Abstract
Background
Dexamethasone is the corticosteroid most commonly used for the management of vasogenic edema and increased intracranial pressure in patients with brain tumours. It is also used after surgery (before embarking on radiotherapy), particularly in patients whose tumours exert significant mass effect. Few prospective clinical trials have set out to determine the optimal dose and schedule for dexamethasone in patients with primary brain tumours, and subsequently, fewer clinical practice guideline recommendations have been formulated.
Methods
A review of the scientific literature published to November 2012 considered all publications that addressed dexamethasone use in adult patients with brain tumours. Evidence was selected and reviewed by a working group comprising 3 clinicians and 1 methodologist. The resulting draft guideline underwent internal review by members of the Alberta Provincial cns Tumour Team, and feedback was incorporated into the final version of the guideline.
Recommendations
Based on the evidence available to date, the Alberta Provincial cns Tumour Team makes these recommendations:
Treatment with dexamethasone is recommended for symptom relief in adult patients with primary high-grade glioma and cerebral edema.
After surgery, a maximum dose of 16 mg daily, administered in 4 equal doses, is recommended for symptomatic patients. This protocol should ideally be started by the neurosurgeon.
A rapid dexamethasone tapering schedule should be considered where appropriate.
Patients who have high-grade tumours, are symptomatic, or have poor life expectancy, can be maintained on a 0.5–1.0 mg dose of dexamethasone daily.
Side effects with dexamethasone are common, and they increase in frequency and severity with increased dose and duration of therapy. Patients should be carefully monitored for endocrine, muscular, skeletal, gastrointestinal, psychiatric, and hematologic complications, and for infections and other general side effects.
Keywords: High-grade glioma, dexamethasone, corticosteroids, clinical practice guidelines
1. INTRODUCTION
Patients with primary brain tumours often develop vasogenic edema and increased intracranial pressure. In three quarters of such patients, corticosteroid therapy improves those symptoms, generally within 48 hours1. As such, corticosteroid therapy is a necessary prerequisite to embarking on radiotherapy after surgery, particularly in patients whose brain tumours exert significant mass effect. Similarly, management of edema and intracranial pressure with corticosteroids forms an integral aspect of treatment in the post-radiotherapy phases of care. Dexamethasone is the usual corticosteroid of choice because of its minimal mineralocorticoid activity, long half-life, and high potency. However, despite the common use of dexamethasone, few prospective clinical trials have set out to determine its optimal dose and schedule in patients with primary brain tumours, and subsequently, fewer clinical practice guideline recommendations have been formulated.
Dexamethasone and all steroids are associated with a variety of side effects, and therefore the risks and benefits must be carefully weighed for each patient. The goals of the present work were to review the evidence for the use of dexamethasone in patients with high-grade glioma, to describe the management of side effects associated with dexamethasone use in that patient population, and to document the recommendations of the Alberta Provincial cns Tumour Team for the use of dexamethasone in patients with high-grade glioma.
2. METHODS
2.1. Questions
When should dexamethasone be considered in adult patients with high-grade glioma?
What are the optimal dexamethasone dose ranges?
What is the optimal schedule for dexamethasone tapering?
What are the most common adverse events associated with dexamethasone therapy, and how are they best managed?
2.2. Target Population
The target population for this guideline is adult patients with primary high-grade glioma.
2.3. Search Strategy
Medical journal articles were searched using the medline (1948 to November, week 3, 2012), PubMed (1950 to November 2012), Cochrane Database of Systematic Reviews (2005 to November 2012), and cinahl (1982 to November 2012) electronic databases. Search terms included “dexamethasone,” “glucocorticoids,” “corticosteroids,” “Decadron,” or “adrenal cortex hormones” and “brain tumour,” “glioma,” “high-grade glioma,” or “brain neoplasm.” The reference lists of relevant articles were hand-searched for additional articles. In addition to the U.S. National Guideline Clearinghouse database, the Web sites of national and international guideline developers were also searched for relevant content. A search of the grey literature was also conducted using Google and Google Scholar. The patient population was limited to adolescents and adults; no limitations by publication type or study design were imposed.
The literature search resulted in eight publications that were used to formulate the final recommendations addressing the first three guideline questions. To address the fourth guideline question, a systematic review of the literature was carried out incorporating the mesh terms already listed, in combination with these key words: “hyperglycemia,” “myopathy,” “osteoporosis,” “avascular necrosis,” “peptic ulceration,” “bowel “perforation,” “anxiety,” “irritability,” “insomnia,” “mania,” “psychosis,” “depression,” “seizures,” “infections,” “Pneumocystis jiroveci pneumonia,” “candidiasis,” “venous thromboembolism,” “hypertension,” “cardiovascular complications,” “weight gain,” “Cushingoid,” “hirsutism,” “fragile skin,” and “skin complications.”
2.4. Development of Recommendations
Evidence was selected and reviewed by a working group consisting of 3 members of the provincial cns Tumour Team and 1 methodologist. The working group developed the draft recommendations and accompanying guideline based on the evidence combined with expert opinion. The draft document was initially reviewed and discussed at a consensus conference attended by 25 provincial Tumour Team members and was subsequently distributed by anonymous electronic survey to members of the cns Tumour Team (n = 30) for additional review and comment. The survey response rate was 33%. All respondents agreed that the document should be approved as a clinical practice guideline, with modifications. The final guideline was reviewed and endorsed in February 2013 by the Alberta Provincial cns Tumour Team.
3. RESULTS
Table i summarizes the results from key publications as they pertain to the first three questions. The quality of the evidence was classified according to criteria used in other Canadian guidelines (Table ii), and the strength of the resulting recommendations from the Alberta Provincial cns Tumour Team were graded based on the evidence classifications10.
TABLE I.
Summary of published literature of dexamethasone use in adult patients with brain tumours

| Study | Level of evidence | Patient characteristics | Dose range | Duration and taper schedule |
|---|---|---|---|---|
| Weissman et al., 19912 | 3 | No prior history of brain metastases, brain tumour, or cranial radiotherapy, no intracranial surgery within 3 weeks of study entry, not using oral or intravenous glucocorticoids | Patients (n = 14) received 8 mg twice daily for 4 days, tapered to 2 mg twice daily until completion of radiotherapy. Patients with progressive neurologic symptoms during radiotherapy (n = 6) had their dose increased to 4 mg 4 times daily for 3 days, then tapered to the minimum dose required to maintain neurologic function. | No patients were documented to have difficulty with the tapering schedule. |
| Vecht et al., 19943 | 2 | ct-proven brain metastasis and brain tumour edema | Series 1: 8 mg daily vs. 16 mg daily Series 2: 4 mg daily vs. 16 mg daily |
Authors recommend that, after radiotherapy or other antitumour therapy, tapering of dexamethasone from 4 mg daily should be temporized over 4 weeks. |
| Wolfson et al., 19944 | 3 | Measurable metastasis on ct imaging, histologically confirmed primary malignancy, granulocytes> 1000/mm 3, serum glucose < 300 mg | All patients given high-dose dexamethasone (24 mg intravenously every 6 hours for 48 hours), then randomized to either 4 mg every 6 hours for 2 weeks → radiotherapy → tapering regimen (group 1), or no further dexamethasone (group 2) | |
| Lagerwaard et al., 19995 | 3 | nr | Steroid of choice, usually dexamethasone Dose range: 4–16 mg daily (mean: 14.6 mg daily) | Tapered slowly and discontinued in the weeks after treatment. |
| Hempen et al., 20026 | 3 | 47 Patients withprimary brain tumours; 91 patients with metastatic disease | Dose of 2–4 mg 3 times daily most common | Mean treatment duration: 6.9 weeks (range: 1–50.5 weeks) for patients with metastases; 23.3 weeks (range: 7.5–52 weeks) for patients with primary tumours and no stop in dexamethasone; 17.3 weeks for patients with primary tumours who stopped dexamethasone after surgery and restarted at beginning of radiotherapy, No information on tapering schedule. |
| Millar et al., 20047 | 2 | nr | Steroid use reported in 18 studies. Of studies with specific details on dexamethasone dosing (n = 4), 2 used 16 mg daily; 1 used 2 mg 4 times daily; and 1 used 10 mg/m2 daily. | 5 Studies reported tapering the dose as tolerated after wbrt. 1 Study reported a tapering period of 2 weeks. |
| Sturdza et al., 20088 | 3 | nr | Of responding oncologists, 45% reported prescribing a dose of 4 mg 4 times daily (16 mg total) for all patients with brain metastases. Of responding oncologists, 60% reported adjusting the dose during radiotherapy. | Survey results: <2 weeks (n = 5) 2–4 weeks (n = 20) 4–6 weeks (n = 5) >6 weeks (n = 4) Chart review: 66% of patients tapered after completion of wbrt. Median treatment duration 45 days (range: 12–206 days). |
| Ryken et al., 20109 | 2 | Symptoms related to increased intracranial pressure and edema secondary to brain metastasis | Starting dose 4–8 mg daily Consider doses such as 16 mg daily or higher in the presence of severe symptoms consistent with increased intracranial pressure. |
Tapered slowly over 2 weeks or longer in symptomatic patients to avoid rebound symptoms |
ct = computed tomography; nr = not reported; wbrt = whole-brain radiotherapy.
TABLE II.
Levels of evidence and consensus10
| Level or grade | Description |
|---|---|
| Level of evidence | |
| 1 | Randomized controlled trials or meta-analyses without important limitations |
| 2 | Randomized controlled trials or meta-analyses with important limitations; observational studies (not including cohort studies) with overwhelming evidence |
| 3 | Prospective cohort studies, case–control studies, case series |
| 4 | Inadequate or no data in the population of interest; anecdotal evidence or clinical experience |
| Grade of recommendation | |
| A | Strong recommendation; applies to most individuals in most circumstances Benefits clearly outweigh risks, or vice versa Level of evidence 1, 2 or 3 |
| B | Intermediate recommendation; application may vary depending on patient characteristics or other circumstances Unclear whether benefits outweigh risks Level of evidence 1, 2, or 3 |
| C | Consensus recommendation Unclear whether benefits outweigh risks Level of evidence 3 or 4 |
3.1. Question 1: Patient Selection
Treatment with dexamethasone is recommended for symptom relief in adult patients with primary high-grade glioma and cerebral edema (recommendation grade: B). In accordance with the American College of Radiology Appropriateness Criteria, there is no evidence to support the routine use of dexamethasone in patients with brain tumours in the absence of neurologic signs or symptoms11.
3.2. Question 2: Dosing
Dexamethasone dosage requirements vary greatly from patient to patient and depend on the diseases being treated. The literature addressing the use of dexamethasone for adult patients with brain tumours is limited and conflicting, and primarily describes patients with brain metastases. In a review describing the prescribing practices of oncologists and palliative care specialists for their patients with brain metastases, Sturdza and colleagues8 reported that 45% of surveyed physicians routinely prescribe dexamethasone at a fixed dose of 16 mg daily for all patients with brain metastases. The presence or absence of symptoms, the types of symptoms, the types of neurologic deficits, and the degree of edema on imaging were all factors cited by the physicians who chose to prescribe a dose other than 16 mg daily8. In an accompanying chart review of 88 patients with brain metastases, the authors reported that, of patients treated with dexamethasone, 91% of those receiving 16 mg or more daily and 65% of those receiving less than 16 mg daily experienced at least one side effect (p=0.006)8. In a systematic review of twenty-one randomized controlled trials of whole-brain radiotherapy for patients with multiple brain metastases, Millar and colleagues7 reported that, of the eighteen studies documenting steroid use, only five provided details on the type and dose of steroid. Dexamethasone was the most commonly used steroid, and doses in four studies ranged from 8 mg to 16 mg daily; one study reported the use of prednisone at 40 mg daily7. In a study reporting the results of two consecutive randomized trials involving 96 patients with brain metastases, Vecht et al.3 compared 4-, 8-, and 16-mg daily oral doses of dexamethasone. More toxic effects were reported in the patients treated with 16 mg daily, with no significant improvement in Karnofsky performance status over that seen at lower doses. The authors concluded that, for most patients, lower doses of 4 mg or 8 mg daily have an equivalent effect on neurologic performance3.
Retrospective studies and reviews report that the most common starting dose of dexamethasone ranges between 8 mg and 16 mg daily5,6,8,12. In a recent review of the role of steroids in the management of brain metastases, Ryken and colleagues concluded that symptomatic patients should be treated with a starting dose of 4–8 mg daily; if the patient exhibits severe symptoms associated with increased intracranial pressure, a dose of 16 mg daily or higher could be considered9. Two recent comprehensive reviews recommend an initial intravenous loading dose of 10–20 mg dexamethasone when a patient presents with acute neurologic symptoms caused by a brain tumour or spinal cord lesion; the loading dose should be followed by maintenance dosing with oral or intravenous dexamethasone in divided doses totalling to 4–24 mg daily13,14.
There is variability in the published literature about how to divide the dexamethasone dose for patients with brain tumours. In their recent survey of 34 Canadian oncologists, Sturdza and colleagues8 reported that, among respondents, the most common starting dose of dexamethasone for patients with brain metastases was 4 mg four times daily; this dose schedule has also been reported in small prospective trials involving patients with brain metastases2,4.
The published guidance on the use of dexamethasone in patients with high-grade glioma is also limited. Table iii summarizes the dosing recommendations from published clinical practice guidelines addressing the use of dexamethasone in patients with primary brain tumours. These recommendations are all based on lower levels of evidence such as case series and retrospective reviews, and they are variable in their advice about starting doses and tapering regimens. However, based on the literature and guidelines published to date, combined with clinical expert opinion, the Alberta Provincial cns Tumour Team members recommend a maximum starting dose of 16 mg daily, administered in 4 equal daily doses, for symptomatic patients after biopsy or surgical resection (recommendation grade: C).
TABLE III.
Recommendations for dexamethasone dosing from published clinical practice guidelines.
| Guideline developer | Recommendations |
|---|---|
| American College of Radiology, 199910 | For patients with minimal neurologic symptoms, the committee recommends starting either with 4–8 mg dexamethasone daily or with 16 mg dexamethasone daily, but tapering after a few days. In all cases, steroids should be tapered as clinically indicated and tolerated. |
| BC Cancer Agency, 200415 | Dexamethasone is most commonly used in a dose range of 2–16 mg daily (divided dosing of 2 or more times daily) depending on symptom severity. In emergent situations, higher doses of dexamethasone may be used, and mannitol may also be used. During radiation therapy, a tapering dose of dexamethasone, as clinically tolerated (to alleviate symptoms of brain edema), is prescribed, and the lowest effective dose is used. After completion of radiation therapy, the dexamethasone is usually tapered and discontinued over 2–4 weeks. |
| Australian Cancer Network, 200916 | The usual starting dose is 16 mg daily. Dexamethasone should gradually be tapered to the lowest dose that controls the patient’s symptoms; dexamethasone should not be discontinued abruptly. |
| U.S. National Comprehensive Cancer Network, 201317 | In general, the lowest dose of steroids should be used for the shortest time possible. Downward titration of the dose should be attempted whenever possible. |
3.3. Question 3: Dexamethasone Tapering
Most patients begin to improve symptomatically within hours of dexamethasone administration, achieving maximum benefit within 24–72 hours13. A high rate of side effects is associated with prolonged dexamethasone use, as is a risk of suppression of the hypothalamic–pituitary–adrenocortical axis. Dexamethasone should therefore be tapered once symptoms begin to improve13,14,18.
Published evidence and recommendations about the optimal tapering schedule are varied. In general, the tapering schedules most commonly reported involve a gradual decrease in the dose or dosing interval over a period of 2–4 weeks to prevent rebound symptoms, with longer periods for symptomatic patients3,7,8,9,15. Some publications favour tapering until a physiologic dose equivalent to 20 mg of cortisol daily is achieved, which might be 0.25 mg dexamethasone daily18,19. Other publications state that the tapering schedule is determined by the patient’s symptoms11,16,17.
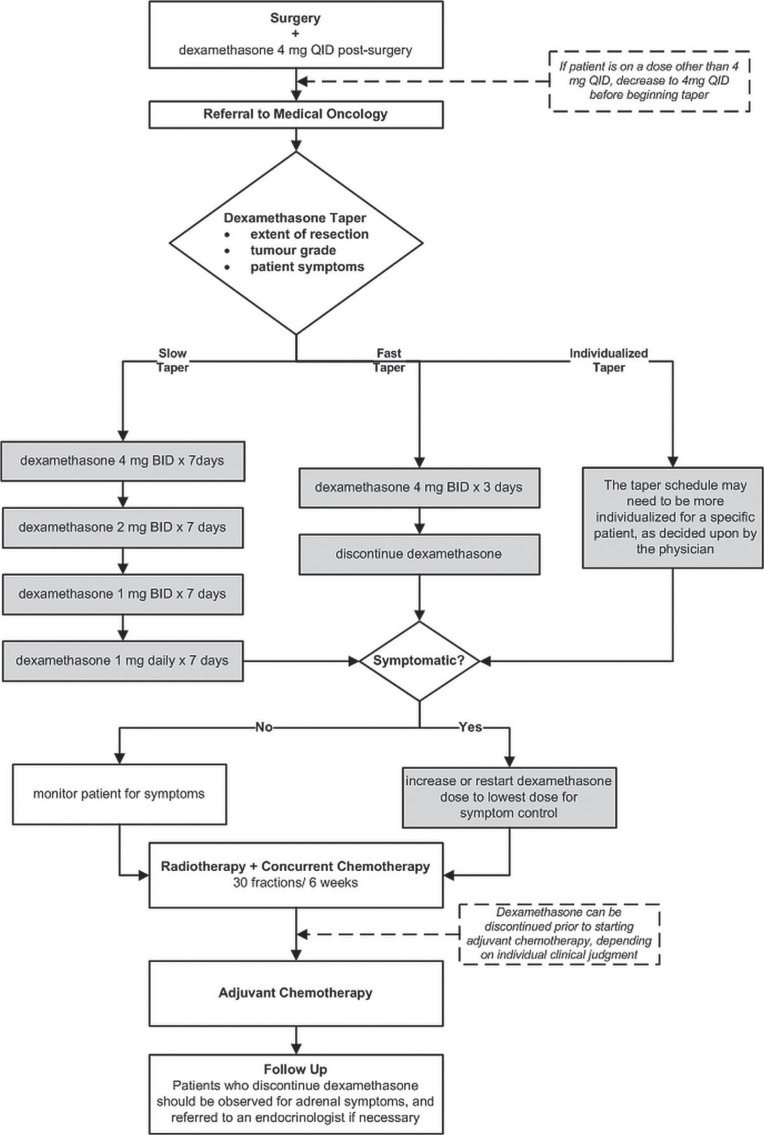
Based on a combination of published guidance and expert opinion, the Alberta Provincial cns Tumour Team members recommend that patients should initially be dosed according to surgeon preference, because those practitioners are typically the first on the treating neuro-oncology team to see the patient. The typical maximum dose should be dexamethasone 4 mg four times daily after resection. A slow, fast, or individualized taper can be initiated on the ward and continued by the medical or radiation oncologist (or both). The slow taper starts with 4 mg twice daily for 7 days, then 2 mg twice daily for 7 days, then 1 mg twice daily for 7 days, then 1 mg once daily for 7 days. The fast taper allows for the dexamethasone to be tapered faster and discontinued 3 days after resection. Patients should be observed during the taper for symptoms of increased intracranial pressure, and if such symptoms occur, the dexamethasone should be increased back to the previous dose in the tapering regimen (recommendation grade: C).
After completion of the tapering regimen, symptomatic patients can be either restarted or continued on dexamethasone 0.5 mg daily throughout radio-therapy. Patients who have high-grade tumours, are symptomatic, or have poor life expectancy can also be maintained on dexamethasone 0.5–1.0 mg daily after radiotherapy.
All patients should be observed for symptoms of adrenal insufficiency if dexamethasone is discontinued abruptly or used for a prolonged time, and advice from an endocrinologist should be sought if needed. Similarly, because dexamethasone is metabolized by the p450 3A4 pathway common to many other drugs, there is potential for interaction with other medications, including rapid catabolism of dexamethasone.
3.4. Question 4: Management of Adverse Events Associated with Dexamethasone Therapy
Side effects of steroid therapy are common. The duration of steroid therapy increases the frequency of side effects, and prolonged treatment (>3 weeks) is associated with greater toxicity20. Hypoalbuminemia also increases the risk of steroid toxicity because the percentage of unbound steroid increases; such toxicity has been observed in patients with albumin levels less than 25 g/L21. Patients should be carefully monitored for potential side effects (grade of recommendation: B).
Table iv summarizes prevention and treatment strategies for complications associated with steroid therapy. Clinically, the most frequently observed complications include hyperglycemia, myopathy, gastrointestinal complications, irritability, anxiety, and insomnia.
TABLE IV.
Prevention and treatment of common complications of dexamethasone therapy
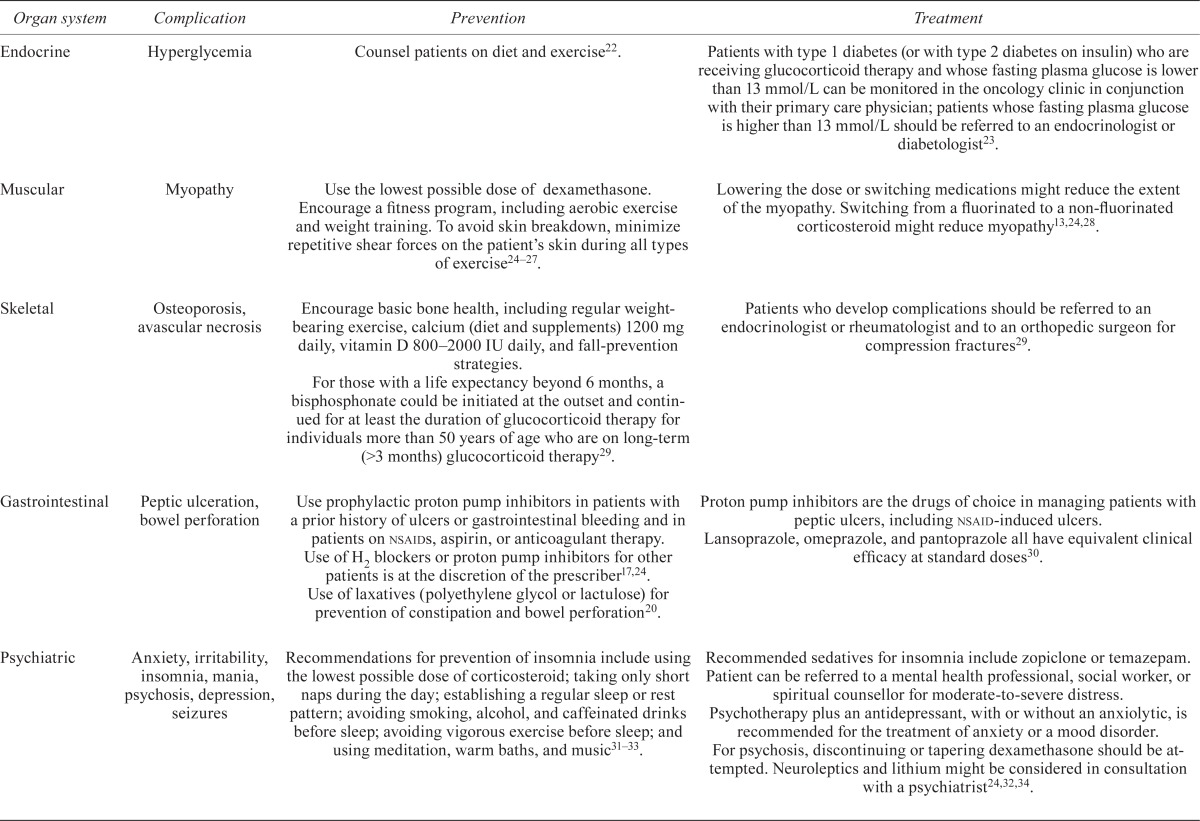
| Organ system | Complication | Prevention | Treatment |
|---|---|---|---|
| Endocrine | Hyperglycemia | Counsel patients on diet and exercise22. | Patients with type 1 diabetes (or with type 2 diabetes on insulin) who are receiving glucocorticoid therapy and whose fasting plasma glucose is lower than 13 mmol/L can be monitored in the oncology clinic in conjunction with their primary care physician; patients whose fasting plasma glucose is higher than 13 mmol/L should be referred to an endocrinologist or diabetologist23. |
| Muscular | Myopathy | Use the lowest possible dose of dexamethasone. Encourage a fitness program, including aerobic exercise and weight training. To avoid skin breakdown, minimize repetitive shear forces on the patient’s skin during all types of exercise24–27. | Lowering the dose or switching medications might reduce the extent of the myopathy. Switching from a fluorinated to a non-fluorinated corticosteroid might reduce myopathy13,24,28. |
| Skeletal | Osteoporosis, avascular necrosis | Encourage basic bone health, including regular weight-bearing exercise, calcium (diet and supplements) 1200 mg daily, vitamin D 800–2000 IU daily, and fall-prevention strategies. For those with a life expectancy beyond 6 months, a bisphosphonate could be initiated at the outset and continued for at least the duration of glucocorticoid therapy for individuals more than 50 years of age who are on long-term (>3 months) glucocorticoid therapy29. |
Patients who develop complications should be referred to an endocrinologist or rheumatologist and to an orthopedic surgeon for compression fractures29. |
| Gastrointestinal | Peptic ulceration, bowel perforation | Use prophylactic proton pump inhibitors in patients with a prior history of ulcers or gastrointestinal bleeding and in patients on nsaids, aspirin, or anticoagulant therapy. Use of H2 blockers or proton pump inhibitors for other patients is at the discretion of the prescriber17,24. Use of laxatives (polyethylene glycol or lactulose) for prevention of constipation and bowel perforation20. |
Proton pump inhibitors are the drugs of choice in managing patients with peptic ulcers, including nsaid-induced ulcers. Lansoprazole, omeprazole, and pantoprazole all have equivalent clinical efficacy at standard doses 30. |
| Psychiatric | Anxiety, irritability, insomnia, mania, psychosis, depression, seizures | Recommendations for prevention of insomnia include using the lowest possible dose of corticosteroid; taking only short naps during the day; establishing a regular sleep or rest pattern; avoiding smoking, alcohol, and caffeinated drinks before sleep; avoiding vigorous exercise before sleep; and using meditation, warm baths, and music31–33. | Recommended sedatives for insomnia include zopiclone or temazepam. Patient can be referred to a mental health professional, social worker, or spiritual counsellor for moderate-to-severe distress. Psychotherapy plus an antidepressant, with or without an anxiolytic, is recommended for the treatment of anxiety or a mood disorder. For psychosis, discontinuing or tapering dexamethasone should be attempted. Neuroleptics and lithium might be considered in consultation with a psychiatrist24,32,34. |
| Infections | Pneumocystis jirovecii pneumonia (pjp), candidiasis | Consider prophylactic therapy with trimethoprim–sulfamethoxazole against pjp in brain cancer patients receiving prolonged corticosteroid therapy24,35. | Treatment of pjp infections with trimethoprim–sulfamethoxazole, trimethoprim plus dapsone, atovaquone suspension, clindamycin plus primaquine, or pentamidine. Treat oropharyngeal candidiasis with nystatin, fluconazole, or itraconazole 24,35. |
| Hematologic | Venous thromboembolism (vte) | Routine prophylaxis with an antithrombotic agent is not recommended. Prophylaxis with low molecular weight heparin (dalteparin, enoxaparin, tinzaparin), fondaparinux, unfractionated heparin, or warfarin might be warranted in individuals considered to be at risk of vte based on assessment of risk factors36,37. | Low molecular weight heparin is the preferred approach for cancer patients with established vte and for long-term anticoagulant therapy36,38. |
| Cardiovascular | Hypertension, cardiovascular risk | There is no clear evidence with respect to the prevention and treatment of corticosteroid-induced hypertension. Screening is recommended, particularly during the first months of therapy and in patients with corticosteroid-induced lipodystrophy26. |
nsaid = nonsteroidal anti-inflammatory drug.
Hyperglycemia has been reported in up to 72% of patients with primary brain tumours receiving dexamethasone6 and might be associated with shorter survival in those patients39,40. The hyperglycemia from corticosteroid therapy usually occurs in the first 6 weeks of therapy and is believed to be secondary to insulin resistance and increased hepatic gluconeogenesis24.
Glucocorticoids also have a catabolic effect on skeletal muscle; they lead to muscle atrophy by decreasing protein synthesis and increasing the rate of protein catabolism25. Symptomatic steroid myopathy occurs in approximately 10% of patients, and the incidence is increased in elderly patients and after prolonged use of high-dose corticosteroids24. Steroid myopathy involves proximal muscle weakness and eventual wasting, especially of the pelvic girdle. The proximal arm muscles and neck may become involved as the myopathy progresses20. The respiratory muscles may also become affected, resulting in symptomatic dyspnea in patients who are severely myopathic20. Steroid myopathy can develop in as little as 2–3 weeks in patients taking 16 mg of dexamethasone daily, and the development of myopathy correlates best with the total steroid dose20. Myopathy is reversible upon discontinuation of the steroid, usually in a few days to several months28.
There is debate about whether steroids cause a higher risk of upper gastrointestinal complications. Two large meta-analyses have been conducted, and they reported conflicting results. A review by Messer et al.41 included 3064 corticosteroid-treated patients evaluated for peptic ulcers. The relative risk of developing peptic ulcers was 2.3 [95% confidence interval (ci): 1.4 to 3.7], and the relative risk of gastrointestinal hemorrhage was 1.5 (95% ci: 1.1 to 2.2). A review by Conn and Poynard42 yielded statistically insignificant results, with a relative risk of 1.3 for peptic ulcers (95% ci: 0.8 to 2.1) and a relative risk of 1.2 for hemorrhage (95% ci: 0.7 to 2.2). The authors noted that the incidence of peptic ulcers tended to increase with the dose and duration of therapy. In patients concomitantly receiving nonsteroidal anti-inflammatory drugs, the risk of peptic ulcers is higher by a factor of 4–726. The incidence of ulcers and bleeding is higher in patients using anticoagulants and in patients who have a history of upper gastrointestinal bleeding20. Bowel perforation is also a serious complication of corticosteroid therapy20. It tends to occur in patients who are receiving high doses of steroids and who have been constipated because of medication, immobility, or neurologic dysfunction.
Mild psychiatric effects associated with dexamethasone use include anxiety, irritability, and insomnia, and the incidence of those side effects ranges from 13% to 62%31. Severe manifestations can include mania, depression, and psychosis, and the incidence of severe reactions is 5.7% (range: 1.6%–50%)31. Most psychiatric reactions occur within the first week of steroid therapy, and the most significant risk factor associated with psychiatric reactions is the corticosteroid dose31. Corticosteroids can also cause cognitive impairment such as memory disturbances; these deficits are dose-dependent and resolve on termination of the corticosteroid31.
4. CONCLUSIONS AND RECOMMENDATIONS
Based on the evidence available to date, the Alberta Provincial cns Tumour Team makes these recommendations:
Treatment with dexamethasone is recommended for symptom relief in patients with primary high-grade glioma and cerebral edema.
After surgery, a maximum dose of 16 mg daily, administered in 4 equal doses, is recommended for symptomatic patients. This protocol should ideally be started by the neurosurgeon.
- Dexamethasone should be tapered in a manner individualized to the patient. One of these taper schedules is recommended:
- Slow taper: Start with 4 mg twice daily for 7 days, then 2 mg twice daily for 7 days, then 1 mg twice daily for 7 days, then 1 mg once daily for 7 days.
- Fast taper: Dexamethasone can be discontinued within 3 days of surgery.
- Individualized taper: Use a taper schedule individualized for the specific patient as decided by the physician.
Patients who have high-grade tumours, are symptomatic, or have poor life expectancy can be maintained on dexamethasone 0.5–1.0 mg daily.
Side effects of dexamethasone are common, and they increase in frequency and severity with increased dose and duration of therapy. Patients should be carefully monitored for endocrine, muscular, skeletal, gastrointestinal, psychiatric, and hematologic complications, and also for infections and other general side effects.
Based on the foregoing recommendations, the members of the Alberta Provincial cns Tumour Team propose an algorithm for the use of dexamethasone in patients with high-grade glioma (Figure 1).
FIGURE 1.
Treatment algorithm for the use of dexamethasone in patients with high-grade gliomas. qid = 4 times daily; bid = twice daily.
5. ACKNOWLEDGMENTS
The authors thank the members of the Alberta Provincial cns Tumour Team for their contributions to the development of this clinical practice guideline.
6. CONFLICT OF INTEREST DISCLOSURES
The authors declare that there are no financial conflicts of interest.
7. REFERENCES
- 1.Drappatz J, Schiff D, Kesari S, Norden AD, Wen PY. Medical management of brain tumor patients. Neurol Clin. 2007;25:1035–71. doi: 10.1016/j.ncl.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Weissman DE, Janjan NA, Erickson B, et al. Twice-daily tapering dexamethasone treatment during cranial radiation for newly diagnosed brain metastases. J Neurooncol. 1991;11:235–9. doi: 10.1007/BF00165531. [DOI] [PubMed] [Google Scholar]
- 3.Vecht CJ, Hovestadt A, Verbiest HB, van Vliet JJ, van Putten WL. Dose–effect relationship of dexamethasone on Karnofsky performance in metastatic brain tumors: a randomized study of doses of 4, 8, and 16 mg per day. Neurology. 1994;44:675–80. doi: 10.1212/WNL.44.4.675. [DOI] [PubMed] [Google Scholar]
- 4.Wolfson AH, Snodgrass SM, Schwade JG, et al. The role of steroids in the management of metastatic carcinoma to the brain. A pilot prospective trial. Am J Clin Oncol. 1994;17:234–8. doi: 10.1097/00000421-199406000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Lagerwaard FJ, Levendag PC, Nowak PJ, Eijkenboom WM, Hanssens PE, Schmitz PI. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys. 1999;43:795–803. doi: 10.1016/S0360-3016(98)00442-8. [DOI] [PubMed] [Google Scholar]
- 6.Hempen C, Weiss E, Hess CF. Dexamethasone treatment in patients with brain metastases and primary brain tumors: do the benefits outweigh the side-effects? Support Care Cancer. 2002;10:322–8. doi: 10.1007/s00520-001-0333-0. [DOI] [PubMed] [Google Scholar]
- 7.Millar BM, Bezjak A, Tsao M, Sturdza A, Laperriere N. Defining the impact and contribution of steroids in patients receiving whole-brain irradiation for cerebral metastases. Clin Oncol (R Coll Radiol) 2004;16:339–44. doi: 10.1016/j.clon.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Sturdza A, Millar BA, Bana N, et al. The use and toxicity of steroids in the management of patients with brain metastases. Support Care Cancer. 2008;16:1041–8. doi: 10.1007/s00520-007-0395-8. [DOI] [PubMed] [Google Scholar]
- 9.Ryken TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:103–14. doi: 10.1007/s11060-009-0057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau DC, Douketis JD, Morrison KM, Hramiak IM, Sharma AM, Ur E, on behalf of the Obesity Canada Clinical Practice Guidelines Expert Panel 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary] CMAJ. 2007;176:S1–13. doi: 10.1503/cmaj.061409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaspar LE, Videtic GMM, Gore EM, et al. on behalf of the Expert Panel on Radiation Oncology–Brain Metastases . American College of Radiology: ACR Appropriateness Criteria. Pre-irradiation Evaluation and Management of Brain Metastases. Reston, VA: American College of Radiology; 2011. [Available online at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Oncology/PreIrradiationEvaluationBrainMetastases.pdf; cited February 27, 2013] [Google Scholar]
- 12.Soffietti R, Ruda R, Mutani R. Management of brain metastases. J Neurol. 2002;249:1357–69. doi: 10.1007/s00415-002-0870-6. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich J, Rao K, Pastorino S, Kesari S. Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol. 2011;4:233–42. doi: 10.1586/ecp.11.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roth P, Wick W, Weller M. Steroids in neurooncology: actions, indications, side-effects. Curr Opin Neurol. 2010;23:597–602. doi: 10.1097/WCO.0b013e32833e5a5d. [DOI] [PubMed] [Google Scholar]
- 15.BC Cancer Agency (bcca) Vancouver, BC: BCCA; 2004. Home > Health Professionals Info > Cancer Management Guidelines > Neuro-Oncology > 5. Management [Web resource] [Available at: http://www.bccancer.bc.ca/HPI/CancerManagementGuidelines/NeuroOncology/ManagementPolicies/default.htm; cited February 27, 2013] [Google Scholar]
- 16.Cancer Council Australia Home > Health professionals > Clinical guidelines > Brain tumours > Clinical Practice Guidelines for the Management of Adult Gliomas: Astrocytomas and Oligodendrogliomas [Web resource] 2009. [Available at: http://www.cancer.org.au/Healthprofessionals/clinicalguidelines/braintumours.htm; cited February 27, 2013]
- 17.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Central Nervous System Tumours. Fort Washington, PA: NCCN; 2013. Ver 2.2013. [Current version available online at: http://www.nccn.org/professionals/physician_gls/pdf/cns.pdf (free registration required); cited April 3, 2013] [Google Scholar]
- 18.Nahaczewski AE, Fowler SB, Hariharan S. Dexamethasone therapy in patients with brain tumors—a focus on tapering. J Neurosci Nurs. 2004;36:340–3. doi: 10.1097/01376517-200412000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Szabo GC, Winkler SR. Withdrawal of glucocorticoid therapy in neurosurgical patients. Surg Neurol. 1995;44:498. doi: 10.1016/0090-3019(95)00252-9. [DOI] [PubMed] [Google Scholar]
- 20.Batchelor TT, Byrne TN. Supportive care of brain tumor patients. Hematol Oncol Clin North Am. 2006;20:1337–61. doi: 10.1016/j.hoc.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Weissman DE, Dufer D, Vogel V, Abeloff MD. Corticosteroid toxicity in neuro-oncology patients. J Neurooncol. 1987;5:125–8. doi: 10.1007/BF02571300. [DOI] [PubMed] [Google Scholar]
- 22.Hoogwerf B, Danese RD. Drug selection and the management of corticosteroid-related diabetes mellitus. Rheum Dis Clin North Am. 1999;25:489–505. doi: 10.1016/S0889-857X(05)70083-1. [DOI] [PubMed] [Google Scholar]
- 23.Clore JN, Thurby–Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15:469–74. doi: 10.4158/EP08331.RAR. [DOI] [PubMed] [Google Scholar]
- 24.Wen PY, Schiff D, Kesari S, Drappatz J, Gigas DC, Doherty L. Medical management of patients with brain tumors. J Neurooncol. 2006;80:313–32. doi: 10.1007/s11060-006-9193-2. [DOI] [PubMed] [Google Scholar]
- 25.Pereira RM, Freire de Carvalho J. Glucocorticoid-induced myopathy. Joint Bone Spine. 2011;78:41–4. doi: 10.1016/j.jbspin.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 26.Fardet L, Kassar A, Cabane J, Flahault A. Corticosteroid-induced adverse events in adults: frequency, screening and prevention. Drug Saf. 2007;30:861–81. doi: 10.2165/00002018-200730100-00005. [DOI] [PubMed] [Google Scholar]
- 27.LaPier TK. Glucocorticoid-induced muscle atrophy. The role of exercise in treatment and prevention. J Cardiopulm Rehabil. 1997;17:76–84. doi: 10.1097/00008483-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Frieze DA. Musculoskeletal pain associated with corticosteroid therapy in cancer. Curr Pain Headache Rep. 2010;14:256–60. doi: 10.1007/s11916-010-0120-z. [DOI] [PubMed] [Google Scholar]
- 29.Papaioannou A, Morin S, Cheung AM, et al. on behalf of the Scientific Advisory Council of Osteoporosis Canada 2010 Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ. 2010;182:1864–73. doi: 10.1503/cmaj.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen B, Regier LD, editors. RxFiles: Drug Comparison Charts. 8th ed. Saskatoon, SK: Saskatoon Health Region; 2010. gerd and peptic ulcer disease: evidence and chart; p. 41. [Google Scholar]
- 31.Poetker DM, Reh DD. A comprehensive review of the adverse effects of systemic corticosteroids. Otolaryngol Clin North Am. 2010;43:753–68. doi: 10.1016/j.otc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 32.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Distress Management. Fort Washington, PA: NCCN; 2013. Ver 2.2013. [Current version available online at: http://www.nccn.org/professionals/physician_gls/pdf/distress.pdf (free registration required); cited February 27, 2013] [Google Scholar]
- 33.BC Cancer Agency (bcca) Vancouver, BC: BCCA; 2006. Home > Patient/Public Info > Coping with Cancer > Managing Symptoms and Side Effects > Fatigue > Professional Management: Managing Your Cancer Treatment–Related Fatigue [Web resource] [Available online at: http://www.bccancer.bc.ca/PPI/copingwithcancer/symptoms/fatigue/pmgt.htm; cited February 27, 2013] [Google Scholar]
- 34.Jensen B, Regier LD, editors. RxFiles: Drug Comparison Charts. 8th ed. Saskatoon, SK: Saskatoon Health Region; 2010. Sleep disorders: sedatives; p. 111. [Google Scholar]
- 35.Gilbert DN, Moellering RC, Eliopoulos GM, Chambers HF, Saag MS, editors. The Sanford Guide to Antimicrobial Therapy. 41st ed. Sperryville, VA: Antimicrobial Therapy; 2011. [Google Scholar]
- 36.Lyman GH, Khorana AA, Falanga A, et al. on behalf of the American Society of Clinical Oncology American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25:5490–505. doi: 10.1200/JCO.2007.14.1283. [DOI] [PubMed] [Google Scholar]
- 37.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Venous Thromboembolic Disease. Fort Washington, PA: NCCN; 2013. Ver 1.2013. [Current version available online at: http://www.nccn.org/professionals/physician_gls/pdf/vte.pdf (free registration required); cited February 27, 2013] [Google Scholar]
- 38.Wen PY, Lee EQ. Boston, MA: Anticoagulant and antiplatelet therapy in patients with brain tumors [Web resource] Up-ToDate; n.d. [Current version available online at: http://www.uptodate.com/contents/anticoagulant-and-antiplatelet-therapy-in-patients-with-brain-tumors (membership required); cited January 2, 2012] [Google Scholar]
- 39.Derr RL, Ye X, Islas MU, Desideri S, Saudek CD, Grossman SA. Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J Clin Oncol. 2009;27:1082–6. doi: 10.1200/JCO.2008.19.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGirt MJ, Chaichana KL, Gathinji M, et al. Persistent outpatient hyperglycemia is independently associated with decreased survival after primary resection of malignant brain astrocytomas. Neurosurgery. 2008;63:286–91. doi: 10.1227/01.NEU.0000315282.61035.48. [DOI] [PubMed] [Google Scholar]
- 41.Messer J, Reitman D, Sacks HS, Smith H, Jr, Chalmers TC. Association of adrenocorticosteroid therapy and peptic-ulcer disease. N Engl J Med. 1983;309:21–4. doi: 10.1056/NEJM198307073090105. [DOI] [PubMed] [Google Scholar]
- 42.Conn HO, Poynard T. Corticosteroids and peptic ulcer: meta-analysis of adverse events during steroid therapy. J Intern Med. 1994;236:619–32. doi: 10.1111/j.1365-2796.1994.tb00855.x. [DOI] [PubMed] [Google Scholar]