Abstract
Background
Concurrent chemoradiation with fluorouracil (5fu) and mitomycin C (mmc) is standard treatment for anal canal carcinoma (acc). The current protocol in Alberta is administration of 5fu and mmc during weeks 1 and 5 of radiation. However, administration of the second bolus of mmc has been based largely on centre preference. Given limited published data on outcomes with different mmc regimens, our objective was to compare the efficacy and toxicity of 1 compared with 2 cycles of mmc in acc treatment.
Methods
Our retrospective study evaluated 169 acc patients treated with radical chemoradiotherapy between 2000 and 2010 at two tertiary cancer centres. All patients were treated with 2 cycles of 5fu and with 1 cycle (mmc1) or 2 cycles (mmc2) of mmc. Acute toxicities, disease-free (dfs) and overall survival (os) were analyzed.
Results
Baseline demographics, performance status, and stage were similar in the groups of patients who received mmc1 (52%) and mmc2 (48%). Before treatment, median hematologic parameters were comparable, except for white blood cell count, which was higher in the mmc2 group, but within normal range. The 5-year os and dfs were similar (75.1% and 54.2% for mmc1 vs. 70.7% and 44.2% for mmc2, p = 0.98 and p = 0.63 respectively). On multivariate analysis, mmc2 was the factor most strongly associated with specific acute toxicities: grade 3+ leukopenia (hazard ratio: 4.82; p < 0.01), grade 3+ skin toxicity (hazard ratio: 4.76; p < 0.001), and hospitalizations secondary to febrile neutropenia (hazard ratio: 9.91; p = 0.001).
Conclusions
In definitive chemoradiotherapy for acc, 1 cycle of mmc appears to offer outcomes similar to those achieved with 2 cycles, with significantly less acute toxicity.
Keywords: Chemoradiotherapy, anal cancer
1. INTRODUCTION
Carcinoma of the anal canal, although relatively rare, has been increasing in incidence since the early 1980s1. Historically, anal canal carcinoma (acc) was treated with abdominal–perineal resection resulting in permanent colostomy. However, since the 1980s, standard management of acc has been definitive concurrent chemoradiation therapy (ccrt) using 5-fluorouracil (5fu) and mitomycin C (mmc), with salvage abdominal–perineal resection for those in whom ccrt fails2–5.
Several pivotal randomized controlled trials have established ccrt with 5fu and mmc as standard treatment for acc. In those trials, 1 and 2 cycles of mmc have both been used concurrently with 2 cycles of 5fu and radiotherapy (rt). The ccrt regimen in trials conducted by the U.K. Co-ordinating Committee on Cancer Research (act i) and the European Organisation for Research and Treatment of Cancer used a single cycle of mmc and, compared with rt alone, demonstrated superiority in locoregional control and disease-free survival (dfs). However, no overall survival (os) benefit was found6–8. The Radiation Therapy Oncology Group (rtog) 87-04 trial similarly demonstrated significant improvements in colostomy-free survival and dfs with the addition of 2 cycles of mmc to 5fu and rt, but again, no survival benefit was observed9.
More recent trials have investigated cisplatinum as an alternative to mmc. However, those phase iii studies have failed to show significant improvements in dfs, os, and progression-free survival, and thus continue to support mmc-based chemotherapy as the standard of care10,11.
Although ccrt with 5fu and mmc remains standard treatment for acc, published data comparing outcomes and toxicities for different mmc regimens are limited. A retrospective study by Cummings et al. compared 6 regimens of ccrt with rt alone, including an uninterrupted course of ccrt with a single cycle of 5fu–mmc and a split course of rt with 2 cycles of 5fu–mmc. Although patients receiving split-course rt received 2 cycles of mmc, they developed less hematologic toxicity than did patients receiving the continuous course (8% vs. 28%), likely because of the mandatory treatment break. Overall 5-year cancer-specific survival was similar in the split and continuous courses, but the sample size was small12. Various mmc regimens in the context of a continuous rt course (which is currently considered standard acc treatment) were not examined.
The current acc treatment protocol in Alberta is administration of mmc and 5fu during weeks 1 and 5 of radiation. However, administration of the second mmc cycle has been based largely on centre preference. The objectives of the present study were to compare efficacy and toxicity of 1 or 2 cycles of mmc in the treatment of acc patients with ccrt.
2. METHODS
2.1. Patient Population
This retrospective study included acc patients treated with definitive ccrt between 2000 and 2010 at Alberta’s two tertiary cancer centres [Tom Baker Cancer Centre (tbcc), Calgary, and Cross Cancer Institute (cci), Edmonton]. Patients were included if they were 18 years of age or older, had a histologic diagnosis of acc and no other active malignancies, and were treated with curative intent. All patients included in the analysis received 2 cycles of 5fu 1000 mg/m2 given over 96 hours starting on day 1 of weeks 1 and 5 of rt, and 1 or 2 cycles of mmc 10 mg/m2 administered on day 1 of 5fu. Patients who received a rt dose of 45 Gy or more were included in the analysis. Patients who received rt less than 45 Gy, rt alone, or chemotherapy other than mmc and 5fu were excluded.
Before treatment, evaluation of all patients included clinical examination, tumour biopsy, baseline complete blood count, and computed tomography imaging of abdomen and pelvis. Tumour size was based on clinical exam (when documented) or imaging. Weekly complete blood count and toxicities (skin, gastrointestinal, genitourinary) while on treatment were documented and graded using the rtog acute scoring index13. All blood counts were retrieved from the provincial clinical database (Alberta Netcare) during ccrt and up to 4 weeks after the last chemotherapy cycle. Hematologic nadirs were recorded and analyzed. Testing for hiv was not routinely performed and was not included in the analysis.
Approval for this study was obtained from the University of Calgary Conjoint Health Research Ethics board.
2.2. Statistical Analysis and Definitions
Patients were classified into two treatment cohorts: those who received 1 cycle of mmc (mmc1) and those who received 2 cycles (mmc2). Group analyses considered dfs, os, colostomy-free survival (cfs), and acute hematologic and non-hematologic toxicities.
Study endpoints were defined based on rtog definitions. Disease-free survival was the interval between diagnosis and evidence of local, regional, or metastatic failure; second primary; death; or last follow-up for patients without treatment failure. Local failure was evidence of persistent local disease or local recurrence. Regional failure was persistence, appearance, or recurrence of regional nodal disease. Patients with persistent disease were considered to be in failure on the day of their first follow-up after ccrt or date of biopsy-proven persistent disease (when available). Failure for the os endpoint was death from any cause, and it was measured from diagnosis to the date of death or last follow-up. Colostomy-free survival was the interval between diagnosis and date of colostomy, including diverting colostomy and colostomy from salvage abdominal–perineal resection, or last follow-up for patients not requiring a colostomy.
Results were analyzed using the Stata software application (version 12.0 for Microsoft Windows: StataCorp LP, College Station, TX, U.S.A.). Chi-square or Fisher exact testing was used to evaluate differences between discontinuous variables. Wilcoxon rank-sum testing was used to evaluate differences between continuous variables. Kaplan–Meier survival analyses with log-rank testing were used to analyze dfs, os, and cfs. Statistical significance was accepted at p values less than 0.05.
Multivariate logistic regression was used to model factors associated with toxicity outcomes. Cox proportional hazards modelling was used for multivariate analysis of survival outcomes.
3. RESULTS
3.1. Demographics
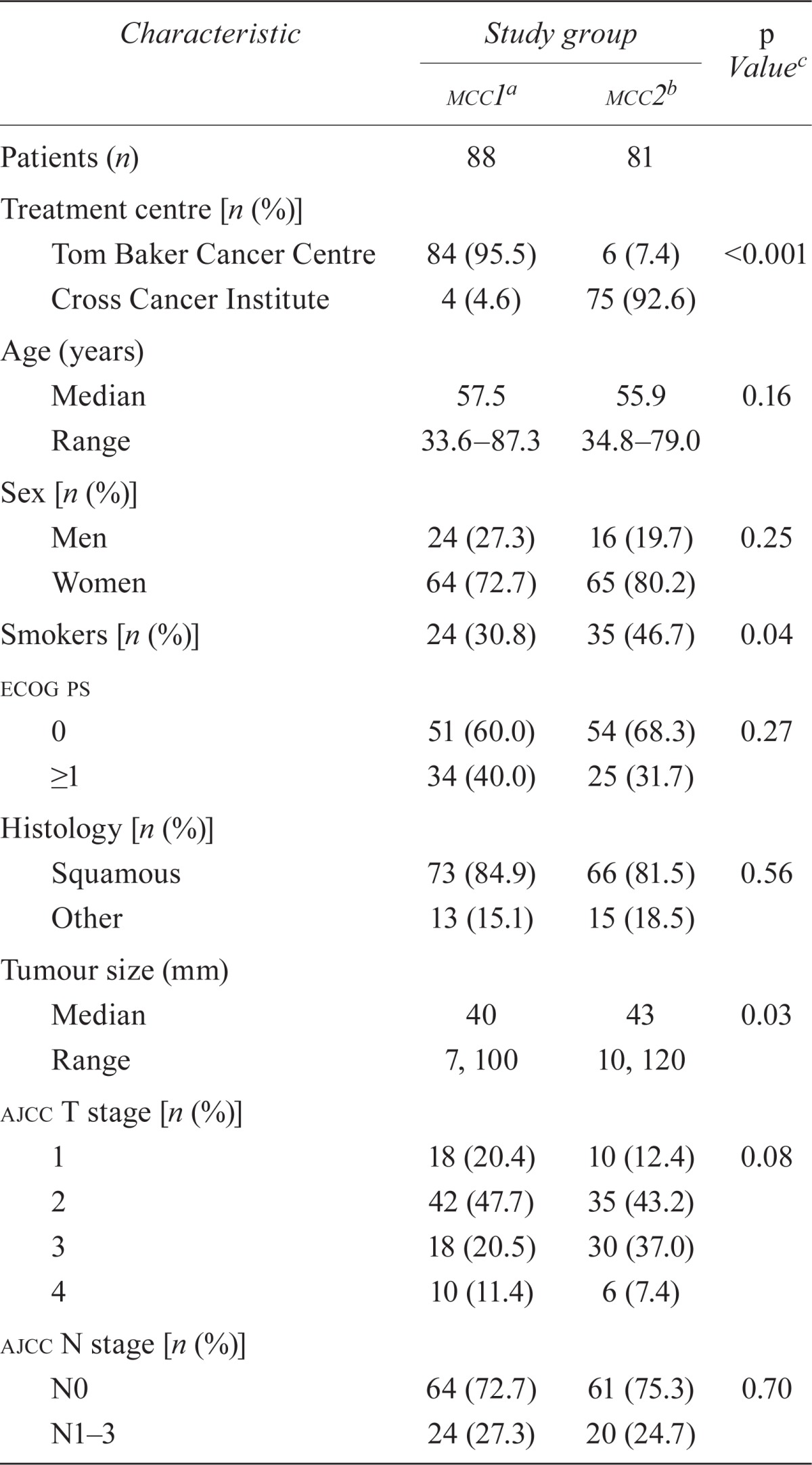
Between 2000 and 2010, 210 patients were diagnosed with acc in Alberta. Of those patients, 41 were excluded from the analysis (21 received rt alone, 4 received an rt dose < 45 Gy, 13 received chemotherapy other than mmc, and 3 had incomplete charts). Of the remaining 169 patients, 88 received 1 cycle of mmc (95.5% tbcc, 4.5% cci), and 81 received 2 cycles (7.4% tbcc, 92.6% cci). Table i summarizes patient, tumour, and treatment characteristics.
TABLE I.
Baseline characteristics of patients with anal canal carcinoma treated with chemoradiation, by treatment cohort
| Characteristic |
Study group
|
p Valuec | |
|---|---|---|---|
| mcc1a | mcc2b | ||
| Patients (n) | 88 | 81 | |
| Treatment centre [n (%)] | |||
| Tom Baker Cancer Centre | 84 (95.5) | 6 (7.4) | <0.001 |
| Cross Cancer Institute | 4 (4.6) | 75 (92.6) | |
| Age (years) | |||
| Median | 57.5 | 55.9 | 0.16 |
| Range | 33.6–87.3 | 34.8–79.0 | |
| Sex [n (%)] | |||
| Men | 24 (27.3) | 16 (19.7) | 0.25 |
| Women | 64 (72.7) | 65 (80.2) | |
| Smokers [n (%)] | 24 (30.8) | 35 (46.7) | 0.04 |
| ecog ps | |||
| 0 | 51 (60.0) | 54 (68.3) | 0.27 |
| ≥1 | 34 (40.0) | 25 (31.7) | |
| Histology [n (%)] | |||
| Squamous | 73 (84.9) | 66 (81.5) | 0.56 |
| Other | 13 (15.1) | 15 (18.5) | |
| Tumour size (mm) | |||
| Median | 40 | 43 | 0.03 |
| Range | 7, 100 | 10, 120 | |
| ajcc T stage [n (%)] | |||
| 1 | 18 (20.4) | 10 (12.4) | 0.08 |
| 2 | 42 (47.7) | 35 (43.2) | |
| 3 | 18 (20.5) | 30 (37.0) | |
| 4 | 10 (11.4) | 6 (7.4) | |
| ajcc N stage [n (%)] | |||
| N0 | 64 (72.7) | 61 (75.3) | 0.70 |
| N1–3 | 24 (27.3) | 20 (24.7) | |
| ajcc stage [n (%)] | |||
| 1 | 18 (20.4) | 9 (11.1) | 0.08 |
| 2 | 37 (42.0) | 49 (60.5) | |
| 3a | 11 (12.5) | 11 (13.6) | |
| 3b | 21 (23.9) | 12 (14.8) | |
| 4 | 1 (1.1) | 0 (0) | |
| Pre-treatment blood counts | |||
| Hemoglobin (g/dL) | |||
| Median | 136 | 137 | 0.49 |
| Range | 87–167 | 72–171 | |
| wbcs (×109/L) | |||
| Median | 6.9 | 8.1 | 0.007 |
| Range | 4.1–17.0 | 3.9–19.9 | |
| Neutrophils (×109/L) | |||
| Median | 4.7 | 5.4 | 0.07 |
| Range | 2.2–11.6 | 2.5–17 | |
| Platelets (×109/L) | |||
| Median | 277 | 292 | 0.41 |
| Range | 98–536 | 133–490 | |
| Radiotherapy | |||
| Dose to primary tumour (Gy) | |||
| Median | 54 | 54 | 0.94 |
| Range | 45–60 | 45–59 | |
| Technique [n (%)] | |||
| imrt | 28 (31.8) | 21 (25.9) | 0.4 |
| Otherd | 60 (68.2) | 60 (74.1) | |
Patient cohort receiving 1 cycle of mitomycin C with radiation.
Patient cohort receiving 2 cycles of mitomycin C with radiation.
By Fisher exact test where any cell n<5; otherwise, by chi-square test.
Two- or three-dimensional conformal radiation therapy.
ecog ps = Eastern Cooperative Oncology Group performance status; ajcc = American Joint Committee on Cancer; wbcs = white blood cells; imrt = intensity-modulated radiotherapy.
Median age and sex distribution were similar in the mmc1 and mmc2 groups. Most tumours were of squamous cell histology; the most common nonsquamous tumours were cloacogenic or basaloid.
In the mmc2 group, the proportion of smokers was higher, and the median tumour size was slightly larger (43 mm vs. 40 mm, p = 0.03). The proportion of clinically node-positive patients was similar, and the stage distribution was not statistically different in the two groups. In the mmc1 group, 1 patient had stage iv disease at diagnosis, with a resectable solitary liver lesion, and was offered radical ccrt (per tumour board consensus). Most patients in both groups were treated with two-dimensional planning or three-dimensional conformal rt. Before treatment, median hematologic parameters were similar in the groups, with the exception of white blood cell count, which was higher in the mmc2 group, but within normal laboratory range.
3.2. Toxicities
Table ii presents acute hematologic and non-hematologic toxicities. The overall grade 3+ toxicity rate was higher in the mmc2 group than in the mmc1 group. The most common non-hematologic toxicity was acute skin reaction, with a higher proportion of mmc2 patients experiencing grade 3+ skin toxicity. On multivariate regression controlling for age, sex, smoking, Eastern Cooperative Oncology Group performance status, histology, T and N stage, and radiation dose, mmc2 group membership remained the only independent predictor of grade 3+ skin toxicity [odds ratio (or): 4.76; p < 0.001].
TABLE II.
Grade 3 or greater toxicities in patients with anal canal carcinoma treated with chemoradiation, by treatment cohort
| Toxicitya |
Study group
|
p Valued | ||
|---|---|---|---|---|
| Overall (N = 169) | mmc1b (n = 88) | mmc2c (n = 81) | ||
| Overall toxicities (%) | 76.9 | 70.5 | 84.0 | 0.04 |
| Hematologic toxicities (%) | ||||
| Leukopenia | 37.9 | 30.7 | 45.7 | 0.045 |
| Neutropenia | 32.0 | 26.1 | 38.3 | 0.09 |
| Thrombocytopenia | 9.5 | 6.8 | 12.4 | 0.22 |
| Anemia | 1.2 | 0 | 2.5 | 0.23 |
| Febrile neutropenia requiring hospitalization | 11.2 | 3.4 | 19.8 | 0.001 |
| Non-hematologic toxicities (%) | ||||
| Skin | 58.0 | 44.3 | 72.8 | <0.001 |
| Gastrointestinal | 15.4 | 14.8 | 16.1 | 0.82 |
| Genitourinary | 1.8 | 3.4 | 0 | 0.25 |
| Unscheduled treatment break | 23.7 | 22.7 | 24.7 | 0.76 |
Using the Radiation Therapy Oncology Group acute radiation morbidity scoring criteria13.
Patient cohort receiving 1 cycle of mitomycin C with radiation.
Patient cohort receiving 2 cycles of mitomycin C with radiation.
By Fisher exact test where any cell n<5; otherwise, by chi-square test.
For hematologic toxicities, rates of grade 3+ leukopenia and febrile neutropenia requiring hospitalization were higher in the mmc2 group. On multivariate regression for grade 3+ leukopenia, mmc2 group membership (or: 4.82; p < 0.001) and having a lower white blood cell count before treatment (or: 1.97; p = 0.003) or a higher pretreatment neutrophil count (or: 1.60; p = 0.03) were significantly associated.
The risk of hospitalization for febrile neutropenia was increased by a factor of 10 for mmc2 patients compared with mmc1 patients on both the univariate and multivariate analyses [hazard ratio (hr): 9.91; p = 0.001]. The only other factor independently associated with hospitalization for febrile neutropenia was a performance status greater than 1 before treatment (hr: 4.11; p = 0.01).
3.3. OS and DFS
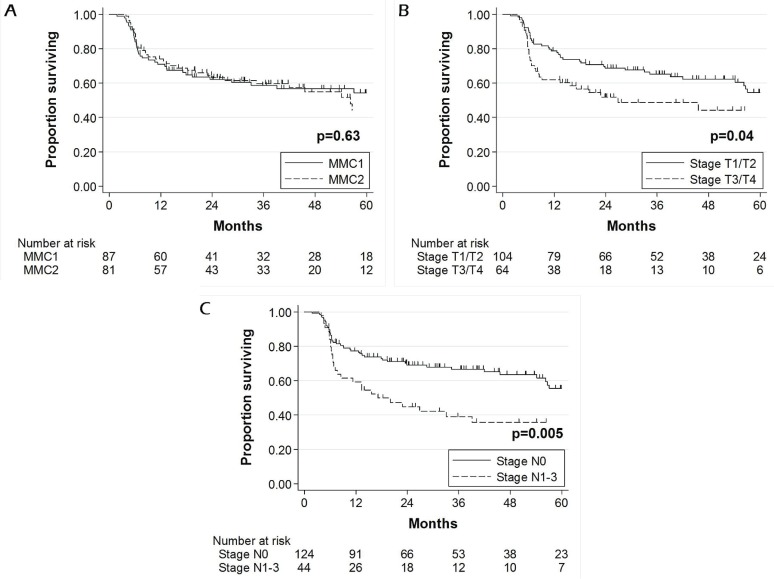
Median follow-up for survivors at the time of analysis was 39 months. Estimated 5-year os [75.1% mmc1 vs. 70.7% mmc2, p = 0.98, Figure 1(A)] and dfs [54.2% mmc1 vs. 44.2% mmc2, p = 0.63, Figure 2(A)] were similar in the two groups. At the time of the present analysis, 36 patients had died (19 deaths in mmc1, 17 deaths in mmc2).
FIGURE 1.
Overall survival of patients with anal canal carcinoma treated with chemoradiation: (A) 1 (mmc1) compared with 2 (mmc2) cycles of mitomycin C; (B) Eastern Cooperative Oncology Group (ecog) performance status 0 compared with ≥1; (C) stage T1/2 compared with T3/4; (D) stage N0 compared with N1–3.
FIGURE 2.
Disease-free survival of patients with anal canal carcinoma treated with chemoradiation: (A) 1 (mmc1) compared with 2 (mmc2) cycles of mitomycin C; (B) stage T1/2 compared with T3/4; (C) stage N0 compared with N1–3.
Figure 1(B–D) shows factors significantly associated with worse 5-year os on univariate Kaplan–Meier analysis. In Cox modelling, only a performance status of 1 or greater and an N stage of 1–3 were associated with worse 5-year os (Table iii). Squamous cell histology approached significance as a predictor of better outcome (hr: 0.44; p = 0.07).
TABLE III.
Cox proportional hazards model for overall survival
| Variable | hr | 95% cl | p Value |
|---|---|---|---|
| Smoker | 1.30 | 0.61, 2.78 | 0.50 |
| ecog ps ≥ 1 | 2.19 | 1.03, 4.66 | 0.04 |
| Squamous cell histology | 0.44 | 0.18, 1.08 | 0.07 |
| N Stage 1–3 | 3.39 | 1.54, 7.49 | 0.002 |
| T Stage 3/4 | 1.30 | 0.56, 3.01 | 0.54 |
| mmc2 chemotherapy | 0.92 | 0.44, 1.91 | 0.83 |
hr = hazard ratio; cl = confidence limits; ecog ps = Eastern Cooperative Oncology Group performance status; mmc2 = patient cohort receiving 2 cycles of mitomycin C with radiation.
Figure 2(B–C) shows factors significantly associated with poorer 5-year dfs on Kaplan–Meier univariate analysis. In Cox modelling, N stage 1–3 was the only factor that remained significant (Table iv).
TABLE IV.
Cox proportional hazards model for disease-free survival
| Variable | hr | 95% cl | p Value |
|---|---|---|---|
| Age > 60 years | 0.71 | 0.41, 1.23 | 0.22 |
| Male | 0.89 | 0.48, 1.65 | 0.72 |
| Smoker | 1.10 | 0.65, 1.87 | 0.71 |
| ecog ps ≥ 1 | 1.42 | 0.84, 2.40 | 0.19 |
| Squamous cell histology | 0.71 | 0.37, 1.36 | 0.34 |
| N Stage 1–3 | 1.88 | 1.08, 3.27 | 0.02 |
| T Stage 3/4 | 1.30 | 0.72, 2.36 | 0.38 |
| Radiation dose > 54 Gy | 0.72 | 0.40, 1.29 | 0.27 |
| mmc2 chemotherapy | 1.17 | 0.69, 2.01 | 0.56 |
| Worst grade 3+ toxicity | 0.71 | 0.40, 1.24 | 0.23 |
hr = hazard ratio; cl = confidence limits; ecog ps = Eastern Cooperative Oncology Group performance status; mmc2 = patient cohort receiving 2 cycles of mitomycin C with radiation.
3.4. CFS
Rates of colostomy (25.0% mmc1 vs. 22.2% mmc2, p = 0.67) and of salvage abdominal–perineal resection (11.4% mmc1 vs. 12.3% mmc2, p = 0.84) were similar in the two groups. The estimated 5-year rate of cfs was also comparable (72.6% mmc1 vs. 77.7% mmc2, p = 0.57). Only lower T stage was associated with better 5-year cfs on both univariate analysis (61.3% T1/2 vs. 83.1% T3/4, p = 0.0004) and Cox modelling (hr: 3.72; p = 0.004).
4. DISCUSSION
For many years, mmc has been used as a radiosensitizer in acc treatment14. A well-established adverse effect of mmc is cumulative myelosuppression resulting in leukopenia and thrombocytopenia15. Since the establishment of ccrt using 5fu and mmc as standard acc treatment, many trials have investigated other chemotherapy regimens in effort to reduce the toxic effects of mmc while maintaining or improving outcomes.
Cisplatinum has frequently been studied as an alternative to mmc10,11,16,17. In large retrospective studies, good results have been shown with cisplatin-based regimens compared with standard mmc treatment16,17. However, two phase iii trials failed to demonstrate superiority for cisplatin compared with mmc for the treatment of acc. In rtog 98-11, ccrt with 2 cycles of mmc and 5fu was compared with a course of neoadjuvant cisplatinum and 5fu followed by ccrt with cisplatinum and 5fu. In the mmc group, 5-year os and dfs were superior, and a trend toward improved cfs was observed. However, the study was criticized for the use of neoadjuvant therapy in the cisplatinum arm, which might have contributed to inferior outcomes because of delays in ccrt10. More recently, the U.K. Co-ordinating Committee on Cancer Research act ii study randomized patients to 5fu-based ccrt with either concurrent mmc or cisplatinum followed by no further therapy or by maintenance chemotherapy. Complete response at 26 weeks and 3-year progression-free survival were similar in the groups. Grade 3+ acute toxicities were also similar, although greater grade 3 hematologic toxicity occurred in the mmc arm than in the cisplatinum arm (26% vs. 16%, p < 0.001)11. Because neither of the prospective trials demonstrated superiority for cisplatinum compared with mmc, ccrt with mmc and 5fu remains the standard treatment for acc.
Results from our retrospective study suggest that a reduction in mmc cycles (1 vs. 2) and a lower dose might be another feasible option for reducing acute toxicities while maintaining similar clinical outcomes. Although the present study was not a randomized comparison of the two regimens, the mmc1 and mmc2 cohorts were almost evenly split between the two tertiary cancer centres. The decision to use 1 or 2 cycles of mmc with ccrt was established historically at each centre (1 cycle at tbcc, 2 cycles at cci). Earlier phase iii trials have used a single bolus of mmc at 12–15 mg/m2 or 2 cycles at 10 mg/m2. In our study, mmc was prescribed at a lower dose of 10 mg/m2, but survival outcomes in the two study arms were similar6–11.
Baseline characteristics in the mmc1 and mmc2 groups were comparable on most factors, but differed slightly in median tumour size. The proportion of node-negative patients and the overall TNM stage were, however, similar. Before treatment, most hematologic parameters were also comparable between the groups—with the exception of white blood cell count, which was slightly higher in the mmc2 group, although within normal limits. Despite that difference, acute leukopenia was significantly greater in the mmc2 group. That observation is not surprising given the cumulative leukopenic effects of mmc.
Although rates of neutropenia were similar, febrile neutropenia requiring hospitalization occurred almost 10 times more often in patients who received 2 cycles of mmc (hr: 9.91; p = 0.001). Grade 3+ acute skin reactions also occurred more frequently in the mmc2 group, but interestingly, did not translate to a higher rate of unintended treatment breaks. That finding might be a result of variation in the threshold at which physicians decide to offer treatment breaks. On multivariate analysis, receiving 2 cycles of mmc was the strongest factor associated with hospitalizations secondary to febrile neutropenia, grade 3+ leukopenia, and dermatologic toxicity.
Development of more-conformal rt techniques have allowed for reductions in toxicity to normal tissue. In rtog 0529, acute toxicities in acc treatment were compared for intensity-modulated rt (imrt) and conventional two-dimensional planning rt, with significantly lower grade 2+ hematologic and grade 3+ gastrointestinal and dermatologic toxicities being demonstrated with imrt18.
Although our study included patients treated with two-dimensional planning rt, three-dimensional conformal rt, and imrt, acute toxicities were comparable to those reported in rtog 052918. Overall grade 3+ toxicity and grade 3+ genitourinary and gastrointestinal toxicities were comparable between the studies (83%, 2%, and 21% respectively in the rtog study vs. 77%, 2%, and 15% in the present study). Total grade 3+ hematologic toxicities were lower in our study (58% rtog vs. 43%). There was a higher proportion of grade 3+ dermatologic toxicities in our study (23% rtog vs. 58%), which might be a result of differences in rt delivery, such as less use of imrt. Interestingly, a treatment break was more often required for patients in the rtog imrt arm than in our patients (49% vs. 24%). That finding might be attributable to rtog guidelines for the introduction of treatment breaks. Similar results are seen when the mmc2 arm of our study is compared with the rtog 0529 trial, which used 2 cycles of mmc. The 5-year os and cfs results from our mmc2 study arm compare favourably with results from other phase iii trials10. The 5-year os and cfs in the standard arm of rtog 98-11 were similar to those in our study (78.3% and 71.9% respectively for rtog vs. 70.7% and 77.7% for mmc2). However, the 5-year dfs in our study was lower (44.2% mmc2 vs. 67.8% rtog), which might be attributable to our smaller sample size and might reflect a more general population of acc patients.
Our study has the inherent limitations of a retrospective study from a single health authority. Also, no formal pathology or radiology review was undertaken. Despite using a standardized toxicity grading scale, there is potential subjectivity in retrospectively grading toxicities. In effort to obtain consistency, a single reviewer graded all toxicities.
5. CONCLUSIONS
In this analysis of radical ccrt for acc, 1 cycle of mmc appears to offer outcomes similar to those achieved with 2 cycles, but with significantly fewer adverse effects, particularly with respect to hospitalization rates for febrile neutropenia and acute dermato-logic toxicities. Our results support the need for a randomized controlled trial to further investigate the optimal dose and number of mmc cycles in ccrt treatment of acc.
6. ACKNOWLEDGMENTS
We are grateful for funding support from the Alberta Cancer Foundation.
7. CONFLICT OF INTEREST DISCLOSURES
KEM reports personal fees from Lilly Canada and Bayer Canada, and nonfinancial support from Novartis Canada, outside of the submitted work. All other authors declare no financial conflicts of interest.
8. REFERENCES
- 1.United States Department of Health and Human Services, National Institutes of Health, National Cancer InstituteSurveillance, Epidemiology, and End Results Program (seer) SEER > Statistical Summaries > Cancer Stat Fact Sheets > More Cancer Types > Cancer of the Anus, Anal Canal, and Anorectum > SEER Stat Fact Sheets: Anal Cancer [Web resource] Bethesda, MD: SEER; 2013. [Available at: http://seer.cancer.gov/statfacts/html/anus.html; cited May 31, 2013] [Google Scholar]
- 2.Nigro ND, Vaitkevicius VK, Buroker T, Bradley GT, Considine B. Combined therapy for cancer of the anal canal. Dis Colon Rectum. 1981;24:73–5. doi: 10.1007/BF02604287. [DOI] [PubMed] [Google Scholar]
- 3.Nigro ND, Seydel HG, Considine B, Vaitkevicius VK, Leichman L, Kinzie JJ. Combined preoperative radiation and chemotherapy for squamous cell carcinoma of the anal canal. Cancer. 1983;51:1826–9. doi: 10.1002/1097-0142(19830515)51:10<1826::AID-CNCR2820511012>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 4.Leichman L, Nigro N, Vaitkevicius VK, et al. Cancer of the anal canal. Model for preoperative adjuvant combined modality therapy. Am J Med. 1985;78:211–15. doi: 10.1016/0002-9343(85)90428-0. [DOI] [PubMed] [Google Scholar]
- 5.Meyer J, Willet C, Czito B. Current and emerging treatment strategies for anal cancer. Curr Oncol Rep. 2010;12:168–74. doi: 10.1007/s11912-010-0100-9. [DOI] [PubMed] [Google Scholar]
- 6.Epidermoid anal cancer: results from the ukcccr randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin ukcccr Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet. 1996;348:1049–54. doi: 10.1016/S0140-6736(96)03409-5. [DOI] [PubMed] [Google Scholar]
- 7.Northover J, Glynne–Jones R, Sebag–Montefiore D, et al. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised ukcccr Anal Cancer Trial (act i) Br J Cancer. 2010;102:1123–8. doi: 10.1038/sj.bjc.6605605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase iii randomized trial of European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040–9. doi: 10.1200/JCO.1997.15.5.2040. [DOI] [PubMed] [Google Scholar]
- 9.Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase iii randomized intergroup study. J Clin Oncol. 1996;14:2527–39. doi: 10.1200/JCO.1996.14.9.2527. [DOI] [PubMed] [Google Scholar]
- 10.Gunderson LL, Winter KA, Ajani JA, et al. Long-term update of US gi Intergroup rtog 98-11 phase iii trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol. 2012;30:4344–51. doi: 10.1200/JCO.2012.43.8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James RD, Glynne–Jones R, Meadows HM, et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (act ii): a randomised, phase 3, open-label, 2×2 factorial trial. Lancet Oncol. 2013;14:516–24. doi: 10.1016/S1470-2045(13)70086-X. [DOI] [PubMed] [Google Scholar]
- 12.Cummings BJ, Keane TJ, O’Sullivan MB, Wong CS, Catton CN. Epidermoid anal cancer: treatment by radiation alone or by radiation and 5-fluorouracil with and without mitomycin C. Int J Radiat Oncol Biol Phys. 1991;21:1115–25. doi: 10.1016/0360-3016(91)90265-6. [DOI] [PubMed] [Google Scholar]
- 13.Radiation Therapy Oncology Group (rtog) RTOG > Research Associates > Adverse Event Reporting > Acute Radiation Morbidity Scoring Criteria [Web resource] Philadelphia, PA: Radiation Therapy Oncology Group; 2012. [Available at: http://www.rtog.org/ResearchAssociates/AdverseEventReporting/AcuteRadiationMorbidityScoringCriteria.aspx; cited February 16, 2012] [Google Scholar]
- 14.Horsman MR, van der Kogel A. Therapeutic approaches to tumor hypoxia. In: Joiner M, van der Kogel A, editors. Basic Clinical Radiobiology. 4th ed. London, U.K: Hodder Arnold; 2009. pp. 233–45. [DOI] [Google Scholar]
- 15.BC Cancer Agency (bcca) BC Cancer Agency > Health Professionals Info > Cancer Drug Manual > Drug Index (Professional) > Mitomycin [Web resource] Vancouver, BC: BCCA; 2013. [Available at: http://www.bccancer.bc.ca/HPI/DrugDatabase/DrugIndexPro/Mitomycin.htm; cited July 8, 2013] [Google Scholar]
- 16.Eng C, Chang GJ, You YN, et al. Long-term results of weekly/daily cisplatin-based chemoradiation for locally advanced squamous cell carcinoma of the anal canal. Cancer. 2013;119:3769–75. doi: 10.1002/cncr.28296. [DOI] [PubMed] [Google Scholar]
- 17.Olivatto LO, Cabral V, Rosa A, et al. Mitomycin-C– or cisplatin-based chemoradiotherapy for anal canal carcinoma: long term results. Int J Radiat Oncol Biol Phys. 2011;79:490–5. doi: 10.1016/j.ijrobp.2009.11.057. [DOI] [PubMed] [Google Scholar]
- 18.Kachnic LA, Winter K, Myerson RJ, et al. rtog 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluroruracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2013;86:27–33. doi: 10.1016/j.ijrobp.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]