Abstract
Evidence shows that both poor physical fitness and obesity are linked to low-grade inflammation and inflammatory diseases. However, their relative roles on inflammation and underlying mechanisms remain unclear. Given the inhibitory effect of catecholamines on inflammatory cytokine production, we speculated that compromised responsiveness of immune cells’ beta adrenergic receptors (β-ARs) to agonists may be associated with constitutively elevated levels of inflammatory cytokines. We examined circulating levels of inflammatory cytokines TNF, IL-1β, IL-6 and β-AR sensitivity of, 70 overweight or obese compared to 26 normal-weight, otherwise healthy individuals in order to investigate the associations among obesity, physical fitness, and low-grade inflammation and to examine the role of β-ARs in these relationships. Cardiorespiratory fitness was determined by VO2peak (ml/kg/min) via a treadmill exercise. Beta-AR sensitivity was evaluated by measuring the degree of inhibition in lipopolysaccharides-stimulated monocytic intracellular TNF production by isoproterenol. In all participants, BMI, which was initially a predictor of IL-1β and IL-6 levels independent of demographic characteristics, no longer significantly predicted them after controlling for fitness levels. Among the overweight or obese participants, greater cardiorespiratory fitness was a strong predictor of lower levels of TNF and IL-1β after controlling for the covariates. When β-AR sensitivity was controlled for, however, fitness was no longer a significant predictor of those cytokines. Monocytic β-AR sensitivity was negatively associated with inflammatory marker levels and diminished in obese individuals; however, when fitness was controlled for, the significant weight group differences in β-AR sensitivity disappeared. Our findings indicate that better cardiorespiratory fitness protects against obesity-related low-grade inflammation and β-AR desensitization. Given the significance of β-AR function in pathogenesis of various diseases, clinical implications of its role in the fitness-inflammation association among the obese are profound.
Keywords: Beta adrenergic receptor, LPS-stimulated TNF production, Monocytes, Obesity, Physical fitness, Sensitivity to isoproterenol
1. Introduction
Obesity is a major risk factor for an array of chronic diseases and functional morbidity (Poirier et al., 2006), coupled with a sedentary lifestyle that a large portion of the population leads. American Medical Association recently classified obesity as a disease, emphasizing its direct impact on the pathogenesis of many other diseases. Conversely, regular exercise and high levels of physical fitness, as demonstrated by large population studies, are inversely related to all-cause and CVD mortality in the general population as well as in individuals with chronic diseases such as type 2 diabetes (Balducci et al., 2012; Fogelholm, 2010).
Obesity and poor physical fitness often, but not always, co-occur, and their relative or independent impact on health is unclear (Davison et al., 2010). The findings from a recent meta-analytic review of the literature suggest that poor cardiorespiratory fitness is an independent and a better predictor of mortality than obesity; the risk for all-cause and cardiovascular mortality is higher in individuals with normal body mass index (BMI) and poor physical fitness, compared to individuals with high BMI and good physical fitness (Fogelholm, 2010). In addition, lower physical fitness significantly predicts mortality across age groups and regardless of chronic illness presence, independent of BMI (Balducci et al., 2012; Pedersen, 2007; Shaw et al., 2006; Sui et al., 2007). Mortality prediction by obesity indices disappears after adjusting for fitness levels or vice versa (Sui et al., 2007).
Individuals with poor physical fitness or obesity exhibit an elevated inflammatory state (O'Connor et al., 2009), which is an underlying factor for increased morbidity and mortality from various diseases; however, it remains unclear whether obesity or poor fitness independently mediates elevated inflammation or if the two share the same pathways leading to the onset and progression of low-grade inflammation, even among initially asymptomatic individuals (Hamer, 2007). Reports of the association between greater cardiorespiratory fitness levels and lower circulating levels of inflammatory markers are somewhat inconclusive depending on the markers examined (O'Connor et al., 2009). The effect of exercise-induced IL-6 and IL-10 was postulated to counteract the actions of proinflammatory cytokines and contribute to the beneficial health effects of exercise training in patients with chronic inflammatory conditions (Petersen and Pedersen, 2006). Given that weight loss leads to reductions in circulating IL-6, TNF, sTNF-R, and CRP levels regardless of the way in which the weight loss was achieved, including hypocaloric dietary intake, exercise, or liposuction, (Nicklas et al., 2005; You and Nicklas, 2006), the “anti-inflammatory” exercise benefit may be primarily through reduced adipose tissue volume.
In obesity, macrophages are thought to switch their differentiation from wound-healing to pro-inflammatory type when they infiltrate adipose tissue (Mosser and Edwards, 2008). Typically, glucocorticoids and Gs-protein-coupled receptor ligands, such as catecholamines, adenosine, dopamine, and histamine, inhibit pro-inflammatory functions of macrophages/monocytes. These hormones also give rise to a population of regulatory macrophages of an anti-inflammatory phenotype that produces high levels of IL-10 and low levels of IL-12 and TNF (Mosser and Edwards, 2008). However, this regulatory effect of neurohormones might be blunted by decreased sensitivity of their cognate receptors on monocytes/macrophages. Catecholamines, which are β-adrenergic receptor (AR) agonists, effectively inhibit TNF production by monocytes (Dimitrov et al., 2013). A decreased β-AR sensitivity of cardiac receptors was found to be linked to higher circulating levels of the inflammatory marker CRP in healthy subjects (Euteneuer et al., 2012). In addition, inflammatory cytokines can further contribute to the desensitisation of β-ARs to their ligands, by an elevation of G protein–coupled receptor kinase-2 (GRK-2) (Eisenhut, 2012). Thus, we speculate that compromised responsiveness of leukocytes’ β-ARs to the inhibitory effect of agonists may be associated with elevated levels of inflammatory cytokine production.
Regular physical exercise is a widely advocated prevention and rehabilitation intervention for cardiovascular health and diseases; exercise training often lead to sympathetic tone reduction, which is associated with resting bradycardia and reduced blood pressure (BP) (Hautala et al., 2008; Mueller, 2007). Although full understanding of regular exercise induced bradycardia or decreased BP is lacking, changes in ARs have been postulated as a potential mechanism. Yet, no consensus exists in the literature regarding changes to the signal transduction pathway and sensitivity or density of β-ARs on cardiac tissue following exercise training (Zanesco and Antunes, 2007). The evidence of the effects of regular physical activity or greater physical fitness on β-ARs on immune cells is even more inconclusive. Physical fitness was shown to be inversely (Butler et al., 1982; Fujii et al., 1998; Kizaki et al., 2008), positively (Lehmann et al., 1984; Maki et al., 1987), or not related to (Eysmann et al., 1996; Frey et al., 1989) the density of β-ARs on immune cells in both human and animal studies.
We examined the circulating levels of inflammatory cytokines TNF, IL-1β, and IL-6 of 96 asymptomatic, normal-weight to obese individuals in order to investigate the associations among obesity, physical fitness, and low-grade inflammation. Furthermore, in the fitness-inflammation relationship among obese vs. normal-weight participants, we focused on the impact of decreased responsiveness of β-ARs expressed by blood monocytes. We hypothesized that obesity will be associated with diminished leukocyte β-AR responses to the inhibitory effect of isoproterenol (Iso) in cytokine production, resulting in increased basal levels of inflammatory cytokines and that better fitness will have a mitigating effect on this pathway.
2. Materials and methods
2.1. Participants
All 96 subjects gave informed consent to the protocol, which was approved by the University of California, San Diego Human Research Protection Program. Seventy overweight or obese and 26 normal-weight otherwise healthy, non-smoking men and women between ages of 18 to 65 years with normal to mildly elevated blood pressure, but without hypertension, were included in this study from a parent trial that investigates prehypertension and immune activation. To confirm eligibility, all subjects underwent blood tests for liver, metabolic, lipid, and thyroid panels, and normal resting electrocardiogram (ECG) was confirmed. Individuals who had a current diagnosis or a history of heart, liver, or renal disease, diabetes, psychiatric and mood disorders, severe asthma, ongoing inflammatory diseases (e.g., rheumatoid arthritis, multiple sclerosis, lupus), acute illness, and current pregnancy were excluded. Criteria for exclusion also included current use of anti-inflammatory medications or other medications that are known to influence the immune or neuroendocrine parameters of interest (e.g., beta blockers), current drug or alcohol abuse, and smoking within 6 months of the enrolment in the study.
2.2. Procedure
Cardiorespiratory fitness was determined by a VO2peak (ml/kg/min) via a treadmill exercise test using the standard Bruce protocol in which treadmill speed and grade were increased gradually from 1.7 mph and 10% grade every 3 minutes (Borg, 1970). Subject’s expired gas was analyzed using a Sensormedics metabolic cart (Sensormedics, Yorba Linda, CA) equipped with Vmax software (Version 6-2A), and the ECG was monitored using Marquette CardioSoft V.3 (GE Medical Systems, Milwaukee, WI). About a week after the peak exercise test, blood was collected between 8 and 10 am through an intravenous catheter inserted into an antecubital vein using minimal tourniquet. Participants fasted for 12 hours prior to the blood sampling. Standard anthropometric data (i.e. height, weight, waist circumference, hip circumference, and waist/hip ratio) and % fat data by Dual-Energy X-Ray Absorptiometry (DEXA) were obtained. Blood pressure was measured using a Dinamap Compact BP® monitor (Critikon, Tempa, FL) and defined as the average of six seated BP measures taken over two separate days.
2.3. Cytokine levels in plasma
Blood for plasma TNF, IL-1 and IL-6 measurement was drawn in EDTA-treated vacutainers and placed on ice. After centrifugation in a refrigerated centrifuge, plasma was stored at −80°C until the assays were performed. Plasma cytokine levels were measured using commercially available immunoassay kits (Meso Scale Discovery, Gaithersburg, MD). The intra- and inter-assay variations were 6.8% and 6.4% for TNF, 8.4% and 6.0% for IL-1β, and 8.5% and 7.8% for IL-6, respectively.
2.4. LPS-stimulated monocytic intracellular TNF production by flow cytometry
Whole blood was analyzed for LPS-stimulated intracellular monocytic TNF production. The dose of 200 pg/mL LPS (E. coli 0111:B4, catalog # L4391, Sigma-Aldrich, St. Louis, MO) was predetermined to be appropriate for significant activation of monocytes in preliminary experiments, with 30 to 90% of cells producing TNF. Peripheral blood cells were incubated in sterile polypropylene plates with or without LPS for 3.5 hours at 37°C with 5% CO2. To stop cytokine excretion (allowing intracellular detection), brefeldin A (10 µg/mL, Sigma-Aldrich) was added for the last 3 hours of LPS incubation.
Intracellular TNF production of monocytes was evaluated by multiparametric flow cytometry using fluorochrome-conjugated antibodies, as described previously (Dimitrov et al., 2013). Briefly, erythrocytes were lysed using ammonium chloride solution followed by centrifugation (5 min at 500 × g). The cell pellet was washed once with PBS, containing 0.1% azide and 0.5% bovine serum albumin, prior to incubation with monoclonal antibodies (15 min) for the monocytes identification: HLA-DR/PE (BD Biosciences, San Jose, CA), and CD14/APC (Biolegend, San Diego, CA). After fixation and permeabilization according to the manufacturer’s instructions (Cytofix/Cytoperm Kit, BD Biosciences), cells were stained intracellularly with TNF/FITC antibody (Biolegend). At least 10,000 gated monocytes were collected for each tube on a dual-laser FACSCalibur (BD Biosciences). Monocytes were distinguished from lymphocytes and granulocytes by means of their forward and side scatter (FSC and SSC) characteristics and were identified as CD14+/dim HLA-DR+ cells as shown previously (Dimitrov et al., 2013). The percentage of the CD14+/dimHLA-DR+ cells that were positive for TNF (“% TNF+ monocytes”) was assessed. The analysis of the flow cytometric data were performed using FlowJo (Tree star, Ashland, OR).
2.5. β-AR responsivity to agonist (β-AR-mediated inhibition of TNF production)
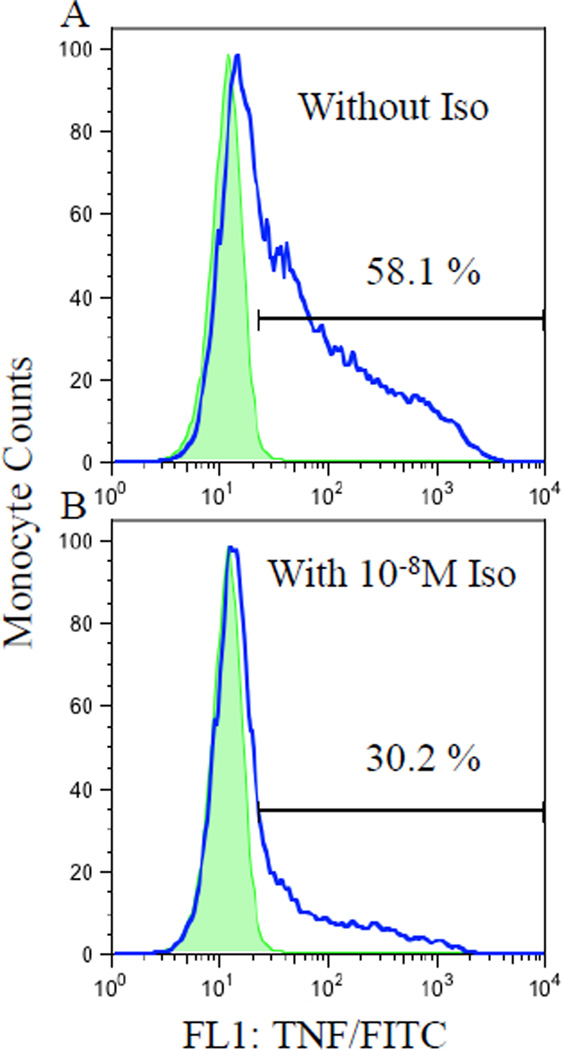
Monocytic β-AR sensitivity to agonist was evaluated based on the inhibitory effect of isoproterenol (Iso), a non-specific β-AR agonist, on the monocytic intracellular TNF production in whole blood stimulated with LPS (Figure 1). In addition to LPS, the whole blood was simultaneously incubated with Iso in final concentrations of 10−10, 10−9, and 10−8 M (Sigma-Aldrich). Intracellular TNF production by monocytes was evaluated by flow cytometry in the same way as the samples treated without Iso as described above. β-AR responsivity in TNF inhibition was calculated as the difference in monocytic %TNF production between LPS only and LPS plus Iso of three concentrations (10−10, 10−9, or 10−8 M). As a β-AR agonist, Iso inhibits stimulated cytokine (i.e., TNF) production, and the degree to which Iso suppresses TNF production relative to the control condition would indicate the sensitivity of β-ARs. As peripheral blood monocytes have been shown to possess about 1600 β2-ARs per cell in previous receptor binding studies (Landmann, 1992; Maisel et al., 1990; Van Tits et al., 1990), they are a good cellular candidate to investigate not only the effects of sympathoadrenal hormones but also the β-AR system of the organism. Systematic assay optimization steps were previously taken to ensure the validity of this method, including LPS dosage and time, establishing response curves to various agonists and antagonists, timing of the agonist treatment, and testing mediation by the cAMP-PKA pathway.
Fig.1.
Flow cytometry histograms of LPS-stimulated intracellular TNF expression by monocytes from a representative subject without (A) and with (B) 10−8 M Iso stimulation in the absence (shaded peaks; as a negative control) and presence (clear peaks) of anti-TNF/FITC antibody. The numbers represent the % TNF-expressing monocytes of total monocytes, showing suppression of TNF production by Iso.
2.6. Statistical analysis
Statistical analyses were performed using SPSS Statistical Software (v 20.0). Descriptive data are presented as means ± SD. Results were considered statistically significant if p≤ 0.05, and all tests were two-tailed. In case of missing data, cases were excluded listwise. BMI was calculated by the formula [weight (kg)/height (m)2]. Mean arterial pressure (MAP) was estimated by the calculation [1/3 × systolic blood pressure + 2/3 diastolic blood pressure]. Normality of the data was determined by the Kolmogorov-Smirnov test, and variables that were not normally distributed were transformed as appropriate: BMI, VO2peak (ml/kg/min), TNF, IL-1β, IL-6 values were natural log transformed. Collinearity statistics were examined for regression analyses.
Univariate correlations among cardiorespiratory fitness (determined by peak oxygen consumption during exercise per minute, given body weight), obesity, inflammatory cytokines, and β-AR sensitivity were measured by Pearson’s r in all 96 participants to examine simple associations. Next, a series of multiple regression analysis were performed to examine the role of fitness in inflammation, controlling for demographic characteristics (age, gender, and race) and MAP. Firstly, in the total cohort, the independent association of BMI (step 3) to each inflammatory cytokine was examined, controlling for the above covariates (step 1), followed by same regression models for which fitness (step 2) was also controlled for in order to examine the role of fitness in the obesity-inflammation relationship (i.e., Does fitness modify/moderate the relationship between obesity and inflammation?). Secondly, to test the hypothesis that fitness will be predictive of lower inflammation in obese individuals, fitness was entered as step 2 for each cytokine, controlling for age, gender, race, and MAP (step 1) in 70 overweight or obese separate from 26 normal-weight participants. Thirdly, the role of β-AR sensitivity (step 2) in fitness-inflammation was examined whether the predictability of fitness (step 3) for inflammatory cytokine levels remained even after controlling for β-AR sensitivity in addition to the aforementioned covariates (step 1). Monocytic β-AR sensitivity was also compared among normal, overweight, and obese individuals using one-way ANCOVA with pairwise comparisons, first controlling for age, gender, race, and MAP, then again with fitness controlled for in order to examine the effects of fitness on the weight-based differences in β-AR sensitivity.
3. Results
3.1. Participants
Of the 96 participants 40 % were overweight and 33% were obese according to the BMI-based weight/obesity classification. Demographic and basic physical characteristics are presented for the separate BMI groups to provide more comprehensive characterization of the study population (Table 1). Age and gender distribution did not differ between the overweight or obese compared to normal-weight individuals. The race distribution in the obese group differed from the other two groups with a greater portion of non-white persons. The average BP was greater, and cardiorespiratory fitness was lower in the overweight or obese compared to normal-weight groups. Overall, cardiorespiratory fitness was associated with self-reported levels of leisure time physical activity levels in all (r= .25, p< .05).
Table 1.
Demographic, basic physical characteristics, and blood cytokine levels of study participants according to the BMI-based weight categories.
| Variable | Normal weight (N=26, 27%) |
Overweight (N=38, 40%) |
Obese (N=32, 33%) |
F/ χ21 |
p- values1 |
|---|---|---|---|---|---|
| Age (years) | 37.4 ± 12.2 | 42.4 ± 9.9 | 40.3 ± 11.2 | 1.5 | 0.22 |
| Gender (male/female) | 10/16 | 21/17 | 15/17 | 1.82 | 0.412 |
| Race (white/others) | 19/7 | 24/14 | 14/18‡2 | 5.52 | 0.062 |
| Systolic BP3 (mmHg) | 114.8 ± 11.9 | 119.4 ± 11.6††† | 129.1 ± 9.9‡‡‡ | 12.9 | <0.001 |
| Diastolic BP (mmHg) | 71.8 ± 8.3 | 73.8 ± 8.2† | 78.8 ± 8.2‡‡ | 5.8 | <0.01 |
| MAP4 (mmHg) | 86.1 ± 9.0 | 89.0 ± 8.4†† | 95.6 ± 7.9‡‡‡ | 10.0 | <0.001 |
| Body mass index (kg/m2) | 22.8 ± 1.7*** | 27.7 ± 1.5††† | 35.5 ± 4.1‡‡‡ | 201 | <0.001 |
| Waist circumference (cm) | 84.2 ± 10.7*** | 96.4 ± 8.4††† | 113.0 ± 10.9‡‡‡ | 50.0 | <0.001 |
| % total fat (%) | 25.1 ± 8.3 | 28.4 ± 8.1†† | 34.8 ± 9.5‡‡‡ | 9.2 | <0.001 |
| % trunk fat (%) | 23.7 ± 8.3** | 29.3 ± 7.8††† | 37.0 ± 7.5‡‡‡ | 20.0 | <0.001 |
| VO2peak (ml/kg/min) | 39.3 ± 10.5* | 33.7 ± 9.2†† | 27.3 ± 9.2‡‡‡ | 13.6 | <0.001 |
| Plasma TNF (pg/ml) | 4.8 ± 2.6 | 5.3 ± 2.9 | 6.5 ± 3.7‡ | 2.3 | 0.10 |
| Plasma IL-1 (pg/ml) | 0.29 ± 0.5 | 0.45 ± 0.5 | 0.56 ± 0.5‡ | 2.5 | 0.09 |
| Plasma IL-6 (pg/ml) | 0.81 ± 0.6 | 0.99 ± 0.6 | 1.11 ± 0.6‡ | 1.8 | 0.18 |
Values are presented as mean ± SD;
*, **, and *** indicate p≤ 0.05, p< 0.01, and p< 0.001 normal weight vs. overweight, respectively;
†, ††, and ††† indicate p≤ 0.05, p< 0.01, and p< 0.001 overweight vs. obese, respectively;
‡, ‡‡, and ‡‡‡ indicate p≤ 0.05, p< 0.01, and p< 0.001 normal weight vs. obese, respectively;
F/ χ2 and p-values are derived from ANOVA or non-parametric χ2 test;
Denotes non-parametric χ2 test;
BP, blood pressure;
MAP, Mean Arterial Pressure
Mean (SD, range) plasma inflammatory cytokine levels for all participants were 5.57 pg/ml (3.14, 1 – 14.7) for TNF, 0.59 pg/ml (0.47, 0.04 – 2.20) for IL-1, and 0.98 pg/ml (0.59, 0.01 – 2.69) for IL-6. Cytokine levels for the separate BMI groups are presented in Table 1. As often seen also in other studies reporting blood levels of IL-1 in asymptomatic individuals, IL-1 levels for 23% of the participants were below the detectable level thus, excluded from the analyses. When these 22 participants were compared with the rest with detectable levels there were no differences in age, BP, BMI, or VO2peak.
3.2. Univariate associations among obesity, fitness, and inflammation in all participants
Firstly, in all 96 participants, there were positive, univariate associations between obesity indices and inflammatory cytokine levels as anticipated. BMI was positively correlated with plasma IL-1β (r= 0.38, p≤ 0.001) and IL-6 (r= 0.27, p≤ 0.01). Specifically for central adiposity, % trunk fat assessed by DEXA was positively associated with plasma IL-1 (r= 0.26, p< 0.05) and IL-6 (r= 0.26, p< 0.05). The correlation coefficients between plasma TNF levels and BMI and % trunk fat were r= 0.17 and 0.16, respectively but were not statistically significant. Waist circumference also showed similar results with BMI and % trunk fat in its association with inflammatory cytokine levels.
Cardiorespiratory fitness determined by VO2peak (ml/kg/min) was negatively associated with obesity indices BMI (r= −0.54, p< 0.0001), % trunk fat (r= −0.74, p< 0.0001), and waist circumference (r= −0.38, p< 0.0001), as hypothesized. Fitness was also negatively correlated with age (r= −0.28, p< 0.01), but its negative correlation with MAP (r= −0.15) was not statistically significant. Negative, univariate correlation between fitness and inflammatory cytokines were at a statistically marginal level of around p= 0.1: TNF (r= −0.15), IL-1β (r= −0.19), and IL-6 (r= −0.17).
3.3. Role of fitness in obesity-related inflammation
BMI was an independent predictor of IL-1β (β= .32, p< .05) and IL-6 (β= .31, p< .01) levels after controlling for demographic variables and BP in all participants (2 step model). However, a three-step multiple regression (step 1: age, race, gender, MAP; step 2: fitness; step 3: BMI) revealed that BMI was no longer a significant predictor of inflammatory cytokine levels after controlling for fitness in addition to the demographic characteristics and BP. Although no longer significant, BMI added 0, 2.6, and 4% R2 in the final model for TNF, IL-1β, and IL-6 levels, respectively. And, the standardized β coefficient for BMI was the greatest among all predictors in the final model for IL-1β (β= .25) and IL-6 (β= .30) levels. For TNF levels, however, the coefficient for fitness (β= −.25) was the largest in the final model. Nonetheless, neither BMI nor fitness was a significant predictor of inflammatory cytokine levels independent of each other (i.e., when the other is controlled for in the model).
In order to further investigate whether physical fitness protects against obesity-related low grade inflammation, the predictability of cardiorespiratory fitness for inflammatory cytokine levels was examined among the 70 overweight or obese separate from the 26 normal-weight participants using multiple regression analyses. After controlling for covariates age, race, gender, and MAP, fitness explained additional 8% of the variance in TNF (p< 0.05), 14% in IL-1β (p< 0.01), and 4% in IL-6 (p= 0.09) levels (Table 2). Among age, race, gender, MAP, and fitness, cardiorespiratory fitness was the strongest predictor of levels of all three inflammatory cytokines with the greatest β coefficient, although gender was also a significant predictor for the levels of TNF and IL-1. Men showed higher levels of TNF and IL-1β than women in our sample. In 26 normal-weight individuals, age, race, gender, MAP, and fitness together explained 25, 10, and 12% of the variance in plasma levels of TNF, IL-1β, and IL-6, respectively, but none of the predictors was significant. Standardized β coefficients of fitness predicting the inflammatory cytokine levels for the normal-weight participants were smaller than those among the overweight or obese, except for IL-6 levels. Curiously, among the normal-weight individuals, greater fitness appeared to predict a greater level of plasma IL-6 (β= 0.35).
Table 2.
Multiple regression analyses examining associations between plasma cytokines and VO2peak (the top half of the table), and plasma cytokines and Δ %TNF by Iso10−8 and VO2peak (the bottom half of the table), after controlling for age, gender, race, and mean arterial pressure (MAP) in the overweight or obese.
| Plasma Cytokines |
Significant Predictors |
Standardized β-coefficient |
t | Final model F |
R2 | ΔR2 | ΔF |
|---|---|---|---|---|---|---|---|
| TNF | Gender | −0.43 | −2.91** | 1.49 | 0.09 | 0.09 | 1.49 |
| VO2peak | −0.35 | −2.45* | 2.49 | 0.17 | 0.08 | 6.01* | |
| IL-1β | Gender | −0.36 | −2.47* | 0.43 | 0.03 | 0.03 | 0.43 |
| VO2peak | −0.47 | −3.27** | 2.53 | 0.17 | 0.14 | 10.66** | |
| IL-6 | --- | ||||||
| TNF | Gender | −0.36 | −2.41* | 1.77 | 0.10 | 0.10 | 1.77 |
| Δ %TNF by Iso | −0.22 | −1.81# | 2.73 | 0.18 | 0.08 | 6.0* | |
| IL-1β | Gender | −0.48 | −3.16** | 3.96 | 0.25 | 0.25 | 3.96** |
| Race | 0.36 | 2.67** | 4.80 | 0.34 | 0.09 | 6.36* | |
| Δ %TNF by Iso | −0.26 | −2.10* | 4.28 | 0.36 | 0.02 | 1.46 | |
| IL-6 | --- | ||||||
The regression models were as follows: (the top half of the table) step 1: age, gender, race, and MAP; step 2: VO2peak; (the bottom half of the table) step 1: age, gender, race, and MAP; step 2: ΔIso10−8; step 3: VO2peak.
Only the significant predictors are shown marked with #, *, and ** to indicate p< 0.10, p< 0.05, and p< 0.01, respectively.
3.4. Role of β adrenergic receptors in the relationship of fitness to inflammation
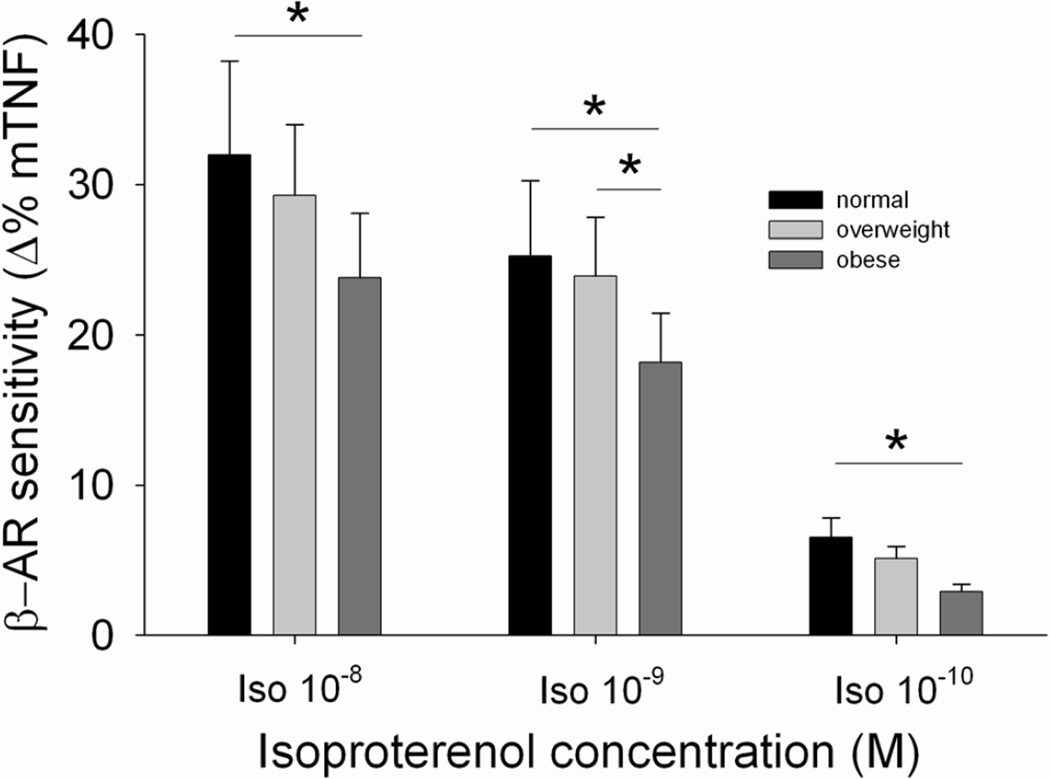
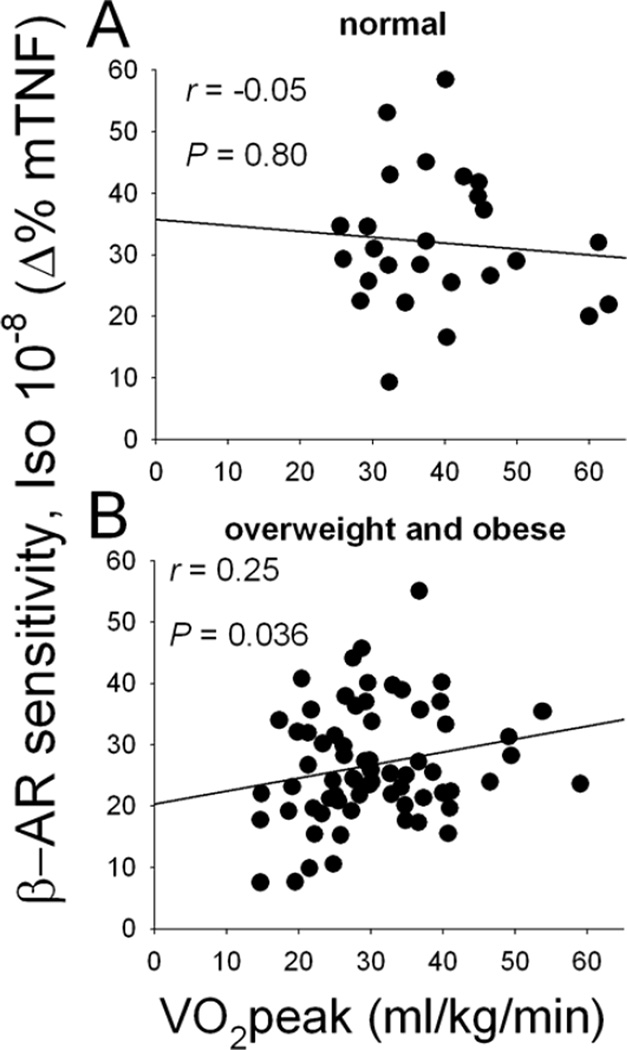
The receptor sensitivity of β-ARs on monocytes was determined based on the degree of inhibition in LPS-stimulated intracellular TNF production by Iso, a β-AR agonist (difference between % TNF-producing monocytes after PBS vs. 10−8 M Iso treatments). Firstly, univariate correlation revealed that monocytic β-AR sensitivity was negatively correlated with BMI (r= −0.36, p< 0.0001) and also with central adiposity as measured by % trunk fat (r= −0.24, p< 0.05) and waist circumference (r= −0.32, p< 0.01) in all participants. Furthermore, monocytic β-AR sensitivity significantly differed among the BMI-based weight groups after controlling for demographic covariates (age, gender, race and MAP) (p< 0.05), and pairwise comparisons revealed that the obese individuals exhibited significantly lower receptor sensitivity than the normal-weight group (Figure 2). The difference between the obese and overweight groups was marginal (p= 0.066). However, when fitness was also controlled for, the significant weight group differences in β-AR sensitivity disappeared, and only the fitness and gender effects were significant (p’s< 0.05). Cardiorespiratory fitness was positively associated with β-AR sensitivity (r= 0.24, p< 0.05). Of interest, this fitness-β-AR sensitivity relationship was only evident among the overweight or obese individuals (Figure 3). Furthermore, β-AR-mediated inhibition of stimulated TNF production was negatively associated with plasma levels of TNF (r= −0.34, p≤ 0.001), IL-1β (r= −0.30, p< 0.01), and IL-6 (r= −0.26, p< 0.05).
Fig.2.
β-AR sensitivity, assessed by the degree of the inhibition of monocytic TNF (mTNF) production by 10−8, 10−9, or 10−10 M Iso in three BMI-based weight categories; normal-weight vs. overweight vs. obese individuals, controlling for age, gender, race, and MAP. Beta AR sensitivity values of three weight groups for three Iso concentrations are depicted to showcase a clear dose response. Values are presented as mean ± SEM. Overall between group differences were significant for all Iso concentrations (p’s< 0.05). *p< 0.05 denotes statistical differences for pairwise comparisons with Bonferroni correction for multiple comparisons.
Fig.3.
Scatterplots showing the correlation between physical fitness (VO2peak) and β-AR sensitivity (measured as inhibition of monocytic TNF (mTNF) production by 10−8 M Iso) in normal-weight (A) vs. overweight or obese (B) individuals. Correlation coefficients and significance are computed from the log-transformed data of VO2peak, while the raw data are presented in the graph.
When β-AR responsivity was entered as the step 2 in regression models with age, race, gender, and MAP in step 1, fitness (step 3) was no longer a significant predictor for plasma levels of cytokines in overweight or obese participants, as was seen in previous analyses (Table 2). This indicated that β-AR responsivity modulated the fitness-inflammation relationship, especially in the overweight or obese. For TNF levels, β-AR responsivity and gender were significant predictors in the final model such that diminished β-AR sensitivity and male gender predicted elevated levels of TNF. Smaller β-AR sensitivity, male gender, and non-White race significantly predicted higher IL-1β levels.
4. Discussion
Mounting evidence shows that obesity/adiposity is directly linked to low-grade inflammation. As inflammation is an underlying condition for various chronic diseases, the obesity-inflammation link is of great clinical significance. The literature also indicates the benefits of cardiorespiratory fitness and regular physical activity against inflammatory diseases. However, the evidence of direct fitness impact on inflammation is less conclusive, and the underlying mechanisms remain unclear. We show in this investigation that cardiorespiratory fitness plays a protective role in lowering systemic levels of inflammatory cytokines in overweight or obese individuals. In addition, we show that these fitness effects on obesity-related inflammation are largely influenced by responsivity of monocytic β-ARs to Iso in inhibition of TNF production and that this β-AR-mediated regulation of TNF production is diminished in obese individuals. Finally, our findings indicate that greater fitness is predictive of greater β-AR responsivity, which is in turn predictive of lower levels of systemic proinflammatory cytokine levels.
Greater cardiorespiratory fitness predicted lower TNF and IL-1β, but not IL-6 levels, after controlling for demographic characteristics and BP in the overweight or obese individuals. Given the studies reporting an increased level of muscle-derived IL-6 with its anti-inflammatory action as a mechanism for the anti-inflammatory effects of exercise (Petersen and Pedersen, 2006; Starkie et al., 2003), the impact of fitness on IL-6 compared to other clearly proinflammatory cytokines may differ. In addition, our finding that the impact of fitness on IL-6 levels appeared to be greater among the normal-weight individuals may indicate a potentially confounding effect of adiposity in the fitness-IL-6 relationship, especially, as IL-6 is also produced by adipocytes. We also found a positive fitness-IL-6 association among the normal-weight participants. Thus, distinguishing whether physical fitness mitigates overall inflammation mainly via lowering proinflammatory, facilitating anti-inflammatory, or affecting both pathways requires further investigation.
We report that the diminished monocytic β-AR-mediated inhibitory control of TNF production among overweight or obese individuals was associated with elevated inflammatory cytokine levels. Given the inhibitory control that β-adrenergic hormones (i.e., epinephrine and norepinephrine) exerts over cellular immune activities, including inflammatory cytokine production, it is highly plausible that desensitized or less (β-agonist) responsive ARs on immune cells would lead to elevated inflammatory cytokine production. Immediate activation of the sympathetic nervous system (SNS) during acute exercise results in markedly increased levels of catecholamines in circulating blood (Epi and NE) (Dimsdale and Moss, 1980; Hong et al., 2004; Hong et al., 2005) and at the vicinity of sympathetic nerve synaptic terminals (NE) (Bellinger et al., 2008). Human leukocytes including monocytes possess substantial numbers of Gαs-coupled β-ARs (Elenkov et al., 2000; Maisel et al., 1989; Maisel et al., 1990), and LPS-stimulated production of inflammatory cytokines (i.e., TNF-α and IL-1β) by monocytes is suppressed in the presence of catecholamines (Severn et al., 1992; van der et al., 1996).
There is equivocal evidence in β-AR density on immune cells in relation to fitness or exercise training (Butler et al., 1982; Eysmann et al., 1996; Frey et al., 1989; Fujii et al., 1998; Kizaki et al., 2008; Lehmann et al., 1984; Maki et al., 1987) and paucity of β-AR sensitivity findings in the current literature. We previously reported that catecholamine responses during acute bout of moderate exercise (Hong et al., 2005) and a laboratory psychological stressor (Hong et al., 2004) were smaller among individuals who were physically fit or regularly exercised. It has also been shown that exercise training and pharmacological treatments using a β-blocker ameliorated age-dependent impairment of β-AR signaling and enhanced cardiac responsiveness to adrenergic stimulation, which was mediated by the suppression of GRK-2 protein levels (Leosco et al., 2007). These results show that re-sensitization of β-ARs can be achieved through regular exercise and/or blocking agonist binding. Sympathoadrenal activation during an acute bout of exercise immediately subsides upon termination of exercise. We postulate that repeated episodes of transient SNS activation during exercise followed by swift recovery and catecholamine clearance after exercise (“SNS efficiency”) may facilitate highly responsive ARs in fit individuals. Thus, we further speculate that regular exercise or improved physical fitness may be an efficacious non-pharmacological therapeutics against β-AR desensitization, given the findings of the β-blocker effects in altering β-AR sensitivity in conditions such as hypertension (Feldman et al., 1984; McAllister, Jr. et al., 1979; Michel et al., 1990), chronic stress (Mills et al., 1999; Rief et al., 2010), and obesity (Eikelis and Esler, 2005; Rahmouni, 2010; Straznicky et al., 2008).
In spite of the mixed findings of β-AR density resulted from exercise training, our findings evidently indicate greater fitness is predictive of greater β-AR responsivity to Iso in inhibiting TNF production, which is in turn predictive of lower levels of systemic proinflammatory cytokine levels. Our findings highlight the importance of building and maintaining physical fitness, especially among overweight or obese individuals, as it appears to mitigate the obesity-related proinflammatory state. Further investigations to confirm the alterations in β-AR sensitivity as a result of obesity/adiposity and physical fitness are warranted to establish temporal and causal relationships. Physical training results in decreased number of myocardial β2-ARs, leading to reduced sympathomimetic effects in animals (Plourde et al., 1991; Sylvestre-Gervais et al., 1982; Werle et al., 1990), but mixed evidence remains, and training effects on inflammation mediated by changes in leukocyte β2-ARs are largely unknown. In addition, whether β-AR sensitivity fully mediates the fitness-inflammation association in obese individuals requires further examination in a larger trial, as it was beyond the capacity of this study. Additionally, our findings that men showed higher inflammatory cytokine levels independent of other factors are in line with the similar findings by others, and lower inflammatory cytokine levels in women is thought to be the role of estrogen (An et al., 1999; Cartier et al., 2009; Ershler and Keller, 2000). The gender effects on inflammation, the mechanisms, including the role of estrogen, and potential gender by fitness or obesity interactions warrant further investigation.
Of note, in our current investigation the relationships among obesity, fitness, and systemic inflammation were as evident, if not more, using a simple index of BMI compared to the indices of central adiposity (i.e., % trunk fat, waist circumference). This does not imply diminished significance of central adiposity in obesity-related inflammation, as overwhelming evidence exists, showing its impact on various diseases. It should be also noted that our findings regarding the protection physical fitness provides against low grade inflammation is not entirely independent of one’s degree of obesity/adiposity. In our group of asymptomatic individuals, the effect of fitness appeared to be greater among the obese, which is an encouraging public health message but does not fully clarify relative impact of fitness vs. obesity on inflammation. In addition, given the complexity of immunological consequences of β2-AR engagement regarding immune cell types, temporality of receptor vs. antigen stimulation, and immune parameters (Sanders, 2012), our findings should be interpreted within its context.
We show that better cardiorespiratory fitness protects against obesity-related low-grade inflammation and also against β-AR desensitization, which was evident among obese individuals. Together with the findings of the association between elevated inflammation and lower β-AR sensitivity, our results indicate that even among overweight or obese individuals, maintaining better cardiorespiratory fitness through regular physical activity would mitigate chronic immune activation and inflammation, leading to reduced risk for various diseases. These findings could send a readily implementable public health message that improvement in physical fitness leads to better health via reduced inflammation among overweight or obese individuals without placing a sole emphasis on weight loss. Given the implications of elevated sympathetic activation and AR dysregulation in CVD (Brodde et al., 1984; Lohse et al., 2003), our findings also may shed light on an underlying mechanism of exercise benefits in patients with CVD.
Research highlight.
Cardiorespiratory fitness protects against obesity-related low-grade inflammation which may be in part via better β adrenergic receptor function.
Acknowledgments
This work was supported by the research grants R01HL090975 (SH) and HL090975S1 (American Recovery and Reinvestment Act grant; SH) and UL1RR031980 for the UCSD Clinical and Translational Science Awards from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
Reference List
- An J, Ribeiro RC, Webb P, Gustafsson JA, Kushner PJ, Baxter JD, Leitman DC. Estradiol repression of tumor necrosis factor-alpha transcription requires estrogen receptor activation function-2 and is enhanced by coactivators. Proc. Natl. Acad. Sci. U. S. A. 1999;96:15161–15166. doi: 10.1073/pnas.96.26.15161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balducci S, Zanuso S, Cardelli P, Salvi L, Mazzitelli G, Bazuro A, Iacobini C, Nicolucci A, Pugliese G. Changes in physical fitness predict improvements in modifiable cardiovascular risk factors independently of body weight loss in subjects with type 2 diabetes participating in the Italian Diabetes and Exercise Study (IDES) Diabetes Care. 2012;35:1347–1354. doi: 10.2337/dc11-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DL, Millar BA, Perez S, Carter J, Wood C, ThyagaRajan S, Molinaro C, Lubahn C, Lorton D. Sympathetic modulation of immunity: relevance to disease. Cell Immunol. 2008;252:27–56. doi: 10.1016/j.cellimm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970;2:92–98. [PubMed] [Google Scholar]
- Brodde OE, Daul A, O'Hara N, Bock KD. Increased density and responsiveness of alpha 2 and beta-adrenoceptors in circulating blood cells of essential hypertensive patients. J. Hypertens. 1984;(Suppl 2):S111–S114. [PubMed] [Google Scholar]
- Butler J, O'Brien M, O'Malley K, Kelly JG. Relationship of beta-adrenoreceptor density to fitness in athletes. Nature. 1982;298:60–62. doi: 10.1038/298060a0. [DOI] [PubMed] [Google Scholar]
- Cartier A, Cote M, Lemieux I, Perusse L, Tremblay A, Bouchard C, Despres JP. Sex differences in inflammatory markers: what is the contribution of visceral adiposity? Am. J. Clin. Nutr. 2009;89:1307–1314. doi: 10.3945/ajcn.2008.27030. [DOI] [PubMed] [Google Scholar]
- Davison K, Bircher S, Hill A, Coates AM, Howe PR, Buckley JD. Relationships between Obesity, Cardiorespiratory Fitness, and Cardiovascular Function. J. Obes. 2010 doi: 10.1155/2010/191253. 2010, 191253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov S, Shaikh F, Pruitt C, Green M, Wilson K, Beg N, Hong S. Differential TNF production by monocyte subsets under physical stress: blunted mobilization of proinflammatory monocytes in prehypertensive individuals. Brain Behav. Immun. 2013;27:101–108. doi: 10.1016/j.bbi.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimsdale JE, Moss J. Plasma catecholamines in stress and exercise. JAMA. 1980;243:340–342. [PubMed] [Google Scholar]
- Eikelis N, Esler M. The neurobiology of human obesity. Exp. Physiol. 2005;90:673–682. doi: 10.1113/expphysiol.2005.031385. [DOI] [PubMed] [Google Scholar]
- Eisenhut M. Inflammation-induced desensitization of beta-receptors in acute lung injury. Am. J. Respir. Crit Care Med. 2012;185:894–895. doi: 10.1164/ajrccm.185.8.894. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu. Rev. Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Euteneuer F, Mills PJ, Rief W, Ziegler MG, Dimsdale JE. Association of in vivo beta-adrenergic receptor sensitivity with inflammatory markers in healthy subjects. Psychosom. Med. 2012;74:271–277. doi: 10.1097/PSY.0b013e318245d762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysmann SB, Gervino E, Vatner DE, Katz SE, Decker L, Douglas PS. Prolonged exercise alters beta-adrenergic responsiveness in healthy sedentary humans. J. Appl. Physiol. 1996;80:616–622. doi: 10.1152/jappl.1996.80.2.616. [DOI] [PubMed] [Google Scholar]
- Feldman RD, Limbird LE, Nadeau J, Robertson D, Wood AJ. Leukocyte beta-receptor alterations in hypertensive subjects. J. Clin. Invest. 1984;73:648–653. doi: 10.1172/JCI111255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelholm M. Physical activity, fitness and fatness: relations to mortality, morbidity and disease risk factors. A systematic review. Obes. Rev. 2010;11:202–221. doi: 10.1111/j.1467-789X.2009.00653.x. [DOI] [PubMed] [Google Scholar]
- Frey MJ, Mancini D, Fischberg D, Wilson JR, Molinoff PB. Effect of exercise duration on density and coupling of beta-adrenergic receptors on human mononuclear cells. J. Appl. Physiol. 1989;66:1494–1500. doi: 10.1152/jappl.1989.66.3.1494. [DOI] [PubMed] [Google Scholar]
- Fujii N, Homma S, Yamazaki F, Sone R, Shibata T, Ikegami H, Murakami K, Miyazaki H. Beta-adrenergic receptor number in human lymphocytes is inversely correlated with aerobic capacity. Am. J. Physiol. 1998;274:E1106–E1112. doi: 10.1152/ajpendo.1998.274.6.E1106. [DOI] [PubMed] [Google Scholar]
- Hamer M. The relative influences of fitness and fatness on inflammatory factors. Prev. Med. 2007;44:3–11. doi: 10.1016/j.ypmed.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Hautala AJ, Kiviniemi AM, Makikallio TH, Tiinanen S, Seppanen T, Huikuri HV, Tulppo MP. Muscle sympathetic nerve activity at rest compared to exercise tolerance. Eur. J. Appl. Physiol. 2008;102:533–538. doi: 10.1007/s00421-007-0618-1. [DOI] [PubMed] [Google Scholar]
- Hong S, Farag NH, Nelesen RA, Ziegler MG, Mills PJ. Effects of regular exercise on lymphocyte subsets and CD62L after psychological vs. physical stress. J. Psychosom. Res. 2004;56:363–370. doi: 10.1016/S0022-3999(03)00134-X. [DOI] [PubMed] [Google Scholar]
- Hong S, Johnson TA, Farag NH, Guy HJ, Matthews SC, Ziegler MG, Mills PJ. Attenuation of T-lymphocyte demargination and adhesion molecule expression in response to moderate exercise in physically fit individuals. J. Appl. Physiol. 2005;98:1057–1063. doi: 10.1152/japplphysiol.00233.2004. [DOI] [PubMed] [Google Scholar]
- Kizaki T, Takemasa T, Sakurai T, Izawa T, Hanawa T, Kamiya S, Haga S, Imaizumi K, Ohno H. Adaptation of macrophages to exercise training improves innate immunity. Biochem. Biophys. Res. Commun. 2008;372:152–156. doi: 10.1016/j.bbrc.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Landmann R. Beta-adrenergic receptors in human leukocyte subpopulations. Eur. J. Clin. Invest. 1992;22(Suppl 1):30–36. [PubMed] [Google Scholar]
- Lehmann M, Dickhuth HH, Schmid P, Porzig H, Keul J. Plasma catecholamines, beta-adrenergic receptors, and isoproterenol sensitivity in endurance trained and non-endurance trained volunteers. Eur. J. Appl. Physiol Occup. Physiol. 1984;52:362–369. doi: 10.1007/BF00943364. [DOI] [PubMed] [Google Scholar]
- Leosco D, Rengo G, Iaccarino G, Filippelli A, Lymperopoulos A, Zincarelli C, Fortunato F, Golino L, Marchese M, Esposito G, Rapacciuolo A, Rinaldi B, Ferrara N, Koch WJ, Rengo F. Exercise training and beta-blocker treatment ameliorate age-dependent impairment of beta-adrenergic receptor signaling and enhance cardiac responsiveness to adrenergic stimulation. Am. J. Physiol Heart Circ. Physiol. 2007;293:H1596–H1603. doi: 10.1152/ajpheart.00308.2007. [DOI] [PubMed] [Google Scholar]
- Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ. Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- Maisel AS, Fowler P, Rearden A, Motulsky HJ, Michel MC. A new method for isolation of human lymphocyte subsets reveals differential regulation of beta-adrenergic receptors by terbutaline treatment. Clin. Pharmacol. Ther. 1989;46:429–439. doi: 10.1038/clpt.1989.161. [DOI] [PubMed] [Google Scholar]
- Maisel AS, Harris T, Rearden CA, Michel MC. Beta-adrenergic receptors in lymphocyte subsets after exercise. Alterations in normal individuals and patients with congestive heart failure. Circulation. 1990;82:2003–2010. doi: 10.1161/01.cir.82.6.2003. [DOI] [PubMed] [Google Scholar]
- Maki T, Kontula K, Myllynen P, Harkonen M. Beta-adrenergic receptors of human lymphocytes in physically active and immobilized subjects: characterization by a polyethylene glycol precipitation assay. Scand. J. Clin. Lab Invest. 1987;47:261–267. doi: 10.1080/00365518709168900. [DOI] [PubMed] [Google Scholar]
- McAllister RG, Jr., Love DW, Guthrie GP, Jr., Dominic JA, Kotchen TA. Peripheral beta-receptor responsiveness in patients with essential hypertension. Arch. Intern. Med. 1979;139:879–881. [PubMed] [Google Scholar]
- Michel MC, Brodde OE, Insel PA. Peripheral adrenergic receptors in hypertension. Hypertension. 1990;16:107–120. doi: 10.1161/01.hyp.16.2.107. [DOI] [PubMed] [Google Scholar]
- Mills PJ, Yu H, Ziegler MG, Patterson T, Grant I. Vulnerable caregivers of patients with Alzheimer's disease have a deficit in circulating C. Psychosom. Med. 1999;61:168–174. doi: 10.1097/00006842-199903000-00008. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller PJ. Exercise training and sympathetic nervous system activity: evidence for physical activity dependent neural plasticity. Clin. Exp. Pharmacol. Physiol. 2007;34:377–384. doi: 10.1111/j.1440-1681.2007.04590.x. [DOI] [PubMed] [Google Scholar]
- Nicklas BJ, You T, Pahor M. Behavioural treatments for chronic systemic inflammation: effects of dietary weight loss and exercise training. CMAJ. 2005;172:1199–1209. doi: 10.1503/cmaj.1040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav. Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK. Body mass index-independent effect of fitness and physical activity for all-cause mortality. Scand. J. Med. Sci. Sports. 2007;17:196–204. doi: 10.1111/j.1600-0838.2006.00626.x. [DOI] [PubMed] [Google Scholar]
- Petersen AM, Pedersen BK. The role of IL-6 in mediating the anti-inflammatory effects of exercise. J. Physiol Pharmacol. 2006;57(Suppl 10):43–51. [PubMed] [Google Scholar]
- Plourde G, Rousseau-Migneron S, Nadeau A. Beta-adrenoceptor adenylate cyclase system adaptation to physical training in rat ventricular tissue. J. Appl. Physiol. 1991;70:1633–1638. doi: 10.1152/jappl.1991.70.4.1633. [DOI] [PubMed] [Google Scholar]
- Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- Rahmouni K. Obesity, sympathetic overdrive, and hypertension: the leptin connection. Hypertension. 2010;55:844–845. doi: 10.1161/HYPERTENSIONAHA.109.148932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rief W, Mills PJ, ncoli-Israel S, Ziegler MG, Pung MA, Dimsdale JE. Overnight changes of immune parameters and catecholamines are associated with mood and stress. Psychosom. Med. 2010;72:755–762. doi: 10.1097/PSY.0b013e3181f367e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders VM. The beta2-adrenergic receptor on T and B lymphocytes: do we understand it yet? Brain Behav. Immun. 2012;26:195–200. doi: 10.1016/j.bbi.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severn A, Rapson NT, Hunter CA, Liew FY. Regulation of tumor necrosis factor production by adrenaline and beta-adrenergic agonists. J. Immunol. 1992;148:3441–3445. [PubMed] [Google Scholar]
- Shaw K, Gennat H, O'Rourke P, Del MC. Exercise for overweight or obesity. Cochrane. Database. Syst. Rev. 2006:CD003817. doi: 10.1002/14651858.CD003817.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003;17:884–886. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- Straznicky NE, Eikelis N, Lambert EA, Esler MD. Mediators of sympathetic activation in metabolic syndrome obesity. Curr. Hypertens. Rep. 2008;10:440–447. doi: 10.1007/s11906-008-0083-1. [DOI] [PubMed] [Google Scholar]
- Sui X, LaMonte MJ, Laditka JN, Hardin JW, Chase N, Hooker SP, Blair SN. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA. 2007;298:2507–2516. doi: 10.1001/jama.298.21.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvestre-Gervais L, Nadeau A, Nguyen MH, Tancrede G, Rousseau-Migneron S. Effects of physical training on beta-adrenergic receptors in rat myocardial tissue. Cardiovasc. Res. 1982;16:530–534. doi: 10.1093/cvr/16.9.530. [DOI] [PubMed] [Google Scholar]
- van der PT, Coyle SM, Barbosa K, Braxton CC, Lowry SF. Epinephrine inhibits tumor necrosis factor-alpha and potentiates interleukin 10 production during human endotoxemia. J. Clin. Invest. 1996;97:713–719. doi: 10.1172/JCI118469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tits LJ, Michel MC, Grosse-Wilde H, Happel M, Eigler FW, Soliman A, Brodde OE. Catecholamines increase lymphocyte beta 2-adrenergic receptors via a beta 2-adrenergic, spleen-dependent process. Am. J. Physiol. 1990;258:E191–E202. doi: 10.1152/ajpendo.1990.258.1.E191. [DOI] [PubMed] [Google Scholar]
- Werle EO, Strobel G, Weicker H. Decrease in rat cardiac beta 1- and beta 2-adrenoceptors by training and endurance exercise. Life Sci. 1990;46:9–17. doi: 10.1016/0024-3205(90)90051-r. [DOI] [PubMed] [Google Scholar]
- You T, Nicklas BJ. Chronic inflammation: role of adipose tissue and modulation by weight loss. Curr. Diabetes Rev. 2006;2:29–37. doi: 10.2174/157339906775473626. [DOI] [PubMed] [Google Scholar]
- Zanesco A, Antunes E. Effects of exercise training on the cardiovascular system: pharmacological approaches. Pharmacol. Ther. 2007;114:307–317. doi: 10.1016/j.pharmthera.2007.03.010. [DOI] [PubMed] [Google Scholar]