Abstract
Purpose
To investigate the potential clinical utility of endorectal MRI-guided biopsy in patients with known or suspected prostate cancer.
Methods
We prospectively recruited 24 men with known or suspected prostate cancer in whom MRI-guided biopsy was clinically requested after multiparametric endorectal MRI showed one or more appropriate targets. One to six 18-gauge biopsy cores were obtained from each patient. Transrectal ultrasound guided biopsy results and post MRI-guided biopsy complications were also recorded.
Results
MRI-guided biopsy was positive in 5 of 7 patients with suspected prostate cancer (including 2 of 4 with prior negative ultrasound-guided biopsies), in 8 of 12 with known untreated prostate cancer (including 5 where MRI-guided biopsy demonstrated a higher Gleason score than ultrasound guided biopsy results), and in 3 of 5 with treated cancer. MRI-guided biopsies had a significantly higher maximum percentage of cancer in positive cores when compared to ultrasound guided biopsy (mean of 37 ± 8% versus 13 ± 4%; p = 0.01). No serious post-biopsy complications occurred.
Conclusion
Our preliminary experience suggests endorectal MRI-guided biopsy may safely contribute to the management of patients with known or suspected prostate cancer by making a new diagnosis of malignancy, upgrading previously diagnosed disease, or diagnosing local recurrence.
Keywords: MR imaging, prostate biopsy, prostate cancer
INTRODUCTION
Prostate cancer can be found in up to 40% of men at autopsy (1). An estimated 17% of North American men will be diagnosed with prostate cancer during their lifetime and 3.4% will die of the disease, with an estimated 28,710 deaths in the United States due to prostate cancer in 2012 (2, 3). The central challenge in managing this common malignancy is accurately determining who has indolent disease that may be appropriate for active surveillance versus who has aggressive disease that needs definitive treatment by surgery or radiation. Another major problem in the rational management of prostate cancer lies in the limitations of ultrasound guided systematic biopsy, which is the standard technique used to make the diagnosis. Transrectal ultrasound is primarily used to localize the prostate prior to blindly obtaining 12 or more biopsy cores from standard locations in the gland. Ultrasound is generally not used to directly visualize the tumor because 37% to 50% of cancers are isoechoic or only slightly hypoechoic (4). Transrectal ultrasound guided biopsy has a false negative rate of up to 30% (5) and under-grades cancers in up to 38% of patients when compared with definitive pathology from radical prostatectomy (5). Despite the substantial sampling error of traditional systematic biopsy, multiple risk stratification schemes and management strategies (7, 8) rely heavily on the histopathological findings of Gleason grade and percentage of tumor in the core biopsy specimens. It is not ideal practice for such critical decision-making algorithms to depend on suboptimal data. The counter-argument that the multifocality of prostate cancer requires systematic biopsy has questionable validity; there is little evidence that small secondary foci of cancer are clinically important (9, 10). One could make a compelling case that the multifocality of prostate cancer is an effect rather than the cause of the currently used diagnostic approach. The multifocality of prostate cancer might never have been so widely recognized if imaging had allowed for identification of a dominant tumor followed by a targeted biopsy of that site alone, as is the case with most other common primary malignancies. This is analogous to breast cancer, where multifocality is primarily determined by how hard one looks, ranging from 9% with standard assessment up to 63% with rigorous serial sectioning (11, 12). The current diagnostic approach to prostate cancer using transrectal ultrasound guided systematic biopsy of the whole gland is arguably the result of historically inadequate imaging of the dominant lesion rather than reflecting any major biological difference from other common cancers. In the last 20 or so years, endorectal multiparametric MR imaging has emerged as an attractive method for the identification of dominant prostate cancer foci (13–17), and more recent advances have allowed for direct MRI-guided biopsies of the dominant lesion (18). The addition of functional and metabolic parameters to standard anatomic T2-weighted imaging has improved the ability of MR imaging to localize and characterize the aggressiveness of the dominant tumor focus (19). This offers a new paradigm in prostate cancer diagnosis, in which a dominant lesion is identified by MR imaging and only that lesion is selectively biopsied under direct imaging guidance. Therefore, we undertook this study to investigate the potential clinical utility of endorectal MRI-guided biopsy in the management of patients with known or suspected prostate cancer.
MATERIALS AND METHODS
Subjects
This was a prospective study approved by our Committee on Human Research, and written informed consent was obtained from all participants. The study was compliant with the requirements of the Health Insurance Portability and Accountability Act. Between December 2010 and July of 2012, we recruited 24 patients from the population undergoing multiparametric endorectal prostate MR imaging at our institution in whom MRI-guided biopsy was considered appropriate, based on a combination of clinical circumstance and imaging findings of a target appropriate for biopsy. We have an active multiparametric endorectal prostate MR imaging program, and scan approximately 400 patients per year with a protocol that includes:
Studies performed on 3.0T scanner (General Electric Healthcare Technologies, Waukesha, WI) using a body coil for excitation and a Medrad (Pittsburgh, PA) inflatable endorectal coil filled with perfluorocarbon (3M, St. Louis, MO) in conjunction with a pelvic phase array coil.
Axial fast 3D SPGR T1-weighted images from the aortic bifurcation to the symphysis pubis using a spin echo sequence with the following parameters: minimum TR/TE, flip angle = 12°, 4.2mm slices, and a 24cm FOV, matrix 256 × 192, transverse frequency direction, and one signal excitation acquired. This T1-weighted sequence allows detection of post-biopsy hemorrhage, nodal enlargement, and bony metastases (since disease status is not always well established at the time of imaging, we do not restrict this sequence to high risk patients only). Thin-section high-spatial-resolution axial and coronal T2-weighted fast spin-echo MR images of the prostate and seminal vesicles obtained using the following parameters: Repetition time/effective echo time of 6000/95 msec, echo train length of 16, 3-mm section thickness, no intersection gap, 14 cm field of view, 256 × 192 acquisition matrix, and 3 signal excitations acquired.
3D MR spectroscopic imaging acquired with MLEV-PRESS sequence (20) that allows the acquisition of completely upright citrate resonance at TE of 85 msec with a 0.16 cm3 nominal spatial resolution with the following parameters: Repetition time/echo time of 2000/85 85 msec; NEX = 2; phase encoding steps = 16 × 12 × 10; FOV = 86 × 65 × 54 mm3; scan time = 8 minutes. An interleaved echo-planar spectroscopic readout was used in the left–right dimension.
Oblique axial diffusion tensor imaging echoplanar imaging with six diffusion gradient directions and a parallel imaging acceleration factor of 2, were acquired in 2.5 minutes with the following parameters: FOV = 24 cm, 256 × 128 matrix, 4 mm thick slices, and b-value of 0 and 600 s/mm2 (8–10 slices typically required to cover the prostate). While diffusion tensor imaging is a routine part of our multiparametric prostate MRI protocol, the anisotropy data was not used for clinical interpretation or biopsy planning in this study.
Multiparametric MR images were reviewed on a picture archiving and communication system workstation (Impax; Agfa, Mortsel, Belgium) by two attending radiologists specializing in abdominal imaging and each with over ten years of experience in endorectal multiparametric prostate MR imaging (AJJ and FVC). By consensus, readers determined if a focal target appropriate for biopsy was present. Tumor was considered present if an ovoid mass-like or crescentic subcapsular region of reduced T2 signal was visible. Tumor had to be sufficiently visible on T2 weighted imaging to allow for adequate targeting (none of these findings were seen in any of the patients in this study). Based on the totality of multiparametric findings, tumor probability was classified as high or low. In general, the probability of tumor was based on co-existent findings; a focus of low T2 signal intensity was considered high probability for tumor if accompanied by clear-cut diffusion and/or spectroscopic abnormality and was considered low probability if not accompanied by clear-cut diffusion and/or spectroscopic abnormality. The option of MRI-guided biopsy was offered, in consultation with the referring physician to those patients with one or more biopsy targets.
MRI-guided biopsy technique
All MRI-guided biopsies were performed by a single attending radiologist (AJJ) with 10 years of experience in prostate MR imaging on a 3.0T scanner (General Electric Healthcare Technologies, Waukesha, WI) with the patient prone. This radiologist had no prior experience performing MRI-guided prostate biopsies. The biopsies were performed as a separate procedure, on a different day from the diagnostic multiparametric MR imaging study described above. A body coil was used for excitation and a pelvic phased array coil for reception. The surface coil was positioned as inferiorly as possible while still allowing access to the rectum. Biopsies were performed using a commercially available prostate biopsy device (DynaTRIM, Invivo, Gainesville, FL) in conjunction with a related software package for device tracking and target localization (DynaCAD, Invivo, Gainesville, FL). An initial digital rectal examination using lidocaine gel was performed to evaluate for anorectal pathology that might preclude or complicate transrectal biopsy (no anorectal abnormalities were detected in the study population) and to approximate the position of the prostate. The needle sleeve of the biopsy system, a fingerlike rigid hollow device that functions both as a guide for the needle and as a fiducial marker for software localization, was then placed in the rectum. The needle sleeve was then attached to the clamp stand of the system. The clamp stand attaches to the table top of the magnet and establishes rigid co-registration between the needle sleeve, the patient, and the scanner. The clamp stand has adjustable marked cogs that allow for repositioning of the needle sleeve in three planes. The patient was then advanced into the bore of the scanner and high resolution T2 sagittal images obtained (with the same parameters as used for the diagnostic multiparametric MR imaging studies described above). The location of the needle sleeve was marked on the images and calibrated to the system. The target lesion was then identified and a cursor placed in the center of the target lesion. The software tracking system then provided the required three-dimensional adjustments needed on the clamp stand to deliver the needle to the target. The technical use of the system is illustrated in Figure 1. Reconfirmation of target and needle track was performed by obtaining oblique axial and true sagittal MR images with a steady-state gradient echo sequences (FOV = 24 cm, TR 5 = sec, TE = 2.3 msec, 4mm thick slice). Oblique axial images were aligned with the needle sleeve, and so did not necessarily correspond to the axial plane of imaging utilized in the preliminary diagnostic study. Retargeting and reconfirmation were repeated until needle track and target were correctly aligned. Once satisfactory alignment was obtained, the patient was removed from the scanner and an average of 2 (range, 1 to 3) biopsy cores were obtained from each target based on certainty of targeting, using an automatic titanium 18G MR imaging-compatible biopsy gun (InVivo, Gainesville, FL). Certainty of targeting was based on subjective clinical assessment of multiple factors, including lesion size and operator satisfaction with lesion visibility and needle alignment. Early attempts to obtain MR imaging with the needle in place (i.e., after firing of the biopsy device) to confirm good needle position were abandoned because blooming artifact limited the utility of the images and because obtaining this extra sequence added to the duration of the procedure for the patient, while providing little benefit. Total table time was approximately 1-2 hours per patient. Patients were observed for thirty minutes to one hour after the procedure for the occurrence of significant post-biopsy complications, defined as any complaint requiring specific treatment, intervention, additional observation, or hospitalization. No post-procedural imaging was performed. All participants were contacted by the study research coordinator within 7 days of the procedure, to ensure no delayed complications had developed.
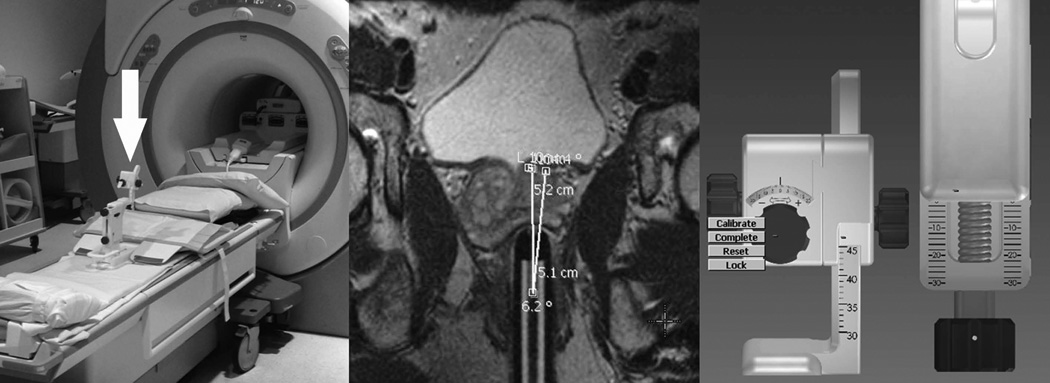
Figure 1.
Photomontage demonstrating the technical use of the MRI-guided biopsy system. The left component of the montage shows the biopsy device (arrow) clamped to the MRI tabletop. The central component of the montage is an oblique axial T2-weighted MR image (obtained in the plane of the needle sleeve, visible in the rectum) showing how the system calculates the three-dimensional adjustments required to bring the trajectory of a biopsy needle introduced through the needle sleeve into alignment with the intended target. The right component of the montage is a software graphic illustrating the three adjustable cogs that position the needle sleeve. After initial sleeve insertion and preliminary imaging, the software automatically calculates and displays the three calibration adjustments required to align the needle sleeve with the target.
Statistical analysis
A single experienced pathologist (JPS) reviewed all cores obtained by MRI-guided biopsy and recorded the presence or absence of cancer in each one. For cancer containing cores, the Gleason grade and score and percentage of tumor seen in each core were recorded. Samples processed at our institution were fixed in formalin immediately after biopsy and subsequently placed in a block of paraffin wax. Microtome sections were then mounted on a glass slide and stained with hematoxylin and eosin. High molecular weight keratin immunoperoxidase staining was also performed on areas suspicious for adenocarcinoma. The presence or absence of cancer in the MRI-guided biopsy specimen was used as the reference standard to determine if the biopsy was positive or negative. Given the small size of our population, simple descriptive statistical techniques were used to analyze the data, specifically the frequency of positive or negative biopsies as related to indication, target size, and target level of suspicion. Student’s t test was used to compare the maximum percentage of cancer in positive cores between ultrasound and transrectal ultrasound guided biopsies. Results of previous transrectal ultrasound guided biopsy were also recorded when available.
RESULTS
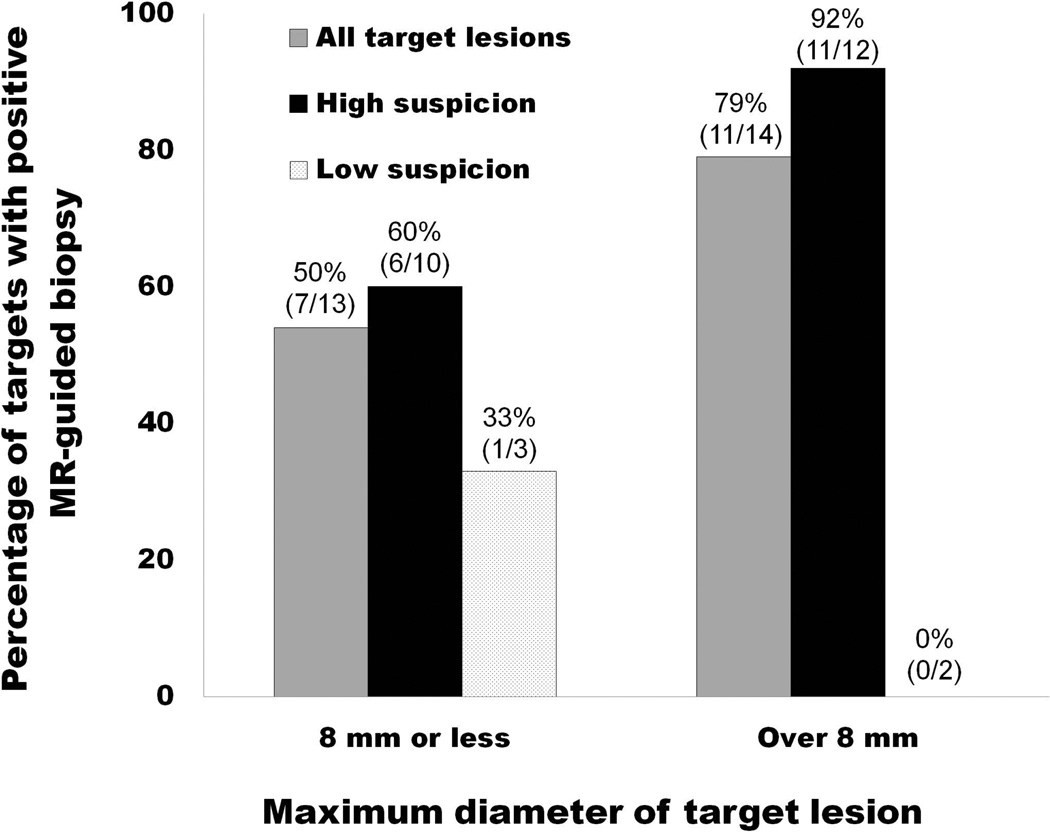
The clinical and imaging characteristics of the 24 patients in the study group are shown in Table 1. The study population consisted of 7 men with suspected prostate cancer, 12 men with known untreated prostate cancer, and 5 men with previously treated prostate cancer. A total of 54 MRI-guided biopsy cores were obtained from 27 target lesions. MRI-guided biopsy yielded a new diagnosis of prostate cancer in 5 of 7 patients with suspected disease (including 2 of 4 patients with prior negative transrectal ultrasound-guided biopsies). One of these patients is illustrated in Figure 2. The other two patients with suspected disease and a negative MRI-guided biopsy result also had a subsequent negative transrectal ultrasound-guided biopsy. MRI-guided biopsy was positive in 8 of 12 patients with known untreated prostate cancer, showing cancer with a higher Gleason score than the baseline transrectal ultrasound guided biopsy in 5 and the same Gleason score in 5. MRI-guided biopsy was positive in 3 of 5 patients with previously treated prostate cancer, showing aggressive Gleason 8 or 9 cancer postradiation recurrence in all three of these patients. The other two patients with known and previously treated prostate cancer had both undergone hormonal treatment only and had negative MRI-guided biopsy results. One of these two patients had a contemporaneous transrectal ultrasound guided biopsy, and this biopsy demonstrated Gleason score 6 cancer. Overall, MRI-guided biopsies had a significantly higher maximum percentage of cancer in positive cores when compared to prior transrectal ultrasound guided biopsy (mean of 37 ± 8% versus 13 ± 4%; p = 0.01). When examined on a per-target basis and stratified into two groups based on the median target diameter of 8 mm, the rate of positive MRI-guided biopsy was highest in the group with a target diameter over 8 mm and a high level of certainty for cancer, as illustrated in Figure 3. No post-biopsy complications occurred. None of the patients progressed to radical prostatectomy during the study period.
Table 1.
Clinical and imaging characteristics of the 24 patients in the study group undergoing MR-guided prostate biopsy.
| Patient | Age (years) |
PSA (ng/ mL) |
Clinical history | Multiparametric MRI biopsy target(s) |
MR-guided biopsy result |
Most recent ultrasound- guided biopsy result |
Interval between biopsies |
Incremental information from MRI-guided biopsy |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Maximum diameter (mm) |
Probability | |||||||||
| SUSPECTED PROSTATE CANCER | 1 | 58 | 6.0 | Elevated PSA with negative prior TRUS biopsy. | 15 | High | Two cores. Gleason 3+4 in up to 4% of core. | Negative. | 5 months | New diagnosis of Gleason 3+4 prostate cancer. |
| 2 | 59 | 10.8 | Elevated PSA with negative prior TRUS biopsy. | 15 | Low | One core, benign. | Negative. | 11 months | Non-contributory. | |
| 3 | 69 | 5.8 | Elevated PSA, declined TRUS biopsy. | 7 | High | Two cores, Gleason 3+3 in up to 45% of core. | No priors. | NA | New diagnosis of Gleason 3+3 prostate cancer. | |
| 9 | High | Two cores, Gleason 3+3 in up to 73% of core. | ||||||||
| 5 | High | Two cores, all benign. | ||||||||
| 4 | 58 | 9.7 | Elevated PSA with negative prior TRUS biopsy. | 5 | High | Three cores, all benign. | Negative. | 2 months | Non-contributory. | |
| 5 | 68 | 23.0 | Elevated PSA with 7 prior negative TRUS biopsies. | 17 | High | Two cores. Gleason 4+3 in up to 100% of core. | Negative. | 2 months | New diagnosis of Gleason 4+3 prostate cancer. | |
| 6 | 57 | 6.6 | Elevated PSA, declined TRUS biopsy | 11 | High | 3 cores. Gleason 3+3 in up to 89% of core. | No priors. | NA | New diagnosis of Gleason 3+3 prostate cancer. | |
| 7 | 73 | 18.4 | Elevated PSA, declined TRUS biopsy | 22 | High | 1 core. Gleason 4+3 in 70% of core. | No priors. | NA | New diagnosis of 4+3 prostate cancer. | |
| KNOWN UNTREATED CANCER | 8 | 67 | 32.1 | Rising PSA with untreated Gleason 3+3 on biopsy 6 years before. | 35 | High | One core. Gleason 4+3 in 100% of core. | Gleason 3+3. | 6 years | Indeterminate. Borderline upgrade from 3+4 to 4+3. |
| 9 | 57 | 8.3 | Rising PSA with untreated Gleason 3+2 on biopsy 8 years before. | 16 | High | Two cores. Gleason 3+4 in up to 17% of core. | Gleason 3+2. | 8 years | Upgrade from Gleason 3+2 to 3+4. | |
| 10 | 68 | 3.9 | Untreated Gleason 3+4 on biopsy 2 years before. | 5 | Low | One core, benign. | Gleason 3+4 | 7 months | Non-contributory. | |
| 11 | 61 | 5.7 | Untreated Gleason 3+3 on biopsy 1 year before. | 7 | Low | Two cores, both benign. | Gleason 3+3 | 5 months | Non-contributory. | |
| 12 | 52 | 2.1 | Untreated Gleason 3+4 on biopsy 6 months before. | 8 | High | Two cores, Gleason 3+4 in up to 40% of core | Gleason 3+4 | 3 months | Non-contributory. | |
| 3 | Low | Atypical low grade cancer in one of two cores. | ||||||||
| 13 | 61 | 8.1 | Untreated Gleason 3+3 on multiple prior biopsies | 12 | High | Two cores. Gleason 3+3 in up to 82% of core. | Gleason 3+3 | 1 day | Non-contributory. | |
| 14 | 68 | 7.9 | Untreated Gleason 3+3 on biopsy 5 years before. | 5 | High | One core, benign. | Gleason 3+3 | 5 years | Non-contributory. | |
| 15 | 52 | 8.0 | Untreated Gleason 3+3 on TRUS 6 months before. | 8 | High | Two cores. Gleason 4+4 in up to 20% of one. | Gleason3+3 | 6 months | Upgrade from Gleason 3+3 to 4+4. | |
| 16 | 69 | 12.8 | Untreated Gleason 3+3 on TRUS 6 years before. | 16 | High | Two cores. Gleason 3+4 in up to 80% of core. | Gleason 3+3. | 3 months | Upgrade from Gleason 3+3 to 3+4. | |
| 17 | 60 | 4.6 | Untreated Gleason 3+3 on biopsy 6 month before. | 5 | High | 3 cores. Gleason 3+3 in up to 25% of core. | Gleason 3+3. | 6 months | Non-contributory. | |
| 18 | 56 | 3.8 | Untreated Gleason 3+4. | 7 | High | 2 cores, all benign. | Gleason 3+4. | 10 months | Non-contributory. | |
| 19 | 56 | 4.3 | Untreated Gleason 3+3 with TRUS 9 months prior. | 9 | High | 2 cores. Gleason 3+4 in up to 8% of core. | Gleason 3+3. | 9 months | Upgrade in Gleason from 3+3 to 3+4. | |
| TREATED CANCER | 20 | 68 | 18.6 | Gleason 6 cancer on biopsy 6 years before, elevated PSA despite intermittent hormonal treatment. | 10 | Low | Three cores, all benign. | Gleason 3+3 | 6 years | Non-contributory. |
| 21 | 76 | 4.0 | Brachytherapy 9 years before, with PSA elevation. | 8 | High | 3 cores. Gleason 4+4 in up to 25% of core | Negative prior TRUS bx. | 15 months | New Gleason 4+4 cancer recurrence. | |
| 22 | 58 | 13.5 | Gleason 3+4. Radiation and hormonal therapy 12 years before. | 11 | High | Two cores. Gleason 4+4 in up to 66% of core. | Negative. | 6 months | New diagnosis of aggressive local recurrence. | |
| 23 | 83 | 4.7 | EBRT 1o years before. Currently on Lupron and Casodex with elevated PSA. | 27 | High | 2 cores. Gleason 4+5 in up to 100% of core. | No priors. | 10 years | New Gleason 4+5 cancer recurrence. | |
| 24 | 68 | 5.3 | Dutasteride treatment, elevated PSA. | 6 | High | 2 cores, all benign. | Gleason 3+4 prior to treatment. | 1 month | Non-contributory. | |
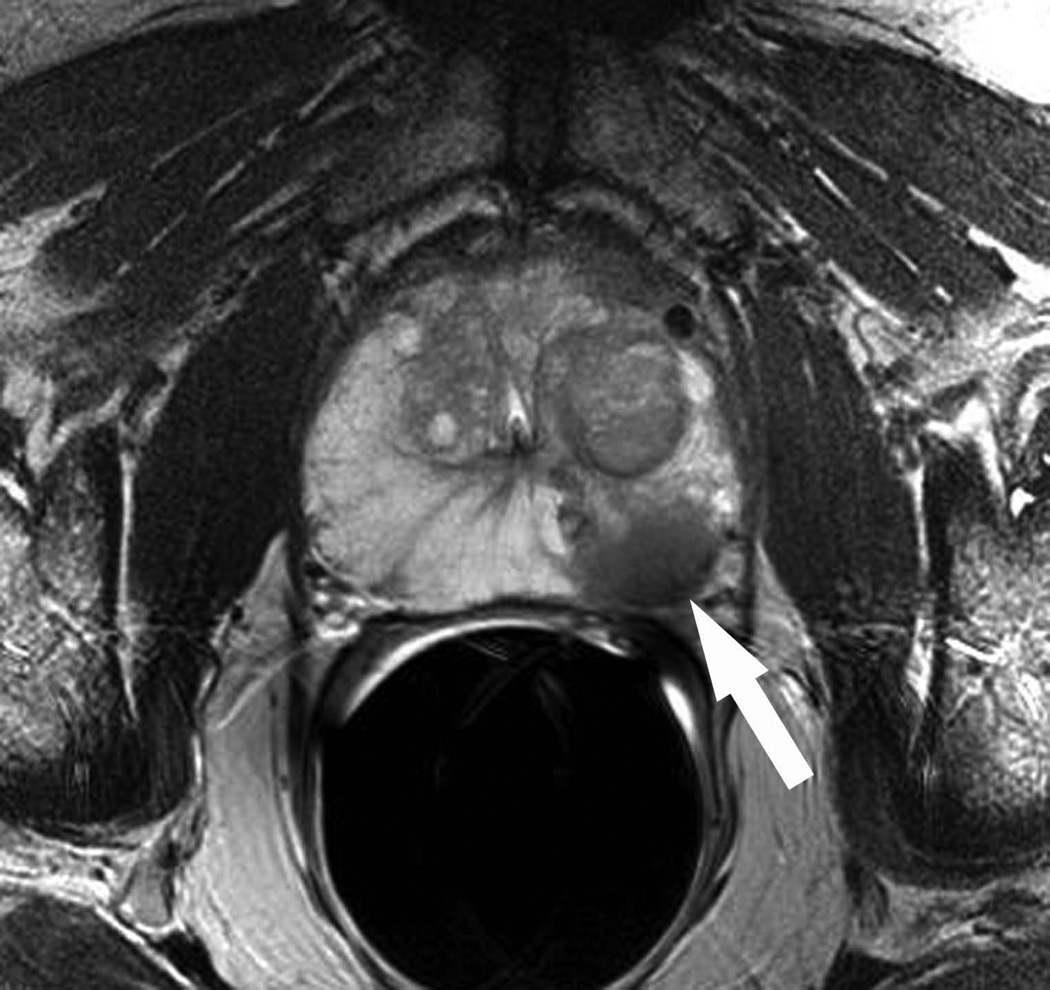
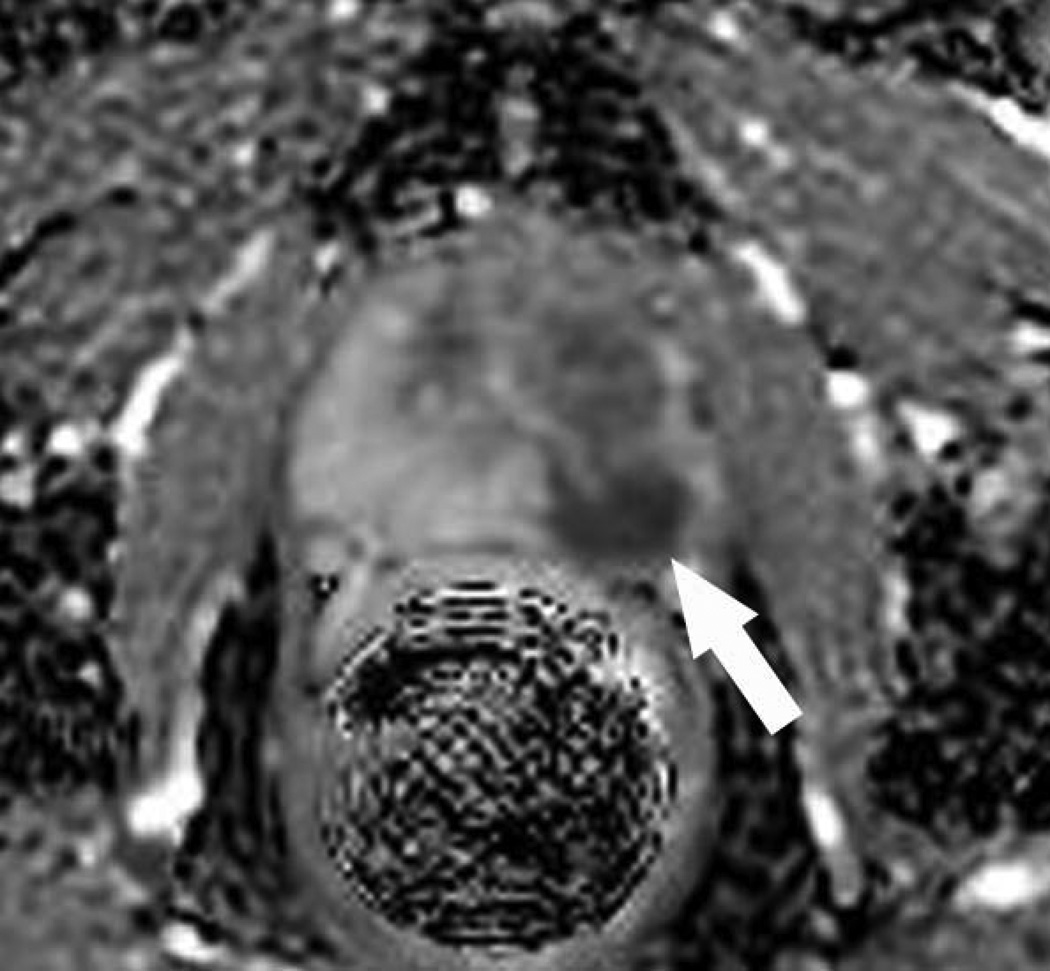
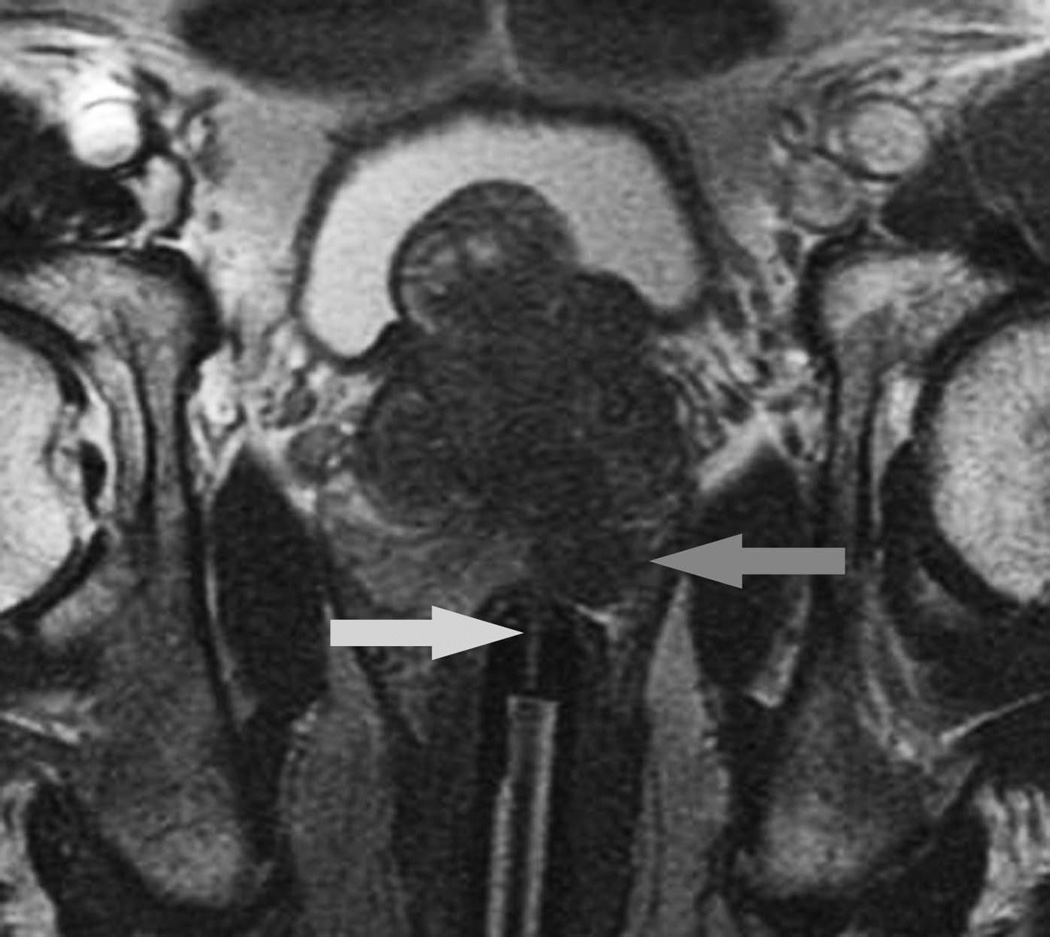
Figure 2.
A: Axial T2-weighted MR image in a 73 year old man who had declined transrectal ultrasound guided biopsy after a screening serum prostatic specific antigen level of 18.4ng/mL (patient #21 in Table 1). A masslike focus (arrow) of low T2 signal is seen in the left mid-gland.
B: Corresponding axial apparent diffusion coefficient map demonstrates a focus (arrow) of restricted diffusion. The findings were considered highly suspicious for prostate cancer by two independent reviewers. The patient consented to undergo MR-guided biopsy.
C: Oblique axial T2-weighted image obtained in the plane of the needle sleeve of the biopsy device confirms the needle tip (horizontal arrow) is targeted towards the suspicious focus (vertical arrow). One core was obtained, and demonstrated Gleason 4+3 cancer in 70% of the tissue.
Figure 3.
Graph illustrating the relationship between the rate of positive MR-guided biopsy and the size and level of suspicion of the target lesion.
DISCUSSION
The results of this preliminary study investigating the potential clinical utility of endorectal MRI-guided biopsy of the prostate suggest the technique might be helpful at several junctures in the management of prostate cancer in selected patients. For men with an elevated serum prostatic specific antigen who have had a prior negative transrectal ultrasound guided biopsy or who have declined a transrectal ultrasound guided biopsy, MRI-guided biopsy of suspicious lesions may yield a first time diagnosis of prostate cancer, as seen in 5 of 7 such patients, and rationally guide treatment. Fewer cores would theoretically result in less discomfort and biopsy associated morbidity (i.e., bleeding or infection) from the presumed decreased number of cores obtained through MRI-guided biopsy. We averaged only 2 cores per patient with no complications. This is in contrast to ultrasound guided prostate biopsy where typically 12 or more cores are routinely obtained. For men with known prostate cancer that have not yet been treated, either because they are undecided as to their preferred treatment or have opted for active surveillance, MRI-guided biopsy may result in cancer upgrading, as seen in 5 of 12 such patients. Finally, in men who have already been treated, MRI-guided biopsy may provide a diagnosis of local recurrence, as seen in 3 of 5 patients in our study. In all these scenarios, MRI-guided biopsy provides incremental clinically useful information with the potential to impact management. The rate of positive MRI-guided biopsy results as a function of target size and level of suspicion is also noteworthy. While small numbers precluded any meaningful or multivariate statistical analyses, we found that larger (8 mm maximum diameter or above) targets with high level of suspicion were most likely to yield a positive biopsy. This result may seem intuitively obvious, but could be helpful when deciding whether to offer an MRI-guided biopsy. An average target diameter of 8 mm corresponds to a tumor volume of 0.26 cm3; and given that the threshold for clinically significant prostate cancer volume is generally regarded as 0.5 cm3 (21), offering to biopsy lesions of 8 mm or more in diameter would seem adequate for detection of clinically important disease. Another finding that speaks to the power of MRI-guided versus standard non-targeted biopsy is the percentage of cancer-containing tissue in positive cores; we found this was significantly higher in MRI-guided versus prior transrectal ultrasound guided cores. This provides provisional support for the notion that multiparametric MR-identified targets are truly cancers and represent “actionable intelligence”.
Our results are broadly aligned with prior similar studies, particularly those investigating the use of MRI-guided biopsy in patients without a diagnosis of prostate cancer. In a study from Nijmegen, MRI-guided biopsy was positive in 108 of 265 (41%) men with a serum prostatic specific antigen level greater than 4.0 ng/mL, a prior negative transrectal ultrasound guided biopsy, and a focal suspicious lesion on T2 and diffusion-weighted MR imaging (22). In another study from Nijmegen, where MRI-guided biopsy was targeted to lesions with restricted diffusion in a group of 98 men, MRI-guided biopsy outperformed ultrasound guided biopsy in determining the highest Gleason grade present, using radical prostatectomy as the reference standard (23). In three similar studies from Germany, MRI-guided biopsy was positive in 52 of 100 (52%), 15 of 27 (56%), and 14 of 37 (38%) of men with suspected prostate cancer and at least one negative prior transrectal ultrasound-guided biopsy (24–26). In an National Institute of Health study of 101 men with some degrees of suspicion for prostate cancer on multiparametric MR imaging, transrectal-ultrasound guided biopsy using fusion software to direct the needle to the MR-identified targets, cancer was detected in 12 of 43 (27.9%), 26 of 39 (66.7%) and 17 of 19 (89.5%) patients with low, moderate and high suspicion, respectively (27). In a similar study from Germany, ultrasound guided biopsy was directed to targets identified by MR imaging in a group of 54 patients with suspected prostate cancer and at least one prior negative ultrasound guided biopsy. Prostate cancer was detected in 21 of 54 patients (53 of 178 targets) when biopsy was directed to sites considered suspicious at MR imaging (28). We demonstrated the role of MRI-guided biopsy in upgrading disease in untreated patients and showing that positive MRI-guided cores are likely to contain more cancer than standard ultrasound-guided cores.
Our study has several limitations. The study population was small, selected, heterogeneous, and performed at a single institution. We do not anticipate or favor the widespread or indiscriminate use of MRI-guided biopsy as a replacement for transrectal ultrasound until our findings have been further substantiated in standardized prospective multi-institutional studies with larger patient cohorts. Such studies will need to address incremental clinical value and cost effectiveness of MR guided biopsy, as stratified by patient risk category. Our study only included patients considered to have target lesions considered appropriate for biopsy, so we cannot estimate the prevalence of such targets in the general population of men with prostate cancer. Our entry criteria were somewhat subjective in that physician and patient preference were factors in driving the decision to perform an MRI-guided biopsy. While we do not know exactly the reasoning that lead to biopsy in these cases, we do know that we only recruited 24 patients over a two year period, despite performing several hundred diagnostic prostate MRI examinations over the same interval. This suggests that referrals for biopsy were made in a highly selective fashion. Because the decision making in these cases was generally made by the referring physician on a case by case basis, and was not systematically available to us, we do not know why repeat biopsies were considered appropriate in patients with biopsy-proven disease. That said, our sense is that the decision to re-biopsy was often driven by concern that tumor had been under-graded at TRUS biopsy or might have progressed since TRUS biopsy, especially in patients considering active surveillance where such under-grading might result in inappropriate management by active surveillance. Similarly, the determination of what constituted a target appropriate for biopsy was qualitative and reflected the judgment of the interpreting radiologist. We did not utilize standardized interpretation schema or quantitative criteria. Future studies incorporating more objective entry criteria will be required to address potential selection bias introduced by this methodology, though we would note that such qualitative approaches are ubiquitous in contemporary practice, where standardized reporting or objective criteria for tumor localization and characterization at prostate MR imaging is not yet established as common practice. Our study lacks follow-up data that might help corroborate the meaning of a negative MRIguided biopsy in a patient with a prior negative transrectal ultrasound-guided biopsy. We do not know if such a “double-negative” result indicates the absence or decreased likelihood of clinically significant cancer, or simply the failure of both studies to detect a cancer that is present. The fact that 2 of 10 patients in our study with known and untreated prostate cancer (as proven by transrectal ultrasound guided biopsy) had a negative MRI-guided biopsy certainly raises a concern regarding the value of a negative MRI-guided biopsy and the risk of false negatives. Both patients had small (under 8 mm) target lesions. It is interesting to note that in one study, cancer was found in 8 of 10 patients undergoing a repeat MRI-guided prostate biopsy after an initial negative MRI-guided prostate biopsy (21). Clearly, like transrectal ultrasound guided biopsy, MRI-guided biopsy can miss cancers. Further studies with radical prostatectomy as the reference standard would be helpful in determining to what extent such differences between biopsy modalities and false negative biopsies are related to true disease burden as against technical difference. Another concern is that tumor upgrading on re-biopsy may reflect disease progression rather than inherently more accurate assessment of Gleason score secondary to targeting of the dominant tumor at MRI. Specifically, in our study, two of the 12 patients with known untreated prostate cancer had intervals between ultrasound and MRI-guided biopsies of 6 and 8 years, and disease progression could reasonably be invoked to explain tumor upgrading in such cases. However, in the remaining 10 patients, the interval was 10 months or less. Particularly since this group generally consisted of low risk patients with indolent disease, tumor progression seems an unlikely explanation of upgrading on re-biopsy. Our pre-biopsy multiparametric MR imaging protocol could be criticized for not adhering with recent guidelines offered by the European Society of Urogenital Radiology (19), but our study predated the publication of these suggested practice parameters. For example, we used a diffusion sequence with only a single b value of 600, which does not conform with the increasing evidence-based trend towards multi b value diffusion imaging and high b value diffusion imaging. The radiologist who performed the biopsies had no prior experience with endorectal MRI-guided biopsies and only obtained a limited number of cores. While these are valid concerns, the fact that we were still able to demonstrate relatively good and promising results supports the concept of MR guided biopsy as a robust and useful technique. We have added text to address this issue in our review of study limitations in the Discussion.
In conclusion, our preliminary experience suggests MRI-guided biopsy offers a potentially new paradigm for the tissue diagnosis, characterization, and post-therapeutic surveillance of prostate cancer and could impact the patient management across the continuum of care. While promising, our results are preliminary and will need to be further substantiated in standardized and prospective trials with larger patient cohorts.
Acknowledgments
Work supported by NIH grant R01 CA137207-01
References
- 1.Stamey TA, McNeal JE. Adenocarcinoma of the prostate. In: Walsh PC, Retik AB, Stamey TA, Vaughan ED, editors. Campbell’s Urology. 6th ed. Vol. 2. Philadelphia: WB Saunders; 1992. pp. 1159–1221. [Google Scholar]
- 2. http://understandingrisk.cancer.gov/learn/whatisrisk.cfmMedicineNet.com.
- 3.American Cancer Society. Cancer Facts and Figures 2012. Atlanta: American Cancer Society; 2012. Page 4. [Google Scholar]
- 4.Vo T, Rifkin MD, Peters TL. Should ultrasound criteria of the prostate be redefined to better evaluate when and where to biopsy. Ultrasound Q. 2001;17:171–176. doi: 10.1097/00013644-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Rabbani F, Stroumbakis N, Kava BR, Cookson MS, Fair WR. Incidence and clinical significance of false-negative sextant prostate biopsies. J Urol. 1998;159:1247–1250. [PubMed] [Google Scholar]
- 6.Kvåle R, Møller B, Wahlqvist R, Fosså SD, Berner A, Busch C, Kyrdalen AE, Svindland A, Viset T, Halvorsen OJ. Concordance between Gleason scores of needle biopsies and radical prostatectomy specimens: A population-based study. BJU Int. 2009;103:1647–1654. doi: 10.1111/j.1464-410X.2008.08255.x. [DOI] [PubMed] [Google Scholar]
- 7.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368–374. [PubMed] [Google Scholar]
- 8.D'Amico AV, Whittington R, Malkowicz SB, Wu YH, Chen M, Art M, Tomaszewski JE, Wein A. Combination of the preoperative PSA level, biopsy Gleason score, percentage of positive biopsies, and MRI T-stage to predict early PSA failure in men with clinically localized prostate cancer. Urology. 2000;55:572–577. doi: 10.1016/s0090-4295(99)00479-3. [DOI] [PubMed] [Google Scholar]
- 9.Meiers I, Waters DJ, Bostwick DG. Preoperative prediction of multifocal prostate cancer and application of focal therapy: Review 2007. Urology. 2007;70(Suppl6A):3–8. doi: 10.1016/j.urology.2007.06.1129. [DOI] [PubMed] [Google Scholar]
- 10.Muezzinoglu B, Frolov A, Ohori M, et al. Clinicopathologic significance of multifocal prostate cancer [abstract] Lab Invest. 2006;86:151A. [Google Scholar]
- 11.Morrow M, Bucci C, Rademaker A. Medical contraindications are not a major factor in the underutilization of breast conserving therapy. J Am Coll Surg. 1998;186:269–274. doi: 10.1016/s1072-7515(97)00153-1. [DOI] [PubMed] [Google Scholar]
- 12.Vaidya JS, Vyas JJ, Chinoy RF, Merchant N, Sharma OP, Mittra I. Multicentricity of breast cancer: whole-organ analysis and clinical implications. Br J Cancer. 1996;74:820–824. doi: 10.1038/bjc.1996.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurhanewicz J, Vigneron DB, Hricak H, Narayan P, Carroll PR, Nelson SJ. Three-dimensional H-1 MR spectroscopic imaging of the in situ human prostate with high (0.24-0.7cm3) spatial resolution. Radiology. 1996;198:795–805. doi: 10.1148/radiology.198.3.8628874. [DOI] [PubMed] [Google Scholar]
- 14.Engelbrecht MR, Huisman HJ, Laheij RJ, et al. Discrimination of prostate cancer from normal peripheral zone and central gland tissue by using dynamic contrastenhanced MR imaging. Radiology. 2003;229:248–254. doi: 10.1148/radiol.2291020200. [DOI] [PubMed] [Google Scholar]
- 15.Kim CK, Park BK, Kim B. Localization of prostate cancer using 3T MRI: comparison of T2-weighted and dynamic contrast-enhanced imaging. J Comput Assist Tomogr. 2006;30:7–11. doi: 10.1097/01.rct.0000185384.27765.09. [DOI] [PubMed] [Google Scholar]
- 16.Vargas HA, Akin O, Franiel T, Mazaheri Y, Zheng J, Moskowitz C, Udo K, Eastham J, Hricak H. Diffusion-weighted endorectal MR imaging at 3 T for prostate cancer: Tumor detection and assessment of aggressiveness. Radiology. 2011;259:775–784. doi: 10.1148/radiol.11102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bittencourt LK, Barentsz JO, de Miranda LC, Gasparetto EL. Prostate MRI: diffusion-weighted imaging at 1.5T correlates better with prostatectomy Gleason Grades than TRUS-guided biopsies in peripheral zone tumours. Eur Radiol. 2012;22:468–475. doi: 10.1007/s00330-011-2269-1. [DOI] [PubMed] [Google Scholar]
- 18.Yacoub JH, Verma S, Moulton JS, Eggener S, Aytekin O. Imaging-guided prostate biopsy: conventional and emerging techniques. Radiographics. 2012;32:819–837. doi: 10.1148/rg.323115053. [DOI] [PubMed] [Google Scholar]
- 19.Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, Rouviere O, Logager V, Fütterer JJ. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen AP, Cunningham CH, Kurhanewicz J, et al. High-resolution 3D MR spectroscopic imaging of the prostate at 3 T with the MLEV-PRESS sequence. Magn Reson Imaging. 2006;24:825–832. doi: 10.1016/j.mri.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Stamey TA, Freiha FS, McNeal JE, Redwine EA, Whittemore AS, Schmid HP. Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer. 1993;71(3 Suppl):933–938. doi: 10.1002/1097-0142(19930201)71:3+<933::aid-cncr2820711408>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 22.Hoeks CM, Schouten MG, Bomers JG, Hoogendoorn SP, Hulsbergen-van de Kaa CA, Hambrock T, Vergunst H, Sedelaar JP, Fütterer JJ, Barentsz JO. Three-tesla magnetic resonance-guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic, transrectal ultrasound biopsies: detection of clinically significant prostate cancers. European Urology. 2012;62:902–909. doi: 10.1016/j.eururo.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 23.Hambrock T, Hoeks C, Hulsbergen-van de Kaa C, Scheenen T, Fütterer J, Bouwense S, van Oort I, Schröder F, Huisman H, Barentsz J. Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. Eur Urol. 2012;61:177–184. doi: 10.1016/j.eururo.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 24.Roethke M, Anastasiadis AG, Lichy M, Werner M, Wagner P, Kruck S, Claussen CD, Stenzl A, Schlemmer HP, Schilling D. MRI-guided prostate biopsy detects clinically significant cancer: analysis of a cohort of 100 patients after previous negative TRUS biopsy. World J Urol. 2012;30:213–218. doi: 10.1007/s00345-011-0675-2. [DOI] [PubMed] [Google Scholar]
- 25.Anastasiadis AG, Lichy MP, Nagele U, Kuczyk MA, Merseburger AS, Hennenlotter J, Corvin S, Sievert KD, Claussen CD, Stenzl A, Schlemmer HP. MRI-guided biopsy of the prostate increases diagnostic performance in men with elevated or increasing PSA levels after previous negative TRUS biopsies. Eur Urol. 2006;50:738–748. doi: 10.1016/j.eururo.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Engelhard K, Hollenbach HP, Kiefer B, Winkel A, Goeb K, Engehausen D. Prostate biopsy in the supine position in a standard 1.5-T scanner under real time MR-imaging control using a MR-compatible endorectal biopsy device. Eur Radiol. 2006;16:1237–1243. doi: 10.1007/s00330-005-0100-6. [DOI] [PubMed] [Google Scholar]
- 27.Pinto PA, Chung PH, Rastinehad AR, Baccala AA, Jr, Kruecker J, Benjamin CJ, Xu S, Yan P, Kadoury S, Chua C, Locklin JK, Turkbey B, Shih JH, Gates SP, Buckner C, Bratslavsky G, Linehan WM, Glossop ND, Choyke PL, Wood BJ. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. Journal of Urology. 2011;186:1281–1285. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franiel T, Stephan C, Erbersdobler A, Dietz E, Maxeiner A, Hell N, Huppertz A, Miller K, Strecker R, Hamm B. Areas suspicious for prostate cancer: MR–guided biopsy in patients with at least one transrectal US-guided biopsy with a negative finding -Multiparametric MR imaging for detection and biopsy planning. Radiology. 2011;259:162–172. doi: 10.1148/radiol.10101251. [DOI] [PubMed] [Google Scholar]