Abstract
Venous thromboembolism (vte) represents a major challenge in the management of patients with cancer. The malignant phenotype is associated with derangements in the coagulation cascade that can manifest as thrombosis, hemorrhage, or disseminated intravascular coagulation. The risk of vte is increased by a factor of approximately 6 in patients with cancer compared with non-cancer patients, and cancer patients account for approximately 20% of all newly diagnosed cases of vte. Postmortem studies have demonstrated rates of vte in patients with cancer to be as high as 50%. Despite that prevalence, vte prophylaxis is underused in hospitalized patients with cancer. Studies have demonstrated that hospitalized patients with cancer are less likely than their non-cancer counterparts to receive vte prophylaxis. Consensus guidelines address the aforementioned issues and emerging concepts in the area, including the use of risk-assessment models, biomarkers to identify patients at highest risk of vte, and use of anticoagulants as anticancer therapy. Despite those guidelines, a gulf exists between current recommendations and clinical practice; greater efforts are thus required to ensure effective implementation of strategies to reduce the incidence of vte in patients with cancer.
Keywords: Venous thromboembolism
1. INTRODUCTION
Armand Trousseau first described thrombophlebitis as a presenting sign of visceral malignancy more than 150 years ago. Since then, the effect of cancer on blood coagulation and management has remained a major challenge for multidisciplinary care providers1. As a consequence, patients with cancer can experience complications including thrombosis, bleeding, and disseminated intravascular coagulation2. Prevention and management of those complications in cancer patients can significantly affect patient treatment, prognosis, and quality of life.
Venous thromboembolism (vte), which includes deep venous thrombosis (dvt) and pulmonary embolism, might precede or coincide with a diagnosis of cancer. In this patient group, vte can potentially complicate surgery, hospitalization, or systemic chemotherapy3–5. Risk for vte is increased by a factor of approximately 6 in patients with cancer compared with non-cancer patients, and patients with cancer account for 20% of all newly diagnosed cases of vte6. Postmortem studies suggest that the incidence of vte in cancer patients might be as high as 50%, in keeping with the finding that, after cancer itself, vte represents the second leading cause of death in hospitalized patients with cancer7–9.
Venous thromboembolism is associated with high morbidity, mortality, and economic burden. Its diagnosis and management can interrupt essential cancer therapy and cause potentially serious bleeding complications10. Moreover, approximately 25% of cancer patients with vte require readmission because of bleeding or recurrent vte11,12.
2. MECHANISMS UNDERLYING THE CANCER-ASSOCIATED PROTHROMBOTIC PHENOTYPE
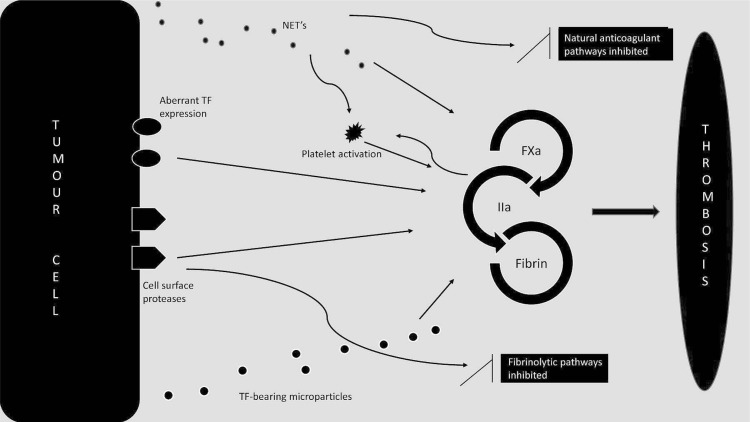
Direct and indirect mechanisms contribute to the pathogenesis of cancer-associated vte13–15 (Figure 1). The prothrombotic state variably results from tumour- and patient-specific factors, including vascular compression, vessel injury, use of intravascular devices, administration of systemic chemotherapy, and blood hypercoagulability16,17. Cancer-mediated hypercoagulability can result from direct activation of procoagulant pathways by cancer cells or from indirect systemic effects of cancer on a variety of cell types, including leukocytes and endothelial cells18.
FIGURE 1.
Cancer-mediated hypercoagulability occurs as a consequence of direct activation of procoagulant pathways by cancer cells [mediated by aberrant expression of tissue factor (tf) by tumour cells; release of tumour cell–derived,tf-expressing microparticles; and cancer procoagulant and other cell-surface proteases] or of indirect systemic effects of cancer on a variety of cell types, including leucocytes, endothelial cells, and platelets. In various malignancies, neutrophils are “primed” to release their contents in the form of neutrophil extracellular traps, resulting in direct activation of procoagulant pathways, platelet activation, and inhibition of naturally occurring anticoagulant pathways, including tf pathway inhibitor. As a consequence of these various direct and indirect mechanisms, patients with cancer have an elevated risk of venous thromboembolism.
2.1. Direct Procoagulant Effects of Cancer Cells
2.1.1. Tissue Factor Expression
Full-length tissue factor (tf) is a 47-kDa transmembrane glycoprotein that binds to coagulation factor vii and activated coagulation factor vii (fviia), triggering blood coagulation19. Under usual circumstances, tf is not exposed to flowing blood, but rather is expressed on cells in the extravascular compartment. Thus, tf forms a hemostatic “envelope” that attenuates bleeding upon exposure to flowing blood during vascular injury19.
However, in certain situations (including sepsis20 and malignancy21), aberrant tf expression on a variety of cell types can result in its exposure to the intravascular compartment, with an associated potential for coagulation activation. Mechanisms underlying aberrant cancer-associated tf expression include hypoxia-induced signalling, epidermalto-mesenchymal transformation, and mutation of tumour suppressor genes including TP53 (reviewed in van den Berg et al.21).
Cancer-associated tf expression has several consequences. The membrane-bound full-length tf–fviia complex, coupled with cell surface integrins, triggers signalling through an interplay between protease-activated receptor 2 cleavage and tf cytoplasmic domain phosphorylation22,23. Those signalling pathways modulate angiogenesis and tumour growth. Emerging evidence suggests that an alternatively-spliced soluble form of tf that lacks a transmembrane region and that has low affinity for fviia can also interact directly with cellular integrins and initiate proangiogenic signalling (reviewed in van den Berg et al.21). Secondly, tf–fviia directly activates coagulation by cleaving and activating factor x, resulting in prothrombin activation and thrombin generation19. Thrombin then activates platelets and feeds back to propagate blood coagulation through activation of factors v and viii (cofactors for factor x and for prothrombin activation respectively). Finally, cell-surface microparticles derived from tumour cells express tf and are also capable of activating blood coagulation (as described next).
2.1.2. Tumour-Derived TF-Bearing Microparticles
Cancer cells of various types, particularly those of epithelial origin, can spontaneously release small (0.1–1 μm) tf-bearing particles, called “microparticles” (reviewed in Geddings and Mackman24). Microparticles promote coagulation because they express not only tf, but also negatively-charged phospholipids, including phosphatidylserine. Tumour-derived microparticles that express tf have been shown to promote thrombosis in vivo24.
2.1.3. Expression of Cell-Surface Proteases
Cancer cells variably express cell-surface enzymes that are capable of direct modulation of procoagulant and fibrinolytic mechanisms. Cancer procoagulant is a cysteine protease expressed on the surface of many cancer cells that directly activates factor x and thus promotes thrombin generation25. Cancer cells can also express proteases that modulate fibrinolytic pathways, including tissue plasminogen activator and plasminogen activator inhibitors i and ii26.
2.2. Indirect Procoagulant Effects of Malignancy
2.2.1. Enhanced Formation of Neutrophil Extracellular Traps
Neutrophils are major effectors of innate immunity, engulfing and phagocytosing invading microorganisms. Recently, the role of innate immune cells in inducing thrombosis has been further characterized by the novel identification of neutrophil extracellular traps (nets). These extracellular fibres are released upon neutrophil degranulation in response to proinflammatory stimuli and are composed of a matrix of granule and nuclear constituents, including dna and histones27. Not only do nets contribute to direct bacterial killing27, but they also directly activate procoagulant mechanisms28. Constituents of nets bind to and activate platelets, enhance the activity of neutrophil elastase (which inactivates the anticoagulant molecule tf pathway inhibitor), and contain components that directly activate the contact pathway of blood coagulation29. Recently published in vivo data indicate that various cancer types are associated with a systemic environment that predisposes peripheral blood neutrophils to netosis and promotes venous thrombosis30.
2.2.2. Other Mechanisms Contributing to the Indirect Procoagulant Effect of Malignancy
In addition to the foregoing recently described mechanisms, other factors that likely contribute to the increased vte risk in cancer patients include altered plasma levels of proinflammatory cytokines and coagulation factors18,31.
3. RISK FACTORS AND BIOMARKERS FOR CANCER-ASSOCIATED VTE
The risk of vte in patients with cancer is highest in patients receiving systemic chemotherapy and in those hospitalized on medical and surgical wards. Clinical risk factors for vte in patients with cancer can be patient-related, cancer-related, and treatment-related (Table i).
TABLE I.
Risk factors for venous thromboembolism in patients with cancer
| Patient-related | Age, ethnicity, comorbidities (for example, obesity, infection, renal and pulmonary disease) |
| Cancer-related | Primary site, histologic subtype, natural history of cancer |
| Treatment-related | Indwelling catheters, systemic chemotherapy, supportive therapies (for example, erythropoiesis-stimulating agents, red blood cell and platelet transfusions) |
Advanced age, obesity, and the presence of comorbidities including infection, anemia, and renal or pulmonary disease modulate individual vte risk32. The vte risk also varies considerably depending on the primary site and histologic subtype of the cancer. The highest vte rates have been reported in patients with pancreatic (19.2%), stomach (15.8%), and lung cancer (13.9%)33. Patients with hematologic malignancies, particularly lymphoma, are also at increased risk. The risk appears to be highest within 3 months of the initial cancer diagnosis33–36. Elevated pre-chemotherapy leukocyte and platelet counts have also been shown to be associated with a higher incidence of vte37,38. Systemic chemotherapy increases the vte risk by a factor of approximately 2–639,40. That risk is compounded by the use of central catheters41. Supportive therapies, including the use of erythropoiesis-stimulating agents and red blood cell and platelet transfusions, further elevate the vte risk42,43. Plasma levels of P-selectin, D-dimer, and tf are currently under investigation as predictive tools for identifying patients at elevated vte risk44.
Recently published consensus guidelines from the American Society of Clinical Oncology discourage the use of single risk factors to guide clinical decision-making45. A risk assessment model for chemotherapy-associated vte that assigns scores to 5 predictive variables has recently been developed and validated (Table ii)46. In addition to identifying patients who are at highest risk of vte, the risk score has also been shown to predict favourable outcomes from chemotherapy. Currently published guidelines recommend that risk assessment in the outpatient setting should use this model45.
TABLE II.
Risk assessment model developed by Khorana et al.46
| Patient characteristic | Score |
|---|---|
| Cancer site | |
| Very high riska | 2 |
| High riskb | 1 |
| Platelet count ≥ 350,000/mm3 | 1 |
| Leukocyte count > 11,000/mm3 | 1 |
| Hemoglobin < 10 g/dL or use of esas | 1 |
| Body mass index ≥ 35 | 1 |
Stomach, pancreas.
Lung, lymphoma, gynecologic, and genitourinary (excluding prostate).
esas = erythropoiesis-stimulating agents.
4. THROMBOPROPHYLAXIS IN CANCER PATIENTS
Despite the well-established prevalence of vte in cancer patients, the Fundamental Research in Oncology and Thrombosis survey demonstrated that fewer than 5% of patients with cancer hospitalized on medical wards receive thromboprophylaxis47. Current consensus guidelines include recommendations for primary thromboprophylaxis in hospitalized cancer patients on medical and surgical wards and in carefully selected ambulatory patients48.
4.1. Thromboprophylaxis in Hospitalized Medical Patients with Cancer
Three large-scale randomized controlled trials (rcts) in acutely ill hospitalized medical patients demonstrated reduced rates of vte in patients receiving prophylactic low molecular weight heparin (lmwh). Those trials explored the clinical efficacy of enoxaparin, dalteparin, and fondaparinux in vte prevention49–51. Although patients with cancer have a significantly higher vte risk than do hospitalized medical patients without cancer, cancer patients constituted only a minority (5%–15%) of the rct populations. To date, no published rct has specifically assessed vte prophylaxis solely in hospitalized patients with cancer.
Several recently published consensus guidelines promote thromboprophylaxis in all patients admitted to hospital with a diagnosis of cancer in the absence of contraindications48,52. Despite the recommendations of those and other guidelines, thromboprophylaxis remains underused in hospitalized patients with cancer53–55. In fact, the probability of receiving appropriate thromboprophylaxis has been reported to be lower in patients with cancer than in non-cancer patients56.
4.2. Thromboprophylaxis in Ambulatory Patients with Cancer
A number of studies have explored the role of primary thromboprophylaxis in ambulatory patients with cancer. Those studies pertain to the period during which cancer patients are not in hospital for surgery or end-of-life care, but are living at home and receiving anticancer therapy as outpatients57. In women with metastatic breast cancer receiving chemotherapy, low-dose warfarin was associated with a vte risk reduction of 85% (compared with placebo)58. Subsequently, the protecht (Prophylaxis Thromboembolic Events Chemotherapy) trial randomly assigned patients with metastatic or locally advanced lung, breast, gastrointestinal, ovarian, or head-and-neck cancer to receive either nadroparin or placebo, and reported a significant reduction in the composite endpoint of arterial and venous thrombosis59.
More recently, the save-onco trial randomly assigned patients with metastatic or locally advanced solid tumours commencing chemotherapy to receive either the ultralow molecular weight heparin semuloparin or placebo. Despite the relatively low incidence of vte in the control arm (3.4%), the study demonstrated a significant reduction in the incidence of vte in patients receiving semuloparin (1.2%), with no apparent increase in the incidence of major bleeding60. Similarly, the fragem and conko 004 trials explored thromboprophylaxis in patients with locally advanced pancreatic cancer undergoing systemic chemotherapy and demonstrated a combined vte risk reduction of 12.5%61,62.
Cumulatively, the randomized trials suggest that thromboprophylaxis with lmwh can be considered in carefully selected outpatients with solid tumours receiving systemic chemotherapy. As discussed earlier, thalidomide and lenalidomide are associated with a very high vte risk when used in combination with dexamethasone. Consequently, published consensus guidelines recommend that patients with multiple myeloma receiving either regimen should receive thromboprophylaxis with aspirin or lmwh48.
4.3. Thromboprophylaxis in Surgical Patients with Cancer
Compared with cancer-free patients, those with cancer undergoing surgery are estimated to have a vte risk that is elevated by a factor of 2–36,63–65. A meta-analysis evaluating clinical trials in which patients were randomly assigned to receive either low-dose unfractionated heparin (ufh) or lmwh revealed similar efficacy and safety in preventing screen-detected vte66. A variety of mechanical thromboprophylactic methods have also been evaluated, but in general, they have been shown to be less effective than pharmacologic prophylaxis67,68.
The ideal duration of vte prophylaxis in patients with cancer undergoing major surgery remains unclear. The enoxacan ii study randomly assigned patients with cancer undergoing laparotomy to receive either short-duration (7–10 days) or extended-duration (28 days) postoperative vte prophylaxis with enoxaparin (40 mg once daily). At the end of the study period, both groups underwent screening venography. The incidence of dvt was significantly lower in the extended-duration therapy group than in the short-duration therapy group (12% and 4.8% respectively, p = 0.02). Follow-up at 3 months demonstrated that the benefit of extended-duration thromboprophylaxis was retained69.
In another study of patients undergoing laparotomy, a subset of whom underwent the procedure for malignancy, patients received open-label in-hospital dalteparin for 7 days postoperatively. At discharge, patients were randomly assigned to a group receiving no further vte prophylaxis and a group receiving dalteparin for a further 20 days. The incidence of dvt was reduced by 55%, to 7.3% in the extended-duration group from 16.3% in the short-duration group (p = 0.012)70.
More recently, the save-abdo trial compared the efficacy of semuloparin started preoperatively with enoxaparin started postoperatively in patients undergoing major surgery. Although a lower incidence of bleeding was reported in the semuloparin group, no difference was observed with respect to vte rates or all-cause mortality48. Similarly, Simonneau et al.71 randomly assigned patients with colorectal cancer undergoing surgery to receive either nadroparin or enoxaparin preoperatively. Despite a reduction in the rates of major bleeding in the nadroparin group, there was no significant difference in symptomatic or asymptomatic vte occurrence.
Current consensus guidelines recommend that “all patients with malignant disease undergoing major surgical intervention should be considered for pharmacologic thromboprophylaxis with either ufh or lmwh” unless contraindications exist and that vte prophylaxis should commence preoperatively. Moreover, it is recommended that pharmacologic thromboprophylaxis be continued for 7–10 days in all patients, with the exception of “patients undergoing major abdominal or pelvic surgery for cancer who have high-risk features such as restricted mobility, obesity, history of vte, or with additional risk factors,” in whom vte prophylaxis should be continued for up to 4 weeks48.
5. VTE TREATMENT AND SECONDARY PROPHYLAXIS IN CANCER PATIENTS
Based on published evidence from randomized trials72, currently published consensus guidelines recommend the use of lmwh in preference to ufh for the initial 5–10 days of anticoagulant therapy in patients with cancer and confirmed vte. After that, lmwh should be continued for 6 months for secondary prophylaxis, and anticoagulant therapy can potentially be extended beyond the initial 6-month period in patients with active malignancy. The insertion of a vena cava filter is recommended only in patients with contraindications to anticoagulant therapy and in whom proximal extension of thrombosis occurs despite maximal lmwh therapy48,73.
A meta-analysis comparing lmwh and ufh in the treatment of vte demonstrated a significant reduction in the rates of vte recurrence and major bleeding in individuals receiving lmwh74. A further meta-analysis of rcts revealed that 3-month mortality was significantly lower in patients receiving lmwh than in those receiving ufh75. No significant difference in mortality has been demonstrated between lmwh and vitamin K antagonists (vkas) in patients with cancer76. However, with respect to vte recurrence, lmwh has been shown to be superior to warfarin as a maintenance therapy in patients with cancer77. The clot rct randomly assigned patients with cancer-associated vte to receive either therapeutic dalteparin or warfarin for 6 months. In patients treated with dalteparin, that study demonstrated an absolute risk reduction of 8% (9% vs. 17%) and a relative risk reduction of 52% (p = 0.002; hazard ratio: 0.48) for recurrent vte72.
Despite the body of evidence supporting the superiority of lmwh over vka in the treatment of cancer-associated vte, long-term treatment with lmwh remains suboptimal with respect to patient preference and cost. Indeed, data from the Swiss Venous Thromboembolism (swivter) and master registries demonstrated that a large proportion of patients with cancer-associated thrombosis still receive warfarin as long-term treatment (51% and 62% respectively)78,79.
5.1. Use of Novel Oral Anticoagulants
Newer anticoagulants, such as the direct factor iia and xa inhibitors, are currently being investigated for use in patients with cancer80. Currently published phase iii trial data suggest that that novel oral direct inhibitor therapy is at least as safe and efficacious as heparin or vka therapy in the prevention and treatment of acute vte in specific clinical scenarios81–85. Furthermore, the new agents offer a number of advantages over conventional therapies, including, in general, no requirement for laboratory monitoring, feasibility of oral administration, and reduced drug and food interactions86.
The einstein dvt study randomly assigned patients with acute dvt to receive either rivaroxaban (15 mg twice daily for 3 weeks, followed by 20 mg once daily) or conventional therapy with enoxaparin followed by vka. In that study, rivaroxaban was demonstrated to be noninferior to standard treatment with respect to vte recurrence (2.1% vs. 3%), and the rate of non-major bleeding was similar in both groups. A subgroup analysis of the trial exploring the safety and efficacy of rivaroxaban in patients with active malignancy demonstrated no significant difference in vte recurrence or bleeding complications between the two groups83. Similarly, the einstein-pe study reported the noninferiority of rivaroxaban compared with lmwh–vka treatment in patients with acute symptomatic pulmonary embolism, with no significant difference in major or non-major bleeding complications reported between the groups84. The recently published amplify trial demonstrated the noninferiority of the oral factor xa inhibitor apixaban compared with conventional therapy in patients with acute vte. Moreover, major bleeding occurred less frequently in the apixaban group (0.06% vs. 1.8%, p < 0.001)81.
The orally administered direct thrombin inhibitor dabigatran has also been studied in patients with acute symptomatic vte. The re-cover study randomly assigned patients with acute vte to receive either dabigatran (150 mg twice daily) or warfarin for 6 months. Rates of vte recurrence were similar in both groups (2.4% in patients receiving dabigatran, 2.1% in patients treated with warfarin), and there was no significant difference in major bleeding. In a subgroup of patients with active cancer, a nonsignificant difference in the risk of vte recurrence (3.1% in the dabigatran group vs. 5.3% in those treated with warfarin) was observed85.
Overall, the safety and efficacy of novel oral direct inhibitor therapy in patients with cancer remains unknown. Given the paucity of data supporting the efficacy of these agents and cognizant of potential bleeding complications, published consensus guidelines do not currently recommend their use in patients with malignancy48.
6. ANTICOAGULANTS AS ANTICANCER THERAPY
A growing body of in vitro and in vivo evidence suggests that not only do anticoagulant medications prevent and treat vte in cancer patients, but that they also influence cancer cell biology and patient survival. The mechanisms by which heparins might exert anticancer effects are complex and comprise anticoagulant and non-anticoagulant mechanisms alike (reviewed in Cunningham et al.87 and Casu et al.88).
The anticoagulant activity of heparin is mediated through a specific interaction with the serine protease inhibitor antithrombin. This heparin–antithrombin interaction results in enhanced inactivation of activated coagulation proteases, particularly factor xa and thrombin. Recently published data demonstrate that not only do these activated coagulation proteases play a role in blood coagulation, but that they also initiate signalling pathways89,90. Downstream effects of coagulation protease–induced signalling might have an impact on cancer biology91,92. Moreover, fibrin protects cancer cells from natural killer cell–mediated immune attack93 and mediates tumour cell adhesion to the vascular wall94.
Heparin and lmwh promote endothelial tf pathway inhibitor release that might impair tf-mediated proangiogenic and proinflammatory properties (reviewed in Mousa and Petersen95). Heparin and lmwh also inhibit the activity of heparanase, an enzyme that is upregulated in cancer and that acts by cleaving cell-surface and extracellular matrix heparan sulphate proteoglycans87,88,96. The resultant extracellular matrix disruption facilitates tumour cell invasion. Moreover, degradation of heparan sulphate proteoglycans promotes the release of growth factors implicated in promoting tumour angiogenesis and growth87,88. In addition to inhibiting growth factor release, heparin also impairs growth factor–mediated mitogenic signalling activity by directly preventing their interaction with their receptor87,88,97. Heparins might directly bind to selectins, cell adhesion molecules whose expression is increased on tumour cells and that are postulated to promote tumour invasion96,98. Finally, heparins have been shown to mediate signalling by direct binding to the surface of a variety of cancer cells in vitro, attenuating cellular proliferation99,100.
The precise effect of heparin on patient survival remains incompletely understood. The famous study randomized patients with advanced malignancy to lmwh or placebo for 12 months. A nonsignificant trend toward increased survival in the group randomized to lmwh was observed. In a subgroup of patients who were alive 17 months after randomization, use of lmwh resulted in significantly improved survival at 2 and 3 years101. A subsequent double-blind rct randomly assigned patients with either metastatic or locally advanced malignancy to a 6-week course of lmwh nadroparin or placebo and demonstrated a significant survival benefit, albeit with an increased risk of major bleeding complications102. In a recent meta-analysis of eleven rcts exploring the use of anticoagulants in patients with cancer, Kuderer et al. reported a significant reduction in mortality in patients treated with anticoagulants103. In contrast, a 2006 study demonstrated no survival benefit in patients with advanced cancer receiving lmwh104. Future prospective trials will potentially shed further light on the magnitude of the heparin-mediated survival benefit in cancer patients.
7. CONCLUSIONS
Despite the fact that risk of vte is greatly increased in patients with cancer compared with cancer-free patients, prophylactic strategies remain underutilized. Moreover, even when thromboprophylaxis guidelines are rigorously implemented, uncertainty remains about precisely which outpatient groups should be specifically targeted to achieve the optimal benefit–risk ratio. While the molecular mechanisms underlying the pathogenesis of cancer-associated vte continue to be unravelled, the precise causation for the prothrombotic state and the interplay between its various mechanisms remain poorly understood. Future research initiatives should focus on translating a detailed knowledge of the underlying mechanisms into predictions about the specific groups of ambulatory patients that are at highest vte risk and that would benefit most from thromboprophylaxis. Finally, a gulf clearly exists between currently published thromboprophylaxis recommendations for hospitalized patients and actual clinical practice. Greater efforts are required to ensure effective implementation of recommended strategies to reduce the incidence of this potentially life-threatening complication in patients with cancer.
8. ACKNOWLEDGMENT
This study was supported by funding received from the Irish Health Research Board (Health Research Award R13728) and from the Friends of the Rotunda charity.
9. CONFLICT OF INTEREST DISCLOSURES
FNA has served on advisory boards for Bayer and Bristol–Myers Squibb. The remaining authors have no financial conflicts of interest to disclose.
10. REFERENCES
- 1.Khorana AA. Malignancy, thrombosis and Trousseau: the case for an eponym. J Thromb Haemost. 2003;1:2463–5. doi: 10.1111/j.1538-7836.2003.00501.x. [DOI] [PubMed] [Google Scholar]
- 2.Rickles FR, Falanga A. Molecular basis for the relationship between thrombosis and cancer. Thromb Res. 2001;102:V215–24. doi: 10.1016/S0049-3848(01)00285-7. [DOI] [PubMed] [Google Scholar]
- 3.Varki A. Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110:1723–9. doi: 10.1182/blood-2006-10-053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rickles FR, Edwards RL. Activation of blood coagulation in cancer: Trousseau’s syndrome revisited. Blood. 1983;62:14–31. [PubMed] [Google Scholar]
- 5.Prandoni P, Lensing AW, Buller HR, et al. Deep-vein thrombosis and the incidence of subsequent symptomatic cancer. N Engl J Med. 1992;327:1128–33. doi: 10.1056/NEJM199210153271604. [DOI] [PubMed] [Google Scholar]
- 6.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133(suppl):381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 7.Peuscher FW. Thrombosis and bleeding in cancer patients. Neth J Med. 1981;24:23–35. [PubMed] [Google Scholar]
- 8.Thompson CM, Rodgers LR. Analysis of the autopsy records of 157 cases of carcinoma of the pancreas with particular reference to the incidence of thromboembolism. Am J Med Sci. 1952;223:469–78. doi: 10.1097/00000441-195205000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Donati MB. Cancer and thrombosis. Haemostasis. 1994;24:128–31. doi: 10.1159/000217092. [DOI] [PubMed] [Google Scholar]
- 10.Alcalay A, Wun T, Khatri V, et al. Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J Clin Oncol. 2006;24:1112–18. doi: 10.1200/JCO.2005.04.2150. [DOI] [PubMed] [Google Scholar]
- 11.Bullano MF, Willey V, Hauch O, Wygant G, Spyropoulos AC, Hoffman L. Longitudinal evaluation of health plan cost per venous thromboembolism or bleed event in patients with a prior venous thromboembolism event during hospitalization. J Manag Care Pharm. 2005;11:663–73. doi: 10.18553/jmcp.2005.11.8.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elting LS, Escalante CP, Cooksley C, et al. Outcomes and cost of deep venous thrombosis among patients with cancer. Arch Intern Med. 2004;164:1653–61. doi: 10.1001/archinte.164.15.1653. [DOI] [PubMed] [Google Scholar]
- 13.Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol. 2005;6:401–10. doi: 10.1016/S1470-2045(05)70207-2. [DOI] [PubMed] [Google Scholar]
- 14.Falanga A, Rickles FR. Pathophysiology of the thrombophilic state in the cancer patient. Semin Thromb Hemost. 1999;25:173–82. doi: 10.1055/s-2007-994919. [DOI] [PubMed] [Google Scholar]
- 15.ten Cate H, Falanga A. Overview of the postulated mechanisms linking cancer and thrombosis. Pathophysiol Haemost Thromb. 2008;36:122–30. doi: 10.1159/000175150. [DOI] [PubMed] [Google Scholar]
- 16.Rickles FR. Mechanisms of cancer-induced thrombosis in cancer. Pathophysiol Haemost Thromb. 2006;35:103–10. doi: 10.1159/000093551. [DOI] [PubMed] [Google Scholar]
- 17.Zwicker JI, Furie BC, Furie B. Cancer-associated thrombosis. Crit Rev Oncol Hematol. 2007;62:126–36. doi: 10.1016/j.critrevonc.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Buller HR, van Doormaal FF, van Sluis GL, Kamphuisen PW. Cancer and thrombosis: from molecular mechanisms to clinical presentations. J Thromb Haemost. 2007;(suppl 1):246–54. doi: 10.1111/j.1538-7836.2007.02497.x. [DOI] [PubMed] [Google Scholar]
- 19.Versteeg HH, Peppelenbosch MP, Spek CA. The pleiotropic effects of tissue factor: a possible role for factor viia-induced intracellular signalling? Thromb Haemost. 2001;86:1353–9. [PubMed] [Google Scholar]
- 20.Pawlinski R, Mackman N. Cellular sources of tissue factor in endotoxemia and sepsis. Thromb Res. 2010;125(suppl 1):S70–3. doi: 10.1016/j.thromres.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Berg YW, Osanto S, Reitsma PH, Versteeg HH. The relationship between tissue factor and cancer progression: insights from bench and bedside. Blood. 2012;119:924–32. doi: 10.1182/blood-2011-06-317685. [DOI] [PubMed] [Google Scholar]
- 22.Dorfleutner A, Hintermann E, Tarui T, Takada Y, Ruf W. Cross-talk of integrin α3β1 and tissue factor in cell migration. Mol Biol Cell. 2004;15:4416–25. doi: 10.1091/mbc.E03-09-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaffner F, Versteeg HH, Schillert A, et al. Cooperation of tissue factor cytoplasmic domain and par2 signaling in breast cancer development. Blood. 2010;116:6106–13. doi: 10.1182/blood-2010-06-289314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geddings JE, Mackman N. Tumor-derived tissue factor–positive microparticles and venous thrombosis in cancer patients. Blood. 2013;122:1873–80. doi: 10.1182/blood-2013-04-460139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falanga A, Gordon SG. Isolation and characterization of cancer procoagulant: a cysteine proteinase from malignant tissue. Biochemistry. 1985;24:5558–67. doi: 10.1021/bi00341a041. [DOI] [PubMed] [Google Scholar]
- 26.Kwaan HC. The plasminogen–plasmin system in malignancy. Cancer Metastasis Rev. 1992;11:291–311. doi: 10.1007/BF01307184. [DOI] [PubMed] [Google Scholar]
- 27.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular dna traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–5. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 30.Demers M, Krause DS, Schatzberg D, et al. Cancers pre-dispose neutrophils to release extracellular dna traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci U S A. 2012;109:13076–81. doi: 10.1073/pnas.1200419109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falanga A, Russo L, Verzeroli C. Mechanisms of thrombosis in cancer. Thromb Res. 2013;131(suppl 1):S59–62. doi: 10.1016/S0049-3848(13)70024-0. [DOI] [PubMed] [Google Scholar]
- 32.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110:2339–46. doi: 10.1002/cncr.23062. [DOI] [PubMed] [Google Scholar]
- 33.Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–22. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 34.Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: determination of frequency and characteristics. Thromb Haemost. 2002;87:575–9. [PubMed] [Google Scholar]
- 35.Khorana AA, Francis CW, Culakova E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104:2822–9. doi: 10.1002/cncr.21496. [DOI] [PubMed] [Google Scholar]
- 36.Park LC, Woo SY, Kim S, et al. Incidence, risk factors and clinical features of venous thromboembolism in newly diagnosed lymphoma patients: results from a prospective cohort study with Asian population. Thromb Res. 2012;130:e6–12. doi: 10.1016/j.thromres.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 37.Khorana AA, Francis CW, Menzies KE, et al. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost. 2008;6:1983–5. doi: 10.1111/j.1538-7836.2008.03156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Starling N, Rao S, Cunningham D, et al. Thromboembolism in patients with advanced gastroesophageal cancer treated with anthracycline, platinum, and fluoropyrimidine combination chemotherapy: a report from the UK National Cancer Research Institute Upper Gastrointestinal Clinical Studies Group. J Clin Oncol. 2009;27:3786–93. doi: 10.1200/JCO.2008.19.4274. [DOI] [PubMed] [Google Scholar]
- 39.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case–control study. Arch Intern Med. 2000;160:809–15. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 40.Blom JW, Vanderschoot JP, Oostindier MJ, Osanto S, van der Meer FJ, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost. 2006;4:529–35. doi: 10.1111/j.1538-7836.2006.01804.x. [DOI] [PubMed] [Google Scholar]
- 41.Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol. 2003;21:3665–75. doi: 10.1200/JCO.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299:914–24. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- 43.Khorana AA, Francis CW, Blumberg N, Culakova E, Refaai MA, Lyman GH. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168:2377–81. doi: 10.1001/archinte.168.21.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ay C, Vormittag R, Dunkler D, et al. D-Dimer and prothrombin fragment 1+2 predict venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol. 2009;27:4124–9. doi: 10.1200/JCO.2008.21.7752. [DOI] [PubMed] [Google Scholar]
- 45.Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2189–204. doi: 10.1200/JCO.2013.49.1118. [DOI] [PubMed] [Google Scholar]
- 46.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–7. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kakkar AK, Levine M, Pinedo HM, Wolff R, Wong J. Venous thrombosis in cancer patients: insights from the frontline survey. Oncologist. 2003;8:381–8. doi: 10.1634/theoncologist.8-4-381. [DOI] [PubMed] [Google Scholar]
- 48.Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2189–204. doi: 10.1200/JCO.2013.49.1118. [DOI] [PubMed] [Google Scholar]
- 49.Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332:325–9. doi: 10.1136/bmj.38733.466748.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leizorovicz A, Cohen AT, Turpie AG, Olsson CG, Vaitkus PT, Goldhaber SZ, on behalf of the prevent Medical Thromboprophylaxis Study Group Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110:874–9. doi: 10.1161/01.CIR.0000138928.83266.24. [DOI] [PubMed] [Google Scholar]
- 51.Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341:793–800. doi: 10.1056/NEJM199909093411103. [DOI] [PubMed] [Google Scholar]
- 52.Khorana AA. The nccn clinical practice guidelines on venous thromboembolic disease: strategies for improving vte prophylaxis in hospitalized cancer patients. Oncologist. 2007;12:1361–70. doi: 10.1634/theoncologist.12-11-1361. [DOI] [PubMed] [Google Scholar]
- 53.Amin A, Stemkowski S, Lin J, Yang G. Thromboprophylaxis rates in US medical centers: success or failure? J Thromb Haemost. 2007;5:1610–16. doi: 10.1111/j.1538-7836.2007.02650.x. [DOI] [PubMed] [Google Scholar]
- 54.Burleigh E, Wang C, Foster D, et al. Thromboprophylaxis in medically ill patients at risk for venous thromboembolism. Am J Health Syst Pharm. 2006;63(suppl 6):S23–9. doi: 10.2146/ajhp060390. [DOI] [PubMed] [Google Scholar]
- 55.Kahn SR, Panju A, Geerts W, et al. on behalf of the curve study investigators Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res. 2007;119:145–55. doi: 10.1016/j.thromres.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 56.Seddighzadeh A, Shetty R, Goldhaber SZ. Venous thromboembolism in patients with active cancer. Thromb Haemost. 2007;98:656–61. [PubMed] [Google Scholar]
- 57.Rana P, Levine MN. Prevention of thrombosis in ambulatory patients with cancer. J Clin Oncol. 2009;27:4885–8. doi: 10.1200/JCO.2009.23.5481. [DOI] [PubMed] [Google Scholar]
- 58.Levine M, Hirsh J, Gent M, et al. Double-blind randomised trial of a very-low-dose warfarin for prevention of thromboembolism in stage iv breast cancer. Lancet. 1994;343:886–9. doi: 10.1016/S0140-6736(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 59.Agnelli G, Gussoni G, Bianchini C, et al. on behalf of the protecht investigators Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009;10:943–9. doi: 10.1016/S1470-2045(09)70232-3. [DOI] [PubMed] [Google Scholar]
- 60.Agnelli G, George DJ, Kakkar AK, et al. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med. 2012;366:601–9. doi: 10.1056/NEJMoa1108898. [DOI] [PubMed] [Google Scholar]
- 61.Maraveyas A, Waters J, Roy R, et al. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur J Cancer. 2012;48:1283–92. doi: 10.1016/j.ejca.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 62.Riess H, Pelzer U, Hilbig A, et al. Rationale and design of prospect–conko 004: a prospective, randomized trial of simultaneous pancreatic cancer treatment with enoxaparin and chemotherapy. BMC Cancer. 2008;8:361. doi: 10.1186/1471-2407-8-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost. 2003;90:446–55. doi: 10.1160/TH03-03-0152. [DOI] [PubMed] [Google Scholar]
- 64.Huber O, Bounameaux H, Borst F, Rohner A. Postoperative pulmonary embolism after hospital discharge. An underestimated risk. Arch Surg. 1992;127:310–13. doi: 10.1001/archsurg.1992.01420030076014. [DOI] [PubMed] [Google Scholar]
- 65.Agnelli G, Bolis G, Capussotti L, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @ristos project. Ann Surg. 2006;243:89–95. doi: 10.1097/01.sla.0000193959.44677.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mismetti P, Laporte S, Darmon JY, Buchmuller A, Decousus H. Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg. 2001;88:913–30. doi: 10.1046/j.0007-1323.2001.01800.x. [DOI] [PubMed] [Google Scholar]
- 67.Roderick P, Ferris G, Wilson K, et al. Towards evidence-based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextran and regional anaesthesia as thromboprophylaxis. Health Technol Assess. 2005;9:iii–iv. ix–x, 1–78. doi: 10.3310/hta9490. [DOI] [PubMed] [Google Scholar]
- 68.Cardiovascular Disease Educational and Research Trust, Cyprus Cardiovascular Disease Educational and Research Trust, European Venous Forum, International Surgical Thrombosis Forum, International Union of Angiology, and Union Internationale de Phlébologie. Prevention and treatment of venous thromboembolism. International consensus statement (guidelines according to scientific evidence) Int Angiol. 2006;25:101–61. [PubMed] [Google Scholar]
- 69.Bergqvist D, Agnelli G, Cohen AT, et al. on behalf of the enoxacanii investigators Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346:975–80. doi: 10.1056/NEJMoa012385. [DOI] [PubMed] [Google Scholar]
- 70.Rasmussen MS, Jorgensen LN, Wille–Jorgensen P, et al. Prolonged prophylaxis with dalteparin to prevent late thromboembolic complications in patients undergoing major abdominal surgery: a multicenter randomized open-label study. J Thromb Haemost. 2006;4:2384–90. doi: 10.1111/j.1538-7836.2006.02153.x. [DOI] [PubMed] [Google Scholar]
- 71.Simonneau G, Laporte S, Mismetti P, et al. on behalf of the FX140 study investigators A randomized study comparing the efficacy and safety of nadroparin 2850 IU (0.3 mL) vs. enoxaparin 4000 IU (40 mg) in the prevention of venous thromboembolism after colorectal surgery for cancer. J Thromb Haemost. 2006;4:1693–700. doi: 10.1111/j.1538-7836.2006.02083.x. [DOI] [PubMed] [Google Scholar]
- 72.Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–53. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 73.Carrier M, Khorana AA, Zwicker J, Noble S, Lee AY, on behalf of the Subcommittee on Haemostasis and Malignancy for the ssc of the isth Management of challenging cases of patients with cancer associated thrombosis including recurrent thrombosis and bleeding: guidance from the ssc of the isth. J Thromb Haemost. 2013;11:1760–5. doi: 10.1111/jth.12338. [DOI] [PubMed] [Google Scholar]
- 74.Siragusa S, Cosmi B, Piovella F, Hirsh J, Ginsberg JS. Low-molecular-weight heparins and unfractionated heparin in the treatment of patients with acute venous thromboembolism: results of a meta-analysis. Am J Med. 1996;100:269–77. doi: 10.1016/S0002-9343(97)89484-3. [DOI] [PubMed] [Google Scholar]
- 75.Hettiarachchi RJ, Smorenburg SM, Ginsberg J, Levine M, Prins MH, Buller HR. Do heparins do more than just treat thrombosis? The influence of heparins on cancer spread. Thromb Haemost. 1999;82:947–52. [PubMed] [Google Scholar]
- 76.Conti S, Guercini F, Iorio A. Low-molecular-weight heparin and cancer survival: review of the literature and pooled analysis of 1,726 patients treated for at least three months. Pathophysiol Haemost Thromb. 2004;33:197–201. doi: 10.1159/000081508. [DOI] [PubMed] [Google Scholar]
- 77.Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162:1729–35. doi: 10.1001/archinte.162.15.1729. [DOI] [PubMed] [Google Scholar]
- 78.Spirk D, Ugi J, Korte W, et al. Long-term anticoagulation treatment for acute venous thromboembolism in patients with and without cancer. The Swiss Venous Thromboembolism Registry (swivter) ii. Thromb Haemost. 2011;105:962–7. doi: 10.1160/TH11-01-0002. [DOI] [PubMed] [Google Scholar]
- 79.Imberti D, Agnelli G, Ageno W, et al. on behalf of the master investigators Clinical characteristics and management of cancer-associated acute venous thromboembolism: findings from the master Registry. Haematologica. 2008;93:273–8. doi: 10.3324/haematol.11458. [DOI] [PubMed] [Google Scholar]
- 80.Levine MN. New antithrombotic drugs: potential for use in oncology. J Clin Oncol. 2009;27:4912–18. doi: 10.1200/JCO.2009.24.7346. [DOI] [PubMed] [Google Scholar]
- 81.Agnelli G, Buller HR, Cohen A, et al. on behalf of the amplify investigators Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808. doi: 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- 82.Cohen AT, Spiro TE, Buller HR, et al. on behalf of the magellan investigators Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513–23. doi: 10.1056/NEJMoa1111096. [DOI] [PubMed] [Google Scholar]
- 83.Bauersachs R, Berkowitz SD, Brenner B, et al. on behalf of the einstein investigators Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 84.Büller HR, Prins MH, Lensin AW, et al. on behalf of the einstein-pe investigators Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–97. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- 85.Schulman S, Kearon C, Kakkar AK, et al. on behalf of the re-cover study group Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–52. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 86.Van Es J, Eerenberg ES, Kamphuisen PW, Buller HR. How to prevent, treat, and overcome current clinical challenges of vte. J Thromb Haemost. 2011;9(suppl 1):265–74. doi: 10.1111/j.1538-7836.2011.04334.x. [DOI] [PubMed] [Google Scholar]
- 87.Cunningham MS, Preston RJ, O’Donnell JS. Does antithrombotic therapy improve survival in cancer patients? Blood Rev. 2009;23:129–35. doi: 10.1016/j.blre.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 88.Casu B, Vlodavsky I, Sanderson RD. Non-anticoagulant heparins and inhibition of cancer. Pathophysiol Haemost Thromb. 2008;36:195–203. doi: 10.1159/000175157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–64. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 90.Gleeson EM, O’Donnell JS, Hams E, et al. Activated factor x signaling via protease-activated receptor 2 suppresses pro-inflammatory cytokine production from lipopolysaccharide-stimulated myeloid cells. Haematologica. 2014;99:185–93. doi: 10.3324/haematol.2013.086918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu J, Schuff–Werner P, Steiner M. Thrombin/thrombin receptor (par-1)–mediated induction of IL-8 and vegf expression in prostate cancer cells. Biochem Biophys Res Commun. 2006;343:183–9. doi: 10.1016/j.bbrc.2006.02.136. [DOI] [PubMed] [Google Scholar]
- 92.Morris DR, Ding Y, Ricks TK, Gullapalli A, Wolfe BL, Trejo J. Protease-activated receptor-2 is essential for factor viia and xa-induced signaling, migration, and invasion of breast cancer cells. Cancer Res. 2006;66:307–14. doi: 10.1158/0008-5472.CAN-05-1735. [DOI] [PubMed] [Google Scholar]
- 93.Palumbo JS, Kombrinck KW, Drew AF, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96:3302–9. [PubMed] [Google Scholar]
- 94.Palumbo JS, Degen JL. Fibrinogen and tumor cell metastasis. Haemostasis. 2001;31(suppl 1):11–15. [PubMed] [Google Scholar]
- 95.Mousa SA, Petersen LJ. Anti-cancer properties of low-molecular-weight heparin: preclinical evidence. Thromb Haemost. 2009;102:258–67. doi: 10.1160/TH08-12-0832. [DOI] [PubMed] [Google Scholar]
- 96.Hostettler N, Naggi A, Torri G, et al. P-selectin– and heparanase-dependent antimetastatic activity of non-anticoagulant heparins. FASEB J. 2007;21:3562–72. doi: 10.1096/fj.07-8450com. [DOI] [PubMed] [Google Scholar]
- 97.Khorana AA, Sahni A, Altland OD, Francis CW. Heparin inhibition of endothelial cell proliferation and organization is dependent on molecular weight. Arterioscler Thromb Vasc Biol. 2003;23:2110–15. doi: 10.1161/01.ATV.0000090671.56682.D7. [DOI] [PubMed] [Google Scholar]
- 98.Nelson RM, Cecconi O, Roberts WG, Aruffo A, Linhardt RJ, Bevilacqua MP. Heparin oligosaccharides bind L- and P-selectin and inhibit acute inflammation. Blood. 1993;82:3253–8. [PubMed] [Google Scholar]
- 99.Balzarotti M, Fontana F, Marras C, et al. In vitro study of low molecular weight heparin effect on cell growth and cell invasion in primary cell cultures of high-grade gliomas. Oncol Res. 2006;16:245–50. doi: 10.3727/000000006783981053. [DOI] [PubMed] [Google Scholar]
- 100.Li HL, Ye KH, Zhang HW, et al. Effect of heparin on apoptosis in human nasopharyngeal carcinoma CNE2 cells. Cell Res. 2001;11:311–15. doi: 10.1038/sj.cr.7290101. [DOI] [PubMed] [Google Scholar]
- 101.Kakkar AK, Levine MN, Kadziola Z, et al. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (famous) J Clin Oncol. 2004;22:1944–8. doi: 10.1200/JCO.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 102.Klerk CP, Smorenburg SM, Otten HM, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130–5. doi: 10.1200/JCO.2005.03.134. [DOI] [PubMed] [Google Scholar]
- 103.Kuderer NM, Khorana AA, Lyman GH, Francis CW. A meta-analysis and systematic review of the efficacy and safety of anticoagulants as cancer treatment: impact on survival and bleeding complications. Cancer. 2007;110:1149–61. doi: 10.1002/cncr.22892. [DOI] [PubMed] [Google Scholar]
- 104.Sideras K, Schaefer PL, Okuno SH, et al. Low-molecular-weight heparin in patients with advanced cancer: a phase 3 clinical trial. Mayo Clin Proc. 2006;81:758–67. doi: 10.4065/81.6.758. [DOI] [PubMed] [Google Scholar]