Abstract
Background
Hepatocellular carcinoma (hcc) is one of the most common causes of cancer-related death worldwide. Overall, liver transplantation and resection are the only available treatments with potential for cure. Various locoregional therapies are widely used to manage patients with advanced hcc or as a bridging therapy for patients with early and intermediate disease. This article reviews and evaluates the role of interventional radiology in the management of such cases by assessing various aspects of each method, such as effect on rates of survival, recurrence, tumour response, and complications.
Methods
A systemic search of PubMed, medline, Ovid Medline In-Process, and the Cochrane Database of Systematic Reviews retrieved all related scientific papers for review.
Results
Needle core biopsy is a highly sensitive, specific, and accurate method for hcc grading. Portal-vein embolization provides adequate expansion of the future liver remnant, making more patients eligible for resection. In focal or multifocal unresectable early-stage disease, radiofrequency ablation tops all other thermoablative methods. However, microwave ablation is preferred in large tumours and in patients with Child–Pugh B disease. Cryoablation is preferred in recurrent disease and in patients who are poor candidates for anesthesia. Of the various transarterial modalities—transarterial chemoembolization (tace), drug-eluting beads, and transarterial radio-embolization (tare)—tace is the method of choice in Child–Pugh A disease, and tare is the method of choice in hcc cases with portal vein thrombosis.
Conclusions
The existing data support the importance of a multidisciplinary approach in hcc management. Large randomized controlled studies are needed to provide clear indication guidelines for each method.
Keywords: Interventional radiology, hepatocellular carcinoma, liver biopsy, ablation, embolization, drug-eluting beads
1. INTRODUCTION
Hepatocellular cancer (hcc) is the 2nd most frequent cause of cancer-related death in men and the 6th in women1. In 2008, the number of new liver cancer cases was estimated to be 748,300 worldwide, and 695,900 deaths were attributed to liver cancer that same year1.
Overall, liver transplantation, surgical resection, and locoregional therapies are the treatment options for hcc. However, only a small proportion of patients are suitable for the first two options2. A variety of image-guided interventions now play a vital role in the management of hcc. A multidisciplinary approach to those cases, with the involvement of interventional radiologists, became the main component in treatment success for patients with hcc.
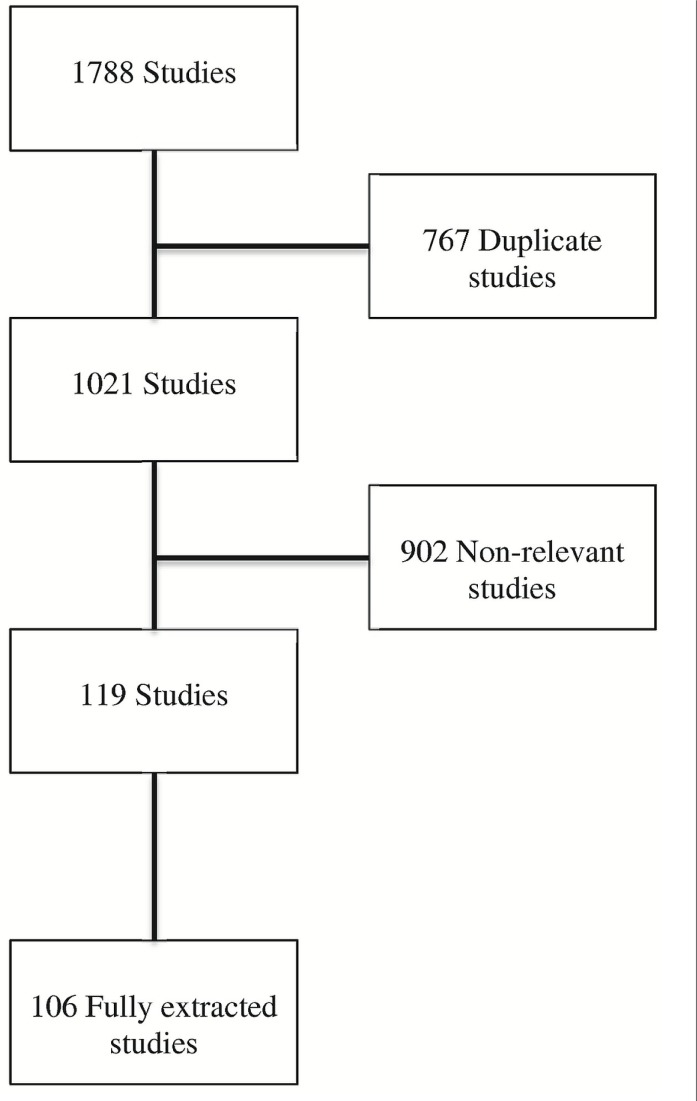
This review summarizes and evaluates the role of the various available radiologic interventions in the management of hcc. A systemic search of PubMed, medline, Ovid Medline In-Process, and the Cochrane Database of Systematic Reviews using the key words “intervention,” “radiology,” “hepatocellular carcinoma,” “liver biopsy,” “ablation,” “embolization,” “chemo-embolization,” “radio-embolization,” “tissue banking,” or “drug-eluting beads” located all English-language scientific papers for retrieval and review. Cross-references and references from reviewed articles were also located and reviewed (Figure 1).
FIGURE 1.
Studies located, excluded, and extracted for the study.
2. ROLE OF INTERVENTIONAL RADIOLOGY IN HCC
2.1. Diagnosis
To plan and assess treatment options, the treating team has to fully understand the pathology of hcc and its imaging characteristics3. In 2010, the American Association for the Study of Liver Diseases based the diagnosis of hcc only on the typical enhancement pattern of the liver lesion in a single contrast-enhanced imaging procedure4. Occasionally, liver biopsies are needed to diagnose hcc and, less frequently, to define prognosis after therapy based on tumour grade and microvascular invasion5. The optimal liver biopsy for grading and staging a liver tumour is either 20–25 mm in length or consists of 11 complete portal tracts, or both6–8.
There are two types of liver biopsy: needle core biopsy (ncb) and fine-needle aspiration (fna). A ncb uses a large-gauge needle (1–3 mm in diameter) and retrieves a thin cylinder of liver tissue9; fna uses needles 0.4–0.6 mm in diameter10. Recent studies indicated that the diagnostic value of these methods is comparable11. With fna, sensitivity is 67%–100%, and specificity is 98%–100%12–15. The sensitivity of fna depends mostly on the technique and skill of the radiologist and the cytopathologist16. Accuracy with ncb is 62%–93%17–19. A study found a 91.4% concordance between preoperative ncbs and final surgical specimens7. However, ncb has an advantage over fna in that the specimen obtained is suitable for an assessment of both architectural and cytologic features in addition to marker studies20. One study showed that the respective diagnostic accuracies of 85.4% and 83% for fna and ncb increased to 89.1% when both methods were used21, and another found an accuracy increase to 88% from 78% with fna and a similar accuracy with ncb22.
The liver biopsy procedure is reported to carry the potential for a number of complications. Minor complications include pain and transient hypotension23. Major complications include intrahepatic hemorrhage, intraperitoneal hemorrhage, hemothorax, pneumothorax23, and injury of adjacent organs such as the pancreas, colon, gallbladder, or right kidney23. Intrahepatic arteriovenous fistula can also occur after liver biopsy23. The overall complication rate is 29%, with 90% of the complications being related to pain24. Tumour seeding is another issue that has been reported in the literature, with a prevalence rate of 0.003%–5%25–30. The mortality rate after liver biopsy is reported to be 0%–0.18%, mostly attributable to significant hemorrhage and bile peritonitis23,31.
2.2. Treatment
In patients with early- or intermediate-stage disease, locoregional modalities play a pivotal role in the management of hcc by controlling disease progression until definitive therapy or by increasing eligibility for a curative treatment. In patients with advanced disease, the main aim of treatment is to control symptoms, prolong survival, and improve quality of life. A number of image-guided therapies are available (Table i), including direct ablation, portal vein embolization (pve), transarterial embolization (tae), transarterial chemoembolization (tace), drug-eluting beads (debs), and transarterial radioembolization (tare)33.
TABLE I.
Summary of interventional procedures used in hepatocellular carcinoma (hcc) management
| Procedure | Aim | Material |
|---|---|---|
| Portal vein embolization | Induce atrophy of embolized area and hypertrophy of contralateral lobe | Glue or alcohol and coil |
| Thermoablation | Induce destruction of tissue through thermal techniques | Radiofrequency ablation Laser ablation Microwave ablation Cryotherapy |
| Chemoablation | Induce coagulative necrosis using chemotherapeutic cytotoxic effects | Percutaneous injection of ethanol or acetic acid |
| Transarterial chemoembolization | Significant necrosis of tumour | Ethiodized oil plus any of various anticancer agents |
| Drug-eluting beads | Induce selective sustained release of chemotherapy over a long period of time | Polyvinyl alcohol hydrogel plus chemotherapy |
| Transarterial radioembolization | Cannulation of the hepatic artery with radiotherapy | 31I-Labelled ethiodized oil 90Y-Loaded microspheres |
2.2.1. Ablative Therapy
Ablation involves the direct application of chemicals or thermal energy to the tumour to achieve necrosis. Two types of thermoablative therapy are available: hyperthermic [radiofrequency ablation (rfa), microwave ablation (mwa), and laser ablation] and hypothermic (cryotherapy). Chemical ablation uses percutaneous ethanol injection (pei) or percutaneous acetic acid injection (pai)33.
Thermoablative Therapy:
Percutaneous thermal ablation is considered the best locoregional treatment option for focal unresectable early-stage hcc33. The available techniques are based on controlling tissue damage by delivering energy to the lesion while minimizing collateral damage to healthy hepatic tissue and adjacent structures34.
In rfa, a needle delivering high-frequency electrical current is introduced as a source of heat that causes cell death. The maximum temperature obtained and the duration of exposure to the heat affect the cell-kill zone35,36, but a major limitation is the possibility of a heat-sink effect produced by large vessels (>3 mm) near the ablation zone. The blood current can prevent complete necrosis of nearby tumour cells, potentially leading to positive margins. In some cases, the tumour’s arterial supply is embolized in advance in an attempt to overcome the heat-sink effect37.
The advantages of rfa include high local efficacy, sparing of normal-liver parenchyma outside the burn zone, and the potential for safe repetition multiple times33. However, rfa puts the patient at a small risk of injury to structures within the burn zone and along the probe’s path of entry: pneumothorax, less than 1%; biliary injury, less than 1%; abscess, 1%; major hemorrhage, less than 1%38,39; and tumour seeding, 1%40. Nevertheless, the major complication rate of rfa ranges from 2.4% to 13.1% compared with a range of 9%–22% for surgical resection38,39,41–43. Treatment of subscapular tumours with rfa might require general anesthesia, because it can cause severe pain44.
Salmi et al. reported a prospective study in which primary ablation after rfa was successful in 96% of patients, with local tumour progression rates of 4% and 14% at 1 and 5 years respectively, and survival rates of 92% at 1 year and 63% at 5 years. The size of ablated tumours in the study ranged between 1.2 cm and 3.5 cm45.
Several studies aimed to evaluate the efficacy of rfa treatment in hcc patients, considering factors such as tumour size, location, and condition of surrounding liver parenchyma. A retrospective study showed no significant difference in the overall survival rate with surgical resection (n = 2857) and rfa (n = 3022) for hcc lesions 3 cm or less in size (2-year survival rate: 94.5% vs. 93%; p = 0.64). However, time to recurrence was significantly lower in the resection group than in the rfa group (p = 0.0001)46.
Livraghi et al.47 tested the efficacy of rfa in medium- and large-size hcc tumours. The trial included 80 tumours 3.1–5 cm in diameter and 46 tumours 5.1–9.5 cm in diameter. The authors reported a significant difference between the groups with respect to tumour necrosis: complete necrosis (defined as 100% necrosis) was found in 61% of medium-sized tumours and in 24% of large-sized tumours (p = 0.001).
In a retrospective study, Ng et al.48 evaluated rfa efficacy in 56 perivascular hcc tumours without hepatic inflow occlusion (tumour situated within 5 mm of a 1st- or 2nd-degree branch of the portal and hepatic veins). Those authors showed no significant difference between perivascular and non-perivascular hcc in terms of rates of complete ablation, local tumour progression, morbidity, and mortality48. Their findings contrast with the results of a study by Lu et al.49 of 31 perivascular hcc tumours, which suggested that the efficacy of rfa is affected by the location of the tumour, which was also suggested in studies by McGahan et al.35 and Lounsberry et al.36.
Theories suggest that cirrhotic tissue could enable better thermal ablation through the “oven effect”50, which posits that the increased thickness of cirrhotic tissue around the nodule works as a thermal insulator, avoiding dispersion of the heat generated around the rfa electrode.
Microwave ablation works by changing the polarity of water molecules within tissues to generate heat. The frequency range used is usually between 915 MHz and 2.45 GHz51. Advantages of mwa include heating that is not limited by desiccation and charring51,52, eliminating the risk of skin burn caused by grounding pads51,53. In mwa, a larger ablation zone is created in a shorter time, meaning that tumours can be treated with fewer applicator insertions than are needed in rfa54. The effect of mwa on perivascular tumours is also better because of the lesser potential for a heat-sink effect51,55. The complication rate reported in mwa is 2.6%–7.5%, which includes the potential for ascites, pleural effusion, liver abscess, and perforation of adjacent organs54,56–58.
Lee et al.54 studied surgical mwa in tumours of 2–6 cm in diameter. All early postoperative computed tomography (ct) imaging showed no residual lesions; however, on follow-up, 42% of patients experienced local tumour progression. As Lee et al.54 noted, high local tumour progression is a downside of mwa and can be attributed to the use of a large applicator (5 mm in diameter), which increases the risk of tumour puncture and therefore tumour seeding.
Cryoablative Therapy:
Ultralow temperature ablation uses argon gas to create tissue injury from temperatures that reach far below the freezing point. Cell death has been confirmed after reaching −20°C or after rapid freeze and slow thaw cycles at higher temperatures59.
Like rfa, cryoablation can be performed under conscious sedation, which makes it a good choice when the patient is a poor candidate for anesthesia60. The technique also has a lesser heat-sink effect than rfa. Cryoablation allows for real-time visualization of an “ice ball,” which permits precise evaluation of the ablated zone61,62 and maintains the cellular integrity of adjacent visceral linings63. Potential adverse effects include disseminated intravascular coagulation, coagulopathy, and multiorgan failure, which are signs of cryoshock syndrome, specific for cryotherapy in the liver64.
Median survival time (7.5 months vs. 3.2 months) and median time to progression (ttp) (3.5 months vs. 1.5 months) were found to be significantly different in cryotherapy and control groups65. Ultrasound-guided percutaneous cryotherapy proved to be effective and safe in patients with unresectable and recurrent hcc (mean tumour size: 2.8 ± 1.7 cm), with 1- and 3-year survival rates calculated to be 81.4% and 60.3% respectively. The disease-free survival rate was 67.6% and 20.8% at 1 and 3 years respectively66 (Table ii).
TABLE II.
Thermal ablation trials in hepatocellular carcinoma (hcc)
| Reference | Study design | Intervention |
Patients
|
Progression
|
cr (%) |
Survival (%)
|
p Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (n) | Characteristics | Median ttp (months) | Rate (%) | 1-Year | 3-Year | 5-Year | |||||
| Salmi et al., 200845 | Prospective | Radiofrequency ablation | 25 | Single nodule <3.5 cm or 3 or fewer nodules <3 cm | 4 (1 year) 14 (3 years) 14 (5 years) |
96 | 92 | 72 | 64 | — | |
| Chang et al., 201165 | Cohort | Cryoablation | 190 | Advanced hcc related to hepatitis B | Intervention: 3.5 Control: 1.5 | — — |
— | — | — | — | ≤0.05 |
| Lee et al., 201254 | Prospective case series | Microwave ablation | 26 | Mean tumour size: 3.8 cm (range: 2–6 cm) | — | — | 65 | — | — | — | — |
ttp = time to progression; cr = complete response.
In a recent meta-analysis comparing cryosurgery with rfa, rfa was found to be superior in terms of the local recurrence rate (odds ratio: 1.96; 95% confidence interval: 1.12 to 3.42)67. To our knowledge, no study has compared survival benefits for these techniques.
Irreversible Electroporation:
Irreversible electro-poration (ire) is a new technology that has recently been applied in hcc treatment. It works by delivering pulses of electrical current up to 3 kV to tumour cells, producing an electrical field that creates nanopores in cell membranes. This irreversible damage affects homeostasis of the cell and causes cell death by apoptosis68. The two advantages of this technology are that it does not affect the extracellular matrix, thus maintaining structural integrity of the adjacent blood vessels and bile ducts69 and that it has no heat-sink effect70. Irreversible electroporation can therefore help in managing tumours in difficult locations. The technique also does not cause fibrosis and scarring after ablation, meaning that the treatment zone can be evaluated earlier than it can with other ablation methods68. However, ire requires the patient to be under general anesthesia and deep neuromuscular block in both open and ct-guided percutaneous procedures71. The use of ire in the management of hcc is still in its early stages, and no long-term results are available.
Chemoablative Therapy:
Percutaneous ethanol injection is a well-established technique for treating hcc tumours less than 3 cm in size33. It is performed with a fine needle under imaging guidance44, and causes coagulative necrosis because of its cytotoxic effects (cytoplasmic dehydration, denaturation of protein, and small-vessel thrombosis)33. Because of cirrhotic margins around hcc tumours, the alcohol is relatively restricted to tumour tissue, sparing normal parenchyma44. Advantages of the procedure include a simple methodology, low cost, and safety33. With pei, more than 4 sessions are required to treat each mass, even tumours smaller than 3 cm. Use of the technique is therefore more limited72 than for other modalities such as rfa, which needs fewer sessions for complete treatment33,41. Risks of hemorrhage, portal vein thrombosis, bowel necrosis, gallbladder injury, and liver necrosis have also been shown to increase with pei40.
Tumour recurrence and survival rates for rfa, pei, and pai in the treatment of hcc 3 cm in size or less, Child–Pugh A and B, were compared in a randomized trial involving 187 patients. Radiofrequency ablation was found to be superior, with local recurrence rates at 1 and 3 years of 10% and 14%, compared with 16% and 34% in the pei group and 14% and 31% in the pai group. Survival rates at 1 and 3 years were 93% and 74%, 88% and 51%, and 90% and 53% in the rfa, pei, and pai groups respectively. Cancer-free survival rates at 1 and 3 years were 74% and 43% in the rfa group, 70% and 21% in the pei group, and 71% and 23% in the pai group. Large tumour size (>2 cm) and high tumour grade were independent factors that correlated with local recurrence41.
2.2.2. Embolization
Venous Embolization:
Portal Vein Embolization: In a retrospective review of patients with hcc who underwent extended liver resection, portal vein embolization (pve) was found to significantly increase the future liver remnant (flr). Before surgical resection, pve is used to induce progressive atrophy of the embolized territory and hypertrophy of the remaining non-tumour-containing parenchyma, allowing for safe extended liver resections33. This technique can be used when the flr is less than 20%–30% of the initial total normal liver volume or less than 50% in fibrotic and cirrhotic livers73–75; however, the flr gain is much less in cirrhotic patients than in those with mild or moderate fibrosis73.
Liquid agents (glue or alcohol) and small-particle embolization materials are used for distal embolization. Some groups also perform large central-vessel occlusion with coils33. Care must be taken not to deploy the coils too close to the hepatic hilum, because surgical ligation can then become more difficult. Observed pve side effects include a transient increase in white blood cell count, fever, and abdominal discomfort76.
Siriwardana et al.77 prospectively studied the effect of pve on hcc recurrence in 34 patients who underwent curative liver resection after pve and in 102 who underwent resection without pve. The use of pve increased the flr from 23% to 34%. The study concluded that pve can increase the resectability rate of hcc tumours considered initially unresectable because of insufficient flr and that pve has no deleterious oncologic effect after major resection of hcc.
Simoneau et al.78 studied the effect of pve on the growth of liver metastases from colorectal tumours. They prospectively followed tumour growth in 109 patients who underwent right pve, and 11 who did not. Tumour growth was significantly different between the groups, with the tumour volume increasing by 33.4% in the right lobe and 49.9% in the left lobe of the liver of the embolized group and decreasing by 34.8% in the right lobe of the liver and by 33.2% in the left lobe of the non-embolized group (p < 0.001 in the right lobe, p = 0.022 in the left lobe)78. Despite those results, resectability was not affected. Similar data in hcc patients are lacking.
Arterial Embolization:
TAE (Bland Embolization): In tae, the hepatic arteries are occluded by injecting an intravascular substrate or by placing a device such as microspheres. The procedure is performed using a catheter under fluoroscopy.
Embolization is effective in the treatment of hcc primarily because hcc relies heavily on the arterial blood supply44. It is believed that intra-arterial administration of various drugs or devices allows for preferential distribution to tumours, and embolization of the feeding arteries results in ischemic tumour necrosis79.
Nicolini et al.80 reported that tae using micro-spheres can achieve a complete response (cr), with evidence of devascularization of the tumour in 89% of cases. The definition of cr is an absence of peripheral enhancement in arterial-phase ct images. However, the authors reported a local recurrence rate of 62% and development of additional tumours in 56% of patients. The time lag between assessment of cr and local recurrence of the tumour ranged from 3 months to 6 months. Nicolini et al.80 suggested the possibility of ct overestimation of tumour response and the small study sample as potential explanations for the high recurrence rate. Still, the study showed that tae is a well-tolerated procedure for patients with early or intermediate hcc, causing no clinically significant deterioration in liver function.
TACE:
Combining embolization and administration of cytotoxic medications, tace is delivered to the tumour by a transarterial route44. The first chemoembolic agent used was iodinated poppy seed oil81, an excellent agent for intra-arterial embolization because of its viscosity and water insolubility. It causes occlusion of the downstream capillaries, has a more lethal effect on tumour cells than on hepatocytes, and is radiopaque, which allows for radiologic visualization during injection81,82. Iodinated poppy seed oil can be used in combination with multiple anticancer agents including cisplatin, doxorubicin, carboplatin, epirubicin, mitoxantrone, and mitomycin C12. The mixture is injected through a catheter placed into the appropriate subsegmental branches of the hepatic artery supplying the tumour33.
The role of tace in neoadjuvant therapy is not entirely clear. A survival advantage might possibly accrue from tace administration before resection (compared with resection alone)83; however, the technique is recommended as first-line palliative therapy for nonsurgical cases with large or multifocal lesions and no vascular invasion of distant metastases4,84. Transarterial chemoembolization can also be used as bridge therapy before transplantation, by downsizing the tumour or controlling its growth33.
When tace and systemic chemotherapy infusion alone were compared in animal trials, tace was found to deliver a drug concentration 1–2 orders of magnitude greater to the target organ and to maintain a markedly longer dwell time85,86. Furthermore, it reduced systemic toxicity because most of the drug was retained in the liver87.
In a French multicentre trial of 127 patients with advanced disease, survival rates in the tace arm were found to be 64% and 38% at 1 and 2 years, compared with just 18% and 6% in matched untreated controls88. In patients with Okuda stage 1 and 2 (but not Okuda stage 3) disease, the survival rate was significantly increased after tace than after no therapy88 (Table iii). A second trial89 compared the mean survival rate in patients with hcc treated with chemoembolization and in control patients treated with symptomatic management: the difference was significant at 2 years, with the former having a 54% survival rate, and the latter, 26%.
TABLE III.
Okuda classification system for hepatocellular carcinoma (hcc)32
| Okuda classification system |
Component
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Tumour sizea | Ascites | Bilirubin | Albumin | |||||
|
|
|
|
|
|||||
| Positive (≥50%) | Negative (<50%) | Positive (present) | Negative (absent) | Positive (≥3 mg/dL) | Negative (<3 mg/dL) | Positive (≤30 g/L) | Negative (>30 g/L) | |
| Stage 1 | X | X | X | X | ||||
| Stage 2 | 1 or 2 positive | |||||||
| Stage 3 | 3 or 4 positive | |||||||
Tumour response to tace treatment is notably affected by tumour size, TNM stage, preserved liver function (Child–Pugh), and serum aspartate aminotransferase. Optimal tace candidates are patients with Child–Pugh A disease, with no extrahepatic spread or vascular invasion (Barcelona Clinic liver cancer stage B)90–92. Hepatic arterial infusion chemotherapy might be a better choice in patients with more advanced stage disease93.
Lee et al. compared tace with surgical resection in a large study94 in which 91 patients underwent resection and 91 received multiple sessions of tace. The survival rate was significantly higher with liver resection than with tace (p = 0.0038), median survival time being 66 months and 37 months respectively. However, the T3N0M0 subgroup of patients showed no significant difference in survival rate (5-year survival: 27% for resection vs. 23% for tace, p = 0.7512). However, there were significant differences in the basic characteristics of the two groups. The resection group had older patients and a higher number of patients who were positive for antibodies to the hepatitis C virus, which might have negatively affected their survival rate.
Contraindications to tace are bilobar tumours or total liver involvement exceeding 50%, Child– Pugh C liver cirrhosis, distant metastasis, glomerular filtration rate less than 40 mL/min/1.73 m2, arterio-portal fistula, or portal vein thrombosis95,96. In the context of defective portal vein flow, the treated zone might develop extensive necrosis because of blocking of the entire blood supply to that specific area97. Its side effects are nausea, vomiting, possible renal failure, cardiac toxicity, bone marrow aplasia, hepatic abscess or cholecystitis, and post-embolization syndrome (which occurs because of tumour necrosis and cytokine release). Manifestations of post-embolization syndrome are fever, nausea, vomiting, and right upper quadrant pain. It usually resolves spontaneously in 48 hours98.
2.2.3. DEBs
Drug-eluting beads, which are made of polyvinyl alcohol hydrogel, provide selective, sustained release of chemotherapy over long periods of time. Their action is based on slow release into the body of doxorubicin adsorbed to their surface. Initial results at follow-up imaging have been positive, as evidenced by a lack of enhancement in the tumour, which is indicative of necrosis99. Beads come in various diameters; the choice of proper bead size depends on vascularity and the size of the lesion. Elution kinetics showed that larger beads release doxorubicin at a slower rate, and vice versa. The calculated half-life of doxorubicin-capable beads is 1730 hours for 700–900 μm beads and 150 hours for 100–300 μm beads. Compared with larger-diameter beads, smaller-diameter beads also cause a larger degree of pan-necrosis of the target lesion and adjacent liver tissue99.
Early studies of hcc response to deb therapy revealed a 75% response rate at 6 months by the Response Evaluation Criteria in Solid Tumors, with 44.4% partial response (pr) and 25.9% stable disease (sd). Progressive disease was seen in 18.5% of patients, and the 1-year survival rate was 92.5%100. More recent evaluation demonstrated a 27% cr rate in patients by the Response Evaluation Criteria in Solid Tumors, with 13% pr and 3% sd. Progressive disease occurred in 40% of patients at 6 months’ follow-up. The overall survival rate was 93% at 6 months101. Boatta et al.102 reported a 42.9% cr rate in patients by the modified Response Evaluation Criteria in Solid Tumors (Table iv), with 29.8% pr, 17.5% sd, and just 9.7% progressive disease; however, the assessment occurred at 1 month of follow-up. The overall survival rate was 92.6% at 6 months.
TABLE IV.
Modified recist criteria, according to guidelines from the American Association for the Study of Liver Diseases and the Journal of the National Cancer Institute103
| Category | Definition |
|---|---|
| Complete response | Disappearance of intratumoural arterial enhancement in all target lesions |
| Partial response | Compared with baseline, 30% or greater decrease in the sum of the diameters of viable target lesions (which enhance in the arterial phase) |
| Stable disease | Any case that shows neither partial response nor progressive disease |
| Progressive disease | 20% or greater increase in the sum of diameters of viable target lesions (compared with the smallest sum recorded since treatment start) |
recist = Response Evaluation Criteria in Solid Tumors.
A blinded randomized trial of 212 patients conducted by Lammer et al.104 compared disease control with debs and with tace—“disease control” being defined as cr, pr, and sd combined. Tumour response was assessed on magnetic resonance images at 6 months by European Association for the Study of the Liver criteria (Table v). The disease control rates were 63.4% for debs and 51.9% for tace, a difference that was not statistically significant. Patients with advanced disease (Child–Pugh B, Eastern Cooperative Oncology Group performance status 1, bilobar disease, and recurrent disease) experienced significantly better disease control and objective response rates with deb treatment104.
TABLE V.
European Association for the Study of the Liver tumour response criteria by magnetic resonance imaging104
| Category | Definition |
|---|---|
| Complete response | All known viable tumours completely disappear (assessed by tumour uptake of contrast in arterial phase) |
| Partial response | Viable tumour area of all measurable lesions is reduced by 50% |
| Progressive disease | Appearance of new lesions or the size of 1 or more measurable lesions increases by 25% |
| Stable disease | All other cases |
With respect to the side effects of debs, Varela et al.100 reported pes in 10 of 27 patients, abdominal pain in 3 patients, mild fever in 6 patients, nausea and vomiting in 3 patients, and liver abscess in 2 patients.
2.2.4. Radioembolization
Radioembolization is a form of interstitial radiotherapy, which merges the interventional radiology technique of hepatic artery cannulation with radiotherapy. It delivers a high radiation dose to selected areas within the liver. The most frequently used compounds are 131I-labelled iodinated poppy seed oil and 90Y-loaded microspheres100. Treatment with 90Y in particular is associated with low toxicity105.
In a study investigating the long-term outcomes of tare therapy in hcc patients, the response rate was found to be 57% after treatment with 90Y (based on size and tumour necrosis criteria); ttp was 7.9 months106. A randomized controlled trial comparing 131I-labelled iodinated poppy seed oil tare with best medical support in hcc patients having portal vein thrombosis showed a 6-month survival of 48% with treatment; in the best medical support arm, there were no survivors at 6 months107. Chaudhury et al.108 reported a case of cr for a 4.7-cm hcc tumour with vascular involvement treated using sorafenib and 90Y radioembolization.
Comparing tace with tare, Salem et al.109 observed no significant difference in survival time (p = 0.42); however, the difference in ttp was found to be clinically significant in favour of radioembolization (13.3 months for tare vs. 8.4 for tace, p = 0.046). When comparing 131I-labelled iodinated poppy seed oil tare with conventional tace for unresectable hcc, survival was similar for both treatments (6-month survival: 69.2% vs. 65.6%), but with fewer side effects in the tare group110.
Possible side effects of tare include fatigue, nausea, vomiting, abdominal pain, and low-grade fever. Lymphopenia not associated with an increased risk of infection is also common. Compared with tace, tare is safe for use in patients with portal vein thrombosis111, and a small study (12 patients) implied its safety in those with lobar or segmental biliary tract obstruction and normal bilirubin levels112,113. However, non-targeted radiation can lead to “bystander organ injury” such as cholecystitis, gastrointestinal ulcers, and pneumonitis106,113–119. For that reason, tare procedures must be preceded by angiographic and scintigraphic mapping studies to evaluate the presence of shunts that might allow the radiosphere particles to bypass the liver and reach other organs. The shunts can then be embolized before therapy begins.
Compared with any other method for treating hcc, tare has, to date, showed no clear survival benefit except for patients with portal vein thrombosis.
3. SUMMARY
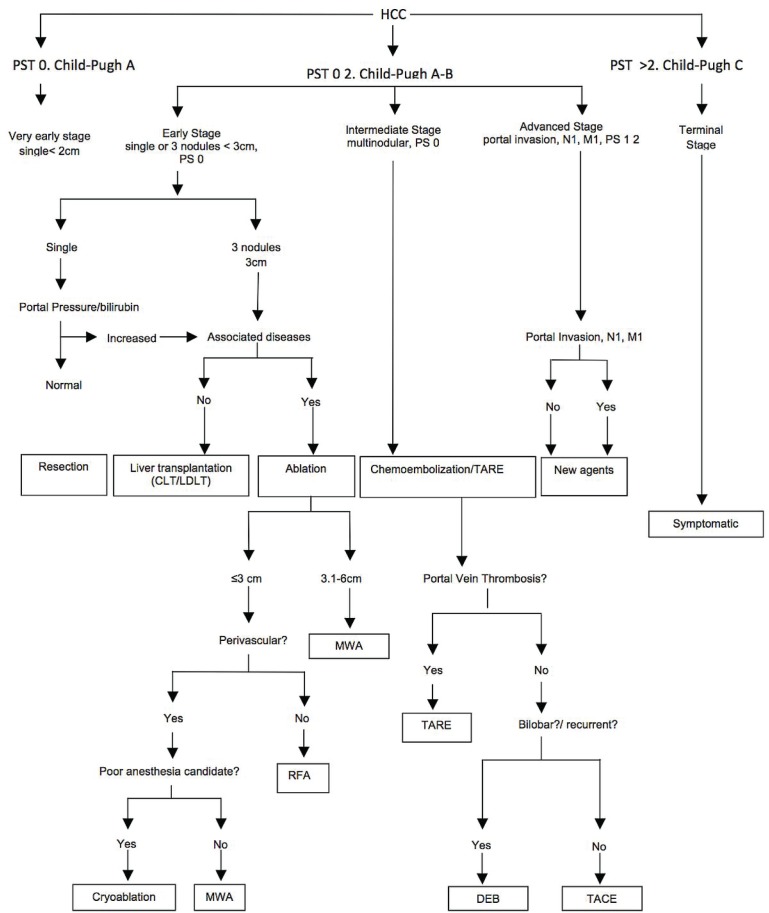
After a thorough review of the literature, we suggest a flow chart (Figure 2) to prioritize the various locoregional treatment methods in specific cases of hcc. In early-stage disease (Child–Pugh A–B, with focal or 1–3 unresectable lesions), rfa tops all other thermoablative methods. In large tumours (up to 6 cm), mwa is preferred, and cryoablation is preferred in recurrent disease. Because of a lesser heat-sink effect, mwa and cryoablation are both preferred to rfa in perivascular disease. Chemoablation can be used in smaller lesions (<3 cm). In intermediate-stage, multifocal lesions (>3), tace, debs, and tare are to be used. In Child–Pugh A patients with T3N0M0 tumours, tace is the method of choice. Drug-eluting beads are preferable in Child–Pugh B patients, with an Eastern Cooperative Oncology Group performance status 1 or bilobar or recurrent disease. In hcc cases with portal vein thrombosis, tare is the method of choice.
FIGURE 2.
Suggested locoregional treatment in specific cases of hepatocellular carcinoma (hcc). ps = performance status; tare = trans-arterial radioembolization; mwa = microwave ablation; rfa = radiofrequency ablation; deb = drug-eluting beads; tace = transarterial chemoembolization.
The efficacy of our approach should be tested in a prospective manner. We believe that hcc patients should be managed in a multidisciplinary fashion.
4. ACKNOWLEDGMENTS
The authors thank the Deanship of Scientific Research at King Saud University for funding the present work (research group project no. RGP-VPP-082). The authors also thank Ms. Isobel Strang and Ms. Maya Wardeh for great help, and Dr. Ahmed Helmy for valuable advice.
5. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
6. REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: globocan 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.N’Kontchou G, Mahamoudi A, Aout M, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology. 2009;50:1475–83. doi: 10.1002/hep.23181. [DOI] [PubMed] [Google Scholar]
- 3.Kondo F. Histological features of early hepatocellular carcinomas and their developmental process: for daily practical clinical application: hepatocellular carcinoma. Hepatol Int. 2009;3:283–93. doi: 10.1007/s12072-008-9107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M on behalf of the American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colecchia A, Scaioli E, Montrone L, et al. Pre-operative liver biopsy in cirrhotic patients with early hepatocellular carcinoma represents a safe and accurate diagnostic tool for tumour grading assessment. J Hepatol. 2011;54:300–5. doi: 10.1016/j.jhep.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 6.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–57. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39:239–44. doi: 10.1016/S0168-8278(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 8.Cholongitas E, Senzolo M, Standish R, et al. A systematic review of the quality of liver biopsy specimens. Am J Clin Pathol. 2006;125:710–21. doi: 10.1309/W3XCNT4HKFBN2G0B. [DOI] [PubMed] [Google Scholar]
- 9.Pathi R, Burnes J. Sydney, Australia: Inside Radiology, Royal Australian and New Zealand College of Radiologists; 2013. Image Guided Liver Biopsy [Web article] [Available at: http://www.insideradiology.com.au/pages/view.php?T_id=85; cited January 17, 2013] [Google Scholar]
- 10.Soderstrom CA, McArdle DQ, Ducker TB, Militello PR. The diagnosis of intra-abdominal injury in patients with cervical cord trauma. J Trauma. 1983;23:1061–5. doi: 10.1097/00005373-198312000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Wee A. Fine needle aspiration biopsy of the liver: algorithmic approach and current issues in the diagnosis of hepatocellular carcinoma. Cytojournal. 2005;2:7. doi: 10.1186/1742-6413-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang P, Meng ZQ, Chen Z, et al. Diagnostic value and complications of fine needle aspiration for primary liver cancer and its influence on the treatment outcome-a study based on 3011 patients in China. Eur J Surg Oncol. 2008;34:541–6. doi: 10.1016/j.ejso.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Hertz G, Reddy VB, Green L, et al. Fine-needle aspiration biopsy of the liver: a multicenter study of 602 radiologically guided fna. Diagn Cytopathol. 2000;23:326–8. doi: 10.1002/1097-0339(200011)23:5<326::AID-DC8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Kuo FY, Chen WJ, Lu SN, Wang JH, Eng HL. Fine needle aspiration cytodiagnosis of liver tumors. Acta Cytol. 2004;48:142–8. doi: 10.1159/000326307. [DOI] [PubMed] [Google Scholar]
- 15.Edoute Y, Tibon–Fisher O, Ben-Haim SA, Malberger E. Imaging-guided and nonimaging-guided fine needle aspiration of liver lesions: experience with 406 patients. J Surg Oncol. 1991;48:246–51. doi: 10.1002/jso.2930480407. [DOI] [PubMed] [Google Scholar]
- 16.Jain D. Diagnosis of hepatocellular carcinoma: fine needle aspiration cytology or needle core biopsy. J Clin Gastroenterol. 2002;35(suppl 2):S101–8. doi: 10.1097/00004836-200211002-00006. [DOI] [PubMed] [Google Scholar]
- 17.Nyman RS, Cappelen–Smith J, Brismar J, von Sinner W, Kagevi I. Yield and complications in ultrasound-guided biopsy of abdominal lesions. Comparison of fine-needle aspiration biopsy and 1.2-mm needle core biopsy using an automated biopsy gun. Acta Radiol. 1995;36:485–90. [PubMed] [Google Scholar]
- 18.Buscarini L, Fornari F, Bolondi L, et al. Ultrasound-guided fine-needle biopsy of focal liver lesions: techniques, diagnostic accuracy and complications. A retrospective study on 2091 biopsies. J Hepatol. 1990;11:344–8. doi: 10.1016/0168-8278(90)90219-H. [DOI] [PubMed] [Google Scholar]
- 19.Rapaccini GL, Pompili M, Caturelli E, et al. Ultrasound-guided fine-needle biopsy of hepatocellular carcinoma: comparison between smear cytology and microhistology. Am J Gastroenterol. 1994;89:898–902. [PubMed] [Google Scholar]
- 20.The International Consensus Group for Hepatocellular Neoplasia Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658–64. doi: 10.1002/hep.22709. [DOI] [PubMed] [Google Scholar]
- 21.McGahan JP, Bishop J, Webb J, et al. Role of fna and core biopsy of primary and metastatic liver disease. Int J Hepatol. 2013;2013:174103. doi: 10.1155/2013/174103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franca AV, Valerio HM, Trevisan M, et al. Fine needle aspiration biopsy for improving the diagnostic accuracy of cut needle biopsy of focal liver lesions. Acta Cytol. 2003;47:332–6. doi: 10.1159/000326529. [DOI] [PubMed] [Google Scholar]
- 23.Al Knawy B, Shiffman M. Percutaneous liver biopsy in clinical practice. Liver Int. 2007;27:1166–73. doi: 10.1111/j.1478-3231.2007.01592.x. [DOI] [PubMed] [Google Scholar]
- 24.Procopet B, Bureau C, Metivier S, et al. Tolerance of liver biopsy in a tertiary care center: comparison of the percutaneous and the transvenous route in 143 prospectively followed patients. Eur J Gastroenterol Hepatol. 2012;24:1209–13. doi: 10.1097/MEG.0b013e328355e2ba. [DOI] [PubMed] [Google Scholar]
- 25.Stigliano R, Marelli L, Yu D, Davies N, Patch D, Burroughs AK. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of hcc. Cancer Treat Rev. 2007;33:437–47. doi: 10.1016/j.ctrv.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Stigliano R, Burroughs AK. Should we biopsy each liver mass suspicious for hcc before liver transplantation?—No, please don’t. J Hepatol. 2005;43:563–8. doi: 10.1016/j.jhep.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Huang GT, Sheu JC, Yang PM, Lee HS, Wang TH, Chen DS. Ultrasound-guided cutting biopsy for the diagnosis of hepatocellular carcinoma—a study based on 420 patients. J Hepatol. 1996;25:334–8. doi: 10.1016/S0168-8278(96)80120-6. [DOI] [PubMed] [Google Scholar]
- 28.Durand F, Regimbeau JM, Belghiti J, et al. Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol. 2001;35:254–8. doi: 10.1016/S0168-8278(01)00108-8. [DOI] [PubMed] [Google Scholar]
- 29.Silva MA, Hegab B, Hyde C, Guo B, Buckels JA, Mirza DF. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008;57:1592–6. doi: 10.1136/gut.2008.149062. [DOI] [PubMed] [Google Scholar]
- 30.Takamori R, Wong LL, Dang C, Wong L. Needle-tract implantation from hepatocellular cancer: is needle biopsy of the liver always necessary? Liver Transpl. 2000;6:67–72. doi: 10.1002/lt.500060103. [DOI] [PubMed] [Google Scholar]
- 31.Fornari F, Civardi G, Cavanna L, et al. Complications of ultrasonically guided fine-needle abdominal biopsy. Results of a multicenter Italian study and review of the literature. The Cooperative Italian Study Group. Scand J Gastroenterol. 1989;24:949–55. doi: 10.3109/00365528909089239. [DOI] [PubMed] [Google Scholar]
- 32.Levy I, Sherman M, on behalf of the Liver Cancer Study Group of the University of Toronto Staging of hepatocellular carcinoma: assessment of the clip, Okuda, and Child–Pugh staging systems in a cohort of 257 patients in Toronto. Gut. 2002;50:881–5. doi: 10.1136/gut.50.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gadahadh R, Valenti D, Aljiffry M, et al. Surgery and interventional radiology collaborate on combination therapy in hepatocellular carcinoma. US Gastroenterol Hepatol Rev. 2011;(7):44–9. [Google Scholar]
- 34.Gervais DA, Goldberg SN, Brown DB, Soulen MC, Millward SF, Rajan DK. Society of Interventional Radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors. J Vasc Interv Radiol. 2009;20(suppl):S342–7. doi: 10.1016/j.jvir.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 35.McGahan JP, Browning PD, Brock JM, Tesluk H. Hepatic ablation using radiofrequency electrocautery. Invest Radiol. 1990;25:267–70. doi: 10.1097/00004424-199003000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Lounsberry W, Goldschmidt V, Linke CA, Walder HJ, Chrzan D. The early histologic changes following electrocoagulation. J Urol. 1961;86:321–9. doi: 10.1016/S0022-5347(17)65169-3. [DOI] [PubMed] [Google Scholar]
- 37.Pompili M, Mirante VG, Rondinara G, et al. Percutaneous ablation procedures in cirrhotic patients with hepatocellular carcinoma submitted to liver transplantation: assessment of efficacy at explant analysis and of safety for tumor recurrence. Liver Transpl. 2005;11:1117–26. doi: 10.1002/lt.20469. [DOI] [PubMed] [Google Scholar]
- 38.Gervais DA, Goldberg SN, Brown DB, Soulen MC, Millward SF, Rajan DK, on behalf of the Interventional Oncology Task Force and Standards Division, Society of Interventional Radiology Society of Interventional Radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors. J Vasc Interv Radiol. 2009;20:3–8. doi: 10.1016/j.jvir.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Koffron AJ, Auffenberg G, Kung R, Abecassis M. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg. 2007;246:385–92. doi: 10.1097/SLA.0b013e318146996c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orlando A, Leandro G, Olivo M, Andriulli A, Cottone M. Radiofrequency thermal ablation vs. percutaneous ethanol injection for small hepatocellular carcinoma in cirrhosis: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2009;104:514–24. doi: 10.1038/ajg.2008.80. [DOI] [PubMed] [Google Scholar]
- 41.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151–6. doi: 10.1136/gut.2004.045203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381–91. doi: 10.1097/00000658-200009000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–51. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 44.Davis CR. Interventional radiological treatment of hepatocellular carcinoma. Cancer Control. 2010;17:87–99. doi: 10.1177/107327481001700204. [DOI] [PubMed] [Google Scholar]
- 45.Salmi A, Turrini R, Lanzani G, Savio A, Anglani L. Efficacy of radiofrequency ablation of hepatocellular carcinoma associated with chronic liver disease without cirrhosis. Int J Med Sci. 2008;5:327–32. doi: 10.7150/ijms.5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hasegawa K, Makuuchi M, Takayama T, et al. Surgical resection vs. percutaneous ablation for hepatocellular carcinoma: a preliminary report of the Japanese nationwide survey. J Hepatol. 2008;49:589–94. doi: 10.1016/j.jhep.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 47.Livraghi T, Goldberg SN, Lazzaroni S, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761–8. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]
- 48.Ng KK, Poon RT, Lam CM, Yuen J, Tso WK, Fan ST. Efficacy and safety of radiofrequency ablation for perivascular hepatocellular carcinoma without hepatic inflow occlusion. Br J Surg. 2006;93:440–7. doi: 10.1002/bjs.5267. [DOI] [PubMed] [Google Scholar]
- 49.Lu DS, Raman SS, Limanond P, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14:1267–74. doi: 10.1097/01.RVI.0000092666.72261.6B. [DOI] [PubMed] [Google Scholar]
- 50.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655–61. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]
- 51.Liang P, Wang Y. Microwave ablation of hepatocellular carcinoma. Oncology. 2007;72(suppl 1):124–31. doi: 10.1159/000111718. [DOI] [PubMed] [Google Scholar]
- 52.Ong SL, Gravante G, Metcalfe MS, Strickland AD, Dennison AR, Lloyd DM. Efficacy and safety of microwave ablation for primary and secondary liver malignancies: a systematic review. Eur J Gastroenterol Hepatol. 2009;21:599–605. doi: 10.1097/MEG.0b013e328318ed04. [DOI] [PubMed] [Google Scholar]
- 53.Liu FY, Yu XL, Liang P, Wang Y, Zhou P, Yu J. Comparison of percutaneous 915 MHz microwave ablation and 2450 MHz microwave ablation in large hepatocellular carcinoma. Int J Hyperthermia. 2010;26:448–55. doi: 10.3109/02656731003717574. [DOI] [PubMed] [Google Scholar]
- 54.Lee KF, Hui JW, Cheung YS, et al. Surgical ablation of hepatocellular carcinoma with 2.45-GHz microwave: a critical appraisal of treatment outcomes. Hong Kong Med J. 2012;18:85–91. [PubMed] [Google Scholar]
- 55.Padma S, Martinie JB, Iannitti DA. Liver tumor ablation: percutaneous and open approaches. J Surg Oncol. 2009;100:619–34. doi: 10.1002/jso.21364. [DOI] [PubMed] [Google Scholar]
- 56.Liang P, Wang Y, Yu X, Dong B. Malignant liver tumors: treatment with percutaneous microwave ablation—complications among cohort of 1136 patients. Radiology. 2009;251:933–40. doi: 10.1148/radiol.2513081740. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y, Zheng Y, Li S, Li B, Zhang Y, Yuan Y. Percutaneous microwave ablation of larger hepatocellular carcinoma. Clin Radiol. 2013;68:21–6. doi: 10.1016/j.crad.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 58.Takami Y, Ryu T, Wada Y, Saitsu H. Evaluation of intraoperative microwave coagulo-necrotic therapy (mcn) for hepatocellular carcinoma: a single center experience of 719 consecutive cases. J Hepatobiliary Pancreat Sci. 2013;20:332–41. doi: 10.1007/s00534-012-0527-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Permpongkosol S, Nicol TL, Link RE, et al. Differences in ablation size in porcine kidney, liver, and lung after cryoablation using the same ablation protocol. AJR Am J Roentgenol. 2007;188:1028–32. doi: 10.2214/AJR.06.0810. [DOI] [PubMed] [Google Scholar]
- 60.Allaf ME, Varkarakis IM, Bhayani SB, Inagaki T, Kavoussi LR, Solomon SB. Pain control requirements for percutaneous ablation of renal tumors: cryoablation versus radiofrequency ablation—initial observations. Radiology. 2005;237:366–70. doi: 10.1148/radiol.2371040829. [DOI] [PubMed] [Google Scholar]
- 61.Permpongkosol S, Nicol TL, Khurana H, et al. Thermal maps around two adjacent cryoprobes creating overlapping ablations in porcine liver, lung, and kidney. J Vasc Interv Radiol. 2007;18:283–7. doi: 10.1016/j.jvir.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 62.Bilchik AJ, Wood TF, Allegra D, et al. Cryosurgical ablation and radiofrequency ablation for unresectable hepatic malignant neoplasms: a proposed algorithm. Arch Surg. 2000;135:657–62. doi: 10.1001/archsurg.135.6.657. [DOI] [PubMed] [Google Scholar]
- 63.Sung GT, Gill IS, Hsu TH, et al. Effect of intentional cryo-injury to the renal collecting system. J Urol. 2003;170:619–22. doi: 10.1097/01.ju.0000068722.22186.66. [DOI] [PubMed] [Google Scholar]
- 64.Seifert JK, Morris DL. World survey on the complications of hepatic and prostate cryotherapy. World J Surg. 1999;23:109–13. doi: 10.1007/PL00013173. [DOI] [PubMed] [Google Scholar]
- 65.Chang XJ, Lu YY, Bai WL, et al. Clinical efficacy and prognostic factors for cryoablation patients with advanced hepatocellular carcinoma [Chinese] Zhonghua Gan Zang Bing Za Zhi. 2011;19:759–63. doi: 10.3760/cma.j.issn.1007-3418.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 66.Chen HW, Lai EC, Zhen ZJ, Cui WZ, Liao S, Lau WY. Ultrasound-guided percutaneous cryotherapy of hepatocellular carcinoma. Int J Surg. 2011;9:188–91. doi: 10.1016/j.ijsu.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 67.Huang YZ, Zhou SC, Zhou H, Tong M. Radiofrequency ablation versus cryosurgery ablation for hepatocellular carcinoma: a meta-analysis. Hepatogastroenterology. 2013;60:1131–5. doi: 10.5754/hge121142. [DOI] [PubMed] [Google Scholar]
- 68.Narayanan G. Irreversible electroporation for treatment of liver cancer. Gastroenterol Hepatol (N Y) 2011;7:313–16. [PMC free article] [PubMed] [Google Scholar]
- 69.Maor E, Ivorra A, Leor J, Rubinsky B. The effect of irreversible electroporation on blood vessels. Technol Cancer Res Treat. 2007;6:307–12. doi: 10.1177/153303460700600407. [DOI] [PubMed] [Google Scholar]
- 70.Charpentier KP, Wolf F, Noble L, Winn B, Resnick M, Dupuy DE. Irreversible electroporation of the liver and liver hilum in swine. HPB (Oxford) 2011;13:168–73. doi: 10.1111/j.1477-2574.2010.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cannon R, Ellis S, Hayes D, Narayanan G, Martin RC., 2nd Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J Surg Oncol. 2013;107:544–9. doi: 10.1002/jso.23280. [DOI] [PubMed] [Google Scholar]
- 72.Ishii H, Okada S, Nose H, et al. Local recurrence of hepatocellular carcinoma after percutaneous ethanol injection. Cancer. 1996;77:1792–6. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1792::AID-CNCR6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 73.Azoulay D, Castaing D, Krissat J, et al. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232:665–72. doi: 10.1097/00000658-200011000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farges O, Belghiti J, Kianmanesh R, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–17. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675–80. doi: 10.1001/archsurg.137.6.675. [DOI] [PubMed] [Google Scholar]
- 76.Tashiro S, Miyake H, Yoshioka K. The role of portal vein embolization prior to hepatic resection for primary and secondary hepatic malignancies. Eur J Clin Med Oncol. 2011;3:1–7. [Google Scholar]
- 77.Siriwardana RC, Lo CM, Chan SC, Fan ST. Role of portal vein embolization in hepatocellular carcinoma management and its effect on recurrence: a case–control study. World J Surg. 2012;36:1640–6. doi: 10.1007/s00268-012-1522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simoneau E, Aljiffry M, Salman A, et al. Portal vein embolization stimulates tumour growth in patients with colorectal cancer liver metastases. HPB (Oxford) 2012;14:461–8. doi: 10.1111/j.1477-2574.2012.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Forner A, Trinchet JC. Transarterial therapies in hcc: does embolization increase survival? J Hepatol. 2009;51:981–3. doi: 10.1016/j.jhep.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 80.Nicolini A, Fasani P, Manini MA, et al. Transarterial embolization with microspheres in the treatment of monofocal hcc. Dig Liver Dis. 2009;41:143–9. doi: 10.1016/j.dld.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 81.Chou FI, Fang KC, Chung C, et al. Lipiodol uptake and retention by human hepatoma cells. Nucl Med Biol. 1995;22:379–86. doi: 10.1016/0969-8051(94)00112-W. [DOI] [PubMed] [Google Scholar]
- 82.Konno T. Targeting cancer chemotherapeutic agents by use of Lipiodol contrast medium. Cancer. 1990;66:1897–903. doi: 10.1002/1097-0142(19901101)66:9<1897::AID-CNCR2820660907>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 83.Gerunda GE, Neri D, Merenda R, et al. Role of transarterial chemoembolization before liver resection for hepatocarcinoma. Liver Transpl. 2000;6:619–26. doi: 10.1053/jlts.2000.8312. [DOI] [PubMed] [Google Scholar]
- 84.de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of hcc. J Hepatol. 2012;56(suppl 1):S75–87. doi: 10.1016/S0168-8278(12)60009-9. [DOI] [PubMed] [Google Scholar]
- 85.Nakamura H, Hashimoto T, Oi H, Sawada S. Transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1989;170:783–6. doi: 10.1148/radiology.170.3.2536946. [DOI] [PubMed] [Google Scholar]
- 86.Egawa H, Maki A, Mori K, et al. Effects of intra-arterial chemotherapy with a new lipophilic anticancer agent, estradiolchlorambucil (KM2210), dissolved in lipiodol on experimental liver tumor in rats. J Surg Oncol. 1990;44:109–14. doi: 10.1002/jso.2930440210. [DOI] [PubMed] [Google Scholar]
- 87.Daniels JR, Sternlicht M, Daniels AM. Collagen chemoembolization: pharmacokinetics and tissue tolerance of cisdiamminedichloroplatinum (ii) in porcine liver and rabbit kidney. Cancer Res. 1988;48:2446–50. [PubMed] [Google Scholar]
- 88.Bronowicki JP, Vetter D, Dumas F, et al. Transcatheter oily chemoembolization for hepatocellular carcinoma. A 4-year study of 127 French patients. Cancer. 1994;74:16–24. doi: 10.1002/1097-0142(19940701)74:1<16::AID-CNCR2820740105>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 89.Barone M, Ettorre GC, Ladisa R, et al. Transcatheter arterial chemoembolization (tace) in treatment of hepatocellular carcinoma. Hepatogastroenterology. 2003;50:183–7. [PubMed] [Google Scholar]
- 90.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–9. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 91.Malagari K, Alexopoulou E, Chatzimichail K, et al. Trans-catheter chemoembolization in the treatment of hcc in patients not eligible for curative treatments: midterm results of doxorubicin-loaded dc bead. Abdom Imaging. 2008;33:512–19. doi: 10.1007/s00261-007-9334-x. [DOI] [PubMed] [Google Scholar]
- 92.Takayasu K, Arii S, Ikai I, et al. on behalf of the Liver Cancer Study Group of Japan Overall survival after transarterial lipiodol infusion chemotherapy with or without embolization for unresectable hepatocellular carcinoma: propensity score analysis. AJR Am J Roentgenol. 2010;194:830–7. doi: 10.2214/AJR.09.3308. [DOI] [PubMed] [Google Scholar]
- 93.Sumie S, Yamashita F, Ando E, et al. Interventional radiology for advanced hepatocellular carcinoma: comparison of hepatic artery infusion chemotherapy and transcatheter arterial lipiodol chemoembolization. AJR Am J Roentgenol. 2003;181:1327–34. doi: 10.2214/ajr.181.5.1811327. [DOI] [PubMed] [Google Scholar]
- 94.Lee HS, Kim KM, Yoon JH, et al. Therapeutic efficacy of transcatheter arterial chemoembolization as compared with hepatic resection in hepatocellular carcinoma patients with compensated liver function in a hepatitis B virus–endemic area: a prospective cohort study. J Clin Oncol. 2002;20:4459–65. doi: 10.1200/JCO.2002.02.013. [DOI] [PubMed] [Google Scholar]
- 95.Brown DB, Cardella JF, Sacks D, et al. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol. 2009;20(suppl):S219–S26. doi: 10.1016/j.jvir.2009.04.033. S26.e1–10. [DOI] [PubMed] [Google Scholar]
- 96.Jelic S, Sotiropoulos GC, on behalf of the esmo Guidelines Working Group Hepatocellular carcinoma: esmo Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v59–64. doi: 10.1093/annonc/mdq166. [DOI] [PubMed] [Google Scholar]
- 97.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–71. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 98.Meza–Junco J, Montano–Loza AJ, Liu DM, et al. Locoregional radiological treatment for hepatocellular carcinoma; which, when and how? Cancer Treat Rev. 2012;38:54–62. doi: 10.1016/j.ctrv.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 99.Lewis AL, Gonzalez MV, Lloyd AW, et al. dc bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol. 2006;17:335–42. doi: 10.1097/01.RVI.0000195323.46152.B3. [DOI] [PubMed] [Google Scholar]
- 100.Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–81. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 101.Kettenbach J, Stadler A, Katzler IV, et al. Drug-loaded microspheres for the treatment of liver cancer: review of current results. Cardiovasc Intervent Radiol. 2008;31:468–76. doi: 10.1007/s00270-007-9280-6. [DOI] [PubMed] [Google Scholar]
- 102.Boatta E, Corona M, Cannavale A, Fanelli F, Cirelli C, de Medici L. Endovascular treatment of hepatocellular carcinoma with drug eluting microparticles (dc Beads): ct evaluation of response to the treatment. Indian J Radiol Imaging. 2013;23:126–33. doi: 10.4103/0971-3026.116564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lencioni R, Llovet JM. Modified recist (mrecist) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 104.Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the precision v study. Cardiovasc Intervent Radiol. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carr BI. Hepatic arterial 90yttrium glass microspheres (Therasphere) for unresectable hepatocellular carcinoma: interim safety and survival data on 65 patients. Liver Transpl. 2004;10(suppl 1):S107–10. doi: 10.1002/lt.20036. [DOI] [PubMed] [Google Scholar]
- 106.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 107.Raoul JL, Guyader D, Bretagne JF, et al. Randomized controlled trial for hepatocellular carcinoma with portal vein thrombosis: intra-arterial iodine-131–iodized oil versus medical support. J Nucl Med. 1994;35:1782–7. [PubMed] [Google Scholar]
- 108.Chaudhury PK, Hassanain M, Bouteaud JM, et al. Complete response of hepatocellular carcinoma with sorafenib and Y radioembolization. Curr Oncol. 2010;17:67–9. doi: 10.3747/co.v17i5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497–507. doi: 10.1053/j.gastro.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Raoul JL, Guyader D, Bretagne JF, et al. Prospective randomized trial of chemoembolization versus intra-arterial injection of 131I-labeled–iodized oil in the treatment of hepatocellular carcinoma. Hepatology. 1997;26:1156–61. doi: 10.1002/hep.510260511. [DOI] [PubMed] [Google Scholar]
- 111.Inarrairaegui M, Thurston KG, Bilbao JI, et al. Radioembolization with use of yttrium-90 resin microspheres in patients with hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol. 2010;21:1205–12. doi: 10.1016/j.jvir.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 112.Gaba RC, Riaz A, Lewandowski RJ, et al. Safety of yttrium-90 microsphere radioembolization in patients with biliary obstruction. J Vasc Interv Radiol. 2010;21:1213–18. doi: 10.1016/j.jvir.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 113.Bilbao JI, de Martino A, de Luis E, et al. Biocompatibility, inflammatory response, and recannalization characteristics of nonradioactive resin microspheres: histological findings. Cardiovasc Intervent Radiol. 2009;32:727–36. doi: 10.1007/s00270-009-9592-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hilgard P, Hamami M, Fouly AE, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52:1741–9. doi: 10.1002/hep.23944. [DOI] [PubMed] [Google Scholar]
- 115.Sangro B, Carpanese L, Cianni R, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868–78. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 116.Chan AO, Yuen MF, Hui CK, Tso WK, Lai CL. A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer. 2002;94:1747–52. doi: 10.1002/cncr.10407. [DOI] [PubMed] [Google Scholar]
- 117.Carretero C, Munoz–Navas M, Betes M, et al. Gastroduodenal injury after radioembolization of hepatic tumors. Am J Gastroenterol. 2007;102:1216–20. doi: 10.1111/j.1572-0241.2007.01172.x. [DOI] [PubMed] [Google Scholar]
- 118.Naymagon S, Warner RR, Patel K, et al. Gastroduodenal ulceration associated with radioembolization for the treatment of hepatic tumors: an institutional experience and review of the literature. Dig Dis Sci. 2010;55:2450–8. doi: 10.1007/s10620-010-1156-y. [DOI] [PubMed] [Google Scholar]
- 119.Leung TW, Lau WY, Ho SK, et al. Radiation pneumonitis after selective internal radiation treatment with intraarterial 90yttrium-microspheres for inoperable hepatic tumors. Int J Radiat Oncol Biol Phys. 1995;33:919–24. doi: 10.1016/0360-3016(95)00039-3. [DOI] [PubMed] [Google Scholar]