Abstract
In the absence of recombination, a mutator allele can spread through a population by hitchhiking with beneficial mutations that appear in its genetic background. Theoretical studies over the past decade have shown that the survival and fixation probability of beneficial mutations can be severely reduced by population size bottlenecks. Here we use computational modeling and evolution experiments with the yeast S. cerevisiae to examine whether population bottlenecks can affect mutator dynamics in adapting asexual populations. In simulation, we show that population bottlenecks can inhibit mutator hitchhiking with beneficial mutations and are most effective at lower beneficial mutation supply rates. We then subjected experimental populations of yeast propagated at the same effective population size to three different bottleneck regimes and observed that the speed of mutator hitchhiking was significantly slower at smaller bottlenecks, consistent with our theoretical expectations. Our results, thus, suggest that bottlenecks can be an important factor in mutation rate evolution and can in certain circumstances act to stabilize or, at least, delay the progressive elevation of mutation rates in asexual populations. Additionally, our findings provide the first experimental support for the theoretically postulated effect of population bottlenecks on beneficial mutations and demonstrate the usefulness of studying mutator frequency dynamics for understanding the underlying dynamics of fitness-effecting mutations.
Keywords: mutation rate, hitchhiking, population bottlenecks, yeast, beneficial mutations, asexual populations
Introduction
Theoretical studies of mutation rate evolution have generally focused on the indirect selection experienced by mutator alleles (defects at mutation rate modifier loci that increase the genomic mutation rate) due to their enhanced associations with fitness-affecting mutations at other loci. Because these associations can be quickly eroded by recombination, indirect selection on mutators is generally expected to be most effective in asexual populations. In fact, a number of modeling (Taddei et al., 1997; Tenaillon et al., 1999; Gerrish et al., 2007) and experimental (Chao & Cox, 1983; Sniegowski et al., 1997; Notley-McRobb et al., 2002; Gentile et al., 2011) studies have shown that mutators can reach high frequencies and even achieve fixation in asexual populations by hitchhiking with beneficial mutations. Natural asexual populations also frequently display evidence of mutator hitchhiking: Clinical populations of pathogenic microbes often contain mutator strains at considerable frequencies (Suarez et al., 1992; LeClerc et al., 1996; Matic et al., 1997; Oliver et al., 2000). Cancers, which are essentially large asexual populations of neoplastic cells, are frequently associated with genomic instability, resulting in extensive genetic and phenotypic heterogeneity within a neoplasm (Sprouffske et al., 2012).
The dynamics of mutator hitchhiking with linked beneficial mutations have been elucidated in experimental studies. Using experimental populations of E. coli, Chao and Cox (1983) showed that mutators could displace isogenic wild-type strains if started above a certain threshold frequency, indicating that mutator success in competition with wild type depends on the supply rate of beneficial mutations to both populations; the significance of the relative beneficial mutation rates was corroborated in experiments of de Visser and Rozen (2006) and Gentile et al. (2011). In a different study, Raynes et al. (2012) showed that mutator hitchhiking could also be affected by the total supply of beneficial mutations to the whole population, so that mutator fixation could be delayed or even inhibited by clonal interference in populations with sufficiently high supply rates of beneficial mutations. Mutators have also been shown to be more successful in populations facing stronger selective pressures in which large-effect beneficial mutations are more likely to be available to accelerate mutator hitchhiking (Mao et al., 1997; Thompson et al., 2006; Gentile et al., 2011).
Notably, while most theory has been developed for populations of constant size and experimental studies have never explicitly considered population size fluctuations, mutators may be expected to face such fluctuations and severe bottlenecks in both experimental and natural populations. In experimental populations, regular population bottlenecks are frequently an inherent feature of the propagation protocol – during serial passaging, for example, samples of experimental populations are repeatedly transferred to fresh medium to allow for continuous growth. In clinical populations, size fluctuations may be more irregular, but bottlenecks are still likely to occur periodically when pathogenic microbes are transmitted from one host to the next or when parts of a tumor are resected in surgery.
In a series of theoretical studies Wahl and colleagues have examined how regular population bottlenecks – severe, quick reductions in population size – may decrease the probability that a new beneficial mutation survives drift and ultimately reaches fixation (Wahl & Gerrish, 2001; Wahl et al., 2002; Campos & Wahl, 2010). Wahl et al. showed that the probability of fixation for a beneficial mutation with fitness advantage s, classically estimated as 2s (Haldane, 1927), is reduced by periodic bottlenecks by a factor of D(lnD)2, where D is the dilution ratio – meaning that more severe bottlenecks are more effective at preventing fixation (Wahl et al., 2002). Given the evidence that mutator hitchhiking depends on linkage with beneficial mutations, it seems likely that population bottlenecks should play an important role in the evolution of mutation rates too. Specifically, we hypothesized that population size bottlenecks can inhibit hitchhiking of a mutator allele by reducing survival of beneficial mutations appearing in its genetic background.
To test this idea, we modeled mutator dynamics in populations subjected to regular bottlenecks. Our simulations show that bottlenecks can severely reduce the probability of mutator fixation while significantly delaying fixation of successful mutators, and that the effect of population bottlenecks depends greatly on population parameters such as population size and mutation rate. We then tested the expectation that smaller bottlenecks should more effectively inhibit mutator hitchhiking in experimental populations of yeast. Consistent with our theoretical results, we show that mutator hitchhiking is significantly delayed in populations subjected to smaller bottlenecks.
Materials and methods
Computer simulations
Wright-Fisher process
We consider a population of constant size N, evolving in discrete, non-overlapping generations according to the Wright-Fisher model (Ewens, 2004). The population is organized into fitness classes consisting of all individuals of the same fitness. Each generation, all fitness classes contribute progeny to form the next generation, with probability proportional to their frequency in the current generation and their relative fitness. Upon reproduction, each fitness class acquires a number of deleterious mutations Xd, randomly drawn from a Poisson distribution with mean NjUd where Nj is the size of the class and Ud is the deleterious mutation rate of the class and a number of beneficial mutations Xb, drawn from a Poisson distribution with mean UbNj, where Ub is the beneficial genomic mutation rate. We assume that fitness effects of deleterious mutations are constant (all reduce fitness by sd, set at 0.01 throughout) while fitness effects of beneficial mutations are additive and independently drawn from an exponential distribution with mean sb. Thus, individuals in a fitness class with x deleterious mutations and y beneficial mutations have a fitness of
Every 10 generations, a random sample of the population, DN, where D is the bottleneck dilution ratio, is chosen for reproduction, resulting in regular population size bottlenecks. Because classes acquire new mutations after reproduction, population bottlenecks do not affect the rates with which new mutations appear in simulated populations.
Each simulation begins with a population of N mutation-free individuals with mutators present at an initial frequency of 50%. In our simulations mutators have no effect on fitness and only act to increase the intrinsic deleterious and beneficial mutation rates by a factor of μ. New mutators cannot appear by mutation and a given simulation run ends when the mutators present at the outset reach fixation or go extinct. Otherwise, a population is allowed to evolve for a predetermined number of generations, G, and whether the mutators have approached fixation (reached a threshold frequency of 95%) by the end of the simulation is assessed. For each combination of parameters we performed 1000 independent runs of the simulation and then calculated the probability of mutator fixation (as the ratio of runs where mutators either fixed during simulation or approached fixation by the end to the total number of runs) and the average time to mutator fixation.
Evolution experiments
Strains, medium, and conditions
Experiments were conducted using wild-type and mutator haploid strains of Saccharomyces cerevisiae. The wild type strain, YPS 3343 (ho::nat, MSH2, MAT a), was originally isolated from Mettler’s Woods, NJ (Kuehne et al., 2007). The otherwise isogenic mutator strain was constructed as previously described (Raynes et al., 2011) by replacing the wild-type MSH2 locus with the msh2::kan allele (ho::nat, msh2::kan, MAT a), resulting in a 60-fold increase in the mutation rate over the wild type (Raynes et al. 2011). The msh2::kan allele (hereafter msh2Δ) also provides resistance to the antibiotic geneticin, which was used as a marker to track mutator frequency during propagation. All populations were propagated in 50 mL Erlenmeyer flasks placed in a shaking incubator at 30°C and 200 rpm. Standard SD minimal medium (1% glucose) was used for all experiments (Rose et al. 1990).
Experimental propagation
Mutator and wild-type strains were streaked onto agar plates from frozen stocks and grown overnight. Individual colonies were randomly chosen to start 24 mutator and 24 wild-type overnight cultures that were then combined at roughly equal frequencies to establish eight replicate populations for each of the three different bottleneck regimes: large, medium, and small. Populations of the large bottleneck regime were propagated in 2 mL cultures by daily 1:100 dilutions, resulting in about 6.6 generations of growth per day. Populations of the medium bottleneck regime were propagated in 10 mL cultures by daily 1:740 dilutions, resulting in about 9.5 generations of growth per day. Populations of the small bottleneck regime were propagated in 20 mL cultures by daily 1:1626 dilutions, resulting in about 10.7 generations of growth per day. All dilutions and culture volumes were chosen to keep similar effective population sizes - 4.38x106, 4.25x106, and 4.33x106 for small, medium, and large bottleneck populations respectively, estimated as Ne = gNo, where g is the number of generations between transfers and No is the initial population size (Lenski et al., 1991). Mutator frequencies were estimated during the course of the experiment by plating population samples on permissive YPD plates and then replica plating about 100–200 colonies onto YPD + geneticin plates. The time to fixation was estimated as the first time when no wild-type colonies were found in the replica plated sample. Mean times to fixation between the three treatments were compared using a two-tailed t-test for each pair of treatments.
Results
Computer simulations
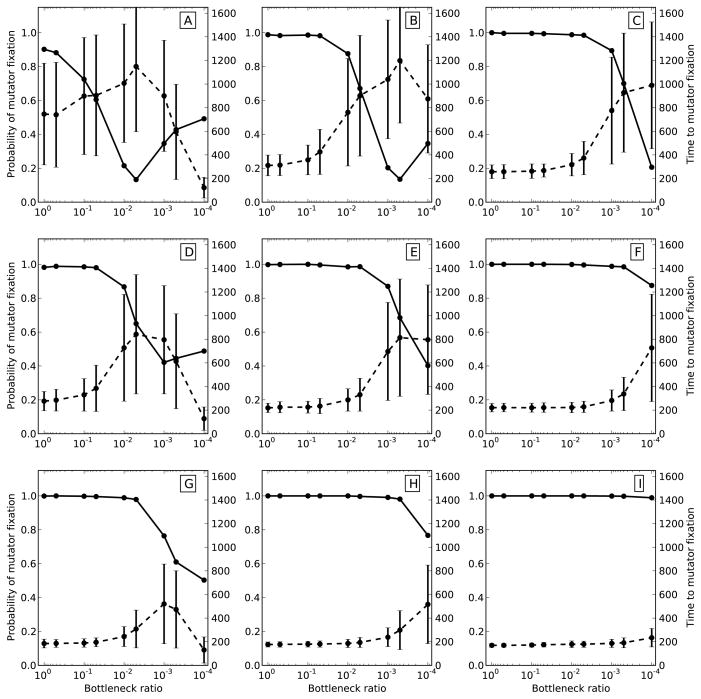
We employed Wright-Fisher simulations to model mutator dynamics in asexual populations subjected to regular population size bottlenecks. Replicate populations were allowed to evolve for 2000 generations (see Figure S1 for further examination of this generation limit) or until the mutator present at the outset of simulation either swept to fixation or went extinct. All runs were started with mutators present at an initial frequency of 50%. Probability of mutator fixation after the 2000 generations of evolution and time to mutator fixation were then assessed over a range of bottleneck ratios (population size to bottleneck size) from 1 to 10−4 and model parameters, including population size, beneficial mutation rate and effect, and mutator mutation rate.
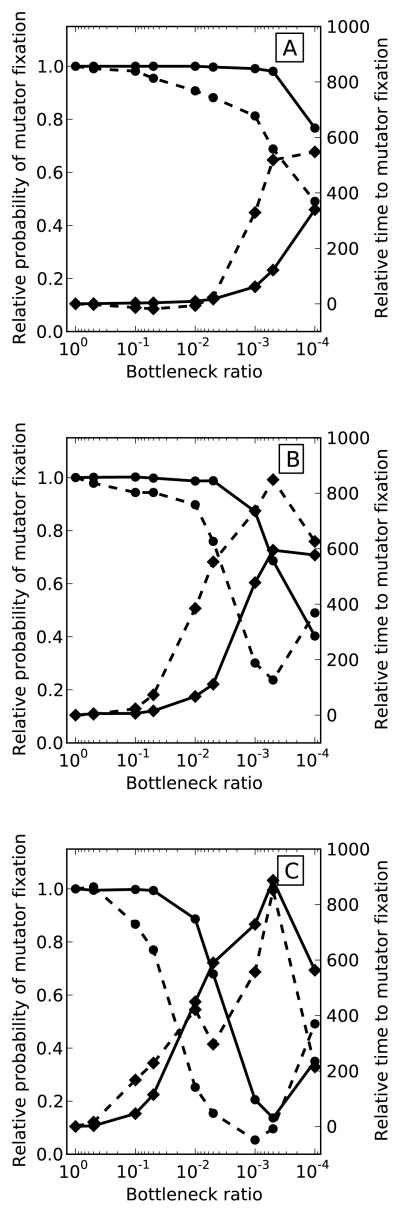
Figure 1 summarizes simulation results for a 50-fold mutator over a range of beneficial mutation supply rates - NUb, which was adjusted by varying the population size (kept constant between bottleneck events), N, from 105 to 107 individuals and the beneficial mutation rate, Ub, from 10−6 to 10−8 mutations per individual per generation. Beneficial mutations were drawn from an exponential distribution with mean sb = 0.05. Generally, reducing bottleneck size decreased the probability of mutator fixation after 2000 generations and increased the average waiting time to fixation of successful mutators. The effect of population bottlenecks was more pronounced at smaller beneficial mutation supply rates (smaller N and Ub). For example, in populations with the smallest NUb (Figure 1A) bottlenecks had a rather dramatic effect, severely reducing mutator fixation probability while in populations with the highest NUb (Figure 1I) bottlenecks had practically no detectable effect. In some populations the probability of mutator hitchhiking increased at very small bottlenecks after declining at larger bottlenecks (e.g. Figures 1A and 1B). This apparent increase in the rate of mutator hitchhiking is likely explained by the effectively random sampling of mutators during the very small bottlenecks. In fact, we observed that fixation probability appeared to approach the starting mutator frequency in severely bottlenecked populations regardless of the strength of the mutator (compare Figures 1 and 2S). Furthermore, when mutators were initialized at 10% rather than 50% in a separate set of simulations, the probability of fixation in populations with small NUb appeared to plateau around 10% rather than 50% (not shown).
Figure 1. The effect of population bottlenecks on mutator dynamics at different supply rates of beneficial mutations in simulated populations.
Probability of mutator fixation (solid lines) generally declined with smaller bottlenecks while the time to mutator fixation (dashed lines) increased and became more variable. Bottlenecks were particularly effective at smaller beneficial mutation supply rates. The supply rate of beneficial mutations, NUb, was adjusted by varying the population size, N, between 105 (Column 1), 106 (Column 2), and 107 (Column 3) individuals and beneficial mutation rate, Ub, between 10−6 (Row 1), 10−7 (Row 2), and 10−8 (Row3) mutations per individual per generation. Beneficial mutations were drawn from a exponential distribution with mean 0.05 and the mutator increased the mutation rate 50-fold. Error bars on the time to fixation represent 1 standard deviation. Results are averaged over 1000 runs of the simulation.
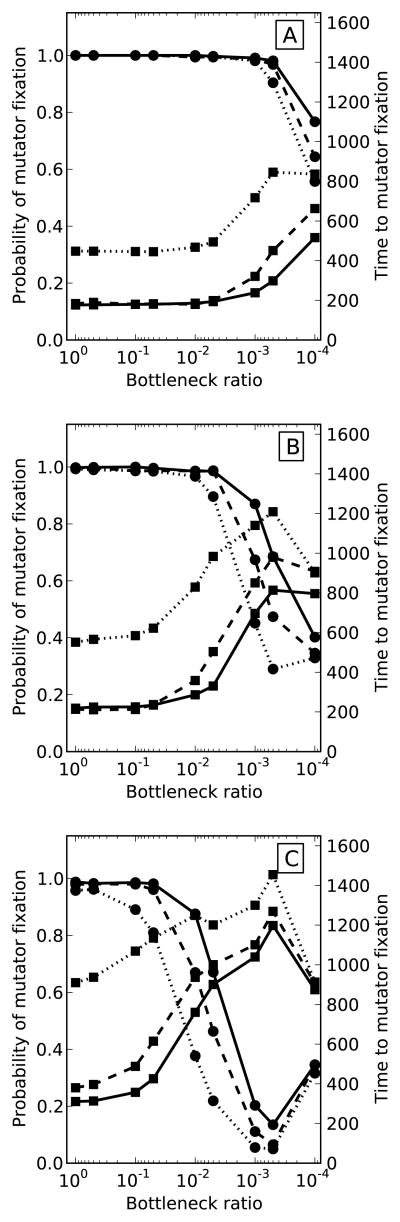
To examine the influence of selection strength on beneficial mutations, we varied the mean selective effect of new beneficial mutations, sb. Figure 2 shows simulation results for a 50-fold mutator in bottlenecked populations with beneficial mutations drawn from exponential distributions with mean sb equal to 0.05, 0.02, or 0.01. Populations were evolved at the same size of 106 individuals and three different beneficial mutation rates: from 10−6 to 10−8 mutations per individual per generation. Despite different mean sb, mutators were able to fix in all non-bottlenecked populations. As before, bottlenecks appeared to inhibit mutator fixation across all parameter combinations. However, as populations were subjected to smaller bottlenecks, mutator hitchhiking was delayed and, consequently, the probability of mutator fixation was reduced to a greater extent in populations with smaller sb.
Figure 2. Selection strength of beneficial mutations and mutator dynamics in bottlenecked populations.
Bottlenecks inhibited mutator fixation across all parameter combinations simulated. The probability of fixation (circles) declined while the average time to fixation (squares) increased faster in populations with smaller mutations. Beneficial mutations were drawn from exponential distributions with means 0.05 (solid), 0.02 (dashed), or 0.01 (dotted). All populations were propagated at N = 106, while Ub varied between 10−6 (A), 10−7 (B), and 10−8 (C). The mutator increased the mutation rate 50-fold. Error bars on the time to fixation represent 1 standard deviation. Results are averaged over 1000 runs of the simulation.
Finally, we looked at the dynamics of a weaker 5-fold mutator in regularly bottlenecked populations. As in figure 2, we simulated populations of size 106 individuals at three different beneficial mutation rates. Weaker mutators are expected to produce fewer beneficial mutations that could facilitate their hitchhiking. Thus, it was expected that they hitchhiked slower than the 50-fold mutators in Figure 1 and, as a result, were less likely to reach fixation in the 2000 generations of the simulation (Figure 2S). To assess whether bottlenecks affected the two mutators differently, though, we compared their hitchhiking dynamics at different bottlenecks relative to their dynamics in non-bottlenecked populations. As seen in Figure 3, hitchhiking of the weaker mutator was, in fact, inhibited to a greater extent than that of the stronger mutator. Clearly, the weaker mutator could be inhibited by larger bottlenecks that did not affect the stronger mutator as much, and at higher supply rates of beneficial mutations.
Figure 3. Mutator strength and population bottlenecks.
Relative fixation probabilities (circles) were calculated as the observed fixation probability over the fixation probability in a non-bottlenecked simulation. Relative average times to fixation (squares) were calculated as the observed average fixation time minus the average fixation time in a non-bottlenecked population. Relative fixation probability of a weaker 5-fold mutator (dashed) was generally lower and declined faster than that of a stronger 50-fold mutator (solid). Relative time to fixation of the 5-fold mutator (dashed) was generally greater than that of the 50-fold mutator and increased faster with smaller bottlenecks. All populations were propagated at N = 106, while Ub varied between 10−6 (A), 10−7 (B), and 10−8 (C). Results are averaged over 1000 runs of the simulation.
Evolution experiments
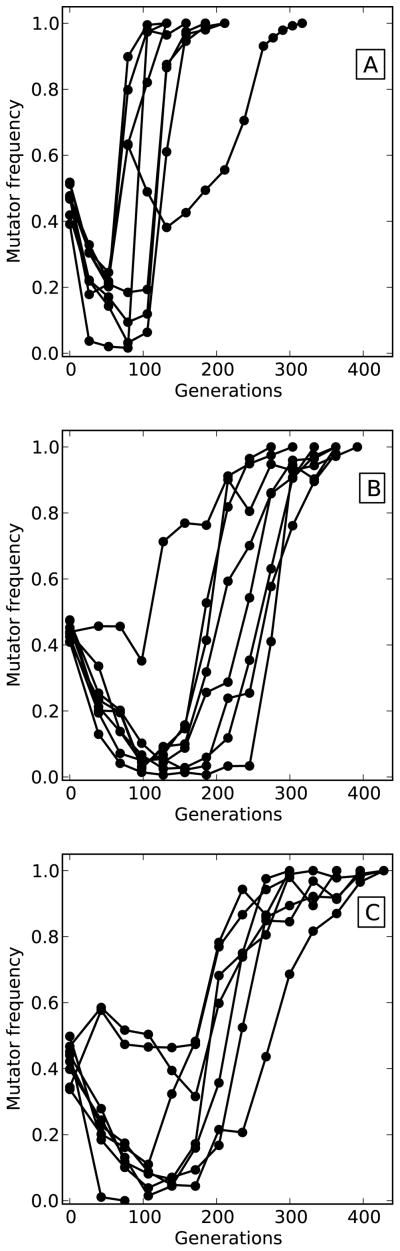
We propagated 24 experimental yeast populations at approximately identical effective population sizes but subjected to three different regular bottleneck regimes (Figure 4). In populations experiencing the large bottlenecks, mutators initially declined in frequency but eventually rebounded and approached fixation in all replicate populations within approximately 150 generations. These dynamics are very similar to those previously observed at this population size (Raynes et al., 2012) and consistent with an earlier postulated cost of msh2Δ (Raynes et al., 2011). Mutator fixation took significantly longer in populations going through the medium size bottlenecks (t14 = 6.10; p = 2.70x10−5) and small size bottlenecks (t13 = 6.89; p = 1.11x10−5) with both the initial mutator decline and the eventual rise taking approximately 100 generations longer than in the large bottleneck populations. There was no significant difference in average mutator fixation time between the medium and small bottleneck populations (t13 = 1.91; p = 0.08).
Figure 4. Mutator dynamics in experimental populations.
Asexual populations of yeast were propagated at a similar effective population size but were subjected to three different bottleneck regimes. A) Large bottlenecks - 2 mL cultures that experienced daily 1:100 dilutions. B) Medium bottlenecks - 10 mL cultures that experienced daily 1:740 dilutions. C) Small bottlenecks - 20 mL cultures that experienced daily 1: 1626 dilutions.
Discussion
Here, we have used computer simulations and evolution experiments to examine the dynamics of mutator hitchhiking in regularly bottlenecked asexual populations. Our work was motivated by theoretical results showing that population size bottlenecks can severely reduce survival and fixation probability of new beneficial mutations and, thus, impede adaptation (Wahl & Gerrish, 2001; Wahl et al., 2002). We postulated that by inhibiting beneficial mutations, population bottlenecks could also slow down mutator hitchhiking and reduce the probability of mutator fixation in asexual populations. In simulations, we investigated a number of population- and individual-level parameters and observed that mutator hitchhiking was possible in all parameter regimes, consistent with initial mutator frequency always being above the mutator-to-wild type threshold ratio theoretically predicted to lead to mutator fixation.
However, mutator hitchhiking was considerably delayed, and the probability of mutator fixation over the course of the simulation was substantially reduced, by regular population bottlenecks. Consistent with the expected impact of bottlenecks on the survival of beneficial mutations (Wahl & Gerrish, 2001) we observed that smaller bottlenecks were more effective at delaying mutator hitchhiking. Furthermore, as bottlenecks got smaller, mutator dynamics became more variable, likely due to bottlenecks randomly eliminating different mutations in different populations. Mutator dynamics were also more affected by bottlenecks in populations with lower supply rates of beneficial mutations (Figure 1) and weaker mutators (Figure 2) – both in accordance with the expected effect of bottlenecks on beneficial mutations of low frequency. Finally, mutator hitchhiking was more influenced by bottlenecks in populations with beneficial mutations of smaller fitness effect (Figure 3), consistent with such mutations being less likely to survive in bottlenecked populations (Wahl et al., 2002). Importantly, the difference in mutator dynamics across dilution ratios could not be explained by different supply rates of beneficial mutations because the size of the mutating population was not affected by bottlenecks (mutations were generated after reproduction).
We then used experimental populations of yeast, all propagated at a similar effective size, to compare rates of mutator fixation at three different bottleneck regimes (Figure 4). In agreement with our modeling results, mutator fixation was significantly faster in populations experiencing large bottlenecks than in populations experiencing medium or small bottlenecks. Interestingly, we did not observe a statistically significant difference between populations subjected to medium and small bottlenecks. According to the Wahl et al. (2002) estimate for the fixation probability of a beneficial mutation of selective advantage s, 2sD(lnD)2, our large bottlenecks reduced this probability by approximate 0.2 while the medium and small bottlenecks reduced it by approximately 0.06 and 0.03 respectively. The less-pronounced difference between the predicted effects of the smaller bottleneck sizes is consistent with the similar mutator dynamics in these populations. On a final note, due to experimental limitations, populations that were subjected to smaller bottlenecks went through more generations of growth between bottleneck events. Because probability of survival for a beneficial mutation increases with time between bottlenecks, the realized difference between mutator dynamics in our populations was, therefore, smaller than it would have likely been in populations subjected to the same bottlenecks but administered at equal intervals.
In summary, our simulation results and evolution experiments provide two independent lines of evidence that random population bottlenecks can inhibit or at least delay mutator hitchhiking by reducing survival probability of incoming beneficial mutations. Earlier work on mutator dynamics in experimental E. coli populations showed that mutators were likely to displace wild-type individuals in an asexual population if present at a sufficiently high frequency, such that the supply rate of new beneficial mutations to the mutator subpopulation was higher than that to the wild type (Chao & Cox, 1983; de Visser & Rozen, 2006). We have previously shown that mutator hitchhiking could be hindered and sometimes completely inhibited by clonal interference even if mutators were present above this threshold frequency (Raynes et al., 2012). Here, we provide evidence for another mechanism that could help delay or even inhibit mutator hitchhiking and potentially help stabilize mutation rate evolution, at least in populations with sufficiently low supply rates of new beneficial mutations. Notably, mutator hitchhiking in bottlenecked populations would clearly be even more difficult when mutator alleles must be generated by mutation rather than being artificially seeded in at relatively high frequencies.
In light of our results, it seems unlikely for natural populations experiencing extreme bottlenecks to evolve elevated mutation rates by mutator hitchhiking. For example, infectious microbial diseases that are easily transmitted between hosts but do not persist in any individual host for long (either because they are rapidly cleared or lethal) may be unlikely to evolve high mutation rates unless large beneficial mutations are available. While tumors do not usually experience multiple bottlenecks, surgery may at least delay the evolution of genomic instability, which could have significant clinical implications as genomic instability is frequently associated with poor outcome (Walther et al., 2008) and multidrug resistance (Lee et al., 2011) in cancer. Importantly, in order to be effective, bottlenecks must be random with respect to the genetic variation in the population. Drug therapies, while inducing severe bottlenecks, may actually help the spread of mutators by selecting for resistance mutations that are more likely to arise in the mutator background. Rapid mutator enrichment in response to hard selection, which mimics such therapy, has been demonstrated in E.coli adapting to switching energy source (Mao et al., 1997) and antibiotic exposure (Mao et al., 1997; Gentile et al., 2011).
Our study provides the first experimental evidence for the theory developed by Wahl et al. (Wahl & Gerrish, 2001; Wahl et al., 2002; Campos & Wahl, 2009; Campos & Wahl, 2010) that bottlenecks can inhibit the spread of beneficial mutations. More generally, our work illustrates the utility of competition studies between mutator and wild-type individuals in elucidating some of the details of the underlying mutational processes. Mutator dynamics in such experiments are driven by multitudes of new mutations and can provide information about the overall rates (Raynes et al., 2012) and effects (Raynes et al., 2011) of fitness-affecting mutations. More fine-grained information can be provided by the analysis of whole-genome re-sequencing approaches to experimental evolution (e.g. Lang et al., 2013), which allow the tracking of large numbers of individual mutations in evolving experimental populations; to date, however, such experiments can still realistically follow only a small fraction of new mutations and cannot easily determine their fitness effects.
Finally, our work has implications for understanding effective population size, Ne, in bottlenecked populations in general, and for practical reasons, in experimental evolution studies that frequently include regular bottlenecks as part of the propagation protocol. It has been generally accepted that Ne in such populations depends strongly on the size of the transferred sample (bottleneck), No, and not on the maximum population size (Kawecki et al., 2012) and so it has been usually estimated as Ne = gNo (Lenski et al., 1991). However, our experimental populations were propagated at approximately the same effective population size as estimated by this conventional formula, yet displayed very different evolutionary dynamics. Thus, our results suggest that the ratio of transfer size to maximum population size, the dilution ratio D, is an important influence in bottlenecked populations and should be accounted for in the definition of the effective population size (Wahl & Gerrish, 2001).
Supplementary Material
Figure S1: The effect of the generation limit on simulation results
Figure S2: Dynamics of a weaker 5-fold mutator
Acknowledgments
We thank P.J. Gerrish and M.R. Gazzara for valuable discussion, D.M. Weinreich for general help with simulations, C.W. Zeyl for sharing yeast strains, and three anonymous reviewers for their constructive comments. This work was supported by National Institutes of Health Grant GM079483-01A2.
References
- Campos PRA, Wahl LM. The Effects of Population Bottlenecks on Clonal Interference, and the Adaptation Effective Population Size. Evolution. 2009;63:950–958. doi: 10.1111/j.1558-5646.2008.00595.x. [DOI] [PubMed] [Google Scholar]
- Campos PRA, Wahl LM. The adaptation rate of asexuals: deleterious mutations, clonal interference and population bottlenecks. Evolution. 2010;64:1973–1983. doi: 10.1111/j.1558-5646.2010.00981.x. [DOI] [PubMed] [Google Scholar]
- Chao L, Cox EC. Competition between high and low mutating strains of Escherichia coli. Evolution. 1983;37:125–134. doi: 10.1111/j.1558-5646.1983.tb05521.x. [DOI] [PubMed] [Google Scholar]
- de Visser JAGM, Rozen DE. Clonal interference and the periodic selection of new beneficial mutations in Escherichia coli. Genetics. 2006;172:2093–2100. doi: 10.1534/genetics.105.052373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewens W. Mathematical Population Genetics. Springer; New York: 2004. [Google Scholar]
- Gentile CF, Yu SC, Serrano SA, Gerrish PJ, Sniegowski PD. Competition between high- and higher-mutating strains of Escherichia coli. Biology Letters. 2011;7:422–424. doi: 10.1098/rsbl.2010.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrish PJ, Colato A, Perelson AS, Sniegowski PD. Complete genetic linkage can subvert natural selection. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6266–6271. doi: 10.1073/pnas.0607280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldane JBS. A methematical theory of natural and artificial selection. Part V. Selection and mutation. Proceedings of the Cambridge Philosophical Society. 1927;23:838–844. [Google Scholar]
- Kawecki TJ, Lenski RE, Ebert D, Hollis B, Olivieri I, Whitlock MC. Experimental evolution. Trends in Ecology & Evolution. 2012;27:547–560. doi: 10.1016/j.tree.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Lang GI, Rice DP, Hickman MJ, Sodergren E, Weinstock GM, Botstein D, Desai MM. Pervasive genetic hitchhiking and clonal interference in forty evolving yeast populations. Nature. 2013;500:571–574. doi: 10.1038/nature12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClerc JE, Li B, Payne WL, Cebula TA. High mutation frequencies among Escherichia coli and Salmonella pathogens. Science. 1996;274:1208–1211. doi: 10.1126/science.274.5290.1208. [DOI] [PubMed] [Google Scholar]
- Lee AJX, Endesfelder D, Rowan AJ, Walther A, Birkbak NJ, Futreal PA, Downward J, Szallasi Z, Tomlinson IPM, Howell M, Kschischo M, Swanton C. Chromosomal Instability Confers Intrinsic Multidrug Resistance. Cancer Research. 2011;71:1858–1870. doi: 10.1158/0008-5472.CAN-10-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenski RE, Rose MR, Simpson SC, Tadler SC. Long-term experimental evolution in Escherichia coli.I. Adaptation and divergence during 2,000 generations. The American Naturalist. 1991;138:1315–1341. [Google Scholar]
- Mao E, Lane L, Lee J, Miller J. Proliferation of mutators in a cell population. J Bacteriol. 1997;179:417–422. doi: 10.1128/jb.179.2.417-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matic I, Radman M, Taddei F, Picard B, Doit C, Bingen E, Denamur E, Elion J. Highly variable mutation rates in commensal and pathogenic Escherichia coli. Science. 1997;277:1833–1834. doi: 10.1126/science.277.5333.1833. [DOI] [PubMed] [Google Scholar]
- Notley-McRobb L, Seeto S, Ferenci T. Enrichment and elimination of mutY mutators in Escherichia coli populations. Genetics. 2002;162:1055–1062. doi: 10.1093/genetics/162.3.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver A, Canton R, Campo P, Baquero F, Blazquez J. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science. 2000;288:1251. doi: 10.1126/science.288.5469.1251. [DOI] [PubMed] [Google Scholar]
- Raynes Y, Gazzara M, Sniegowski P. Mutator dynamics in sexual and asexual experimental populations of yeast. BMC Evolutionary Biology. 2011;11:158. doi: 10.1186/1471-2148-11-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynes Y, Gazzara MR, Sniegowski PD. Contrasting dynamics of a mutator allele in asexual populations of differing size. Evolution. 2012;66:2329–2334. doi: 10.1111/j.1558-5646.2011.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sniegowski PD, Gerrish PJ, Lenski RE. Evolution of high mutation rates in experimental populations of E. coli. Nature. 1997;387:703–705. doi: 10.1038/42701. [DOI] [PubMed] [Google Scholar]
- Sprouffske K, Merlo, Lauren MF, Gerrish Philip J, Maley Carlo C, Sniegowski Paul D. Cancer in Light of Experimental Evolution. Current biology : CB. 2012;22:R762–R771. doi: 10.1016/j.cub.2012.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez P, Valcarcel J, Ortin J. Heterogeneity of the mutation rates of influenza A viruses: isolation of mutator mutants. J Virol. 1992;66:2491–2494. doi: 10.1128/jvi.66.4.2491-2494.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei F, Radman M, Maynard-Smith J, Toupance B, Gouyon PH, Godelle B. Role of mutator alleles in adaptive evolution. Nature. 1997;387:700–702. doi: 10.1038/42696. [DOI] [PubMed] [Google Scholar]
- Tenaillon O, Toupance B, Le Nagard H, Taddei F, Godelle B. Mutators, population size, adaptive landscape and the adaptation of asexual populations of bacteria. Genetics. 1999;152:485–493. doi: 10.1093/genetics/152.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DA, Desai MM, Murray AW. Ploidy controls the success of mutators and nature of mutations during budding yeast evolution. Current Biology. 2006;16:1581–1590. doi: 10.1016/j.cub.2006.06.070. [DOI] [PubMed] [Google Scholar]
- Wahl LM, Gerrish PJ. The Probability That Beneficial Mutations Are Lost in Populations with Periodic Bottlenecks. Evolution. 2001;55:2606–2610. doi: 10.1111/j.0014-3820.2001.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Wahl LM, Gerrish PJ, Saika-Voivod I. Evaluating the impact of population bottlenecks in experimental evolution. Genetics. 2002;162:961–971. doi: 10.1093/genetics/162.2.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther A, Houlston R, Tomlinson I. Association between chromosomal instability and prognosis in colorectal cancer: a meta-analysis. Gut. 2008;57:941–950. doi: 10.1136/gut.2007.135004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: The effect of the generation limit on simulation results
Figure S2: Dynamics of a weaker 5-fold mutator