Abstract
Although metastases are common in patients with renal cell carcinoma (rcc), it is extremely rare for patients to present with metastatic rcc (mrcc) without evidence of a primary mass in the kidney. Two cases of mrcc with no detectable primary renal mass are reported here. Both patients had bilateral native kidneys in situ and no significant prior urologic history. The first patient presented with a hip fracture and was found to have multiple radiologic bony and lung metastases. Biopsy of a mass involving the pubic bone demonstrated clear cell mrcc. Multiple scans by computed tomography (ct) and confirmatory imaging by magnetic resonance demonstrated no renal mass. This first patient had disease stabilization for 18 months on sunitinib and was still alive at last follow-up. The second patient was diagnosed with clear-cell mrcc after thickened synovium was discovered and biopsied during a knee arthroplasty. Multiple scans by ct in this second patient demonstrated no primary renal mass. Sunitinib and radiotherapy to the knee lesion were initiated, but unfortunately, the patient deteriorated clinically and passed away from disease progression shortly after diagnosis. Because of the rare nature of these cases, a standardized course of action has not yet been established. However, we hypothesize that it is reasonable to manage metastases in these patients by following established mrcc protocols.
Keywords: Metastatic renal cell carcinoma, kidney cancer, no primary tumour
1. INTRODUCTION
Renal cell carcinomas (rccs) originate from the renal cortex and constitute 90% of all primary renal neoplasms1. The incidence of rcc varies globally, with the highest rates being found in the Czech Republic and North America1. In the United States, 65,000 new cases of rcc and 13,500 deaths every year are attributed to this type of renal cancer1. In recent decades, the incidence of rcc has been increasing, and the average tumour size at diagnosis has been declining2. Those trends are partially attributable to the widespread use of noninvasive abdominal imaging modalities, which have allowed for more incidental findings of asymptomatic tumours. Renal cell carcinoma is about 50% more common in men than in women, and it is unusual in patients under 40 years of age1. Several distinct subtypes of rcc have been identified, including the clear cell, papillary, chromophobe, and collecting duct variants3. Clear cell rcc, the most common subtype, accounts for 75% of all rcc tumours2.
Metastatic rcc (mrcc) generally presents with evidence of a primary renal mass, except in cases of metachronous mrcc after nephrectomy for rcc. Here, we present two unusual cases of metastatic clear cell rcc with no evidence of a primary renal tumour.
2. CASE DESCRIPTIONS
2.1. Patient 1
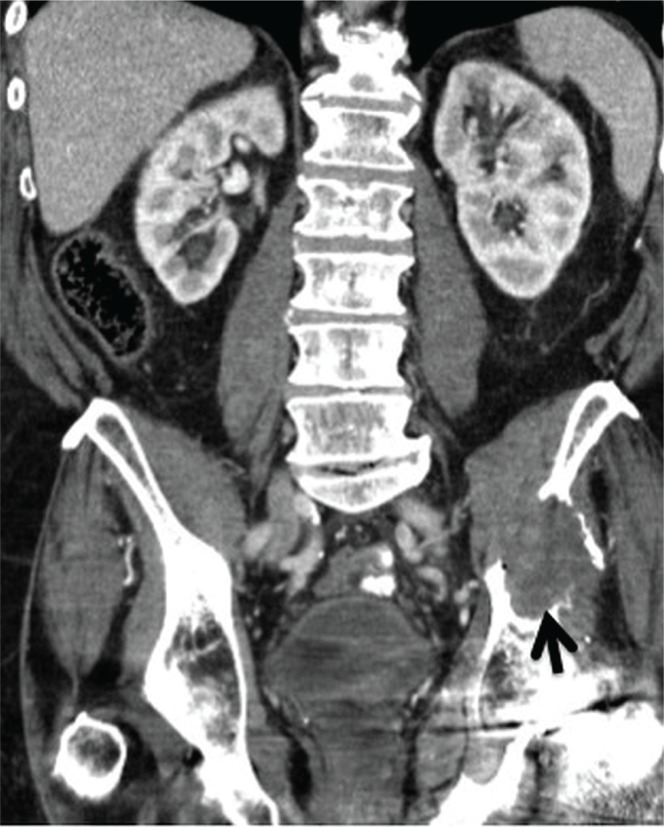
A 70-year-old man was admitted for a fall-induced hip fracture and subsequently underwent arthroplasty. He was readmitted to hospital 8 months later for hypercalcemia and confusion. At that time, computerized tomography (ct) imaging showed bilateral native kidneys in situ; bony lesions in the left scapula, seventh right rib, fifth left rib, and left sternoclavicular joint; and two nodules in the upper lobe of the left lung. There was also destruction of the left pubo-iliac bone from a multi-lobulated lesion (measuring 11 cm) replacing bone, and no evidence of soft-tissue disease (Figure 1).
FIGURE 1.
Abdominal computed tomography imaging of patient 1 shows unremarkable kidneys and metastases in the left pelvic iliac bone.
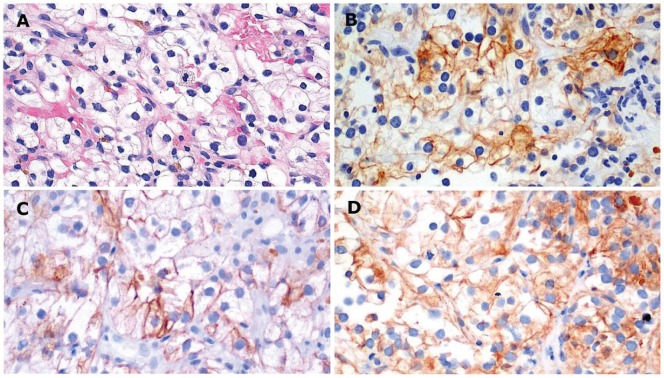
An ultrasound-guided biopsy of the left pelvic mass revealed clear cells with central hyperchromatic nuclei and morphology indicative of clear cell rcc, Fuhrman nuclear grade 1–2 [Figure 2(A)]. Immunohistochemical analysis of the tumour cells indicated positive staining for cam5.2, vimentin, and cd10 [Figure 2(B–D)]. Based on those findings, a diagnosis of clear cell mrcc was made. Multiple scans by ct and confirmatory imaging by magnetic resonance demonstrated no signs of a primary tumour.
FIGURE 2.
Immunohistochemical analysis of a biopsy specimen from patient 1. (A) Tumour with vessels shows clear cells with a central hyperchromatic nucleus, consistent with clear cell renal cell carcinoma, Fuhrman nuclear grade 1–2 (hematoxylin and eosin stain, 20× original magnification). Tumour cells are positive for (B) cytokeratin cam5.2, (C) vimentin, and (D) cd10.
The patient was started on sunitinib 50 mg daily, 4 weeks on and 2 weeks off. The patient also received a zoledronic acid infusion every 4 weeks. After 12 months of therapy, minimal side effects and disease stabilization were observed. Follow-up ct imaging demonstrated a decrease in the size of multiple bone lesions, regression of the lung nodules, and still no primary renal tumour. The patient continues to make regular follow-up and oncologic review visits, with stable disease at 18 months and no evidence of a primary renal mass on regular surveillance.
2.2. Patient 2
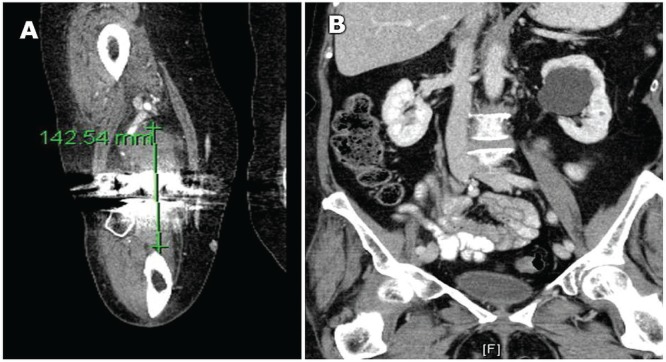
A 69-year-old woman presented with significant right knee pain. After a diagnosis of osteoarthritis, a total right knee arthroplasty was conducted, during which thickened synovium was found and biopsied [Figure 3(A)].
FIGURE 3.
Computed tomography imaging in patient 2 shows (A) bony metastases in the right knee and (B) a 4-cm cyst on the left kidney.
Initial histologic examination of the tissue suggested a malignant neoplasm of epithelial origin, which was confirmed by consultation with an outside expert. Additional immunostaining demonstrated lesional cells positive for epithelial membrane antigen, vimentin, and cd10, and negative for cytokeratins 7 and 20, calretinin, and cd34, leading to a diagnosis of metastatic clear cell rcc. Negative staining for S100 and thyroid transcription factor 1 ruled out the possibility of a melanoma or neoplasm arising from the lung.
Based on a physical examination and ct imaging, no metastatic lesions were present elsewhere. No parenchymal solid renal lesions of note were identified. However, a large (4 cm) benign parapelvic cyst was found on the left kidney [Figure 3(B)]. The patient was diagnosed with rcc metastatic to the right knee, with no detectable kidney mass.
The patient was advised to consider a below-knee amputation to prevent further metastases, but ct imaging revealed multiple new bilateral pulmonary nodules. Sunitinib 50 mg daily (4 weeks on, 2 weeks off) and radiotherapy to the knee lesion were commenced. However, disease progression led to clinical deterioration and the patient’s death 8 months after presentation.
3. DISCUSSION
Patients presenting clinically with symptoms highly suspicious for rcc typically undergo evaluation by ultrasonography or ct imaging for the presence of a renal mass. The imaging techniques can confirm the presence of a renal mass, distinguish suspected rcc from a benign cyst, and assess the extent of disease. Approximately 30% of patients with rcc have distant metastases or advanced local disease at presentation4. The most common sites of metastases include the lungs (75%), lymph nodes (36%), bones (20%), and liver (18%)5. Biopsy of the primary renal mass allows for histologic investigation and immunohistochemical staining to establish a diagnosis in correlation with clinical and radiologic findings.
Tumour type-specific immunohistochemical profiles for primary rccs are largely conserved in their metastatic deposits6. Positive staining for cd10 and a combination of vimentin and cytokeratins is helpful in distinguishing renal cell tumours from non-renal-cell tumours6. All rccs also stain negatively for cytokeratin 206. Distinguishing the histologic subtype of rcc is crucial in guiding the choice of an appropriate molecularly targeted therapy6. The clear cell rcc subtype stains negatively for cytokeratin 7 and is positive for periodic acid–Schiff and negative for periodic acid–Schiff diastase6.
The cases presented here are unusual because the diagnosis of mrcc was reached after immunohistochemical and histologic analysis of nonrenal lesions. As previously mentioned, histologic analysis of patient 1’s biopsy specimens collected from the left pelvic mass showed clear cells with central hyperchromatic nuclei and morphology indicative of clear cell rcc, Fuhrman nuclear grade 1–2. When rcc presents with typical morphology, immunohistochemical staining is not necessary to establish a diagnosis6. Nonetheless, positive staining for cd10, vimentin, and cam5.2 aided in confirming a diagnosis highly suspicious for mrcc.
Histologic analysis of patient 2’s biopsy specimens from the right knee lesion did not yield findings typical of rcc, necessitating extensive staining. Lesional cells were positive for epithelial membrane antigen, vimentin, and cd10, and negative for cytokeratins 7 and 20, calretinin, cd34, S100, and thyroid transcription factor 1. Together, those findings are indicative of mrcc. Epithelioid sarcoma, part of the differential diagnosis for this patient, was deemed less likely because the presence of tumour emboli within endothelium-lined vascular spaces in the bone marrow were suggestive of a metastatic deposit rather than a primary tumour site.
Although the biopsy results of the observed masses in our patients were suggestive for mrcc, there was no detectable radiologic evidence of a primary renal tumour. Patients with mrcc presenting in this manner are extremely rare; to the best of our knowledge, only 5 other cases have been reported to date worldwide7–10. It remains unclear how mrcc develops in the absence of a primary renal tumour. One theory is that a renal mass, although present, might be too small for detection by current imaging modalities. The small size of renal masses often prevents adequate radiologic evaluation because of volume artifacts or an inability to determine whether enhancement is present. In light of those limitations, patient 1 will be followed with regular abdominal ct imaging to address the possibility of a primary renal mass becoming radiologically apparent in the future. Another possibility is the spontaneous regression of a once-present primary renal tumour. Renal cell carcinoma is one of the few cancers in which cases of spontaneous tumour regression in the absence of therapy have been well documented. Lastly, it is possible that the patients initially developed rcc in ectopic renal tissue that eventually metastasized to other regions. Renal cell carcinoma in ectopic renal tissue could present as mrcc without a renal tumour.
Given the absence of established treatment guidelines, management of these patients has proved to be challenging. Our patients were both managed with the administration of sunitinib regimens. Sunitinib, a tyrosine kinase inhibitor, and other targeted systemic therapies have become the standard of care for mrcc10. These agents significantly prolong progression-free survival and overall survival, and improve quality of life for patients with mrcc11. Patient 1 responded well to treatment, displaying disease regression with minimal side effects. However, response to therapy in patient 2 could not be adequately assessed because she died shortly after initiation of the treatment regimen.
4. SUMMARY
We report two rare cases of mrcc without evidence of a primary renal tumour. Previously published cases of this type have shown that, in the context of single-site metastasis, a standard mrcc protocol of aggressive local therapy (that is, resection or radiotherapy) should be followed. Our cases suggest that patients with multiple-site metastases from rcc might also benefit from a sunitinib regimen. This case series highlights the importance of distinguishing primary neoplasms from metastases and of considering mrcc when clear cell neoplasms of an unknown primary are encountered. Until further research is conducted, we hypothesize that it is reasonable to use currently established mrcc treatment guidelines for the management of patients with mrcc without evidence of a renal primary mass.
5. ACKNOWLEDGMENTS
An abstract reporting these cases was accepted and presented as a moderated poster at the 2013 Canadian Urological Association annual meeting.
6. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
7. REFERENCES
- 1.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7:245–57. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–90. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 3.Linehan WM, Srinivasan R, Schmidt LS. The genetic basis of kidney cancer: a metabolic disease. Nat Rev Urol. 2010;7:277–85. doi: 10.1038/nrurol.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol. 2001;166:1611–23. doi: 10.1016/S0022-5347(05)65640-6. [DOI] [PubMed] [Google Scholar]
- 5.Maldazys JD, deKernion JB. Prognostic factors in metastatic renal carcinoma. J Urol. 1986;136:376–9. doi: 10.1016/s0022-5347(17)44873-7. [DOI] [PubMed] [Google Scholar]
- 6.Shen SS, Truong LD, Scarpelli M, Lopez–Beltran A. Role of immunohistochemistry in diagnosing renal neoplasms: when is it really useful? Arch Pathol Lab Med. 2012;136:410–17. doi: 10.5858/arpa.2011-0472-RA. [DOI] [PubMed] [Google Scholar]
- 7.Choi YR, Han HS, Lee OJ, et al. Metastatic renal cell carcinoma in a supraclavicular lymph node with no known primary: a case report. Cancer Res Treat. 2012;44:215–18. doi: 10.4143/crt.2012.44.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawakita M, Kawamura J, Hida S, Ooishi K, Okada K, Yoshida O. Renal cell carcinoma, primary lesion which was not easily identified: report of two cases [Japanese] Hinyokika Kiyo. 1985;31:463–73. [PubMed] [Google Scholar]
- 9.Bhatia S, Ng S, Hodder SC. Metastatic cutaneous head and neck renal cell carcinoma with no known primary: case report. Br J Oral Maxillofac Surg. 2010;48:214–15. doi: 10.1016/j.bjoms.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Wayne M, Wang W, Bratcher J, Cumani B, Kasmin F, Cooperman A. Renal cell cancer without a renal primary. World J Surg Oncol. 2010;8:18. doi: 10.1186/1477-7819-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mihaly Z, Sztupinszki Z, Surowiak P, Gyorffy B. A comprehensive overview of targeted therapy in metastatic renal cell carcinoma. Curr Cancer Drug Targets. 2012;12:857–72. doi: 10.2174/156800912802429265. [DOI] [PMC free article] [PubMed] [Google Scholar]