Abstract
Background
To date, few studies of preoperative chemotherapy or chemoradiotherapy (crt) in gastroesophageal junction (gej) cancer have been statistically powered; indeed, gej tumours have thus far been grouped with esophageal or gastric cancer in phase iii trials, thereby generating conflicting results.
Methods
We studied 41 patients affected by locally advanced Siewert type i and ii gej adenocarcinoma who were treated with a neoadjuvant crt regimen [folfox4 (leucovorin–5-fluorouracil–oxaliplatin) for 4 cycles, and concurrent computed tomography–based three-dimensional conformal radiotherapy delivered using 5 daily fractions of 1.8 Gy per week for a total dose of 45 Gy], followed by surgery. Completeness of tumour resection (performed approximately 6 weeks after completion of crt), clinical and pathologic response rates, and safety and outcome of the treatment were the main endpoints of the study.
Results
All 41 patients completed preoperative treatment. Combined therapy was well tolerated, with no treatment-related deaths. Dose reduction was necessary in 8 patients (19.5%). After crt, 78% of the patients showed a partial clinical response, 17% were stable, and 5% experienced disease progression. Pathology examination of surgical specimens demonstrated a 10% complete response rate. The median and mean survival times were 26 and 36 months respectively (95% confidence interval: 14 to 37 months and 30 to 41 months respectively). On multivariate analysis, TNM staging and clinical response were demonstrated to be the only independent variables related to long-term survival.
Conclusions
In our experience, preoperative chemoradiotherapy with folfox4 is feasible in locally advanced gej adenocarcinoma, but shows mild efficacy, as suggested by the low rate of pathologic complete response.
Keywords: Preoperative chemoradiotherapy, gastroesophageal junction cancer, folfox4
1. INTRODUCTION
Gastric cancer is the 4th most commonly diagnosed cancer and the 2nd most common cause of cancer-related death worldwide1,2. The incidence of distal gastric cancer has declined in the past few decades, but the incidence of gastroesophageal junction (gej) cancer has increased dramatically3,4. In 1998, Siewert described for the first time three different tumour types originating from 5 cm proximal to 5 cm distal of the anatomic cardia:
Type i, adenocarcinoma of the distal esophagus, arises from an area with specialized intestinal metaplasia (“Barrett’s esophagus”), located 1–5 cm above the gej.
Type ii, true carcinoma of the cardia, arises from the cardiac epithelium or an area of Barrett’s esophagus at the gej. It is also called “junctional carcinoma,” and it localizes within 1 cm proximal and 2 cm distal to the gej.
Type iii, subcardial gastric carcinoma, lies 2–5 cm distal to the gej, infiltrating it from below5.
Differences in the epidemiologic, biologic, clinical, and pathology characteristics of these tumour subtypes have since been elucidated, and the adequate surgical approach has been defined6–9. Surgery remains the mainstay of treatment for gej cancer patients, but results are still unsatisfactory10, which in part explains the increasing use of chemotherapy alone or chemoradiation therapy (crt) in combination with surgical resection to improve outcomes.
To date, few studies of preoperative chemotherapy or crt in gej cancer have been statistically powered; indeed, gej tumours have thus far been grouped with esophageal or gastric cancer in phase iii trials, thereby generating conflicting results, controversial interpretations, and no consensus about the best therapy to add to surgery. As a result, U.S. multidisciplinary teams tend more frequently to use preoperative crt rather than chemotherapy alone, as in the United Kingdom11. Recently, however, Stahl et al. reported a survival advantage for preoperative crt compared with preoperative chemotherapy in gej adenocarcinoma. Specifically, preoperative crt was shown to improve the 3-year survival rate to 47.4% from 27.7%, with median overall survival rising to 33.1 from 21.1 months12. Based on those results, we planned a pilot study to confirm the feasibility and safety of preoperative crt in the treatment of patients with locally advanced Siewert type i and ii gej cancer. In light of our previous experience in the adjuvant treatment of gastric cancer with folfox4 [leucovorin–5-fluorouracil (5fu)–oxaliplatin] and radiotherapy, we decided to use the same schedule in the preoperative therapy of locally advanced gej cancer13.
2. METHODS
2.1. Patient Selection and Pretreatment Assessment
Untreated patients with locally advanced (stages ib T2–3 through iiic, according to the current TNM staging system14) Siewert type i and ii gej adenocarcinoma were eligible for the study. Other inclusion criteria were age greater than 18 years; Eastern Cooperative Oncology Group performance status less than 2; evaluable or measurable disease; no previous treatment with chemotherapy or radiotherapy; adequate bone marrow, renal, and hepatic function; and no concomitant illness or medical conditions. Initial evaluation included a detailed clinical history, careful physical examination, recording of concomitant medications, assessment of performance status, hematologic and biochemical profiles, and electrocardiography.
From July 2008 to April 2010, 41 consecutive patients (16 Siewert type i and 25 Siewert type ii tumours) from 3 sites met the study enrollment criteria. Disease extension was assessed by esophagogastroduodenoscopy; abdominal ultrasonography; computed tomography (ct) imaging of neck, chest, and abdomen; and endoscopic ultrasonography. Specifically, pretreatment or basal cancer staging was determined by comparing evaluation of tumour parietal growth (T parameter) and lymph node status (N parameter) by means of ct and endoscopic ultrasonography; the worse scenario was considered. All patients gave informed consent, and the study was approved by the Department of Clinical and Experimental Medicine and Surgery of the Second University of Naples.
2.2. Chemoradiation Schedule and Toxicity Assessment
Chemotherapy (folfox4) consisted of oxaliplatin 85 mg/m2 on day 1; and leucovorin 200 mg/m2 as a 2-hour infusion, followed by bolus 5fu 400 mg/m2 and a 22-hour infusion of 5fu 600 mg/m2 on days 1 and 2 every 2 weeks. Use of central venous catheters and disposable pumps allowed for chemotherapy administration on an outpatient basis. Administration of folfox4 was planned for 4 cycles at full dose; chemotherapy withdrawal was considered in cases of grade 3 or 4 gastrointestinal toxicity. Concurrent ct-based three-dimensional conformal radiotherapy was delivered by a linear accelerator as multiple shaped beams of 6–20 MV X-rays in 5 daily fractions of 1.8 Gy per week for 5 weeks (total dose: 45 Gy). Radiation targets included the entire stomach, any perigastric extension, and lymph nodes (gastric, celiac, porta hepatis, gastroduodenal, splenic–suprapancreatic, and retropancreatic–duodenal), with adequate margins.
Cranially, a 5-cm margin of the esophagus was included. Toxic effects were assessed before treatment start and at each 2-week cycle using the U.S. National Cancer Institute’s Common Toxicity Criteria, version 2.0, and the Radiation Therapy Oncology Group criteria for gastric cancer15,16. Treatment deferral and dose modification were based on results of a complete hematologic evaluation performed on the day of planned treatment. When grade 3 or 4 thrombocytopenia or neutropenia or other significant nonhematologic toxic effects occurred, chemotherapy was delayed for up to 2 weeks. The 5fu dose was reduced in cases of grade 4 diarrhea, stomatitis, and dermatitis.
Peripheral neuropathy was graded according to this oxaliplatin-specific scale:
Grade 1: paresthesia or hypoesthesia of short duration, with complete recovery before the next cycle
Grade 2: paresthesia or hypoesthesia persisting between 2 cycles without functional impairment
Grade 3: permanent paresthesia or hypoesthesia resulting in functional impairment
The oxaliplatin dose was reduced for grade 3 or 4 neutropenia or thrombocytopenia and in cases of persistent (>14 days) paresthesia or temporary (7–14 days) painful paresthesia or functional impairment. In cases of persistent (>14 days) painful paresthesia or functional impairment, oxaliplatin was omitted from treatment until recovery. Nutrition counselling was provided in all cases. Death resulting from toxicity was considered if it occurred during the treatment period or during the 30 days after treatment completion. During crt, no attempt was made to evaluate therapeutic activity. Four weeks after the end of crt, clinical response was assessed by ct imaging and endoscopic ultrasonography, as during basal tumour staging.
2.3. Surgery
Surgery was planned for approximately 6 weeks after completion of crt. In all 25 Siewert type ii and in 11 of the Siewert type i patients, tumour resection was performed by means of conventional open surgery with appropriate bilateral subcostal laparotomy. In resectable patients, an extended total gastrectomy—including a formal abdominal D2 lymphadenectomy, wide splitting of the diaphragmatic hiatus, transhiatal resection of the distal esophagus, and en bloc lymph-adenectomy of the lower posterior mediastinum (namely, node stations 19, 20, 110, and 111)—was performed17. In the remaining 5 Siewert type i gej cancers invading more than 3 cm of esophagus, a subtotal esophagectomy with proximal gastric resection was performed by the abdominothoracic approach6,17,18.
2.4. Pathology Assessment
Pathologic response was evaluated in all resected patients according to the criteria set out by Mandard et al.19, and pathologic complete response [rate of pcr or tumour regression grade (trg) 1 (no residual tumour in the surgical specimen)] was analyzed on an intention-to-treat basis.
An R0 resection was defined as removal of all gross tumour, with no malignant cells found more than 2 mm from the edge of the proximal, distal, or circumferential margins. No further therapy was given if a patient had an R0 resection. For patients with evidence of residual disease after surgery, 2 additional cycles of chemo-therapy with folfox4 every 2 weeks were planned.
2.5. Statistical Analysis
Assessment of the clinical and pathologic response rates to crt was the primary study endpoint. Secondary endpoints included determination of the toxicity of the treatment, completeness of tumour resection, lymph node ratio (the ratio of metastatic to resected nodes), and overall survival. The latter was calculated from the start of neoadjuvant treatment to the date of death or last follow-up.
Statistical analysis was carried out using the SPSS software application (SPSS, Chicago, IL, U.S.A.), integrated with the MedCalc software application (version 9.4.2.0: MedCalc Software, Ostend, Belgium). In all analyses, significance was accepted at p < 0.05. Data are expressed as means ± standard deviation, ranges, and medians. Equality of group means was assessed using the paired Student t-test. Comparisons between proportions, particularly to determine interactions between the variables and the type of clinical response, were analyzed using the chi-square test. Multiple regression analysis was used to test correlations for all variables with outcome; in addition, linear regression analysis was carried out to determine possible relationships between pairs of variables.
Univariate analysis related to overall survival used the log-rank test (Mantel–Cox). Curves were plotted using the product-limit method (Kaplan– Meier)20; p values and hazard ratios with 95% confidence intervals were recorded. The independent significance of prognostic variables (p < 0.1 on univariate analysis) was determined by multivariate analysis, using the Cox proportional hazards model. The model was also tested for possible interaction effects between covariates correlated with each other to determine if those variables could independently influence survival21. No patient was lost to follow-up, and the analysis was complete by December 31, 2012.
3. RESULTS
Table i details baseline patient characteristics. Notably, a large number of patients were found to be node-positive or to have advanced tumour parietal growth, or both.
TABLE I.
Patient characteristics
| Characteristic | Value |
|---|---|
| Patients (n) | 41 |
| Age (years) | |
| Median | 61 |
| Mean | 60±7 |
| Range | 40–70 |
| Sex (n) | |
| Men | 32 |
| Women | 9 |
| ecog ps (n) | |
| 0 | 25 |
| 1 | 13 |
| 2 | 3 |
| Grade (n) | |
| 1 | 5 |
| 2 | 20 |
| 3 | 16 |
| T Stagea (n before crt) | |
| 2 | 10 |
| 3 | 27 |
| 4 | 4 |
| N Stagea (n after crt) | |
| 0 | 3 |
| 1 | 30 |
| 2 | 8 |
| Lauren classification (n) | |
| Intestinal | 19 |
| Diffuse | 22 |
| Siewert type (n) | |
| i | 16 |
| ii | 25 |
Assessed by computed tomography imaging and endoscopic ultrasonography.
ecog = Eastern Cooperative Oncology Group; ps = performance status; crt = chemoradiotherapy.
3.1. Chemoradiation Toxicity and Efficacy
All 41 patients completed preoperative treatment. Combined therapy was well tolerated, with no treatment-related deaths. The total number of folfox4 cycles administered was 159 (median: 4; range: 3–4). Chemotherapy was delayed in 6 patients (14.6%), and dose reduction was necessary in 8 patients (19.5%). The most common grades 3 and 4 toxicities were esophagitis (21%), neutropenia (12%), diarrhea (12%), asthenia (12%), thrombocytopenia (9%), and anemia (9%). Two episodes of symptomatic thrombophlebitis were observed.
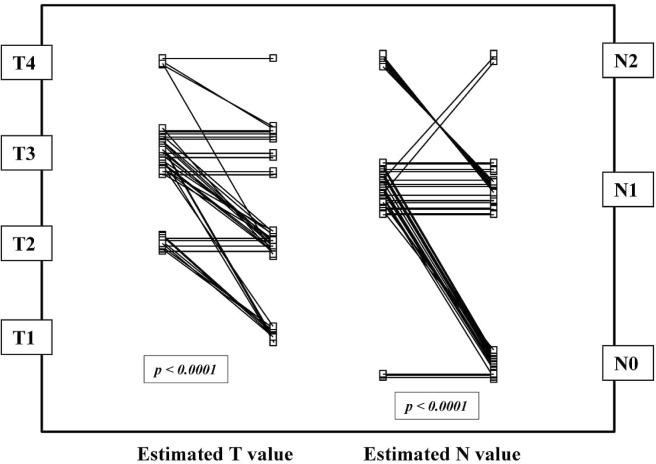
After post-crt ct imaging and endoscopic ultrasonography evaluation, 32 patients (78%) were found to have had a partial clinical response, 7 (17%) to be stable, and 2 (5%) to have experienced disease progression. As Table ii shows, no variable was able to predict clinical response before crt treatment. Interestingly, the neoadjuvant treatment was demonstrated to be highly effective in lowering T and N stage (Figure 1). Comparisons between pre- and post-crt T stage showed a decrease in 27 patients (66%) and no change in 14 (p < 0.0001). The N stage decreased in 21 patients (51%); it remained unchanged in 15 patients (37%), was negative (N0 pre and post-crt) in 3 patients (7%), and progressed in 2 patients (5%, p < 0.0001).
TABLE II.
Prognostic significance related to clinical response
| Variable |
Response type (n)
|
|||
|---|---|---|---|---|
| Partial response | Stable disease | Progressive disease | p Valuea | |
| Age | ||||
| ≤60 Years | 13 | 4 | 2 | 0.216 |
| >60 Years | 19 | 3 | 0 | |
| Sex | ||||
| Men | 26 | 4 | 2 | 0.281 |
| Women | 6 | 3 | 0 | |
| ecog ps | ||||
| 0 | 19 | 4 | 2 | 0.684 |
| 1 | 10 | 3 | 0 | |
| 2 | 3 | 0 | 0 | |
| Grade | ||||
| 1 | 5 | 0 | 0 | 0.336 |
| 2 | 17 | 2 | 1 | |
| 3 | 10 | 5 | 1 | |
| T Stageb (before crt) | ||||
| 2 | 9 | 1 | 0 | 0.238 |
| 3 | 20 | 6 | 1 | |
| 4 | 3 | 0 | 1 | |
| N Stageb (before crt) | ||||
| 0 | 2 | 1 | 0 | 0.836 |
| 1 | 23 | 5 | 2 | |
| 2 | 7 | 1 | 0 | |
| Lauren classification | ||||
| Intestinal | 17 | 1 | 1 | 0.100 |
| Diffuse | 15 | 6 | 1 | |
| Siewert type | ||||
| i | 12 | 3 | 1 | 0.915 |
| ii | 20 | 4 | 1 | |
By chi-square test.
Assessed by computed tomography imaging and endoscopic ultrasonography.
ecog = Eastern Cooperative Oncology Group; ps = performance status.
FIGURE 1.
Estimated T and N stage before and after chemoradiotherapy in 41 patients with esophagogastric junction cancer (p values obtained by paired Student t-test).
3.2. Surgery and Pathologic Response
Of the 41 patients, 40 underwent a resection judged to be radical, with no residual disease (that is, 40 resections were R0). The remaining patient was not operated on because of misdiagnosed liver metastases. All surgical patients recovered, and no postoperative deaths were recorded. Anastomotic leaks occurred in 3 patients (7.5%); pneumonia, in 5 (12.5%); and minor wound complications successfully treated with conservative management, in 6 (15%).
Pathology examination of the surgical specimens identified 4 patients with no residual tumour in the resected specimen and regional lymph nodes (trg 1), corresponding to a 10% pcr rate. Of the remaining 36 patients, 34 (85%) were shown to have a trg 2, 3, or 4; 2 patients (5%) had no disease regression (trg 5).
3.3. Analysis of Overall Survival
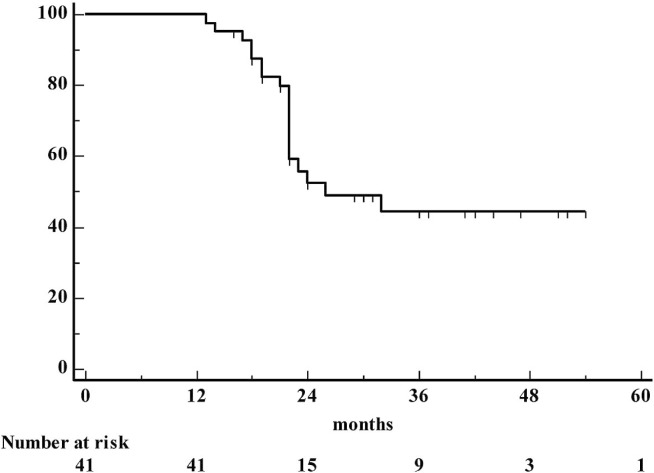
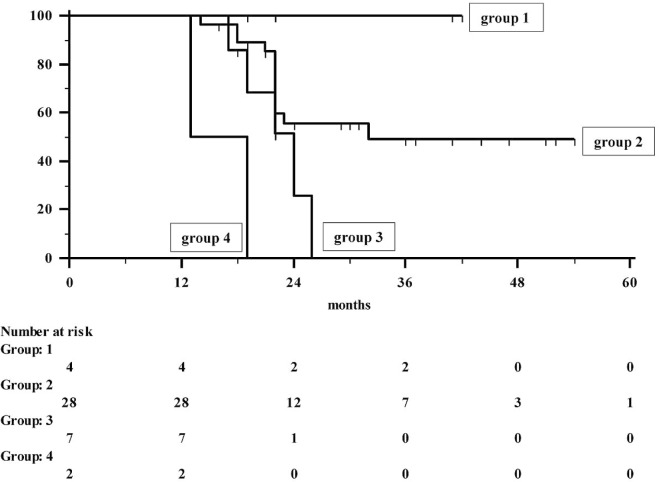
The mean follow-up for the 41 patients was 27 ± 11 months (range: 13–54 months; median: 22 months; 95% confidence interval for the mean: 23 to 30 months). At the end of the study, 23 patients (56%) had experienced tumour recurrence, and of those 23, 19 (46%) died because of the cancer itself. At the time of writing, the remaining 4 patients were undergoing chemotherapy. Distant failure occurred in 12 patients; locoregional failure in 1; and both locoregional and distant recurrence in the remaining 10. The median and mean survival times were 26 and 36 months respectively (95% confidence interval: 14 to 37 months for the median, 30 to 41 months for the mean). The 12-, 24-, 36-, and 48-month survival rates were 100%, 52.5%, 44.5%, and 44.5% respectively (Figures 2 and 3).
FIGURE 2.
Actuarial survival in 41 patients with esophagogastric junction cancer.
FIGURE 3.
Actuarial survival in 41 patients with esophagogastric junction cancer, by response to chemoradiotherapy.
On univariate analysis, the only variables related to overall survival were Lauren classification, post-crt T and N assessment, clinical response, trg, pathologic stage, and lymph node ratio (Table iii). On multiple regression analysis, only clinical response and TNM stage were shown to be related to survival (p = 0.0023 and p = 0.0028 respectively). In addition, both variables were demonstrated to correlate with each other (r = 0.6558, slope = 0.2550, p = 0.001)—that is, responders were observed to have a significantly lower stage. Notably, a 77.7% death rate was observed in 9 non-responders (5 of 7 with stable and 2 of 2 with progressive disease), but all 4 patients with a partial clinical response (100%) were still alive at the time of writing. Of the remaining 28 patients, 16 were alive, for a 5-year survival rate of 49%. Thus, neoadjuvant crt was observed to be able to downstage the tumour, and clinical response appeared to be significantly predictive of long-term outcome.
TABLE III.
Univariate analysis of survival
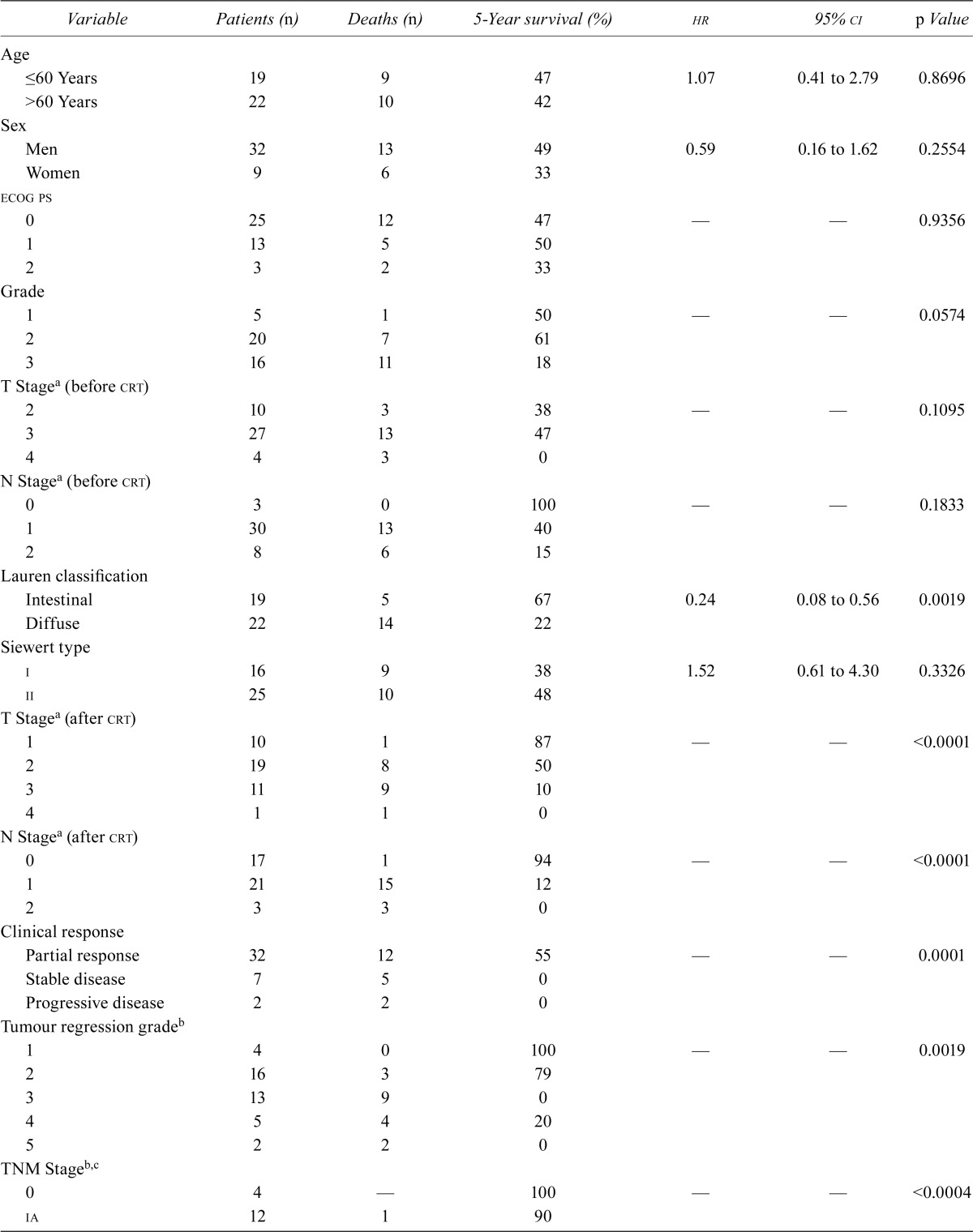
| Variable | Patients (n) | Deaths (n) | 5-Year survival (%) | hr | 95% ci | p Value |
|---|---|---|---|---|---|---|
| Age | ||||||
| ≤60 Years | 19 | 9 | 47 | 1.07 | 0.41 to 2.79 | 0.8696 |
| >60 Years | 22 | 10 | 42 | |||
| Sex | ||||||
| Men | 32 | 13 | 49 | 0.59 | 0.16 to 1.62 | 0.2554 |
| Women | 9 | 6 | 33 | |||
| ecog ps | ||||||
| 0 | 25 | 12 | 47 | — | — | 0.9356 |
| 1 | 13 | 5 | 50 | |||
| 2 | 3 | 2 | 33 | |||
| Grade | ||||||
| 1 | 5 | 1 | 50 | — | — | 0.0574 |
| 2 | 20 | 7 | 61 | |||
| 3 | 16 | 11 | 18 | |||
| T Stagea (before crt) | ||||||
| 2 | 10 | 3 | 38 | — | — | 0.1095 |
| 3 | 27 | 13 | 47 | |||
| 4 | 4 | 3 | 0 | |||
| N Stagea (before crt) | ||||||
| 0 | 3 | 0 | 100 | — | — | 0.1833 |
| 1 | 30 | 13 | 40 | |||
| 2 | 8 | 6 | 15 | |||
| Lauren classification | ||||||
| Intestinal | 19 | 5 | 67 | 0.24 | 0.08 to 0.56 | 0.0019 |
| Diffuse | 22 | 14 | 22 | |||
| Siewert type | ||||||
| i | 16 | 9 | 38 | 1.52 | 0.61 to 4.30 | 0.3326 |
| ii | 25 | 10 | 48 | |||
| T Stagea (after crt) | ||||||
| 1 | 10 | 1 | 87 | — | — | <0.0001 |
| 2 | 19 | 8 | 50 | |||
| 3 | 11 | 9 | 10 | |||
| 4 | 1 | 1 | 0 | |||
| N Stagea (after crt) | ||||||
| 0 | 17 | 1 | 94 | — | — | <0.0001 |
| 1 | 21 | 15 | 12 | |||
| 2 | 3 | 3 | 0 | |||
| Clinical response | ||||||
| Partial response | 32 | 12 | 55 | — | — | 0.0001 |
| Stable disease | 7 | 5 | 0 | |||
| Progressive disease | 2 | 2 | 0 | |||
| Tumour regression gradeb | ||||||
| 1 | 4 | 0 | 100 | — | — | 0.0019 |
| 2 | 16 | 3 | 79 | |||
| 3 | 13 | 9 | 0 | |||
| 4 | 5 | 4 | 20 | |||
| 5 | 2 | 2 | 0 | |||
| TNM Stageb,c | ||||||
| 0 | 4 | — | 100 | — | — | <0.0004 |
| ia | 12 | 1 | 90 | |||
| TNM Stageb,c continued | ||||||
| ib | 6 | 2 | 55 | |||
| iia | 5 | 4 | 20 | |||
| iib | 10 | 8 | 0 | |||
| iiia | 3 | 3 | 0 | |||
| Mean ln ratiob | ||||||
| <0.07 | 24 | 4 | 79 | 0.15 | 0.04 to 0.33 | 0.0001 |
| >0.07 | 16 | 14 | 0 |
Assessed by computed tomography imaging and endoscopic ultrasonography.
Calculated in 40 resected patients.
According to the 2009 TNM staging system.
hr = hazard ratio; ci = confidence interval; ecog = Eastern Cooperative Oncology Group; ps = performance status; ln =lymph node.
On multivariate analysis, clinical response and TNM staging were demonstrated to be the only independent variables related to long-term survival (Table iv). However, because those prognostic factors were shown to correlate with each other, a Cox test with activation of an interaction term was performed to better evaluate the weight of each factor in statistical analyses. Clinical response was again confirmed to be an independent variable predictive of long-term outcome.
TABLE IV.
Multivariate analysis of overall survival by Cox proportional hazards modelling
| Prognostic factor | Coefficient | se | hra | 95%ci | p Value |
|---|---|---|---|---|---|
| Grade | 0.5645 | 1.0455 | 1.75 | 0.22 to 13.50 | 0.5892 |
| Lauren classification | 0.3436 | 0.5690 | 1.41 | 0.46 to 4.27 | 0.5459 |
| T Stage (after crt)b | 0.6855 | 0.5438 | 1.98 | 0.68 to 5.73 | 0.2075 |
| N Stage (after crt)b | 1.1310 | 0.9143 | 3.09 | 0.52 to 18.42 | 0.2161 |
| Clinical response | 1.3017 | 0.5698 | 3.67 | 1.21 to 11.16 | 0.0223 |
| Tumour regression grade | 0.3759 | 0.5484 | 1.45 | 0.49 to 4.24 | 0.4931 |
| TNM stagec | 2.6420 | 0.8871 | 14.04 | 2.41 to 79.19 | 0.0028 |
| Mean ln ratio | 0.6204 | 0.7993 | 1.85 | 0.39 to 8.83 | 0.4376 |
| Stage/responsed | 0.0150 | 0.3962 | 1.01 | 0.46 to 2.20 | 0.9698 |
For cancer-related death.
Assessed by computed tomography imaging and endoscopic ultrasonography.
According to the 2009 TNM staging system.
Analysis with interaction. The interaction term is nonsignificant, which implies that regardless of tumour stage, clinical response is an independent variable20.
se = standard error; hr = hazard ratio; ci = confidence interval; ln = lymph node.
4. DISCUSSION
Preoperative crt has potential advantages for patients with gej cancer. However, the scientific evidence currently supporting the benefit of this therapeutic strategy is derived only from subgroup analysis of four different phase iii trials in which patients with all types of esophageal cancer were randomized either to preoperative crt followed by surgery or to surgery alone22–25. The statistical power of this approach to correctly evaluate specific outcomes is weak because of the low numbers of true gej cancer patients. On the other hand, some authors warned that crt might be associated with increased treatment-related death26,27. Thus far, the main study evaluating the role of preoperative chemoradiotherapy in gej cancer was conducted by Stahl et al.12. Although their trial was closed early and statistical significance was not reached, crt was shown to be superior to preoperative chemotherapy alone, resulting in a 20% increase in the 3-year survival rate and a 17.5% decrease in 3-year local tumour progression. A further confirmation of the effectiveness of preoperative crt compared with surgery alone comes from the cross trial. In that study, preoperative crt significantly improved overall survival (hazard ratio: 0.657; p = 0.003) among patients with potentially curable esophageal or esophagogastric junction cancer28. However, unlike our study and the study by Stahl and colleagues, the cross trial had a mixed population, with only 20% of the patients having a gej cancer. Our results therefore confirm the role of preoperative crt in a patient population representing only gej cancers.
In our series, toxicity was acceptable, with most adverse events being grade 1 or 2, and no treatment-related deaths being recorded. Dose reduction was required in 8 patients, and rates of grades 3 and 4 hematologic and nonhematologic toxicities were similar to those reported in other phase ii trials of preoperative crt (including oxaliplatin and 5fu–based chemotherapy) in esophageal or gej cancer29–32 and also by our group in earlier work involving resected high-risk gastric cancer patients13. Those results are particularly interesting because our study used a higher radiotherapy dose than that reported in the German study (45 Gy vs. 30 Gy).
Partial responses and stabilization of disease were observed in 32 (78%) and 7 (17%) patients respectively. No pretreatment variable was shown to be predictive of clinical response. In our study population, 4 patients (10%) achieved complete tumour regression (trg 1). In randomized trials, rates of pcr have been reported to range from 9% to 40%, although the relative rate for each site has not been available12,22–25. A 9% pcr in 23 gastric cancer patients (10 with gej) was reported by the Spanish Cooperative Group for Digestive Tumor Therapy in a phase ii trial of preoperative chemotherapy induction with irinotecan and cisplatin followed by concurrent crt33. More attractive results were recorded in the Radiation Therapy Oncology Group 9904 trial by Ajani et al., who used paclitaxel, 5fu, and cisplatin as induction chemotherapy, followed by crt, in gastric cancer patients34. Our results were less favourable than those in other studies and also in our group’s previous experiences of the use of cisplatin and paclitaxel in combination with radiotherapy35. However, differences in treatment and patients make interpretation and comparison of our results with those of published studies challenging. In most studies, pcr appears to be a prognostic factor independently correlated with survival. In our study, trg was shown to be a covariate related to overall survival on univariate analysis, but not on multivariate analysis. This apparent inconsistency could be a result of the small number of patients in our series and also of the inclusion, in the trg classification, of patients with a pcr (trg 1) and those with the presence of residual disease (trg 2–5). However, the prognostic role of pcr is also demonstrated in our experience of 4 patients achieving trg 1 being alive at 5 years without recurrence.
Although there was difficulty in discerning tumour from fibrosis after crt, preoperative staging modalities were able to fairly evaluate response to treatment and, consequently, to accurately predict the postoperative pathologic tumour stage. However, accuracy was better for N- than for T-stage estimation (26 vs. 19 exact correlations, 6 vs. 6 underestimations, and 8 vs. 15 overestimations).
Gastroesophageal junction cancer behaves differently from distal gastric cancers. Radical resection and pathologic N stage remain the main predictors of longer survival6,7. Preoperative chemotherapy improves survival36 and, probably through the addition of radiation therapy, might improve resectability, the rates of pcr and local recurrence—thus ultimately improving outcomes.
In our experience, median survival was 26 months, with 44.5% of patients being alive at 48 months, results that accord with the findings of Stahl et al.12 concerning the efficacy of preoperative chemoradiotherapy in gej tumours. In addition, we observed no significant differences in terms of crt efficacy or survival between gej Siewert i or ii tumours, which accords with data from a recent meta-analysis17. Post-treatment TNM stage and clinical response were the only covariates related to better survival.
5. CONCLUSIONS
Management of gej cancer is complex, with surgery still being the main choice for routine practice. However, our work here shows that preoperative crt can be feasible and active in this setting. Specifically, in locally advanced gej adenocarcinoma, folfox4 with concurrent radiation is a safe and modestly active regimen. However, further studies are warranted before concluding that this regimen should be used as standard treatment.
6. CONFLICT OF INTEREST DISCLOSURES
All authors declare that there are no potential conflicts of interest, including no financial interests, relationships, or affiliations relevant to the subject of this manuscript. No financial support was received for the present work.
7. REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2009;71:127–64. doi: 10.1016/j.critrevonc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Botterweck AA, Schouten LJ, Volovics A, Dorant E, van Den Brandt PA. Trends in incidence of adenocarcinoma of the oesophagus and gastric cardia in ten European countries. Int J Epidemiol. 2000;29:645–54. doi: 10.1093/ije/29.4.645. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–92. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 5.Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457–9. doi: 10.1046/j.1365-2168.1998.00940.x. [DOI] [PubMed] [Google Scholar]
- 6.Siewert JR, Stein HJ, Feith M. Adenocarcinoma of the esophagogastric junction. Scand J Surgery. 2006;95:260–9. doi: 10.1177/145749690609500409. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson NW, Howe J, Gay G, Patel–Parekh L, Scott–Conner C, Donohue J. Differences in the pattern of presentation and treatment of proximal and distal gastric cancer: results of the 2001 gastric patient care evaluation. Ann Surg Oncol. 2008;15:1644–50. doi: 10.1245/s10434-008-9877-2. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths EA, Pritchard SA, Mapstone NP, Welch IM. Emerging aspects of oesophageal and gastrooesophageal junction cancer histopathology—an update for the surgical oncologist. World J Surg Oncol. 2006;4:82. doi: 10.1186/1477-7819-4-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dresner SM, Lamb PJ, Bennet MK, Hayes N, Griffin SM. The pattern of metastatic lymph node dissemination from adenocarcinoma of the esophagogastric junction. Surgery. 2001;129:103–9. doi: 10.1067/msy.2001.110024. [DOI] [PubMed] [Google Scholar]
- 10.Wajman J, Bennett MK, Raimes SA, Griffin SM. The pattern of recurrence of adenocarcinoma of the oesophagogastric junction. Br J Cancer. 2002;86:1223–9. doi: 10.1038/sj.bjc.6600252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hingorani M, Crosby T, Maraveyas A, Dixit S, Bateman A, Roy R. Neoadjuvant chemoradiotherapy for resectable oesophageal and gastrooesophageal junction cancer. Do we need another randomized trial? Clin Oncol (R Coll Radiol) 2011;23:696–705. doi: 10.1016/j.clon.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Stahl M, Walz MK, Stuschke M, et al. Phase iii comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851–6. doi: 10.1200/JCO.2008.17.0506. [DOI] [PubMed] [Google Scholar]
- 13.Orditura M, De Vita F, Muto P, et al. Adjuvant chemoradiotherapy in patients with stage iii or iv radically resected gastric cancer: a pilot study. Arch Surg. 2010;145:233–8. doi: 10.1001/archsurg.2010.2. [DOI] [PubMed] [Google Scholar]
- 14.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer–Verlag; 2009. [Google Scholar]
- 15.United States, Department of Health and Human Services, National Institutes of Health, National Cancer Institute Cancer Therapy Evaluation Program (ctep) Common Toxicity Criteria (CTC) Bethesda, MD: CTEP; 1999. Ver. 2.0. [Available online at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf; cited May 12, 2011] [Google Scholar]
- 16.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (rtog) and the European Organization for Research and Treatment of Cancer (eortc) Int J Radiat Oncol Biol Phys. 1995;31:1341–6. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 17.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–23. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 18.Yang K, Chen HN, Chen XZ, et al. Transthoracic resection versus non-transthoracic resection for gastroesophageal junction cancer: a meta-analysis. PLoS One. 2012;7:e37698. doi: 10.1371/journal.pone.0037698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Cancer. 1994;73:2680–6. doi: 10.1002/1097-0142(19940601)73:11<2680::AID-CNCR2820731105>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 21.Chan YH. Biostatistics 203. Survival analysis. Singapore Med J. 2004;45:249–56. [PubMed] [Google Scholar]
- 22.Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for adenocarcinoma. N Engl J Med. 1996;335:462–7. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 23.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase iii trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer. calgb 9781. J Clin Oncol. 2008;26:1086–92. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305–13. doi: 10.1200/JCO.2001.19.2.305. [DOI] [PubMed] [Google Scholar]
- 25.Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase iii trial. Lancet Oncol. 2005;6:659–68. doi: 10.1016/S1470-2045(05)70288-6. [DOI] [PubMed] [Google Scholar]
- 26.Fiorica F, Di Bona D, Schepis F, et al. Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta-analysis. Gut. 2004;53:925–30. doi: 10.1136/gut.2003.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds JV, Ravi N, Hollywood D, et al. Neoadjuvant chemoradiation may increase the risk of respiratory complications and sepsis after transthoracic esophagectomy. J Thorac Cardiovasc Surg. 2006;132:549–55. doi: 10.1016/j.jtcvs.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 29.Pera M, Gallego R, Montagut C, et al. Phase ii trial of preoperative chemoradiotherapy with oxaliplatin, cisplatin, and 5-fu in locally advanced esophageal and gastric cancer. Ann Oncol. 2012;23:664–70. doi: 10.1093/annonc/mdr291. [DOI] [PubMed] [Google Scholar]
- 30.Wahba HA, El-Hadaad HA, Abd-Ellatif EA. Neoadjuvant concurrent chemoradiotherapy with capecitabine and oxaliplatin in patients with locally advanced esophageal cancer. Med Oncol. 2012;29:1693–8. doi: 10.1007/s12032-011-0001-2. [DOI] [PubMed] [Google Scholar]
- 31.Chiarion–Sileni V, Innocente R, Cavina R, et al. Multi-center phase ii trial of chemoradiotherapy with 5-fluorouracil, leucovorin and oxaliplatin in locally advanced esophageal cancer. Cancer Chemother Pharmacol. 2009;63:1111–19. doi: 10.1007/s00280-008-0834-3. [DOI] [PubMed] [Google Scholar]
- 32.De Vita F, Orditura M, Martinelli E, et al. A multicenter phase ii study of induction chemotherapy with folfox-4 and cetuximab followed by radiation and cetuximab in locally advanced oesophageal cancer. Br J Cancer. 2011;104:427–32. doi: 10.1038/sj.bjc.6606093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivera F, Galan M, Tabernero J, et al. Phase ii trial of preoperative irinotecan–cisplatin followed by concurrent irinotecan–cisplatin and radiotherapy for resectable locally advanced gastric and esophagogastric junction adenocarcinoma. Int J Radiat Oncol Biol Phys. 2009;75:1430–6. doi: 10.1016/j.ijrobp.2008.12.087. [DOI] [PubMed] [Google Scholar]
- 34.Ajani JA, Winter K, Okawara GS, et al. Phase ii trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (rtog 9904): quality of combined modality therapy and pathological response. J Clin Oncol. 2006;24:3953–8. doi: 10.1200/JCO.2006.06.4840. [DOI] [PubMed] [Google Scholar]
- 35.Orditura M, Galizia G, Napolitano V, et al. Weekly chemotherapy with cisplatin and paclitaxel and concurrent radiation therapy as preoperative treatment in locally advanced esophageal cancer: a phase ii study. Cancer Invest. 2010;28:820–7. doi: 10.3109/07357901003630926. [DOI] [PubMed] [Google Scholar]
- 36.Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an fnclcc and ffcd multicenter phase iii trial. J Clin Oncol. 2011;29:1715–21. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]