Abstract
Objective
We evaluated the survival benefit of providing concurrent chemoradiotherapy (ccrt) plus adjuvant chemotherapy compared with ccrt alone to patients with locally advanced nasopharyngeal carcinoma.
Methods
This retrospective study included 130 patients with nasopharyngeal carcinoma treated with ccrt plus adjuvant chemotherapy from June 2005 to December 2010. Another 130 patients treated with ccrt alone during the same period were matched on age, sex, World Health Organization histology, T stage, N stage, and technology used for radiotherapy. The endpoints included overall survival, locoregional failure-free survival, distant metastasis failure-free survival, and failure-free survival.
Results
At a mean follow-up of 42.1 months (range: 8–85 months), the observed hazard ratios for the group receiving ccrt plus adjuvant chemotherapy compared with the group receiving ccrt alone were: for overall survival, 0.77 [95% confidence interval (ci): 0.37 to 1.57]; for locoregional failure-free survival, 1.00 (95% ci: 0.37 to 2.71); for distant metastasis failure-free survival, 1.15 (95% ci: 0.56 to 2.37); and for failure-free survival, 1.26 (95% ci: 0.69 to 2.28). There were no significant differences in survival between the groups. After stratification by disease stage, ccrt plus adjuvant chemotherapy provided a borderline significant benefit for patients with N2–3 disease (hazard ratio: 0.35; 95% ci: 0.11 to 1.06; p = 0.052). Multivariate analyses indicated that only tumour stage was a prognostic factor for overall survival.
Conclusions
Patients with locally advanced nasopharyngeal carcinoma received no significant survival benefit from the addition of adjuvant chemotherapy to ccrt. However, patients with N2–3 disease might benefit from the addition of adjuvant chemotherapy to ccrt.
Keywords: Nasopharyngeal carcinoma, concurrent chemoradiotherapy, adjuvant chemotherapy
1. INTRODUCTION
Nasopharyngeal carcinoma (npc) is endemic in southern China, southeast Asia, the Arctic, and the mid-East and North Africa1. The prevalence of npc is reported to be highest in southern China, where an average of 80 cases per 100,000 population are reported each year2. Because the early clinical symptoms of npc are not obvious, at least 60% of patients present with locally advanced disease, and about 5%–8% present with distant metastases at diagnosis3,4. Radiation therapy is the main treatment for npc, and the 5-year survival rate had been reported to be about 85% for stage i–ii disease. However, patients with locoregionally advanced npc (stage iii or iv disease) are reported to have 5-year survival rates of only 55%5.
Results of the Intergroup 0099 study showed that, for patients with locally advanced npc, concurrent chemoradiotherapy (ccrt) administered in conjunction with adjuvant chemotherapy (ac) provided a 31% increase in the 3-year overall survival rate6. Although ccrt administered with or without ac has become standard therapy for advanced npc, the benefits of adding ac to ccrt for treatment of locally advanced npc have not been established.
Recently, several randomized controlled trials (rcts)7–9 compared the benefits of using ccrt followed by ac with the benefits of using ccrt alone, and most showed no survival benefit from the addition of ac to ccrt. Additionally, our previous meta-analysis10 showed that, compared with use of ccrt alone, ccrt followed by ac not only did not significantly improve survival, but also produced greater levels of toxicity during treatment of locally advanced npc. However, guidelines from the U.S. National Comprehensive Cancer Network have recommended use of ccrt plus ac as standard treatment for npc (category 2A)11. In earlier rcts, the number of patients receiving ac has been low and might have influenced the reported efficacy of ac12. For example, in a trial by Chen et al.7, 91 of 251 patients (36.3%) did not receive ac or discontinued ac because of refusal or adverse events.
To further analyze the survival benefit of ac, we retrospectively compared parameters of overall survival (os), locoregional failure-free survival (lffs), distant metastasis failure-free survival (dmfs), and failure-free survival (ffs) in patients with locally advanced npc who received treatment with ccrt+ac or with ccrt alone.
2. METHODS
2.1. Patients
This retrospective analysis considered a group of 130 patients with npc who were treated with ccrt+ac at the Cancer Institute of Guangxi Zhuang Autonomous Region, Nanning, PR China, from June 2005 to December 2010. A second group of 130 patients with npc treated with ccrt alone during the same period and at the same institute were matched 1:1 with the first group on age, sex, World Health Organization histology, T stage, N stage, and technology used for radiotherapy. Patients were 13–70 years of age and had been histologically diagnosed with untreated nonmetastatic npc. All participants had Karnofsky scores of 70 or better. The exclusion criteria for the study were a tumour type other than npc, presence of severe infection or complications such as unstable cardiac disease requiring treatment, pregnancy or lactation, previous receipt of 1 cycle of concomitant chemotherapy or ac, and previous receipt of radiotherapy or chemotherapy. There were no significant differences between the groups in age, sex, Karnofsky score, histologic type, T stage, N stage, overall stage, or chemotherapy administered (Table i). The protocol for this retrospective study was approved by the Ethics Committee of the Cancer Hospital of Guangxi Medical University.
Table I.
Characteristics of the study patients
| Variable |
Patient group
|
p Value | |
|---|---|---|---|
| ccrt+ac | ccrt only | ||
| Patients (n) | 130 | 130 | |
| Sex [n (%) men] | 94 | 97 | 0.673 |
| Age (years) | |||
| Median | 43 | 44 | 0.472 |
| Range | 18–66 | 13–70 | |
| Karnofsky ps score | 0.099 | ||
| 70–80 | 45 | 58 | |
| 90–100 | 85 | 72 | |
| who histologya | 0.561 | ||
| i | 2 | 1 | |
| ii–iii | 128 | 129 | |
| T Classificationb | 1.000 | ||
| T1 | 3 | 3 | |
| T2 | 58 | 58 | |
| T3 | 30 | 30 | |
| T4 | 39 | 39 | |
| N Classificationb | 1.000 | ||
| N0 | 16 | 16 | |
| N1 | 57 | 57 | |
| N2 | 50 | 50 | |
| N3 | 7 | 7 | |
| Overall stageb | 1.000 | ||
| ii | 37 | 37 | |
| iii | 48 | 48 | |
| iva–b | 45 | 45 | |
| Radiotherapy (rt) | 0.167 | ||
| Two-dimensional rt | 70 | 81 | |
| Intensity-modulated rt | 60 | 49 | |
By who criteria.
By American Joint Committee on Cancer criteria (7th edition).
ccrt = concurrent chemoradiotherapy; ac = adjuvant chemotherapy; ps = performance status; who = World Health Organization.
2.2. Radiotherapy
2.2.1. Two-Dimensional Radiotherapy
Two-dimensional radiotherapy was administered using a 6 MV linear accelerator. All patients were immobilized in the supine position with a thermo-plastic head–neck–shoulder mold and a head-and-neck immobilization board. Follow-up computed tomography imaging (2.5-mm slice thickness) of the head and neck, extending from the calvarium to the supraclavicular region, was conducted by a radiologic technician. The radiotherapy field arrangement and coverage were generally divided into 3 phases. Patients were first treated with two lateral opposing facial–cervical fields, with the lower cervical supraclavicular anterior tangential fields set to deliver 36 Gy. Patients were then treated with two shrinking lateral opposing facial–cervical fields, with the lower cervical supraclavicular anterior tangential and posterior cervical fields administering beta irradiation. In the third phase, preauricular portal and posterior cervical beta irradiation fields were used to avoid further irradiation of the spinal cord. A booster dose of irradiation (6–10 Gy in 3–5 fractions) was delivered to the base of the skull if the skull base and intracranial extension showed tumour involvement. In cases in which the patient showed nasal and ethmoidal involvement, an anterior facial electron field was added.
In summary, the cumulative radiation doses were 68–78 Gy to the primary tumour, 60–74 Gy to involved areas of the neck, and 50 Gy to low-risk local areas. Patients were treated with 2.0-Gy fractions, at 5 fractions per week.
2.2.2. Intensity-Modulated Radiotherapy
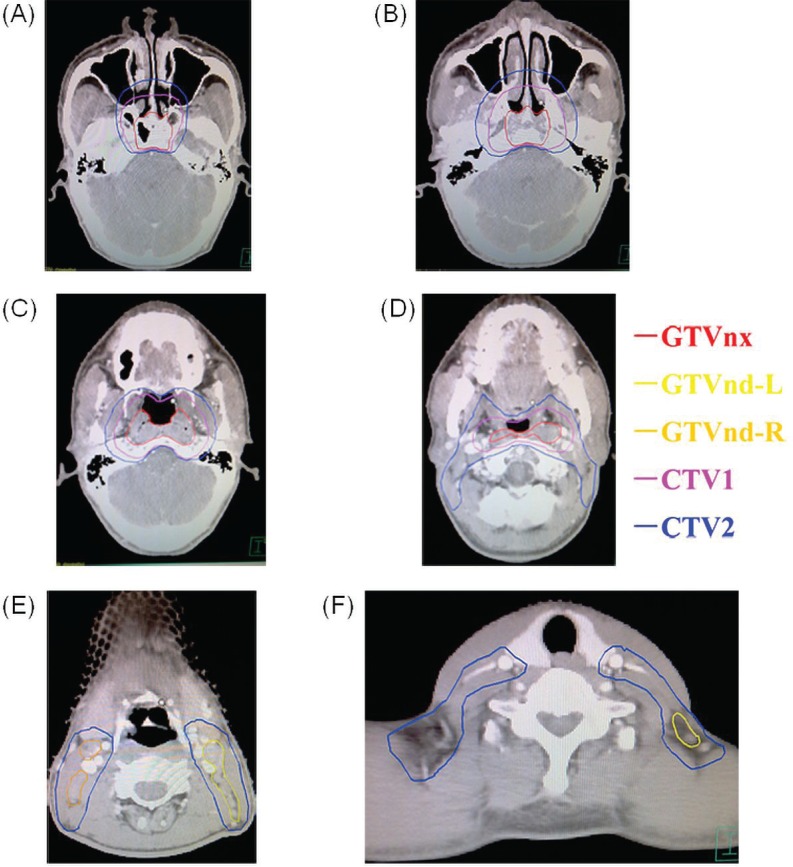
The immobilization methods in intensity-modulated radiotherapy (imrt) were the same as those used in two-dimensional radiotherapy. For patients undergoing imrt, 4 target volumes—the gtvnx (gross tumour volume in the nasopharynx), gtvnd (gross tumour volume of involved lymph nodes), and ctv1 and ctv2 (clinical target volumes 1 and 2)—were contoured on magnetic resonance images (Figure 1). The ctv1 included the gtvnx plus 5- to 10-mm margins (forward, both sides, up and down) and a 3- to 5-mm margin (back). The ctv2 included the gtvnd, the lymphatic regions (including the retropharyngeal nodes levels ii, iii, iv, and v bilaterally), and the ctv1 with 5- to 10-mm margins (forward, both sides, up and down) and a 3- to 5-mm margin (back). A 3-mm margin was added to each of the target volumes to produce 4 planning gross target volumes (pgtvs). Total radiation doses of 68–74 Gy, 66–71 Gy, 60–64 Gy, and 54–60 Gy were delivered to the pgtvnx, pgtvnd, pctv1, and pctv2 respectively, in 30–32 fractions at 5 fractions per week during a period of 6–7 weeks.
FIGURE 1.
Selected computed tomography images showing delineations of gross target volume of tumour (gtvnx), involved nodes left (gtvnd-l) and right (gtvnd-r), and clinical target volumes 1 (ctv1) and 2 (ctv2) in patients receiving intensity-modulated radiotherapy.
2.3. Chemotherapy
All patients in both groups were treated with 2–3 cycles of chemotherapy concomitant with their radiotherapy, with or without the addition of 2–3 cycles of ac. The 3 regimens used in ccrt and ac consisted of cisplatin-based chemotherapy administered as follows:
Cisplatin alone: 75–100 mg/m2 on days 1–3 every 3 weeks
Cisplatin 80 mg/m2 on days 1–3 and 5-fluorouracil (5fu) 1000 mg/m2 daily on days 1–5 (or continuous intravenous infusion for 120 hours) every 3 weeks
Mixed regimen: 1 or 2 cycles of cisplatin alone, plus 1 or 2 cycles of cisplatin–5fu
Table ii shows the chemotherapy regimens used in the two groups.
TABLE II.
Chemotherapy administered to the study patients
| Regimen |
Patient group
|
|
|---|---|---|
| ccrt+ac (n=130) | ccrt-only (n=130) | |
| Concurrent chemotherapy | ||
| Cisplatin alone | 104 | 59 |
| Cisplatin and 5fu | 7 | 59 |
| Mixed regimens | 19 | 13 |
| Adjuvant chemotherapy | ||
| Cisplatin alone | 3 | — |
| Cisplatin and 5fu | 121 | — |
| Mixed regimens | 6 | — |
ccrt = concurrent chemoradiotherapy; ac = adjuvant chemotherapy; 5fu = 5-fluorouracil.
2.4. Follow-Up
After completion of treatment, patients were assessed every 3 months during the first 2 years, every 6 months for the 3 subsequent years, and annually thereafter in clinic visits, telephone interviews, or written correspondence. The information obtained was used to assess patient survival, patterns of relapse, incidence of distant metastasis, and other clinical symptoms. Follow-up examinations included chest radiography or computed tomography, ultrasonography of liver and abdomen, whole-body bone scan, computed tomography or magnetic resonance imaging of the head and neck, and fibrotic endoscopy with or without biopsy.
2.5. Statistical Analysis
All analyses were performed using the SPSS software application (version 16.0: SPSS, Chicago, IL, U.S.A.). The chi-square test was used for comparisons of categorical data such as sex, Karnofsky score, histologic type, T stage, N stage, overall stage, and chemotherapy regimen. The t-test was used for comparisons of continuous variables. The study endpoints of os, lffs, dmfs, and ffs were detemined by patient death, relapse of a local or nodal tumour, occurrence of distant metastasis, and occurrence of relapse or distant metastasis respectively. The time-to-event for each endpoint was calculated from the date of diagnosis to the occurrence date of the event using the Kaplan–Meier method. Statistical differences in endpoints were estimated using the log-rank test. Multivariate analysis used the Cox proportional hazards model. All p values were two-sided, and p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Patient Outcomes
At a mean follow-up of 42.1 months (range: 8–85 months), 40 patients had died, including 12 in the ccrt+ac group and 28 in the ccrt-only group. In the ccrt+ac group, 7 patients had developed a local, regional, or neck relapse, and 15 had developed a distant metastasis. In the ccrt-only arm, 10 patients experienced a local, regional, or neck relapse, and 17 had developed a distant metastasis.
3.1.1. OS
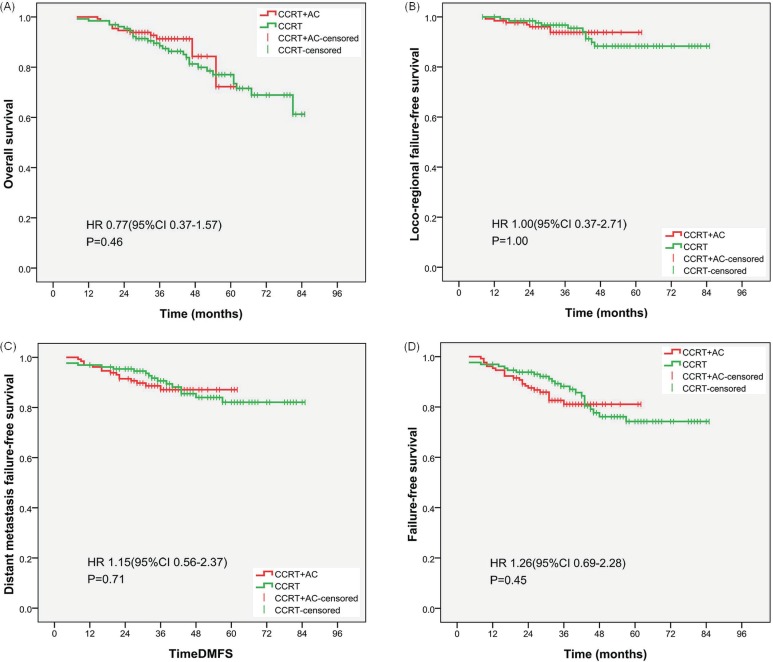
Figure 2(A) shows os curves for the patients in both study groups. At 2 years, os rates were 94.6% for the ccrt+ac group and 95.4% for the ccrt-only group, a difference that was not statistically significant [hazard ratio (hr): 0.77; 95% confidence interval (ci): 0.37 to 1.57; p = 0.46]. After stratification by disease stage, the os rates in the ccrt+ac group were 100% for stage ii, 93.8% for stage iii, and 91.1% for stage iv disease. The 2-year os rates were 96.7% for T1–2, 92.8% for T3–4, 94.5% for N0–1, and 94.7% for N2–3 disease. In contrast, after stratification by disease stage, the comparable os rates in the ccrt-only group were 100%, 97.9%, and 88.9% respectively, and the comparable 2-year os rates 96.7%, 94.2%, 100%, and 89.5% respectively. These results showed a borderline significant difference in os favouring treatment with ccrt+ac in patients with N2–3 disease (hr: 0.35; 95% ci: 0.11 to 1.06; p = 0.052; Table iii).
FIGURE 2.
Kaplan–Meier curves for (A) overall survival, (B) locoregional failure-free survival, (C) distant failure-free survival, and (D) failure-free survival for the study groups. ccrt = concurrent chemoradiotherapy; ac = adjuvant chemotherapy; hr = hazard ratio; ci = confidence interval.
TABLE III.
Survival of nasopharyngeal cancer patients by stage and study group
| Stagea | Survival type | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Overall survival | Locoregional ffs | Distant metastasis ffs | ffs | |||||||||
|
|
|
|
|
|||||||||
| hr | 95% ci | p Value | hr | 95% ci | p Value | hr | 95% ci | p Value | hr | 95% ci | p Value | |
| Overall | 0.77 | 0.37 to 1.57 | 0.46 | 1.00 | 0.37 to 2.71 | 1.00 | 1.15 | 0.56 to 2.37 | 0.71 | 1.26 | 0.69 to 2.28 | 0.45 |
| ii | 0.52 | 0.05 to 5.70 | 0.58 | 3.10 | 0.32 to 29.81 | 0.30 | 1.34 | 0.26 to 6.89 | 0.72 | 1.93 | 0.53 to 7.01 | 0.31 |
| iii | 0.70 | 0.21 to 2.37 | 0.56 | 0.78 | 0.14 to 4.46 | 0.78 | 1.07 | 0.31 to 3.73 | 0.91 | 0.94 | 0.35 to 2.56 | 0.90 |
| iva–b | 0.85 | 0.33 to 2.21 | 0.74 | 0.58 | 0.11 to 3.09 | 0.52 | 1.13 | 0.40 to 3.18 | 0.82 | 1.24 | 0.49 to 3.16 | 0.65 |
| T1–2 | 0.46 | 0.12 to 1.75 | 0.24 | 1.97 | 0.41 to 9.49 | 0.39 | 0.93 | 0.29 to 3.03 | 0.91 | 1.24 | 0.49 to 3.16 | 0.65 |
| T3–4 | 0.98 | 0.41 to 2.33 | 0.96 | 0.62 | 0.16 to 2.48 | 0.50 | 1.28 | 0.52 to 3.17 | 0.59 | 1.24 | 0.57 to 2.69 | 0.59 |
| N0–1 | 1.66 | 0.59 to 4.69 | 0.33 | 2.26 | 0.52 to 9.73 | 0.26 | 1.61 | 0.60 to 4.35 | 0.34 | 2.10 | 0.89 to 4.92 | 0.08 |
| N2–3 | 0.35 | 0.11 to 1.06 | 0.052 | 0.41 | 0.08 to 2.04 | 0.26 | 0.78 | 0.27 to 2.23 | 0.64 | 0.71 | 0.29 to 1.72 | 0.45 |
By American Joint Committee on Cancer criteria (7th edition).
ffs = failure-free survival; hr = hazard ratio; ci = confidence interval.
3.1.2. LFFS
Figure 2(B) shows the lffs curves for both study groups. No significant difference in lffs was observed between the groups (hr: 1.00; 95% ci: 0.37 to 2.71; p = 1.00). The lffs rates at 2 years were 96.2% in the ccrt+ac group and 98.5% in the ccrt-only group. After stratification by disease stage, the lffs rates in the ccrt+ac group were 94.6% for stage ii, 95.8% for stage iii, and 97.8% for stage iv disease. The 2-year lffs rates were 95.1% for T1–2, 97.1% for T3–4, 95.9% for N0–1, and 96.5% for N2–3 disease. In contrast, the comparable lffs rates in the ccrt-only group were 97.3%, 97.9%, and 100% respectively, and the comparable 2-year lffs rates were 98.4%, 98.6%, 98.6%, and 98.2% respectively. These results were not significantly different between the groups (Table iii).
3.1.3. DMFS
Figure 2(C) shows the dmfs curves for the study groups. There was no significant difference between the groups (hr: 1.15; 95% ci: 0.56 to 2.37; p = 0.71). The 2-year dmfs rates were 91.5% in the ccrt+ac group and 95.4% in the ccrt-only group. After stratification by disease stage, the dmfs rates in the ccrt+ac group were 94.6% for stage ii, 93.8% for stage iii, and 86.7% for stage iv disease. The 2-year dmfs rates in the ccrt+ac group were 93.4% for T1–2, 89.9% for T3–4, 90.4% for N0–1, and 93.0% for N2–3 disease. Comparable rates in the ccrt-only group were 100%, 91.7%, and 95.6% respectively, and 96.7%, 94.2%, 97.3%, and 93.0% respectively. These results were not significantly different between the groups (Table iii).
3.1.4. FFS
Figure 2(D) shows the ffs curves for the study groups. No significant difference was found in ffs (hr: 1.26; 95% ci: 0.69 to 2.28; p = 0.45). The 2-year ffs rates were 87.7% in the ccrt+ac group and 93.8% in the ccrt-only group. After stratification by disease stage, the 2-year ffs rates in the ccrt+ac group were 89.2% for stage ii, 89.6% for stage iii, and 84.4% for stage iv disease. The 2-year ffs rates in the ccrt+ac group were 88.5% for T1–2, 87.0% for T3–4, 86.3% for N0–1, and 89.5% for N2–3 disease. In the ccrt-only group, the comparable rates were 97.3%, 89.6%, and 95.6% respectively, and 95.1%, 92.8%, 95.9%, and 91.2% respectively. These results were not significantly different between the groups (Table iii).
3.2. Multivariate Analyses
Multivariate analysis with the Cox proportional hazards model was used to evaluate the prognostic values of age, sex, World Health Organization histology, disease stage, T stage, N stage, chemotherapy regimen, and radiotherapy technology. Multivariate analyses indicated that only disease stage was a prognostic factor for os (Table iv).
TABLE IV.
Summary of multivariate analysis of prognostic factors
| Factor | Survival type | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Overall survival | Locoregional ffs | Distant metastasis ffs | ffs | |||||||||
|
|
|
|
|
|||||||||
| hr | 95% ci | p Value | hr | 95% ci | p Value | hr | 95% ci | p Value | hr | 95% ci | p Value | |
| ccrt+ac vs. ccrt | 0.79 | 0.39 to 1.61 | 0.51 | 1.11 | 0.40 to 3.06 | 0.84 | 1.19 | 0.58 to 2.43 | 0.64 | 1.28 | 0.70 to 2.34 | 0.42 |
| imrt vs. 2D rt | 0.44 | 0.18 to 1.08 | 0.07 | 1.67 | 0.61 to 4.58 | 0.32 | 0.96 | 0.45 to 2.05 | 0.91 | 1.13 | 0.61 to 2.10 | 0.70 |
| Sex | ||||||||||||
| Women vs. men | 0.87 | 0.41 to 1.84 | 0.72 | 0.16 | 0.02 to 1.21 | 0.08 | 0.95 | 0.42 to 2.15 | 0.90 | 0.65 | 0.31 to 1.36 | 0.25 |
| Age | 1.03 | 0.99 to 1.06 | 0.12 | 1.01 | 0.96 to 1.06 | 0.76 | 1.00 | 0.97 to 1.04 | 1.00 | 1.00 | 0.97 to 1.03 | 1.00 |
| Karnofsky ps | ||||||||||||
| 90–100 vs. 70–80 | 0.74 | 0.35 to 1.57 | 0.44 | 0.54 | 0.18 to 1.66 | 0.29 | 0.67 | 0.29 to 1.52 | 0.33 | 0.66 | 0.34 to 1.31 | 0.23 |
| Stage group | ||||||||||||
| iii vs. ii | 3.52 | 0.76 to 16.35 | 0.11 | 0.51 | 0.07 to 3.88 | 0.51 | 1.37 | 0.31 to 6.04 | 0.68 | 1.06 | 0.32 to 3.56 | 0.93 |
| iv vs. ii | 5.49 | 1.11 to 27.16 | 0.04 | 0.55 | 0.06 to 4.77 | 0.59 | 1.99 | 0.40 to 9.97 | 0.41 | 1.23 | 0.33 to 4.63 | 0.76 |
| T stage | ||||||||||||
| T3–T4 vs. T1–T2 | 0.69 | 0.29 to 1.67 | 0.41 | 1.37 | 0.32 to 5.79 | 0.67 | 0.90 | 0.30 to 2.70 | 0.85 | 0.99 | 0.40 to 2.42 | 0.98 |
| N stage | ||||||||||||
| N2–N3 vs. N0–N1 | 1.43 | 0.65 to 3.16 | 0.37 | 2.51 | 0.69 to 9.18 | 0.16 | 1.05 | 0.43 to 2.57 | 0.92 | 1.29 | 0.60 to 2.79 | 0.52 |
ffs= failure-free survival; hr= hazard ratio; ci= confidence interval; ccrt= concurrent chemoradiotherapy; ac = adjuvant chemotherapy; imrt= intensity-modulated radiotherapy; 2D rt= two-dimensional radiotherapy; ps= performance status.
4. DISCUSSION
In this retrospective controlled study, no significant benefit in 2-year os, lffs, dmfs, or ffs was shown after ccrt+ac treatment was compared with ccrt alone in patients with npc. However, after stratification by disease stage, we observed a borderline significant difference in os favouring ccrt+ac treatment in patients with N2–3 disease.
In the present study, addition of ac did not significantly improve 2-year os, lffs, dmfs, or ffs, results that are consistent with findings in earlier published studies7–9,13. In 2012, Chen et al.7 reported results from a phase iii multicentre rct performed at 7 different institutions in China. In that study, 251 patients were assigned to a ccrt+ac group and another 257 were assigned to a ccrt-only group. At a median follow-up of 37.8 months, no significant survival benefit could be demonstrated for the use of ac. Recently, several rcts and meta-analyses have compared the benefits of using ccrt alone or radiotherapy alone in treatment of advanced npc. In a trial by Lin et al.14, 284 patients with stages iii–iv (M0) npc (1992 American Joint Committee on Cancer staging guidelines) were randomly assigned to one of two study arms. Patients in the investigational arm received radiotherapy combined with 2 cycles of cisplatin–5fu chemotherapy (ccrt arm). Patients in the control arm received radiotherapy only. At a median follow-up of 65 months, the authors reported significant differences in os, lffs, or dmfs that favoured ccrt treatment. In 2010, Zhang et al.15 performed a meta-analysis examining the clinical benefits of ccrt compared with radiation alone in treatment of npc in endemic geographic areas. Relative risks of 0.66 (95% ci: 0.48 to 0.92) in the ccrt group and 0.71 (95% ci: 0.58 to 0.88) in the radiotherapy-only group were observed for 3-year os and dmfs. Similar results have been reported in other rcts16,17 and meta-analyses18. A greater improvement in the results achieved with ccrt treatment might have lessened any potential gains in os, lffs, and dmfs provided by the addition of ac.
Treatment with cisplatin–5fu might not be effective for eradicating micrometastases in npc. In 1992, Jacobs et al.19 reported that use of cisplatin–5fu failed to demonstrate any survival benefit compared with the use of cisplatin and 5fu as single agents in treatment of advanced squamous cell carcinoma of the head and neck. New drugs have to be explored for inclusion in ac treatment regimens. Paclitaxel, docetaxel, gemcitabine, and capecitabine are new cytotoxic agents that have shown activity in treating both npc and non-nasopharyngeal head-and-neck cancers20–23. In a trial by He et al.22, 54 patients with locoregionally advanced npc were treated with 2 cycles of cisplatin–gemcitabine as neoadjuvant chenotherapy, followed by 2 cycles of the same regimen administered as ac. At a median follow-up of 30 months, the 3-year locoregional control, metastasis-free survival, and os rates were 94.9%, 86.2%, and 87.7% respectively. An ongoing phase ii study will provide new information about whether npc patients with stage N2–3M0 need ac consisting of paclitaxel and platinum after ccrt (search for NCT01694576 at http://clinicaltrials.gov/).
After treatment with ccrt, stage N2–3 npc might also require further treatment with ac. In 2011, Xu et al.24 reported results of a retrospective study comparing treatment outcomes achieved with the use of various chemotherapy sequences during ccrt treatment of N3 npc. All patients were restaged according to the American Joint Committee on Cancer’s 2002 classification system and were treated using two-dimensional radiotherapy (37 patients received a ccrt regimen, and 15 received ccrt+ac). At a median follow-up of 54 months (range: 3–117 months), the 5-year os and dmfs rates were 71% and 80% respectively in the ccrt+ac group and 51% and 54% respectively in the ccrt-only group. Those results showed that the ccrt+ac regimen was more effective than the ccrt-only regimen for treating N3 npc. However, the study had limitations, in that it was not designed as a rct, and it included only a small number of patients. Large multicentre rcts are needed to assess whether ccrt+ac is superior to ccrt alone for the treatment of locoregionally advanced N2–3 npc.
After primary radiotherapy or chemoradiotherapy, plasma levels of Epstein–Barr viral dna have been used to predict poor patient survival and might serve as a biomarker for subclinical residual disease. Use of ac in such patients might provide survival benefits. To explore this possibility, Chan et al.25 used plasma levels of Epstein–Barr viral dna to select high-risk npc patients for treatment with ac; low-risk npc patients received only clinical follow-up. The results showed that delivery of 6 cycles of ac after administration of full-dose radiotherapy or chemoradiotherapy was feasible and carried acceptable toxicity; however, the survival benefit of such additional treatment requires further verification.
Although improvements in radiotherapy technology, and especially the use of imrt, have produced significant improvements in lffs26–28, effective treatment of distant metastases remains an important problem to be solved. In the present study, 17 patients experienced a locoregional or neck relapse, and 32 patients developed distant metastasis. Development of distant metastases was the primary reason for treatment failure, and similar results have been found in other trials29,30. Chemotherapy might be useful in treating such metastases. Several rcts and meta-analyses demonstrated that ccrt without ac significantly improved dmfs15,16,18. Additionally, trials that compared the efficacy of radiotherapy plus ac with radiotherapy alone found that the use of ac provided no survival benefit31,32. Studies on the effect of adding ac to ccrt also showed no significant dmfs benefit7,9. However, it was unclear whether neoadjuvant chemotherapy can reduce distant metastasis. In a trial by Chen et al.33, a significant dmfs benefit accrued to patients receiving induction chemotherapy with ccrt compared with patients receiving ccrt alone. However, another study34 showed no significant difference in 3-year distant metastasis control rates in advanced npc patients treated either with induction chemotherapy plus ccrt or with ccrt alone.
Targeted therapy has become a popular method of tumour treatment, and its addition to standard chemotherapy might reduce treatment failures from distant metastases. A pilot study conducted by Roychowdhury et al.35 identified significant correlations between microvessel density, the risk of distant metastasis, and rates of patient survival in irradiated npc patients. These data suggest that antiangiogenic agents might play a role in the treatment of distant metastases. Recently, Chen et al.36 reported results of a multicentre safety study examining the effects of using cetuximab plus imrt and concurrent chemotherapy with cisplatin in the treatment of locoregionally advanced npc. They found that the combined treatment modality was well tolerated and manageable. The addition of antiangiogenic agents to a primary treatment has also been speculated to possibly result in sterilization of distant micrometastases.
Our retrospective analysis has several limitations. First, being a nonrandomized controlled study, it included patients only if they met specific selection criteria. Selection bias might therefore have occurred. Second, the relatively short follow-up period might limit proper prediction of long-term results. Finally, the small sample size might have resulted in an inadequate number of events for a proper analysis of results.
5. CONCLUSIONS
The present study suggests that addition of ac to ccrt in patients with locally advanced npc does not provide a benefit in 2-year os, lffs, dmfs, or ffs. It is essential that large rcts with long follow-up periods be conducted to properly evaluate any survival benefit that might accrue to patients with npc from the addition of ac to ccrt.
6. ACKNOWLEDGMENTS
We thank Ying Chen from the Department of Administration, Office of Disease Process, for help with follow-up. This work was sponsored by the Fund for Guangxi Science (no. 0832229), Major Research Projects of Guangxi Universities (no. 201101ZD004), and the Key Project of Guangxi Heath Care (no. 2011076). Zhongguo Liang and Xiaodong Zhu contributed equally to this study and should be considered co– first authors of this report.
7. CONFLICT OF INTEREST DISCLOSURES
The authors declare no financial conflicts of interest regarding the design or results of this study.
8. REFERENCES
- 1.Yoshizaki T, Ito M, Murono S, Wakisaka N, Kondo S, Endo K. Current understanding and management of nasopharyngeal carcinoma. Auris Nasus Larynx. 2012;39:137–44. doi: 10.1016/j.anl.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Chan AT, Teo PM, Johnson PJ. Nasopharyngeal carcinoma. Ann Oncol. 2002;13:1007–15. doi: 10.1093/annonc/mdf179. [DOI] [PubMed] [Google Scholar]
- 3.Fong KW, Chua EJ, Chua ET, et al. Patient profile and survival in 270 computer tomography–staged patients with nasopharyngeal cancer treated at the Singapore General Hospital. Ann Acad Med Singapore. 1996;25:341–6. [PubMed] [Google Scholar]
- 4.Heng DM, Wee J, Fong KW, et al. Prognostic factors in 677 patients in Singapore with nondisseminated nasopharyngeal carcinoma. Cancer. 1999;86:1912–20. doi: 10.1002/(SICI)1097-0142(19991115)86:10<1912::AID-CNCR6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 5.Teo P, Yu P, Lee WY, et al. Significant prognosticators after primary radiotherapy in 903 nondisseminated nasopharyngeal carcinoma evaluated by computer tomography. Int J Radiat Oncol Biol Phys. 1996;36:291–304. doi: 10.1016/S0360-3016(96)00323-9. [DOI] [PubMed] [Google Scholar]
- 6.Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase iii randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–17. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Hu CS, Chen XZ, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2012;13:163–71. doi: 10.1016/S1470-2045(11)70320-5. [DOI] [PubMed] [Google Scholar]
- 8.Huang HC, Xu HY, Wu SX. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma [Chinese] Jilin Med J. 2012;33:2037–40. doi: 10.1016/S1470-2045(11)70320-5. [DOI] [PubMed] [Google Scholar]
- 9.Kwong DL, Sham JS, Au GK, et al. Concurrent and adjuvant chemotherapy for nasopharyngeal carcinoma: a factorial study. J Clin Oncol. 2004;22:2643–53. doi: 10.1200/JCO.2004.05.173. [DOI] [PubMed] [Google Scholar]
- 10.Liang ZG, Zhu XD, Zhou ZR, Qu S, Du YQ, Jiang YM. Comparison of concurrent chemoradiotherapy followed by adjuvant chemotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a meta-analysis of 793 patients from 5 randomized controlled trials. Asian Pac J Cancer Prev. 2012;13:5747–52. doi: 10.7314/APJCP.2012.13.11.5747. [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers. Fort Washington, PA:: NCCN; 2013. Ver 1.2013. [Google Scholar]
- 12.Lee AWM, Tung SY, Ngan RK, et al. Factors contributing to the efficacy of concurrent–adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: combined analyses of npc-9901 and npc-9902 trials. Eur J Cancer. 2011;47:656–66. doi: 10.1016/j.ejca.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Maeng CH, Park S, Jung HA, et al. Comparison of clinical outcomes between concurrent chemoradiotherapy followed by adjuvant chemotherapy and concurrent chemoradiotherapy alone for patients associated with locally advanced nasopharyngeal carcinoma: a retrospective analysis of single center experience [abstract e16003] J Clin Oncol. 2012;30 [Available online at: http://meetinglibrary.asco.org/content/96127-114; cited March 31, 2014] [Google Scholar]
- 14.Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY. Phase iii study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. 2003;21:631–7. doi: 10.1200/JCO.2003.06.158. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Zhao C, Ghimire B, et al. The role of concurrent chemoradiotherapy in the treatment of locoregionally advanced nasopharyngeal carcinoma among endemic population: a meta-analysis of the phase iii randomized trials. BMC Cancer. 2010;10:558. doi: 10.1186/1471-2407-10-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Zhao C, Peng PJ, et al. Phase iii study comparing standard radiotherapy with or without weekly oxaliplatin in treatment of locoregionally advanced nasopharyngeal carcinoma: preliminary results. J Clin Oncol. 2005;23:8461–8. doi: 10.1200/JCO.2004.00.3863. [DOI] [PubMed] [Google Scholar]
- 17.Yu HS, Wang X, Song AQ, Liu N, Zhang W, Yu L. Concurrent chemoradiotherapy versus radiotherapy alone for locoregionally advanced nasopharyngeal carcinoma. Asian Pac J Cancer Prev. 2012;13:3961–5. doi: 10.7314/APJCP.2012.13.8.3961. [DOI] [PubMed] [Google Scholar]
- 18.Ghimire BR, Lin LP, Guo Y, Zhang L. The role of concurrent chemoradiation to radiotherapy alone in the treatment of locally advanced nasopharyngeal carcinoma: a meta-analysis of the phase iii randomized trials. J Evid Based Med. 2007;7:158–66. [Google Scholar]
- 19.Jacobs C, Lyman G, Velez–Garcia E, et al. A phase iii randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck. J Clin Oncol. 1992;10:257–63. doi: 10.1200/JCO.1992.10.2.257. [DOI] [PubMed] [Google Scholar]
- 20.Airoldi M, Pedani F, Marchionatti S, et al. Carboplatin plus Taxol is an effective third-line regimen in recurrent undifferentiated nasopharyngeal carcinoma. Tumori. 2002;88:273–6. doi: 10.1177/030089160208800405. [DOI] [PubMed] [Google Scholar]
- 21.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–15. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 22.He X, Ou D, Ying H, Zhu G, Hu C, Liu T. Experience with combination of cisplatin plus gemcitabine chemotherapy and intensity-modulated radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol. 2012;269:1027–33. doi: 10.1007/s00405-011-1720-x. [DOI] [PubMed] [Google Scholar]
- 23.Chua D, Wei WI, Sham JS, Au GK. Capecitabine monotherapy for recurrent and metastatic nasopharyngeal cancer. Jpn J Clin Oncol. 2008;38:244–9. doi: 10.1093/jjco/hyn022. [DOI] [PubMed] [Google Scholar]
- 24.Xu TT, Hu CS, Wang XS, Wu YR, He XY, Ying HM. Comparing treatment outcomes of different chemotherapy sequences during radio-chemotherapy for stage N3 nasopharyngeal carcinoma. Chin J Radiat Oncol. 2011;20:181–5. [Google Scholar]
- 25.Chan ATC, Ngan RKC, Hui EP, et al. A multicenter randomized controlled trial (rct) of adjuvant chemotherapy (ct) in nasopharyngeal carcinoma (npc) with residual plasma ebv dna (ebv dna) following primary radiotherapy (rt) or chemoradiotherapy (crt) [abstract 5511]. J Clin Oncol. 2012;30 [Available online at: http://meetinglibrary.asco.org/content/95163-114; cited March 31, 2014] [Google Scholar]
- 26.Li GS, Chen SJ, Huang HX, Ning SH. A randomized clinical study of intensity-modulated radiotherapy vs conventional radiotherapy combined with chemotherapy in treating local/regional advanced nasopharyngeal carcinoma. Tumor. 2011;31:343–7. [Google Scholar]
- 27.Peng G, Wang T, Yang KY, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012;104:286–93. doi: 10.1016/j.radonc.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Huang YC, Liu YJ, Xiao L, Ho WQ. Intensity modulated conformal radiation therapy and clinical efficacy of conventional radiotherapy combined with chemotherapy in the treatment of locally advanced nasopharyngeal carcinoma [Chinese] Jilin Med J. 2012;33:2470–1. [Google Scholar]
- 29.Huang PY, Cao KJ, Guo X, et al. A randomized trial of induction chemotherapy plus concurrent chemoradiotherapy versus induction chemotherapy plus radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Oral Oncol. 2012;48:1038–44. doi: 10.1016/j.oraloncology.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Cheng SH, Jian JJ, Tsai SY, et al. Long-term survival of nasopharyngeal carcinoma following concomitant radiotherapy and chemotherapy. Int J Radiat Oncol Biol Phys. 2000;48:1323–30. doi: 10.1016/S0360-3016(00)00779-3. [DOI] [PubMed] [Google Scholar]
- 31.Rossi A, Molinari R, Boracchi P, et al. Adjuvant chemotherapy with vincristine, cyclophosphamide, and doxorubicin after radiotherapy in local-regional nasopharyngeal cancer: Results of a 4-year multicenter randomized study. J Clin Oncol. 1988;6:1401–10. doi: 10.1200/JCO.1988.6.9.1401. [DOI] [PubMed] [Google Scholar]
- 32.Chi KH, Chang YC, Guo WY, et al. A phase iii study of adjuvant chemotherapy in advanced nasopharyngeal carcinoma patients. J Radiat Oncol Biol Phys. 2002;52:1238–44. doi: 10.1016/S0360-3016(01)02781-X. [DOI] [PubMed] [Google Scholar]
- 33.Chen XJ, Qiu RL, Yang CL, Liu YQ. Study of tpf neoadjuvant chemotherapy followed by concurrent intensity modulated radiochemotherapy in the treatment for 30 cases with locally advanced nasopharyngeal carcinoma. Chin J Clin Oncol Rehabil. 2012;19:201–4. [Google Scholar]
- 34.Huang S, Deng G, Huang G, Li Y, Meng Y, Chen J. Efficacy of induction chemotherapy combined with concurrent chemoradiotherapy for advanced nasopharyngeal carcinoma. Chin J Clin Oncol. 2012;39:788–91. [Google Scholar]
- 35.Roychowdhury DF, Tseng A, Jr, Fu KK, Weinburg V, Weidner N. New prognostic factors in nasopharyngeal carcinoma. Tumor angiogenesis and C-erbB2 expression. Cancer. 1996;77:1419–26. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1419::AID-CNCR1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 36.Chen CY, Zhao C, Gao L, et al. Multicenter safety study on cetuximab combined with intensity modulated radiotherapy and concurrent chemotherapy of cisplatin in locoregionally advanced nasopharyngeal carcinoma. Chin J Radiat Oncol. 2012;21:201–4. [Google Scholar]