In 2008, cancer overtook cardiovascular disease as the leading cause of death in Canada. In 2014 alone, an estimated 187,600 Canadians will be diagnosed with cancer, and approximately 75,500 will die from their disease. Between 80% and 90% of those individuals will succumb to their malignancy as a consequence of metastatic dissemination.
Historically, most patients diagnosed with meta-static solid tumours have been deemed incurable. Treatments are generally palliative in nature and can include any combination of systemic therapy, surgery, radiotherapy, or supportive care. Measured outcomes vary widely, because they depend on tumour size, location, number, and histology, as well as on patient-related factors such as pre-existing comorbidities, functional status, and willingness to accept treatment. Furthermore, the biology and response of individual tumours are significantly heterogeneous. Goals of therapy are also variable and can range from increasing overall survival (os) to improving quality of life (qol). Although outcomes with systemic agents have improved over time, the ability of those agents to completely eradicate metastatic disease remains improbable. That improbability, coupled with the potential morbidity of systemic agents, emphasizes the emerging role of minimally invasive local therapies to aid in the treatment of metastatic disease.
Stemming from research exploring the natural history of breast cancer, the concept of a clinically significant state of oligometastatic disease was first proposed in 19951. The suggestion was that many cancers develop a limited number of metastases before acquiring the potential for widespread dissemination. In select instances, it is plausible that a window of opportunity might exist in which local therapies directed at a limited number of metastases might achieve long-term disease control—and possibly even cure. Furthermore, the potential reprieve from systemic therapies might not only serve to improve qol, but also to dampen some of the enormous financial burden placed on governments funding systemic agents.
Numerous studies support the surgical management of oligometastatic disease. Although almost all are nonrandomized in nature, prolonged local control (lc), disease-free survival (dfs), and os have consistently been demonstrated. Based on those data, the surgical resection of limited hepatic metastases in colorectal cancer, pulmonary metastases in soft-tissue sarcoma, and brain metastases in non-small-cell lung cancer have been adopted as common practice in many centres worldwide. Unfortunately, because of either technical or general medical issues, many patients are unable to receive surgery. The recent development and availability of noninvasive techniques, including stereotactic ablative radiotherapy (sabr), might allow for significantly more patients to benefit from local management of their oligometa-static disease while treatment-related morbidity is minimized. The potential result is maximization of the therapeutic ratio in patients with advanced disease who were previously deemed incurable.
The Canadian Association of Radiation Oncology defines sabr as “the precise delivery of highly conformal and image-guided hypofractionated external beam radiotherapy, delivered in a single or few fraction(s)”2. The technique differs drastically from conventional radiotherapy, which typically requires a patient to undergo 25–35 daily treatments in a 5- to 7-week course to achieve biologically equivalent doses capable of permanent tumour control. Basic requirements for the delivery of sabr include a modern linear accelerator, reproducible patient immobilization devices, three-dimensional or intensity-modulated radiotherapy planning, and state-of-the-art image guidance at the time of treatment. Rigorous quality assurance is essential to ensure the accurate delivery of multiple small beams of radiation from multiple angles, which summate onto malignant targets with millimetric precision. The steep dose gradient achieved minimizes the volume of adjacent healthy tissue receiving clinically significant amounts of radiation, allowing for dose escalation far beyond conventional limits (Figure 1). Image guidance further increases the accuracy and precision of radiotherapy delivery, ensuring that each treatment is being delivered as planned.
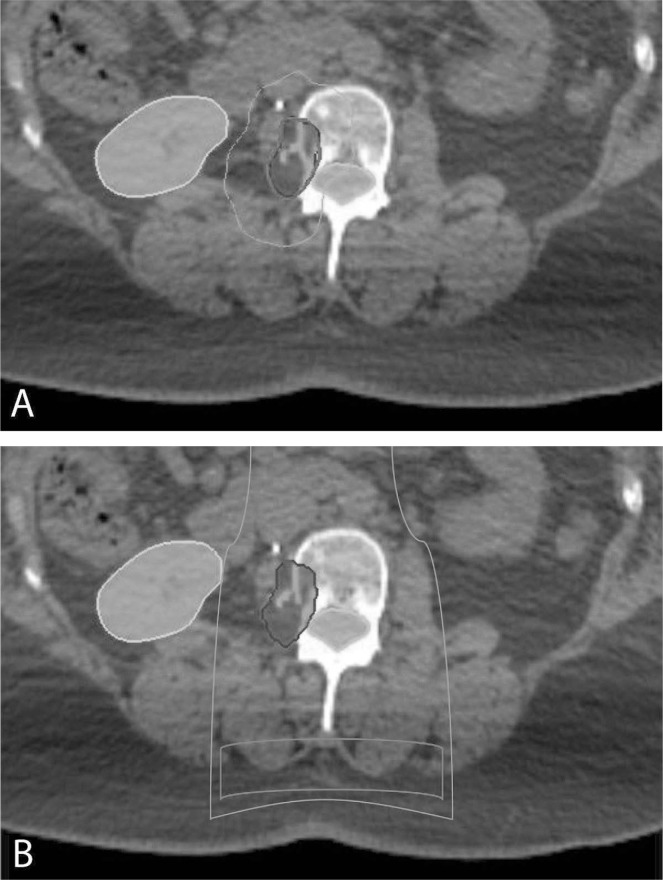
FIGURE 1.
A dosimetric comparison between (A) stereotactic ablative radiotherapy (sabr) and (B) conventional radiotherapy in the palliative management of a painful metastasis of the lumbar spine, as represented on an axial computed tomography slice. The planned target volume (dark blue), spinal canal (turquoise), and right kidney (yellow) are shaded. The radiotherapy dose is indicated by solid lines: 16 Gy (red); 10 Gy (magenta); 8 Gy (green). Note the ability of sabr to deliver twice the dose that conventional techniques (16 vs 8 Gy) can deliver to a malignant target, while simultaneously reducing the dose to adjacent spinal cord and soft tissues.
Initially termed “stereotactic radiosurgery,” the technology was first developed in 1951 as a noninvasive method to manage both benign and malignant intracranial lesions. It provided a therapeutic option for lesions that were otherwise inaccessible to or inappropriate for conventional neurosurgical techniques. Incremental advances in radiotherapy planning systems and delivery platforms, particularly within the past 10–15 years, have allowed the original concepts to be incorporated into the treatment of extracranial targets, essentially revolutionizing the field of radiation oncology. Today, sabr can be applied to the treatment of multiple intraand extracranial targets, delivering ablative doses of ionizing radiation to brain, hepatic, pulmonary, adrenal, skeletal, and lymph node metastases.
The safety of sabr in the treatment of several metastatic sites has been well documented in multiple prospective phase i/ii and large retrospective studies. If carefully performed, the incidence of serious treatment-related toxicities (≥grade 3) is less than 5% in a high-risk population typically deemed medically inoperable3. However, in contrast to conventional radiotherapy, sabr can result in rare toxicities, including irreversible damage to central airways, luminal gastrointestinal organs, and the spinal cord. Clearly, there is strong justification to ensure that the treatment of all patients with sabr occurs within the context of well-established institutional protocols or prospective studies that continue to assess risks on a long-term basis. Currently, patients thought to receive maximal benefit from aggressive management of their oligometastatic disease with sabr are those with 5 or fewer metastases; 3 or fewer involved organ sites; controlled primary disease; prolonged dfs before the development of metastatic disease; and possibly, specific cancer sub-types, including, but not limited to, breast, colorectal, and prostate carcinomas.
A review of 24 retrospective and prospective studies of sabr in the management of oligometastatic disease was published in 20134. Despite considerable heterogeneity in tumour histology, location or locations of metastases, and sabr dose and fractionation schemes, most studies demonstrated excellent 2-year lc rates (between 70% and 90%)—dramatically higher than those observed with conventional radiation. Nevertheless, patients remained at high risk for distant progression, with dfs at 2–5 years approaching 20%. The largest prospective series, which included 121 patients who had 5 or fewer metastases confined to 3 or fewer organs, demonstrated sustained 6-year lc (65%), with maintenance of long-term safety (just a single grade 3 toxicity having been identified)5.
The role of sabr in the palliation of oligometa-static disease has also been reviewed within the context of specific treatment sites, including both intrahepatic and paraspinal locations6,7. With respect to hepatic sabr, a review of 7 prospective phase i/ii and 5 retrospective studies suggested that the ideal candidates are those with good performance status (Eastern Cooperative Oncology Group 0–1), adequate liver function, freedom from extrahepatic disease, and 700 mL or more of uninvolved liver. A clear dose–response for lc was observed, although an optimal threshold was not established. The 2-year lc and os rates were as high as 90% and 83% respectively, with prognosis being associated with tumour size, timing of metastases in relation to the primary disease site, and previous exposure to systemic therapies. Interestingly, patients with noncolorectal histologies appeared to have better outcomes than those with colorectal histology, which is perhaps simply a reflection of the heavier use of systemic agents within the latter population. Unfortunately, regardless of lc efficacy, nearly all patients subsequently developed out-of-field progression, leading the authors to highlight the continued need for systemic agents. Grade 1 and 2 toxicities were frequently encountered, but severe toxicities (≥grade 3) were rare (0%–10%) and related both to the treatment volume and to the dose received by adjacent loops of bowel.
A review of sabr in the management of more than 900 vertebral metastases estimated crude lc rates between 77%–100%, while achieving complete pain relief in 54% of patients 6 months after treatment. In comparison, conventional palliative radiotherapy achieved 1-year lc rates of 86% for non-bulky and 46% for mass-like metastases. At 6 months, complete pain relief was observed in only a small number of patients (0%–20%). However, caution should be exercised before widespread acceptance, because vertebral compression fractures—side effects that are potentially devastating and debilitating—were more commonly observed after stereotactic body radiation therapy than after conventional techniques (11%–39% vs. <5%).
The introduction of any new medical intervention requires that health care professionals ensure its safe delivery within an appropriate patient population. To do otherwise would be ethically unsound and could possibly lead to the infliction of unnecessary harm to patients. Careful consideration is perhaps even more crucial in vulnerable populations, such as those with guarded prognoses, limited treatment options, or functional limitations. To offer sabr to a patient with disseminated metastases who is expected to survive only a few more weeks would almost certainly decrease their qol, regardless of localized efficacy, given the time that treatment would take away from the patient’s ability to be with friends and family toward the end of life. To use sabr to treat a painful thoracic vertebral metastasis adjacent to the spinal cord at a dose deemed safe for peripheral skeletal metastases might be inappropriate, and might result in avoidable toxicities, including paralysis.
The current evidence suggests that, although sabr appears feasible and safe in the treatment of oligometastatic disease, its exact role has not been clearly defined. It is quite plausible that improvements seen with use of sabr are based on analysis of highly selected patients, with favourable tumour biology and performance status, in an era of increasingly accurate staging and effective systemic therapy. It is therefore imperative that sabr be rigorously evaluated in comparison with standard therapies. Although the desired goal of a new treatment or technology is to improve os, other relevant outcomes might be just as important, especially in a population of patients with metastatic disease. Those outcomes include dfs, qol, avoidance of chemotherapy, and cost-effectiveness. In Canada, ongoing efforts are being made to evaluate sabr in phase ii prospective cohort and randomized studies assessing those specific endpoints8. Ideally, that work will lead to high-quality phase iii randomized controlled trials that will enable definitive conclusions about the patients and circumstances for which sabr will be effective in the local treatment of oligometastatic disease.
CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
REFERENCES
- 1.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Sahgal A, Roberge D, Schellenberg D, et al. The Canadian Association of Radiation Oncology scope of practice guidelines for lung, liver and spine stereotactic body radiotherapy. Clin Oncol (R Coll Radiol) 2012;24:629–39. doi: 10.1016/j.clon.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Carey Sampson M, Katz A, Constine LS. Stereotactic body radiation therapy for extracranial oligometastases: does the sword have a double edge? Semin Radiat Oncol. 2006;16:67–76. doi: 10.1016/j.semradonc.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Tree AC, Khoo VS, Eeles RA, et al. Stereotactic body radio-therapy for oligometastases. Lancet Oncol. 2013;14:e28–37. doi: 10.1016/S1470-2045(12)70510-7. [DOI] [PubMed] [Google Scholar]
- 5.Milano MT, Katz AW, Zhang H, Okunieff P. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys. 2012;83:878–86. doi: 10.1016/j.ijrobp.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 6.Hoyer M, Swaminath A, Bydder S, et al. Radiotherapy for liver metastases: a review of evidence. Int J Radiat Oncol Biol Phys. 2012;82:1047–57. doi: 10.1016/j.ijrobp.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Sahgal A, Whyne CM, Ma L, Larson DA, Fehlings MG. Vertebral compression fracture after stereotactic body radiotherapy for spinal metastases. Lancet Oncol. 2013;14:310–20. doi: 10.1016/S1470-2045(13)70101-3. [DOI] [PubMed] [Google Scholar]
- 8.Palma DA, Haasbeek CJ, Rodrigues GB, et al. Stereotactic ablative radiotherapy for comprehensive treatment of oligometastatic tumors (sabr-comet): study protocol for a randomized phase ii trial. BMC Cancer. 2012;12:305. doi: 10.1186/1471-2407-12-305. [DOI] [PMC free article] [PubMed] [Google Scholar]