Abstract
Background
Patients with alpha-fetoprotein (afp)–producing gastric cancer have a high incidence of liver metastasis and poor prognosis. There is some controversy about clinical manifestations in these patients.
Methods
Our study enrolled patients who, before surgery, had gastric cancer with serum afp exceeding 20 ng/mL [afp>20 (n = 58)] and with serum afp 20 ng/mL or less [afp≤20 (n = 1236)]. Clinical manifestations were compared between the groups.
Results
Early gastric cancer was more frequent (30.1% vs. 4%) and advanced gastric cancer was less frequent (69.9% vs. 96%) in the afp≤20 group than the afp>20 group (p < 0.001). Liver and lymph node metastasis occurred less frequently in the afp≤20 group (4.4% vs. 27.6%, p < 0.001, and 60.7% vs. 91.4%, p < 0.001, respectively). The 1-, 3-, 5-, and 10-year survival rates of afp≤20 patients were 75.2%, 53.4%, 45.8%, and 34.6% respectively. The 1-, 3-, 5-, and 10-year survival rates of patients with afp greater than 20 ng/mL, but 300 ng/mL or less, were 46.7%, 28.9%, 17.8%, and 13.3% respectively. The 1-, 3-, and 5-year survival rates of patients with serum afp greater than 300 ng/mL were 15.4%, 7.7%, and 0% respectively. The independent predictors for survival time were afp concentration, age, peritoneal seeding, liver metastasis, lymph node metastasis, vascular invasion, TNM stage, curative surgery, serosal invasion, and Lauren classification.
Conclusions
Patients with high serum afp had a high frequency of liver and lymph node metastasis and very poor prognosis. More aggressive management with multimodal therapy (for example, chemotherapy, radiotherapy) might be needed when treating such patients.
Keywords: Gastric cancer, alpha-fetoprotein, early gastric cancer, metastasis
1. INTRODUCTION
Alpha-fetoprotein (afp) is a glycoprotein that is normally produced during gestation by the fetal liver and yolk sac1. Elevation of serum afp is considered to be abnormal in adults and is often used as a tumour marker in hepatocellular carcinoma and tumours of gonadal origin2. However, a variety of other malignancies also produce afp, of which gastric cancer is the most common3. Elevated serum afp can occur in patients without hepatocellular carcinoma but with chronic liver disease such as viral hepatitis or cirrhosis4,5. The influence of serum afp on the prognosis of patients with gastric cancer remains unclear.
Alpha-fetoprotein–producing gastric cancer (afpgc) is rare, constituting only about 1%–6% of all gastric cancers6. Poor prognosis is usually associated with afpgc because of liver and lymph node metastasis3,7.
Few studies to date have addressed the clinicopathologic features and long-term survival of patients with afpgc. Controversy exists about the clinical manifestations in these patients. Therefore, in this retrospective study, we reviewed clinicopathologic findings for 58 Chinese patients with afpgc and 1236 patients with normal serum afp attending a single centre. We also correlated survival time with serum afp concentration.
2. METHODS
We reviewed the medical records of 3172 consecutive patients with gastric adenocarcinoma who received surgical intervention at the Veterans General Hospital–Taipei between June 1988 and December 2011. Preoperative serum afp was assessed by radioimmunoassay (normal value: <20 ng/mL) in 1331 patients. The analysis excluded 37 patients with acute or chronic hepatitis, cirrhosis, or hepatocellular carcinoma. Surgical and pathologic findings for the remaining 1294 patients were recorded using the Japanese classification of gastric carcinoma and the Lauren classification8,9. Nodal status and disease stage were assessed using the tumour–node–metastasis (TNM) system of the Union for International Cancer Control10. Sex, age, tumour size (mucosal size of the tumour), peritoneal seeding, liver metastasis, lymph node metastasis, location of the main tumour, lymphatic and vascular invasion, clinical staging, curative surgery, cause of death, morphologic appearance and depth of cancer involvement, cancer cell differentiation, and survival time were recorded.
Statistical analysis was performed using the SPSS software application (SPSS for Windows, version 10.0: SPSS, Chicago, IL, U.S.A.). The chi-square test with Yates correction for continuity was used in comparisons of categorical data. The Fisher exact test was used when the numbers were less than 5. Survival curves were estimated by the Kaplan–Meier method, and differences were examined using the log-rank test. A multivariate analysis of prognostic factors was evaluated using the Cox proportional hazards model (forward stepwise method). Differences were considered significant when the p value was less than 0.05.
3. RESULTS
Of the 1294 eligible patients, 58 (4.5%) were found to have high serum afp (>20 ng/mL), with preoperative concentrations ranging from 20.6 ng/mL to 9999.9 ng/mL (median: 90.4 ng/mL). Median follow-up was 43.2 months.
Table i compares the clinicopathologic features of patients with a serum afp of 20 ng/mL or less [afp≤20 (n = 1236)] and those with a serum afp exceeding 20 ng/mL [afp>20 (n = 58)]. Sex, age, tumour size, peritoneal seeding, and tumour location were similar in the two groups. Compared with the afp≤20 group, the afp>20 group had higher incidences of vascular invasion (17.2% vs. 3.8%, p < 0.001), lymphatic invasion (70.7% vs. 59.7%, p < 0.001), liver metastasis (27.6% vs. 4.4%, p < 0.001), and lymph node metastasis (91.4% vs. 60.7%, p < 0.001). Compared with patients having normal serum afp, those in the afp>20 group had more stage iv disease and less stage i or ii disease (p < 0.001). Fewer patients in the afp>20 group received curative surgery (10.3% vs. 37.9% in the afp≤20 group, p < 0.001). More patients in the afp>20 group died of their gastric cancer (58.6% vs. 27.1% in the afp≤20 group, p < 0.001).
TABLE I.
Serum alpha-fetoprotein (afp) and clinicopathologic features in patients with gastric cancer
| Variable |
afp concentration
|
p Value | |
|---|---|---|---|
| ≤20 ng/mL | >20 ng/mL | ||
| Patients (n) | 1236 | 58 | |
| Sex [n (%) men] | 976 (78.2) | 51 (87.9) | 0.099a |
| Mean age (years) | 66.2±11.7 | 68.0±9.5 | 0.257 |
| Mean tumour size (cm) | 5.7±4.1 | 6.7±3.4 | 0.063 |
| Peritoneal seeding [n (%) yes] | 179 (14.5) | 8 (13.8) | 1.000a |
| Peritoneal seeding present [n (%)] | 0.728b | ||
| Proximal to transverse colon | 83 (46.4) | 3 (37.5) | |
| Distal to transverse colon | 96 (53.6) | 5 (62.5) | |
| Metastasis [n (%) yes] | |||
| Liver | 53 (4.4) | 16 (27.6) | <0.001a |
| Lymph nodes | 750 (60.7) | 53 (91.4) | <0.001a |
| Location of main tumour | 0.932a | ||
| Cardia | 195 (15.8) | 10 (17.2) | |
| Body | 395 (32.0) | 19 (32.8) | |
| Antrum | 646 (52.3) | 29 (50.0) | |
| Invasion [n (%) yes] | |||
| Lymphatic | 738 (59.7) | 41 (70.7) | <0.001a |
| Vascular | 47 (3.8) | 10 (17.2) | <0.001a |
| Stage | <0.001a | ||
| ia | 319 (25.2) | 2 (3.3) | |
| ib | 114 (9.0) | 1 (1.6) | |
| ii | 158 (12.5) | 3 (4.9) | |
| iiia | 190 (15.0) | 12 (19.7) | |
| iiib | 153 (12.1) | 11 (18.0) | |
| iv | 333 (26.3) | 32 (52.5) | |
| Curative surgery [n (%)] | 468 (37.9) | 6 (10.3) | <0.001 |
| Cause of death [n (%) cancer] | 330 (27.1) | 34 (58.6) | <0.001a |
By chi-square test.
By Fisher exact test.
Table ii analyzes the depth of cancer involvement in the gastric wall. The afp>20 group included fewer cases of early gastric cancer (egc: 4% vs. 30.1%; p < 0.001) and more cases of advanced gastric cancer (96% vs. 69.9%, p < 0.001). In the 2 egc patients of the afp>20 group, cancer cells had involved the intramucosal and muscularis mucosa layers. In the afp≤20 group, 349 patients (30.1%) had egc, with cancer cells confined to the intramucosa in 97 cases (27.8%), to the muscularis mucosa in 94 cases (26.9%), and to the submucosal layer in 158 cases (45.3%). Cancer cells penetrated to serosal layer and beyond in more patients of the afp>20 group [40 patients (80%) vs. 628 patients (54.2%) in the afp≤20 group, p < 0.001].
TABLE II.
Serum alpha-fetoprotein (afp) and depth of cancer involvement of the gastric wall
| Variable |
afp concentration [n (%)]
|
p Value | |
|---|---|---|---|
| ≤20 ng/mL | >20 ng/mL | ||
| Patientsa | 1158 | 50 | <0.001b |
| Gastric cancer | |||
| Early | 349 (30.1) | 2 (4.0) | |
| Advanced | 809 (69.9) | 48 (96.0) | |
| Depth of involvement | <0.001c | ||
| Intramucosa | 97 (8.4) | 1 (2.0) | |
| Muscularis mucosa | 94 (8.1) | 1 (2.0) | |
| Submucosa | 158 (13.6) | 0 (0) | |
| Muscularis propria | 133 (11.5) | 2 (4.0) | |
| Subserosa α | 10 (0.9) | 2 (4.0) | |
| Subserosa β | 18 (1.6) | 3 (6.0) | |
| Subserosa γ | 20 (1.7) | 1 (2.0) | |
| Serosal penetration | 518 (44.7) | 32 (62.7) | |
| Invasion of adjacent structures | 110 (9.5) | 8 (16) | |
Data not available for 78 patients in the ≤20 ng/mL group, and 7 in the >20 ng/mL group.
By Fisher exact test.
By chi-square test.
Table iii summarizes the histologic classification of cancer cells in the two groups. Poorly differentiated cancers (por 1, por 2, signet-ring cell, mucinous adenocarcinoma) were not statistically different between the groups (afp≤20: n = 558, 48.7%; afp>20: n = 23, 50%; p = 0.87). The Lauren classification was not statistically significantly different between the afp≤20 and afp>20 groups.
TABLE III.
Serum alpha-fetoprotein (afp) and cell differentiation in gastric cancer
| Variable |
afp concentration [n (%)]
|
p Value | |
|---|---|---|---|
| ≤20 ng/mL | >20 ng/mL | ||
| Patientsa | 1145 | 46 | 0.002b |
| Papillary adenocarcinoma | 19 (1.7) | 1 (2.2) | |
| Tubular adenocarcinoma | |||
| Well-differentiated | 113 (9.9) | 1 (2.2) | |
| Moderately differentiated | 446 (39.0) | 18 (39.1) | |
| Adenocarcinoma, poorly differentiated | |||
| Solid type | 43 (3.8) | 4 (8.7) | |
| Non-solid type | 387 (33.8) | 16 (34.8) | |
| Signet-ring cell carcinoma | 89 (7.8) | 3 (6.5) | |
| Mucinous adenocarcinoma | 39 (3.4) | 0 (0) | |
| Adenosquamous carcinoma | 1 (0.1) | 1 (2.2) | |
| Undifferentiated | 3 (0.3) | 1 (2.21) | |
| Miscellaneous | 5 (0.4) | 1 (2.2) | |
| Lauren classification | 0.802b | ||
| Intestinal | 590 (51.5) | 25 (54.3) | |
| Diffuse | 350 (30.6) | 16 (34.8) | |
| Mixed | 205 (17.9) | 5 (10.9) | |
Data not available for 91 patients in the ≤20 ng/mL group, and 12 in the >20 ng/mL group.
By chi-square test.
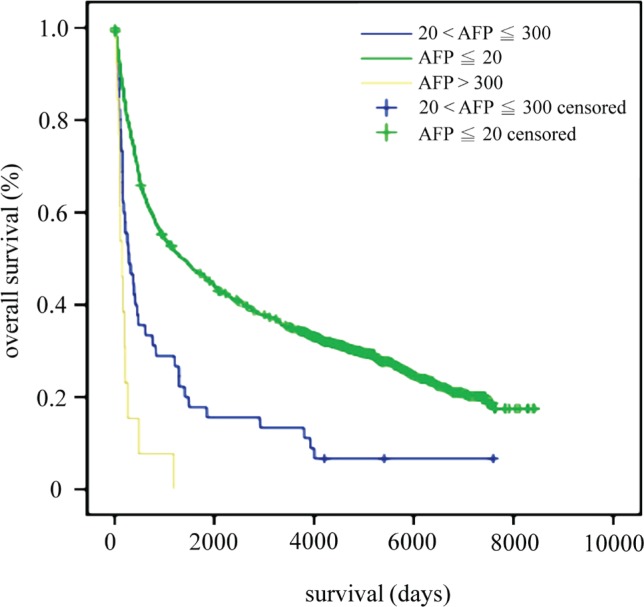
Survival time was further analyzed by various levels of high serum afp. In 45 patients, serum afp was greater than 20 ng/mL but less than or equal to 300 ng/mL (20<afp≤300); in 13 patients, serum afp exceeded 300 ng/mL (afp>300). The 1-, 3-, 5-, and 10-year survival rates for patients in the afp≤20 group were 75.2%, 53.4%, 45.8%, and 34.6% respectively. The 1-, 3-, 5-, and 10-year survival rates for 20<afp≤300 patients were 46.7%, 28.9%, 17.8%, and 13.3% respectively. The 1-, 3-, and 5-year survival rates for patients with afp>300 were 15.4%, 7.7%, and 0% respectively. The patients in the afp≤20 group had the best survival time, and the patients in the 20<afp≤300 group had the poorest survival (p < 0.001, Figure 1).
FIGURE 1.
Overall survival by serum alpha-fetoprotein (afp) concentration in nanograms per milliliter.
In univariate analysis, serum afp greater than 20 ng/mL, male sex, age greater than 60, tumour size greater than 7 cm, peritoneal seeding, liver metastasis, lymph node metastasis, lymphatic and vascular invasion, tumour stage iv, no curative surgery, serosal invasion, and poorly differentiated and diffuse cell types were associated with poor survival time (Table iv).
TABLE IV.
Univariate analysis of all patients by the Kaplan–Meier method
| Variable | Pts (n) | 5-Year survival | Log-rank p value |
|---|---|---|---|
| Alpha-fetoprotein | |||
| ≤20 ng/mL | 1236 | 0.458 | <0.001 |
| >20 ng/mL | 58 | 0.138 | |
| Sex | |||
| Men | 1018 | 0.423 | <0.001 |
| Women | 276 | 0.519 | |
| Age | |||
| <60 Years | 281 | 0.535 | <0.001 |
| ≥60 Years | 1013 | 0.418 | |
| Tumour size | |||
| <7 cm | 862 | 0.577 | <0.001 |
| ≥7 cm | 431 | 0.176 | |
| Peritoneal seeding | |||
| Yes | 187 | 0.032 | <0.001 |
| No | 1107 | 0.513 | |
| Liver metastasis | |||
| Yes | 71 | 0.042 | <0.001 |
| No | 1223 | 0.467 | |
| Lymph node metastasis | |||
| Yes | 803 | 0.228 | <0.001 |
| No | 491 | 0.849 | |
| Location of main tumour | |||
| Cardia | 205 | 0.402 | 0.056 |
| Body | 414 | 0.488 | |
| Antrum | 675 | 0.429 | |
| Lymphatic invasion | |||
| Yes | 803 | 0.321 | <0.001 |
| No | 491 | 0.795 | |
| Vascular invasion | |||
| Yes | 57 | 0.140 | < 0.001 |
| No | 1237 | 0.457 | |
| TNM stage | |||
| i | 314 | 0.843 | <0.001 |
| ii | 280 | 0.687 | |
| iii | 299 | 0.313 | |
| iv | 400 | 0.055 | |
| Curative surgery | |||
| Yes | 474 | 0.790 | <0.001 |
| No | 820 | 0.243 | |
| Serosal invasion | |||
| Yes | 754 | 0.206 | <0.001 |
| No | 540 | 0.775 | |
| Differentiation | |||
| Papillary adenocarcinoma | 20 | 0.450 | <0.001 |
| Tubular adenocarcinoma | 578 | 0.569 | |
| Poorly differentiateda | 554 | 0.403 | |
| Mucinous | 39 | 0.237 | |
| Lauren classification | |||
| Intestinal | 614 | 0.558 | 0.004 |
| Diffuse | 367 | 0.392 | |
| Mixed | 208 | 0.403 |
Includes signet-ring cell carcinoma.
Pts = patients.
In multivariate analysis, the independent prognostic factors for survival were serum afp, patient age, peritoneal seeding, liver metastasis, lymph node metastasis, vascular invasion, TNM stage, curative surgery, serosal invasion, and Lauren classification (Table v).
TABLE V.
Independent prognostic factors by Cox modelling
| Variable | Coefficient | hr | 95% ci | p Value |
|---|---|---|---|---|
| afp group | 0.575 | 1.777 | 1.297 to 2.437 | <0.001 |
| Sex | −0.0.87 | 0.917 | 0.759 to 1.717 | 0.366 |
| Age | 0.028 | 1.029 | 1.021 to 1.036 | <0.001 |
| Tumour size | 0.003 | 1.003 | 0.989 to 1.017 | 0.656 |
| Peritoneal seeding | 0.628 | 1.874 | 1.490 to 2.356 | <0.001 |
| Liver metastasis | 0.575 | 1.776 | 1.253 to 2.519 | 0.001 |
| Lymph node metastasis | 0.267 | 1.306 | 1.035 to 1.649 | 0.025 |
| Lymphatic invasion | 0.003 | 1.003 | 0.809 to 1.244 | 0.979 |
| Vascular invasion | −0.313 | 0.731 | 0.550 to 0.974 | 0.032 |
| TNM stage | 0.417 | 1.518 | 1.310 to 1.759 | <0.001 |
| Curative surgery | 0.265 | 1.304 | 1.054 to 1.612 | 0.015 |
| Serosal invasion | 0.356 | 1.427 | 1.142 to 1.783 | 0.002 |
| Differentiation | 0.070 | 1.073 | 0.928 to 1.240 | 0.343 |
| Lauren classification | −0.122 | 0.885 | 0.789 to 0.992 | 0.037 |
hr = hazard ratio; ci = confidence interval; afp = alpha-fetoprotein.
4. DISCUSSION
In our study, 58 patients with afpgc had a high percentage of lymph node and liver metastasis and a poor prognosis. The prevalence of afpgc is reported to be 0.17%–8.4% in patients with gastric cancer3,6,11–14. Clinical manifestations in patients with afpgc have rarely been observed because of that small incidence15. Furthermore, controversy exists about those manifestations.
We observed that 4.5% of gastric cancer patients (58 of 1294) had an abnormal serum afp reading (>20 ng/mL), a proportion that is comparable with those in other reports. To avoid confounding factors in patients with afpgc, we excluded 37 patients with liver disease (cirrhosis, hepatoma, acute hepatitis) from the analysis.
Liver metastasis (14.3%–75.6%) is one of the main features of afpgc or hepatoid adenocarcinoma of the stomach (has)12–14,16. In our series, 16 patients (27.6%) in the afpgc group were found to have liver metastasis during follow-up. That group more frequently had liver metastasis than did patients with normal serum afp (n = 53, 4.4%, p < 0.001). However, the related literature describes some different observations. Nakajima et al.17 reported that there was no correlation between preoperative afp values and histopathology, lymph node metastasis, vessel invasion, and liver metastasis.
Lymph node involvement has been reported to be present in 62.9%–100% patients with afpgc11–15. Lymph node metastasis was found more often in our afpgc patients than in patients with normal serum afp (91.4% vs. 60.7%, p < 0.001). Vascular invasion is very common in afpgc, occurring in 63.5%–75.6% patients with afpgc or has3,6,11,16. In our series, it occurred in 10 patients (17.2%) with afpgc and in 47 patients (3.8%) with normal serum afp (p < 0.001). Lymphatic invasion has been reported to occur in 71.4%–86.7% of patients with afpgc or has11,12,16,18. In our series, it occurred in 41 patients (70.7%) with afpgc and in 738 (59.7%) patients with normal serum afp (p < 0.001).
The lower one third of the stomach is most common location for afpgc or has, in the range 40%–61.5%3,6,11–13,16. Our observation was similar. In our series, the primary cancer was above the antrum in 29 patients with afpgc (50%) and in 646 patients (52.3%) with normal serum afp.
With respect to clinical staging, the literature reports a number of different observations. In one large series (270 patients), Adachi et al.15 showed that most patients with afpgc had serosal invasion, lymph node metastasis, and liver metastasis; three quarters had stage iii or iv disease. Those authors found that the 5-year survival rate after gastrectomy was only 22%. The poor prognosis was attributable mostly to simultaneous metastases or early recurrence in the liver. However, Chun et al.13 reported that 74% (n = 26) of their afpgc patients had stage i or ii disease. In our study, patients with normal serum afp were observed to have more stage i disease (25.2% vs. 3.3% in patients with afpgc) and less stage iv disease (27.1% vs. 58.6% in patients with afpgc, p < 0.001).
In our study, more patients died of gastric cancer in the afpgc group than in the afp≤20 group (58.6% vs. 27.1%, p < 0.001), an observation that might be explained by a low rate of curative surgery and greater rates of recurrent gastric cancer and liver metastasis in the patients with afpgc. In patients with has or afpgc, the rate of egc has been reported to be 0%–42.9%3,6,11–14,16,19, with most publications reporting rates of less than 10%6,12,14. However, different observations have also been published. Chun et al.13 found that 42.9% of their patients with afpgc (n = 15) had early-stage disease. In our series, egc was found in 4% of patients with afpgc (2 of 50), which is a rate lower than that seen in the patients with normal afp (349 of 1158, 30.1%, p < 0.001). Our finding is compatible with those in most other reports (0%–19.4%)6,11,14, which found that advanced gastric cancer was present in most patients with has or afpgc3,6,12,14. In our study, patients with afpgc more often had advanced gastric cancer than did patients with normal serum afp (96% vs. 69.9%, p < 0.001).
Poorly differentiated cancer cells have been reported to predominate in patients with afpgc or has (48.6%–64.4%)3,12,13,16; however, different findings have also been reported. In one large analysis of pooled data from Japan, Adachi et al.15 found that well-differentiated cancers was predominated in patients with afpgc (n = 218, 87.2%). In our study, the incidence of poorly differentiated cancer cells (por 1, por 2, signet-ring cells, mucinous adenocarcinoma) was similar in both patient groups [50% in the afpgc group (n = 23) and 48.7% in the normal serum afp group (n = 558), p = 0.87].
Surgery is the currently the main therapy for gastric cancer. However, radical surgery was successful in only 6 patients of our afpgc group (10.3%). In contrast, radical surgery was much more successful in patients with normal serum afp (n = 468, 37.9%, p < 0.001). That difference might explain why the afpgc group had more liver metastasis and a worse prognosis than did patients with normal serum afp.
The 5-year survival rate in patients with afpgc has been reported to be 9%–66%6,12,13,16. However, there has been some controversy about the link between afp and survival duration. Survival duration after surgery has been found not to be linked to preoperative serum afp6. Inoue and colleagues observed that 1 patient with high serum afp (25,400 ng/mL) was still living 12 years after diagnosis of gastric cancer6. The large Japanese study using pooled data also showed similar results: Adachi et al.15 found that 5-year survival rates were not different for patients with gastric cancer and a serum afp less than 1000 ng/mL (42.7%) or greater than 1000 ng/mL (39.4%). Nagai et al.20 also reported that the 5-year survival rate was 40% in patients with lower afp and 38% in patients with higher afp.
Other authors found that patients with afpgc had a shorter survival duration. In one large series, Liu et al.3 found that the 1-, 3-, and 5-year survival rates for patients with afpgc were 53%, 35%, and 28% respectively. Those authors also found that patients with afpgc or has had a poorer prognosis than did patients who had lower afp concentrations (p < 0.01) or non-has disease (p < 0.05)3. Chun et al.13 found that the 5-year survival rate in patients with afp-producing disease was significantly poorer than that in non-afp-producing group (66% vs. 80%, p = 0.002); however, their reported 5-year survival rate was extremely high compared with that in other reports. In our study, we found that 1-, 3-, 5-, and 10-year survival rates for 20<afp≤300 patients were 46.7%, 28.9%, 17.8%, and 13.3% respectively. The 1-, 3-, and 5-year survival rates for afp>300 patients were 15.4%, 7.7%, and 0% respectively. Patients in the afp≤20 group had the best survival time, and patients in the 20<afp≤300 group had the poorest survival time (p < 0.001, Figure 1).
5. CONCLUSIONS
Patients with afp-producing gastric cancer had a low rate of successful surgery, a high rate of liver and lymph node metastasis, and very poor prognosis. More aggressive management with multimodal therapy (for example, chemotherapy, radiotherapy) might be needed when treating such patients.
6. ACKNOWLEDGMENT
This study was supported by the Tomorrow Medical Foundation (grant no. 101-2) and was presented as a poster during Digestive Disease Week at Orlando, Florida, U.S.A., in May 2013. The authors express their gratitude to Miss Betty Tzu-En Lin, Mr. Austin Jen-Liang Lin, and Mr. Alex Jen-Hao Lin for their assistance.
7. CONFLICT OF INTEREST DISCLOSURES
The authors declare that they have no financial conflicts of interest.
8. REFERENCES
- 1.Bergstrand CG, Czar B. Demonstration of a new protein fraction in serum from the human fetus. Scand J Clin Lab Invest. 1956;8:174. doi: 10.3109/00365515609049266. [DOI] [PubMed] [Google Scholar]
- 2.El-Bahrawy M. Alpha-fetoprotein–producing non-germ cell tumours of the female genital tract. Eur J Cancer. 2010;46:1317–22. doi: 10.1016/j.ejca.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Cheng Y, Sheng W, et al. Clinicopathologic features and prognostic factors in alpha-fetoprotein-producing gastric cancers: analysis of 104 cases. J Surg Oncol. 2010;102:249–55. doi: 10.1002/jso.21624. [DOI] [PubMed] [Google Scholar]
- 4.Sterling RK, Wright EC, Morgan TR, et al. Frequency of elevated hepatocellular carcinoma (hcc) biomarkers in patients with advanced hepatitis C. Am J Gastroenterol. 2012;107:64–74. doi: 10.1038/ajg.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collier J, Sherman M. Screening for hepatocellular carcinoma. Hepatology. 1998;27:273–8. doi: 10.1002/hep.510270140. [DOI] [PubMed] [Google Scholar]
- 6.Inoue M, Sano T, Kuchiba A, Taniguchi H, Fukagawa T, Katai H. Long-term results of gastrectomy for alpha-fetoprotein-producing gastric cancer. Br J Surg. 2010;97:1056–61. doi: 10.1002/bjs.7081. [DOI] [PubMed] [Google Scholar]
- 7.Chang YC, Nagasue N, Kohno H, et al. Clinicopathologic features and long-term results of alpha-fetoprotein-producing gastric cancer. Am J Gastroenterol. 1990;85:1480–5. [PubMed] [Google Scholar]
- 8.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 9.Lauren PA, Nevalainen TJ. Epidemiology of intestinal and diffuse types of gastric carcinoma. A time-trend study in Finland with comparison between studies from high- and low-risk areas. Cancer. 1993;71:2926–33. doi: 10.1002/1097-0142(19930515)71:10<2926::AID-CNCR2820711007>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 10.Sobin LH, Wittekind C. TNM Classification of Malignant Tumours. 6th ed. Hoboken, NJ: John Wiley and Sons; 2002. [Google Scholar]
- 11.Baek SK, Han SW, Oh DY, Im SA, Kim TY, Bang YJ. Clinicopathologic characteristics and treatment outcomes of hepatoid adenocarcinoma of the stomach, a rare but unique subtype of gastric cancer. BMC Gastroenterol. 2011;11:56. doi: 10.1186/1471-230X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang JF, Shi SS, Shao YF, Zhang HZ. Clinicopathological and prognostic features of hepatoid adenocarcinoma of the stomach. Chin Med J (Engl) 2011;124:1470–6. [PubMed] [Google Scholar]
- 13.Chun H, Kwon SJ. Clinicopathological characteristics of alpha-fetoprotein–producing gastric cancer. J Gastric Cancer. 2011;11:23–30. doi: 10.5230/jgc.2011.11.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ucar E, Semerci E, Ustun H, Yetim T, Huzmeli C, Gullu M. Prognostic value of preoperative cea, ca 19-9, ca 72-4 and afp levels in gastric cancer. Adv Ther. 2008;25:1075–84. doi: 10.1007/s12325-008-0100-4. [DOI] [PubMed] [Google Scholar]
- 15.Adachi Y, Tsuchihashi J, Shiraishi N, Yasuda K, Etoh T, Kitano S. afp-producing gastric carcinoma: multivariate analysis of prognostic factors in 270 patients. Oncology. 2003;65:95–101. doi: 10.1159/000072332. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Cheng Y, Sheng W, et al. Analysis of clinicopathologic features and prognostic factors in hepatoid adenocarcinoma of the stomach. Am J Surg Pathol. 2010;34:1465–71. doi: 10.1097/PAS.0b013e3181f0a873. [DOI] [PubMed] [Google Scholar]
- 17.Nakajima K, Ochiai T, Suzuki T, et al. Impact of preoperative serum carcinoembryonic antigen, ca 19-9 and alpha-fetoprotein levels in gastric cancer patients. Tumor Biol. 1998;19:464–9. doi: 10.1159/000030038. [DOI] [PubMed] [Google Scholar]
- 18.Ishikura H, Fukasawa Y, Ogasawara K, Natori T, Tsukada Y, Aizawa M. An afp-producing gastric carcinoma with features of hepatic differentiation. A case report. Cancer. 1985;56:840–8. doi: 10.1002/1097-0142(19850815)56:4<840::AID-CNCR2820560423>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 19.Kinjo T, Taniguchi H, Kushima R, et al. Histologic and immunohistochemical analyses of α-fetoprotein–producing cancer of the stomach. Am J Surg Pathol. 2012;36:56–65. doi: 10.1097/PAS.0b013e31823aafec. [DOI] [PubMed] [Google Scholar]
- 20.Nagai E, Ueyama T, Yao T, Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach. A clinicopathologic and immunohistochemical analysis. Cancer. 1993;72:1827–35. doi: 10.1002/1097-0142(19930915)72:6<1827::AID-CNCR2820720606>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]