7/8 agonist imiquimod is the only TLR agonist approved for cancer therapy. As TLR7 and TLR8 both recognize ssRNA, many synthetic agonists activate both receptors. Several classes of TLR7/8 agonists have been developed 160: Imidazoquinoline, which resemble adenosine analogues, include the TLR7 agonist imiquimod 138 and the TLR7/8 agonist resiquimod; guanosine analogues such as the TLR7 ligand loxoribine; and ssRNA that has been stabilized to prevent its rapid degradation. Modifications of their RNA sequence can direct ligands specifically towards TLR7 or TLR8 142. Among all TLR7/8 ligands, imiquimod is the best studied 136 and in clinical use: Topical administration of Aldara™ (5% imiquimod cream) has been approved by the FDA and the European Union for the treatment of basal cell carcinoma, and achieves a clearance rate of 42–100% 161,162. Imiquimod is also being used to locally treat other cutaneous tumors including melanoma with well-documented and >85% success rates for lentigo maligna 163 (Fig. 3B). Evidence is accumulating that it may also be effective in more advanced stages such as invasive cutaneous and locally recurrent melanoma 164–166 Topical imiquimod treatment is also utilized in a phase II clinical trial in breast cancer patients with chest wall recurrence or cutaneous metastasis 22,167 (Fig. 3B). Despite success with topical treatment, the role of TLR7/8 agonists for systemic therapy remains uncertain. Unlike imiquimod, the TLR7 agonist 852A and the TLR8 agonist VTX-2337 can be applied systemically and are currently being evaluated in phase I and phase II clinical trials for various cancers including melanoma breast, ovarian, endometrial, cervical and head and neck cancers (Fig. 3B).
5.1.2. TLR9
Although a large number of clinical trials have been performed using TLR9 agonists, there is currently no FDA-approved TLR9 agonist for tumor therapy. A number of CpG-containing TLR9 agonists have been tested in animal models, including cervical carcinoma, neuroblastoma and colon cancer 143–147, and in clinical trials 22. Despite promising preclinical studies, several clinical trials have been disappointing, e.g. failure of TLR9 agonists CGP 7909 in a Phase III trial in non-small cell lung cancer, and safety concerns of IMO-2055 in combination with platinum-based therapies Phase II trial in recurrent or metastatic squamous cell carcinoma of the head and neck cancer, respectively 159. Results from other ongoing studies of TLR9 agonist MGN1703 for metastatic colorectal cancer and TLR9 agonist EMD1201081 for head and neck cancer (Fig. 3B) need to be awaited to judge the role of TLR9 in cancer therapy. Because of these somewhat discouraging clinical studies, focus has shifted towards the ability of TLR9 agonists to activate DC and improve responses to tumor vaccines. The combination of the TLR9 agonist CPG7909, the TLR4 agonist MPL, and the melanoma antigen MAGE-A3 is currently being tested in a Phase III clinical trial (Fig. 3B).
5.1.3. TLR5
Although there is significant data in mice showing anti-tumor effects by TLR5 agonists flagellin 82,135,168 and TLR5-agonistic polyethylenimine-based nanoparticles, that are selectively engulfed by tumor-associated tolerogenic DC 86, translation into clinical practice has not been achieved. CBLB502 (Entolimod) is a TLR5 agonist that is derived from Salmonella flagellin 169 that is currently studied in phase I clinical trials in patients with squamous cell head and neck cancer, and in locally advanced or metastatic solid tumors (Fig. 3B).
5.1.4 TLR3
Despite evidence of tumor-suppressing effects of TLR3 ligand poly I:C in several mouse studies 129,130, there is little data on anti-tumor effects of TLR3 ligands in humans. As poly I:C is inactivated rapidly in humans, several TLR3 ligands have been developed for clinical applications. Poly-ICLC (Hiltinol™) is a derivative of poly I:C that has been stabilized with poly-lysine, and is currently studied in phase II trials for solid tumors (Fig. 3B). Rintatolimod (Ampligen™) is a derivate of poly I:C in which cytidine is replaced by uridine at a ratio of 1:12 (polyI:polyC12U). Rintatolimod is employed in combination with vaccines for the treatment of ovarian and fallopian tube cancer, as well as brain tumors (Fig. 3B). The dsRNA mimic IPH-3102 is another TLR3 agonist, but there is no available data on animal studies or clinical trials.
5.1.5 TLR2, TLR4 and mixed agonists
Although TLR2 and TLR4 mediate tumor promotion in many types of mouse cancers, they may exert anti-tumor effects in others, and have been investigated for tumor therapy as well as adjuvants for vaccination. Although strictly not a pure TLR agonist, Bacillus Calmette-Guérin (BCG) mediates many of its immunomodulatory effects through TLR2 and TLR4 24. Anti-tumor effects of BCG are known for over 40 years 170–173. BCG is effective in the treatment of bladder cancer 174–176 (Fig. 3B) and has been approved by the FDA for this purpose. BCG remains the only known effective tumor therapeutic with TLR2/4 agonistic effects. Although BCG has been evaluated in clinical trials for a large number of other tumors, these have been largely disappointing 25. OM-174 (CXR-526), a derivative from Escherichia coli lipid A, is a mixed TLR2/TLR4 agonist. OM-174 has been evaluated in a Phase I study in patients with solid tumors, and is an adjuvant for an autologous DC vaccine for melanoma in a Phase I/II clinical trial (Fig. 3B). Stimuvax is a cancer vaccine against the tumor antigen MUC1 that contains TLR4 agonist monophosphoryl lipid A (MPL), but failed to improve non-small cell lung cancer 177. In summary, with the exception of the non-specific TLR2/TLR4-agonistic BCG, there is currently no data showing clear anti-tumor activity of TLR2 or TLR4 agonists in clinical trials.
5.2. TLR antagonism for tumor prevention
Based on recent data on tumor promoting effects of TLRs in organs such as the colon, liver and pancreas, inhibition of TLR signaling may represent a novel strategy for tumor prevention. However, knowledge from animal models has not been translated into the clinic and there are currently no ongoing trials. Here we summarize potential therapeutic strategies for tumor prevention:
5.2.1. Modulation of gut microbiome and bacterial translocation by probiotics and antibiotics
The gut microbiome is the main source for bacterial TLR ligands, which may contribute to carcinogenesis in the stomach, colon and liver. Modulating the gut microbiome and/or bacterial translocation by probiotics or antibiotics may represent clinically feasible approaches to reduce TLR-mediated inflammatory and tumor promoting signals. 178. In murine models of colon cancer, administration of synbiotics but not pro- or prebiotics alone, prevented aberrant crypt foci formation in AOM-induced colon cancer 179,180. In a rat model of liver carcinogenesis, administration of the probiotic VSL#3 strongly reduced liver tumor formation 106. In a genetic mouse model of colorectal cancer short-term antibiotic treatment reduces the pro-tumorigenic expression of IL-23 in TAM while long-term depletion of gut microflora, reduces tumor number and size 181. A recent study confirmed these findings in a different model of colon cancer, where gut sterilization limits the development of colonic dysplasia 182. In liver cancer, gut sterilization by oral antibiotics results in drastically reduced tumor burden in mouse and rat models 9,51. Notably, effects of antibiotics are more pronounced when given at late stages of hepatocarcinogenesis, suggesting that gut microbiome modulation by antibiotics might be useful in clinical settings, where early prevention treatment typically is not possible. While the above used antibiotics cannot be applied in patients long-term, recent data also suggests that well tolerated non-absorbable antibiotics such as Rifaximin reduce liver tumors in murine hepatocarcinogenesis 9. Rifaximin is approved for the treatment of hepatic encephalopathy and well-tolerated 183. However, neither probiotics nor antibiotics have yet been studied for primary or secondary tumor prevention in patients. In advanced liver disease, Rifaximin could be used to hit “two birds with one stone” by reducing hepatic encephalopathy and preventing liver cancer development.
5.2.1. TLR2 and TLR4 antagonist for tumor prevention
The TLR4 inhibitors CRX-526 and E5564 are synthetic analogs of the lipid A portion of LPS, and prevent binding of LPS to the TLR4-MD2 complex and TLR4 activation. TAK-242 is a TLR4 antagonist that acts on the intracellular domain of TLR4. Although these TLR antagonists effectively prevent LPS-mediated inflammation and lethality 184–186, they have not been investigated in animal models or clinical trials for tumor prevention. Likewise, the TLR2-inhibitory humanized monoclonal antibody OPN305, reduces inflammation in vivo 187 but has not been investigated for tumor prevention.
Conclusion
Knowledge about the contribution of TLRs to cancer as well as clinical trials employing TLR agonists for anti-tumor therapy have increased tremendously. The most relevant scientific discoveries in the field of TLRs and cancer biology in the past decade has been the realization that TLRs may promote tumor formation in certain organs such as the colon, stomach, pancreas and liver. This knowledge needs to be integrated into current future clinical trials. Although only few approaches have been found to be successful, including the local TLR7 agonist imiquimod and the non-specific TLR2/4 agonist BCG, testing of new TLR agonists, combinations and indications for TLR-based anti-tumor therapy is still ongoing. Integration of TLR agonists as adjuvants into DC-based cancer vaccination has become a new focus 77 that may constitute a new and efficient modality of tumor therapy in the future. Moreover, the tumor-promoting actions of some TLRs may open up new possibilities for primary or secondary tumor prevention. The fact that TLRs may also promote chronic inflammation and cancer in some settings should not deter from successes with TLR agonists as anti-tumor therapy, and requires further mechanistic investigations shedding light on the “Yin and Yang” of TLRs in tumor biology.
Acknowledgments
This work was supported by NIH grants U54CA163111, R01DK076920 and R01AA020211 (to RFS). Dianne H. Dapito was supported by NIH grant F31DK091980.
References
- 1.Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu Rev Immunol. 2012;30:677–706. doi: 10.1146/annurev-immunol-020711-075008. [DOI] [PubMed] [Google Scholar]
- 2.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 4.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lotze MT, et al. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 6.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 7.Goldszmid RS, Trinchieri G. The price of immunity. Nat Immunol. 2012;13:932–938. doi: 10.1038/ni.2422. [DOI] [PubMed] [Google Scholar]
- 8.Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dapito DH, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukata M, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–1881. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tye H, et al. STAT3-driven upregulation of TLR2 promotes gastric tumorigenesis independent of tumor inflammation. Cancer cell. 2012;22:466–478. doi: 10.1016/j.ccr.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Ochi A, et al. Toll-like receptor 7 regulates pancreatic carcinogenesis in mice and humans. J Clin Invest. 2012;122:4118–4129. doi: 10.1172/JCI63606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ochi A, et al. MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. J Exp Med. 2012;209:1671–1687. doi: 10.1084/jem.20111706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuraishy A, Karin M, Grivennikov SI. Tumor promotion via injury- and death-induced inflammation. Immunity. 2011;35:467–477. doi: 10.1016/j.immuni.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 16.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 17.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 18.Bruns P. Die Heilwirkung des Erysipelas auf Geschwülste. Beitr Klin Chir. 1888;3:443. [Google Scholar]
- 19.Busch W. Aus der Stizung der medizinischen Section vom 13. November 1867. Berl Klin Wochenschr. 1868;5:137. [Google Scholar]
- 20.Coley WB. Treatment of inoperable malignant tumors with the toxins of erysipelas and the Bacillus prodigiosus. Trans Amer Surg Assn. 1894;12:183–212. [Google Scholar]
- 21.Starnes CO. Coley’s toxins in perspective. Nature. 1992;357:11–12. doi: 10.1038/357011a0. [DOI] [PubMed] [Google Scholar]
- 22.Goutagny N, Estornes Y, Hasan U, Lebecque S, Caux C. Targeting pattern recognition receptors in cancer immunotherapy. Targeted oncology. 2012;7:29–54. doi: 10.1007/s11523-012-0213-1. [DOI] [PubMed] [Google Scholar]
- 23.Hennessy EJ, Parker AE, O’Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov. 2010;9:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- 24.Galluzzi L, et al. Trial Watch: Experimental Toll-like receptor agonists for cancer therapy. Oncoimmunology. 2012;1:699–716. doi: 10.4161/onci.20696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vacchelli E, et al. Trial watch: FDA-approved Toll-like receptor agonists for cancer therapy. Oncoimmunology. 2012;1:894–907. doi: 10.4161/onci.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 27.Beutler B, et al. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24:353–389. doi: 10.1146/annurev.immunol.24.021605.090552. [DOI] [PubMed] [Google Scholar]
- 28.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 29.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 30.Gilmore TD. The Rel/NF-kappaB signal transduction pathway: introduction. Oncogene. 1999;18:6842–6844. doi: 10.1038/sj.onc.1203237. [DOI] [PubMed] [Google Scholar]
- 31.Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis. 2011;70 (Suppl 1):i104–108. doi: 10.1136/ard.2010.140145. [DOI] [PubMed] [Google Scholar]
- 32.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 33.Apte RN, Voronov E. Is interleukin-1 a good or bad ‘guy’ in tumor immunobiology and immunotherapy? Immunol Rev. 2008;222:222–241. doi: 10.1111/j.1600-065X.2008.00615.x. [DOI] [PubMed] [Google Scholar]
- 34.Grivennikov S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Popivanova BK, et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naugler WE, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 37.Park EJ, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakurai T, et al. Hepatocyte Necrosis Induced by Oxidative Stress and IL-1alpha Release Mediate Carcinogen-Induced Compensatory Proliferation and Liver Tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cataisson C, et al. IL-1R-MyD88 signaling in keratinocyte transformation and carcinogenesis. The Journal of experimental medicine. 2012;209:1689–1702. doi: 10.1084/jem.20101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tu S, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim S, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Karin M. NF-kappaB and cancer: mechanisms and targets. Mol Carcinog. 2006;45:355–361. doi: 10.1002/mc.20217. [DOI] [PubMed] [Google Scholar]
- 44.Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011;208:417–420. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.West XZ, et al. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature. 2010;467:972–976. doi: 10.1038/nature09421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cotter TG. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer. 2009;9:501–507. doi: 10.1038/nrc2663. [DOI] [PubMed] [Google Scholar]
- 47.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 48.Dutta J, Fan Y, Gupta N, Fan G, Gelinas C. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene. 2006;25:6800–6816. doi: 10.1038/sj.onc.1209938. [DOI] [PubMed] [Google Scholar]
- 49.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 50.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Yu LX, et al. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. 2010;52:1322–1333. doi: 10.1002/hep.23845. [DOI] [PubMed] [Google Scholar]
- 52.Jiang D, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 53.Fukata M, et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862–877. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cherfils-Vicini J, et al. Triggering of TLR7 and TLR8 expressed by human lung cancer cells induces cell survival and chemoresistance. The Journal of clinical investigation. 2010;120:1285–1297. doi: 10.1172/JCI36551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang B, et al. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer research. 2005;65:5009–5014. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- 56.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 57.Dolberg DS, Hollingsworth R, Hertle M, Bissell MJ. Wounding and its role in RSV-mediated tumor formation. Science. 1985;230:676–678. doi: 10.1126/science.2996144. [DOI] [PubMed] [Google Scholar]
- 58.Seki E, et al. Contribution of Toll-like receptor/myeloid differentiation factor 88 signaling to murine liver regeneration. Hepatology. 2005;41:443–450. doi: 10.1002/hep.20603. [DOI] [PubMed] [Google Scholar]
- 59.Campbell JS, et al. Proinflammatory cytokine production in liver regeneration is Myd88-dependent, but independent of Cd14, Tlr2, and Tlr4. J Immunol. 2006;176:2522–2528. doi: 10.4049/jimmunol.176.4.2522. [DOI] [PubMed] [Google Scholar]
- 60.Cornell RP, Liljequist BL, Bartizal KF. Depressed liver regeneration after partial hepatectomy of germ-free, athymic and lipopolysaccharide-resistant mice. Hepatology. 1990;11:916–922. doi: 10.1002/hep.1840110603. [DOI] [PubMed] [Google Scholar]
- 61.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci U S A. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Macedo L, et al. Wound healing is impaired in MyD88-deficient mice: a role for MyD88 in the regulation of wound healing by adenosine A2A receptors. Am J Pathol. 2007;171:1774–1788. doi: 10.2353/ajpath.2007.061048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neufert C, et al. Tumor fibroblast–derived epiregulin promotes growth of colitis-associated neoplasms through ERK. J Clin Invest. 2013;123:1428–1443. doi: 10.1172/JCI63748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paik YH, et al. Hepatic stellate cells primed with cytokines upregulate inflammation in response to peptidoglycan or lipoteichoic acid. Lab Invest. 2006;86:676–686. doi: 10.1038/labinvest.3700422. [DOI] [PubMed] [Google Scholar]
- 65.Pierer M, et al. Chemokine secretion of rheumatoid arthritis synovial fibroblasts stimulated by Toll-like receptor 2 ligands. J Immunol. 2004;172:1256–1265. doi: 10.4049/jimmunol.172.2.1256. [DOI] [PubMed] [Google Scholar]
- 66.Kurt-Jones EA, et al. Use of murine embryonic fibroblasts to define Toll-like receptor activation and specificity. J Endotoxin Res. 2004;10:419–424. doi: 10.1179/096805104225006516. [DOI] [PubMed] [Google Scholar]
- 67.Wolf G, et al. Angiotensin II upregulates toll-like receptor 4 on mesangial cells. J Am Soc Nephrol. 2006;17:1585–1593. doi: 10.1681/ASN.2005070699. [DOI] [PubMed] [Google Scholar]
- 68.Otte JM, Rosenberg IM, Podolsky DK. Intestinal myofibroblasts in innate immune responses of the intestine. Gastroenterology. 2003;124:1866–1878. doi: 10.1016/s0016-5085(03)00403-7. [DOI] [PubMed] [Google Scholar]
- 69.Paik YH, et al. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 70.Watanabe A, et al. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology. 2007;46:1509–1518. doi: 10.1002/hep.21867. [DOI] [PubMed] [Google Scholar]
- 71.Seki E, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 72.Isayama F, et al. LPS signaling enhances hepatic fibrogenesis caused by experimental cholestasis in mice. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1318–1328. doi: 10.1152/ajpgi.00405.2005. [DOI] [PubMed] [Google Scholar]
- 73.Csak T, et al. Deficiency in myeloid differentiation factor-2 and toll-like receptor 4 expression attenuates nonalcoholic steatohepatitis and fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. 2011;300:G433–441. doi: 10.1152/ajpgi.00163.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Minmin S, et al. Single nucleotide polymorphisms of Toll-like receptor 4 decrease the risk of development of hepatocellular carcinoma. PLoS One. 2011;6:e19466. doi: 10.1371/journal.pone.0019466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huebener P, Schwabe RF. Regulation of wound healing and organ fibrosis by toll-like receptors. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbadis.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kirkwood JM, et al. Immunotherapy of cancer in 2012. CA Cancer J Clin. 2012;62:309–335. doi: 10.3322/caac.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 79.Diamond MS, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fuertes MB, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208:2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet. 2008;371:771–783. doi: 10.1016/S0140-6736(08)60241-X. [DOI] [PubMed] [Google Scholar]
- 82.Garaude J, Kent A, van Rooijen N, Blander JM. Simultaneous targeting of toll- and nod-like receptors induces effective tumor-specific immune responses. Science translational medicine. 2012;4:120ra116. doi: 10.1126/scitranslmed.3002868. [DOI] [PubMed] [Google Scholar]
- 83.Drobits B, et al. Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cells. The Journal of clinical investigation. 2012;122:575–585. doi: 10.1172/JCI61034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fiebiger E, et al. Serum IgG autoantibodies directed against the alpha chain of Fc epsilon RI: a selective marker and pathogenetic factor for a distinct subset of chronic urticaria patients? The Journal of clinical investigation. 1995;96:2606–2612. doi: 10.1172/JCI118325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stary G, et al. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. The Journal of experimental medicine. 2007;204:1441–1451. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cubillos-Ruiz JR, et al. Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity. The Journal of clinical investigation. 2009;119:2231–2244. doi: 10.1172/JCI37716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nierkens S, et al. Immune adjuvant efficacy of CpG oligonucleotide in cancer treatment is founded specifically upon TLR9 function in plasmacytoid dendritic cells. Cancer Res. 2011;71:6428–6437. doi: 10.1158/0008-5472.CAN-11-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Trinchieri G, Santoli D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. The Journal of experimental medicine. 1978;147:1314–1333. doi: 10.1084/jem.147.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ebihara T, et al. Identification of a polyI:C-inducible membrane protein that participates in dendritic cell-mediated natural killer cell activation. J Exp Med. 2010;207:2675–2687. doi: 10.1084/jem.20091573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 92.Peng G, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 93.Shime H, et al. Toll-like receptor 3 signaling converts tumor-supporting myeloid cells to tumoricidal effectors. Proc Natl Acad Sci U S A. 2012;109:2066–2071. doi: 10.1073/pnas.1113099109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Damiano V, et al. A novel toll-like receptor 9 agonist cooperates with trastuzumab in trastuzumab-resistant breast tumors through multiple mechanisms of action. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:6921–6930. doi: 10.1158/1078-0432.CCR-09-1599. [DOI] [PubMed] [Google Scholar]
- 95.De Palma M, et al. Tumor-targeted interferon-alpha delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer cell. 2008;14:299–311. doi: 10.1016/j.ccr.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 96.Pfeffer LM. Biologic activities of natural and synthetic type I interferons. Seminars in oncology. 1997;24:S9-63–S69–69. [PubMed] [Google Scholar]
- 97.Sidky YA, Borden EC. Inhibition of angiogenesis by interferons: effects on tumor- and lymphocyte-induced vascular responses. Cancer research. 1987;47:5155–5161. [PubMed] [Google Scholar]
- 98.Reddy BS, Watanabe K. Effect of intestinal microflora on 2,2′-dimethyl-4-aminobiphenyl-induced carcinogenesis in F344 rats. J Natl Cancer Inst. 1978;61:1269–1271. doi: 10.1093/jnci/61.5.1269. [DOI] [PubMed] [Google Scholar]
- 99.Reddy BS, Weisburger JH, Narisawa T, Wynder EL. Colon carcinogenesis in germ-free rats with 1,2-dimethylhydrazine and N-methyl-n′-nitro-N-nitrosoguanidine. Cancer Res. 1974;34:2368–2372. [PubMed] [Google Scholar]
- 100.Lofgren JL, et al. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology. 2011;140:210–220. doi: 10.1053/j.gastro.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fukata M, et al. Innate immune signaling by Toll-like receptor-4 (TLR4) shapes the inflammatory microenvironment in colitis-associated tumors. Inflammatory bowel diseases. 2009;15:997–1006. doi: 10.1002/ibd.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fukata M, et al. Constitutive activation of epithelial TLR4 augments inflammatory responses to mucosal injury and drives colitis-associated tumorigenesis. Inflammatory bowel diseases. 2011;17:1464–1473. doi: 10.1002/ibd.21527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 104.Li Y, et al. Gut microbiota accelerate tumor growth via c-jun and STAT3 phosphorylation in APCMin/+ mice. Carcinogenesis. 2012;33:1231–1238. doi: 10.1093/carcin/bgs137. [DOI] [PubMed] [Google Scholar]
- 105.Li Y, et al. Constitutive TLR4 signalling in intestinal epithelium reduces tumor load by increasing apoptosis in APC(Min/+) mice. Oncogene. 2013 doi: 10.1038/onc.2012.581. [DOI] [PubMed] [Google Scholar]
- 106.Zhang HL, et al. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. Journal of hepatology. 2012;57:803–812. doi: 10.1016/j.jhep.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 107.Wang Z, et al. TLR4 activity protects against hepatocellular tumorigenesis and progression via regulating the expression of DNA repair protein Ku70(1) Hepatology. 2013 doi: 10.1002/hep.26234. [DOI] [PubMed] [Google Scholar]
- 108.Mittal D, et al. TLR4-mediated skin carcinogenesis is dependent on immune and radioresistant cells. EMBO J. 2010;29:2242–2252. doi: 10.1038/emboj.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yusuf N, et al. Protective role of Toll-like receptor 4 during the initiation stage of cutaneous chemical carcinogenesis. Cancer research. 2008;68:615–622. doi: 10.1158/0008-5472.CAN-07-5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bauer AK, et al. Toll-like receptor 4 in butylated hydroxytoluene-induced mouse pulmonary inflammation and tumorigenesis. Journal of the National Cancer Institute. 2005;97:1778–1781. doi: 10.1093/jnci/dji403. [DOI] [PubMed] [Google Scholar]
- 111.Ahmed A, Wang JH, Redmond HP. Silencing of TLR4 Increases Tumor Progression and Lung Metastasis in a Murine Model of Breast Cancer. Annals of surgical oncology. 2012 doi: 10.1245/s10434-012-2595-9. [DOI] [PubMed] [Google Scholar]
- 112.Naseemuddin M, et al. Cell mediated immune responses through TLR4 prevents DMBA-induced mammary carcinogenesis in mice. International journal of cancer Journal international du cancer. 2012;130:765–774. doi: 10.1002/ijc.26100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Apetoh L, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature medicine. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 114.Srivastava K, Srivastava A, Kumar A, Mittal B. Significant association between toll-like receptor gene polymorphisms and gallbladder cancer. Liver Int. 2010;30:1067–1072. doi: 10.1111/j.1478-3231.2010.02268.x. [DOI] [PubMed] [Google Scholar]
- 115.Rigoli L, et al. TLR4 and NOD2/CARD15 genetic polymorphisms and their possible role in gastric carcinogenesis. Anticancer research. 2010;30:513–517. [PubMed] [Google Scholar]
- 116.Kutikhin AG. Impact of Toll-like receptor 4 polymorphisms on risk of cancer. Human immunology. 2011;72:193–206. doi: 10.1016/j.humimm.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 117.Lin H, et al. Loss of immunity-supported senescence enhances susceptibility to hepatocellular carcinogenesis and progression in Toll-like receptor 2-deficient mice. Hepatology. 2013;57:171–182. doi: 10.1002/hep.25991. [DOI] [PubMed] [Google Scholar]
- 118.Salcedo R, et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. The Journal of experimental medicine. 2010;207:1625–1636. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lowe EL, et al. Toll-like receptor 2 signaling protects mice from tumor development in a mouse model of colitis-induced cancer. PloS one. 2010;5:e13027. doi: 10.1371/journal.pone.0013027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.de Oliveira JG, Rossi AF, Nizato DM, Miyasaki K, Silva AE. Profiles of Gene Polymorphisms in Cytokines and Toll-Like Receptors with Higher Risk for Gastric Cancer. Dig Dis Sci. 2013 doi: 10.1007/s10620-012-2460-5. [DOI] [PubMed] [Google Scholar]
- 121.Pandey S, et al. Impact of Toll-like receptors [TLR] 2 (−196 to −174 del) and TLR 4 (Asp299Gly, Thr399Ile) in cervical cancer susceptibility in North Indian women. Gynecologic oncology. 2009;114:501–505. doi: 10.1016/j.ygyno.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 122.Ngo VN, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Treon SP, et al. MYD88 L265P somatic mutation in Waldenstrom’s macroglobulinemia. The New England journal of medicine. 2012;367:826–833. doi: 10.1056/NEJMoa1200710. [DOI] [PubMed] [Google Scholar]
- 124.Lohr JG, et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc Natl Acad Sci U S A. 2012;109:3879–3884. doi: 10.1073/pnas.1121343109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Swann J, et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci U S A. 2008;105:652–656. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wald D, et al. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003;4:920–927. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 127.Xiao H, et al. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity. 2007;26:461–475. doi: 10.1016/j.immuni.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 128.Yu P, et al. Nucleic acid-sensing Toll-like receptors are essential for the control of endogenous retrovirus viremia and ERV-induced tumors. Immunity. 2012;37:867–879. doi: 10.1016/j.immuni.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 129.Chin AI, et al. Toll-like receptor 3-mediated suppression of TRAMP prostate cancer shows the critical role of type I interferons in tumor immune surveillance. Cancer research. 2010;70:2595–2603. doi: 10.1158/0008-5472.CAN-09-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Forte G, et al. Polyinosinic-polycytidylic acid limits tumor outgrowth in a mouse model of metastatic lung cancer. J Immunol. 2012;188:5357–5364. doi: 10.4049/jimmunol.1103811. [DOI] [PubMed] [Google Scholar]
- 131.Chew V, et al. Toll-like receptor 3 expressing tumor parenchyma and infiltrating natural killer cells in hepatocellular carcinoma patients. Journal of the National Cancer Institute. 2012;104:1796–1807. doi: 10.1093/jnci/djs436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hasan UA, et al. Cell proliferation and survival induced by Toll-like receptors is antagonized by type I IFNs. Proc Natl Acad Sci U S A. 2007;104:8047–8052. doi: 10.1073/pnas.0700664104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee J, et al. Activation of innate immunity is required for efficient nuclear reprogramming. Cell. 2012;151:547–558. doi: 10.1016/j.cell.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rhee SH, Im E, Pothoulakis C. Toll-like receptor 5 engagement modulates tumor development and growth in a mouse xenograft model of human colon cancer. Gastroenterology. 2008;135:518–528. doi: 10.1053/j.gastro.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cai Z, et al. Activation of Toll-like receptor 5 on breast cancer cells by flagellin suppresses cell proliferation and tumor growth. Cancer research. 2011;71:2466–2475. doi: 10.1158/0008-5472.CAN-10-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schon MP, Schon M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190–199. doi: 10.1038/sj.onc.1210913. [DOI] [PubMed] [Google Scholar]
- 137.Sidky YA, et al. Inhibition of murine tumor growth by an interferon-inducing imidazoquinolinamine. Cancer research. 1992;52:3528–3533. [PubMed] [Google Scholar]
- 138.Hemmi H, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 139.Gibson SJ, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cellular immunology. 2002;218:74–86. doi: 10.1016/s0008-8749(02)00517-8. [DOI] [PubMed] [Google Scholar]
- 140.Fabbri M, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109:E2110–2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bourquin C, et al. Immunostimulatory RNA oligonucleotides induce an effective antitumoral NK cell response through the TLR7. J Immunol. 2009;183:6078–6086. doi: 10.4049/jimmunol.0901594. [DOI] [PubMed] [Google Scholar]
- 142.Lan T, et al. Stabilized immune modulatory RNA compounds as agonists of Toll-like receptors 7 and 8. Proc Natl Acad Sci U S A. 2007;104:13750–13755. doi: 10.1073/pnas.0706059104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Krieg AM. Development of TLR9 agonists for cancer therapy. The Journal of clinical investigation. 2007;117:1184–1194. doi: 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Baines J, Celis E. Immune-mediated tumor regression induced by CpG-containing oligodeoxynucleotides. Clinical cancer research: an official journal of the American Association for Cancer Research. 2003;9:2693–2700. [PubMed] [Google Scholar]
- 145.Brignole C, et al. Therapeutic targeting of TLR9 inhibits cell growth and induces apoptosis in neuroblastoma. Cancer research. 2010;70:9816–9826. doi: 10.1158/0008-5472.CAN-10-1251. [DOI] [PubMed] [Google Scholar]
- 146.Carpentier A, Chen L, Maltonti F, Delattre J. Oligodeoxynucleotides containing CpG motifs can induce rejection of a neuroblastoma in mice. Cancer research. 1999;59:5429–5432. [PubMed] [Google Scholar]
- 147.Heckelsmiller K, et al. Peritumoral CpG DNA elicits a coordinated response of CD8 T cells and innate effectors to cure established tumors in a murine colon carcinoma model. Journal of immunology (Baltimore, Md: 1950) 2002;169:3892–3899. doi: 10.4049/jimmunol.169.7.3892. [DOI] [PubMed] [Google Scholar]
- 148.Kortylewski M, et al. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat Biotechnol. 2009;27:925–932. doi: 10.1038/nbt.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Krieg AM. Toll-like receptor 9 (TLR9) agonists in the treatment of cancer. Oncogene. 2008;27:161–167. doi: 10.1038/sj.onc.1210911. [DOI] [PubMed] [Google Scholar]
- 150.Ballas ZK, Rasmussen WL, Krieg AM. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J Immunol. 1996;157:1840–1845. [PubMed] [Google Scholar]
- 151.Hartmann G, Weiner GJ, Krieg AM. CpG DNA: a potent signal for growth, activation, and maturation of human dendritic cells. Proc Natl Acad Sci U S A. 1999;96:9305–9310. doi: 10.1073/pnas.96.16.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kadowaki N, Antonenko S, Liu YJ. Distinct CpG DNA and polyinosinic-polycytidylic acid double-stranded RNA, respectively, stimulate CD11c- type 2 dendritic cell precursors and CD11c+ dendritic cells to produce type I IFN. J Immunol. 2001;166:2291–2295. doi: 10.4049/jimmunol.166.4.2291. [DOI] [PubMed] [Google Scholar]
- 153.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nature reviews Drug discovery. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 154.So EY, Ouchi T. The application of Toll like receptors for cancer therapy. International journal of biological sciences. 2010;6:675–681. doi: 10.7150/ijbs.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Droemann D, et al. Human lung cancer cells express functionally active Toll-like receptor 9. Respiratory research. 2005;6:1. doi: 10.1186/1465-9921-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ren T, et al. TLR9 signaling promotes tumor progression of human lung cancer cell in vivo. Pathology oncology research: POR. 2009;15:623–630. doi: 10.1007/s12253-009-9162-0. [DOI] [PubMed] [Google Scholar]
- 157.Ren T, et al. Functional expression of TLR9 is associated to the metastatic potential of human lung cancer cell: functional active role of TLR9 on tumor metastasis. Cancer biology & therapy. 2007;6:1704–1709. doi: 10.4161/cbt.6.11.4826. [DOI] [PubMed] [Google Scholar]
- 158.Merrell M, et al. Toll-like receptor 9 agonists promote cellular invasion by increasing matrix metalloproteinase activity. Molecular cancer research: MCR. 2006;4:437–447. doi: 10.1158/1541-7786.MCR-06-0007. [DOI] [PubMed] [Google Scholar]
- 159.Guha M. Anticancer TLR agonists on the ropes. Nature reviews Drug discovery. 2012;11:503–505. doi: 10.1038/nrd3775. [DOI] [PubMed] [Google Scholar]
- 160.Smits EL, Ponsaerts P, Berneman ZN, Van Tendeloo VF. The use of TLR7 and TLR8 ligands for the enhancement of cancer immunotherapy. Oncologist. 2008;13:859–875. doi: 10.1634/theoncologist.2008-0097. [DOI] [PubMed] [Google Scholar]
- 161.Geisse J, et al. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from two phase III, randomized, vehicle-controlled studies. Journal of the American Academy of Dermatology. 2004;50:722–733. doi: 10.1016/j.jaad.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 162.Love WE, Bernhard JD, Bordeaux JS. Topical imiquimod or fluorouracil therapy for basal and squamous cell carcinoma: a systematic review. Arch Dermatol. 2009;145:1431–1438. doi: 10.1001/archdermatol.2009.291. [DOI] [PubMed] [Google Scholar]
- 163.Rajpar SF, Marsden JR. Imiquimod in the treatment of lentigo maligna. Br J Dermatol. 2006;155:653–656. doi: 10.1111/j.1365-2133.2006.07476.x. [DOI] [PubMed] [Google Scholar]
- 164.Bong AB, et al. Imiquimod, a topical immune response modifier, in the treatment of cutaneous metastases of malignant melanoma. Dermatology. 2002;205:135–138. doi: 10.1159/000063904. [DOI] [PubMed] [Google Scholar]
- 165.Wolf IH, et al. Topical imiquimod in the treatment of metastatic melanoma to skin. Arch Dermatol. 2003;139:273–276. doi: 10.1001/archderm.139.3.273. [DOI] [PubMed] [Google Scholar]
- 166.Smyth EC, et al. Treatment of locally recurrent mucosal melanoma with topical imiquimod. J Clin Oncol. 2011;29:e809–811. doi: 10.1200/JCO.2011.36.8829. [DOI] [PubMed] [Google Scholar]
- 167.Adams S, et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin Cancer Res. 2012;18:6748–6757. doi: 10.1158/1078-0432.CCR-12-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sfondrini L, et al. Antitumor activity of the TLR-5 ligand flagellin in mouse models of cancer. J Immunol. 2006;176:6624–6630. doi: 10.4049/jimmunol.176.11.6624. [DOI] [PubMed] [Google Scholar]
- 169.Burdelya LG, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bekierkunst A, Levij IS, Yarkoni E. Suppression of urethan-induced lung adenomas in mice treated with trehalose-6,6-dimycolate (cord factor) and living bacillus Calmette Guerin. Science. 1971;174:1240–1242. doi: 10.1126/science.174.4015.1240. [DOI] [PubMed] [Google Scholar]
- 171.Sjogren HO, Ankerst J. Effect of BCG and allogeneic tumor cells on adenovirus type 12 tumorigenesis in mice. Nature. 1969;221:863–864. doi: 10.1038/221863a0. [DOI] [PubMed] [Google Scholar]
- 172.Zbar B, Bernstein I, Tanaka T, Rapp HJ. Tumor immunity produced by the intradermal inoculation of living tumor cells and living Mycobacterium bovis (strain BCG) Science. 1970;170:1217–1218. doi: 10.1126/science.170.3963.1217. [DOI] [PubMed] [Google Scholar]
- 173.Zbar B, Tanaka T. Immunotherapy of cancer: regression of tumors after intralesional injection of living Mycobacterium bovis. Science. 1971;172:271–273. doi: 10.1126/science.172.3980.271. [DOI] [PubMed] [Google Scholar]
- 174.Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180–183. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 175.Hall MC, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007;178:2314–2330. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 176.Babjuk M, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol. 2011;59:997–1008. doi: 10.1016/j.eururo.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 177.Kroemer G, Zitvogel L, Galluzzi L. Victories and deceptions in tumor immunology: Stimuvax. Oncoimmunology. 2013;2:e23687. doi: 10.4161/onci.23687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kolida S, Gibson GR. Synbiotics in health and disease. Annual review of food science and technology. 2011;2:373–393. doi: 10.1146/annurev-food-022510-133739. [DOI] [PubMed] [Google Scholar]
- 179.Le Leu RK, Hu Y, Brown IL, Woodman RJ, Young GP. Synbiotic intervention of Bifidobacterium lactis and resistant starch protects against colorectal cancer development in rats. Carcinogenesis. 2010;31:246–251. doi: 10.1093/carcin/bgp197. [DOI] [PubMed] [Google Scholar]
- 180.Rowland IR, Rumney CJ, Coutts JT, Lievense LC. Effect of Bifidobacterium longum and inulin on gut bacterial metabolism and carcinogen-induced aberrant crypt foci in rats. Carcinogenesis. 1998;19:281–285. doi: 10.1093/carcin/19.2.281. [DOI] [PubMed] [Google Scholar]
- 181.Grivennikov SI, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Couturier-Maillard A, et al. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. The Journal of clinical investigation. 2013 doi: 10.1172/JCI62236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bass NM, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 184.Fort MM, et al. A synthetic TLR4 antagonist has anti-inflammatory effects in two murine models of inflammatory bowel disease. Journal of immunology. 2005;174:6416–6423. doi: 10.4049/jimmunol.174.10.6416. [DOI] [PubMed] [Google Scholar]
- 185.Rossignol DP, Lynn M. Antagonism of in vivo and ex vivo response to endotoxin by E5564, a synthetic lipid A analogue. J Endotoxin Res. 2002;8:483–488. doi: 10.1179/096805102125001127. [DOI] [PubMed] [Google Scholar]
- 186.Sha T, et al. Therapeutic effects of TAK-242, a novel selective Toll-like receptor 4 signal transduction inhibitor, in mouse endotoxin shock model. Eur J Pharmacol. 2007;571:231–239. doi: 10.1016/j.ejphar.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 187.Arslan F, et al. Treatment with OPN-305, a humanized anti-Toll-Like receptor-2 antibody, reduces myocardial ischemia/reperfusion injury in pigs. Circ Cardiovasc Interv. 2012;5:279–287. doi: 10.1161/CIRCINTERVENTIONS.111.967596. [DOI] [PubMed] [Google Scholar]