Abstract
Purpose of review
This review summarizes and discusses the current knowledge about the physiological roles of the sweet taste receptor in oral and extraoral tissues.
Recent findings
The expression of a functional sweet taste receptor has been reported in numerous extragustatory tissues, including the gut, pancreas, bladder, brain and, more recently, bone and adipose tissues. In the gut, this receptor has been suggested to be involved in luminal glucose sensing, the release of some satiety hormones, the expression of glucose transporters, and the maintenance of glucose homeostasis. More recently, the sweet taste receptor was proposed to regulate adipogenesis and bone biology.
Summary
The perception of sweet taste is mediated by the T1R2/T1R3 receptor, which is expressed in the oral cavity, wherein it provides input on the caloric and macronutrient contents of ingested food. This receptor recognizes all the chemically diverse compounds perceived as sweet by human beings, including natural sugars and sweeteners. Importantly, the expression of a functional sweet taste receptor has been reported in numerous extragustatory tissues, wherein it has been proposed to regulate metabolic processes. This newly recognized role of the sweet taste receptor makes this receptor a potential novel therapeutic target for the treatment of obesity and related metabolic dysfunctions, such as diabetes and hyperlipidemia.
Keywords: carbohydrate, diabetes, insulin, obesity, sweetener, taste receptor function
INTRODUCTION
Obesity and overweight, which have reached epidemic proportions in industrialized and developing countries, lead to major risks for chronic diseases, including type 2 diabetes, hypertension, and cardiovascular diseases. Although reduced physical activity is partly responsible for obesity, the increased consumption of energy-dense foods with high contents of carbohydrates and saturated fats makes a large contribution. Our sense of taste acts as a major determinant for our strong preference for sweet foods and their overconsumption. The detection of sweet-tasting compounds provides input on the caloric and macronutrient contents of ingested foods. Sweet-tasting compounds are detected by a heterodimeric receptor composed of two subunits, T1R2 and T1R3, expressed in taste buds in the mouth. All sweet-tasting molecules, including carbohydrates and noncaloric sweeteners, are detected through the activation of this single receptor. Interestingly, since its discovery in the tongue, the T1R2/T1R3 sweet taste receptor, along with several taste signal transduction molecules, has been demonstrated to be expressed not only in the oral cavity but also in a variety of nontaste organs, including the gastrointestinal tract, pancreas, bladder, adipose tissues, and brain. Although its essential physiological role in numerous tissues remains to be established, it has been demonstrated that the sweet taste receptor expressed in pancreatic and gastrointestinal tissues is involved in glucose sensing, the expression of glucose transporters, and the maintenance of glucose homeostasis. In this review, the physiological functions of the sweet taste receptor in both oral and extraoral tissues are summarized and discussed.
Box 1.
no caption available
THE SWEET TASTE RECEPTOR
The physiological role of taste is to provide the ability to detect key nutrients before ingestion and to avoid bitter-tasting, potentially noxious molecules, such as plant alkaloids. Among the five basic taste modalities (sweet, umami, bitter, salty, and sour), sweet taste is one of the major determinants of dietary choice. A sweet taste indicates the presence of energy-rich carbohydrates, such as glucose, which increases the hedonic tone of food and strongly influences our eating behavior. A major discovery occurred in the early 2000s, when it was discovered that sweetness detection is largely mediated by a single receptor. This receptor is composed of two distinct G protein-coupled receptors (GPCRs): type 1, member 2 (T1R2) and type 1, member 3 (T1R3). The T1R2/T1R3 sweet taste receptor (Fig. 1) responds to various chemically distinct compounds, such as natural sugars, noncaloric artificial and natural sweeteners, some D-amino acids, and sweet-tasting proteins [1]. In addition, it has been shown that T1R3 has the ability to form a T1R3/T1R3 homodimer that is sensitive to monoaccharides and disaccharides, but only at high concentrations [1]. Interestingly, the T1R3 subunit has been shown to assemble with T1R1 to form the heterodimeric umami receptor (T1R1/T1R3), which is sensitive to L-amino acids, such as monosodium glutamate and aspartate. The functional heterologous expression of T1R1 and T1R2 alone revealed that these subunits are not active on their own. The T1R1, T1R2, and T1R3 subunits are members of the small family of class C GPCRs. The most studied class C GPCRs include metabotropic glutamate receptors, the calcium-sensing receptor, and the metabotropic gamma aminobutyric acid metabotropic type B (GABAB) receptor [2]. Class C GPCRs share a common architecture, including a large aminoterminal domain (ATD). This ATD contains a Venus flytrap domain (VFT) and a short cysteine-rich domain, which connects the ATD to the α-helical transmembrane domain characteristic of GPCRs (Fig. 1).
FIGURE 1.
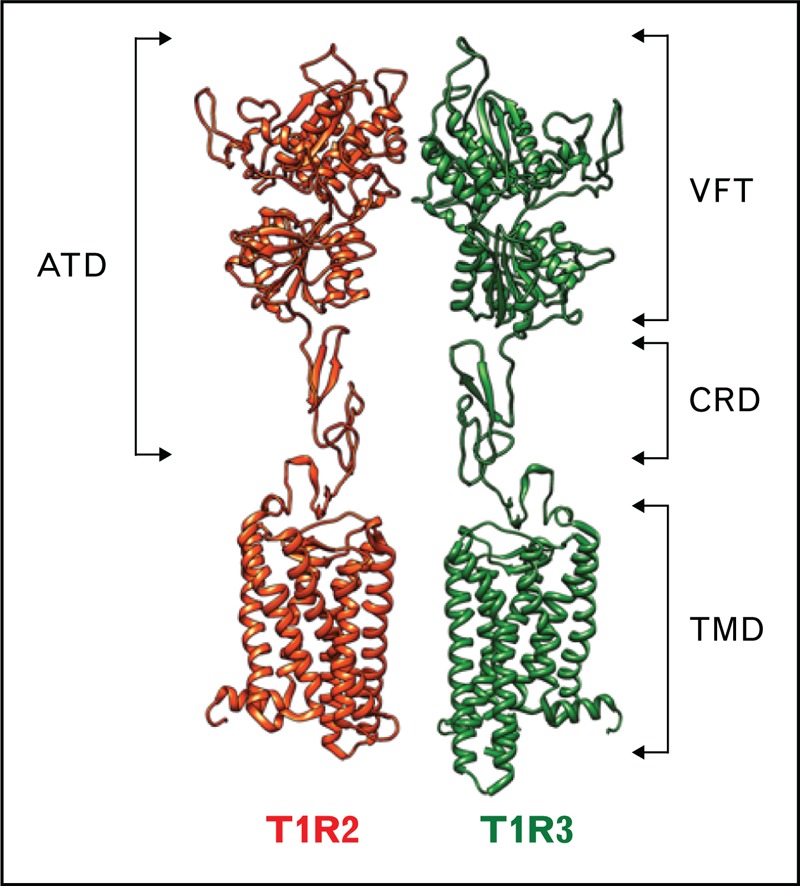
Schematic model of the sweet taste receptor. The sweet taste receptor is composed of two subunits, T1R2 and T1R3. The two subunits belong to the class C GPCRs. T1R2 and T1R3 possess a large aminoterminal domain (ATD) that includes a Venus flytrap domain (VFT) connected to a helical transmembrane domain (TMD) (characteristic of GPCRs) by a short cysteine-rich domain (CRD). The VFT is composed of two lobes separated by a large cleft, in which most sweeteners bind. GPCRs, G protein-coupled receptors.
Although T1Rs are highly conserved (with approximately 70% amino acid sequence identity between humans and rodents), behavioral studies have revealed differences in sweet taste perception between species. For instance, artificial sweeteners, including aspartame, neotame, cyclamate, and neohesperidin dihydrochalcone, as well as sweet-tasting proteins, such as brazzein, monellin, and thaumatin, are perceived by humans and not by rodents [1].
Using rodent–human chimeric T1R2/T1R3 receptors, functional studies of the sweet taste receptor have revealed at least four binding sites for sweet-tasting compounds [1]. Cellular assays and knockout mouse studies have established that T1R2/T1R3 is the primary sweet taste receptor. However, knockout mouse studies in which the T1R3 gene was disrupted suggested that additional sugar-sensing mechanisms might exist [3].
In the oral cavity, sweet compounds (similar to other basic taste stimuli) interact with taste detectors expressed on the apical membranes of taste receptor cells. These taste cells are found as aggregates in onion-shaped structures called taste buds. The activation of the sweet taste receptor in taste buds by sweet compounds induces an intracellular transduction cascade, leading to the depolarization of the taste receptor cells (Fig. 2). The main signal transduction events have been identified. The binding of sweet compounds to the T1R2/T1R3 receptor results in the dissociation of the heterotrimeric G protein (α-gustducin, Gβ3, and Gγ13), leading to an increase in phospholipase C-β2 (PLC-β2) activity, which results in the inositol 1,4,5-triphosphate (IP3) receptor, type 3-mediated release of Ca2+ from intracellular stores and the opening of a transient potential ion channel, transient receptor potential cation channel subfamily M member 5 (TRPMP5) [4]. This transduction mechanism, which is common to the detection of sweet, umami, and bitter tastes, leads to membrane depolarization, generating an action potential and leading to the release of adenosine triphosphate (ATP) as a transmitter to activate gustatory afferents (Fig. 2). Behavioral and electrophysiological experiments have shown that knockout mice in which α-gustducin (a Gαi family member) is disrupted are defective in the detection of sweet, umami, and bitter compounds [5]. However, other Gα proteins, including Gα14 (a Gαq family member), Gαs, Gαi2, and Gαi3, have been detected in taste receptor cells. Interestingly, the Gα protein Gα14 has been shown to be coexpressed with the T1R3 subunit in some taste cells. However, the functional roles of these additional Gα proteins in sweet taste transduction in the tongue remain to be established.
FIGURE 2.
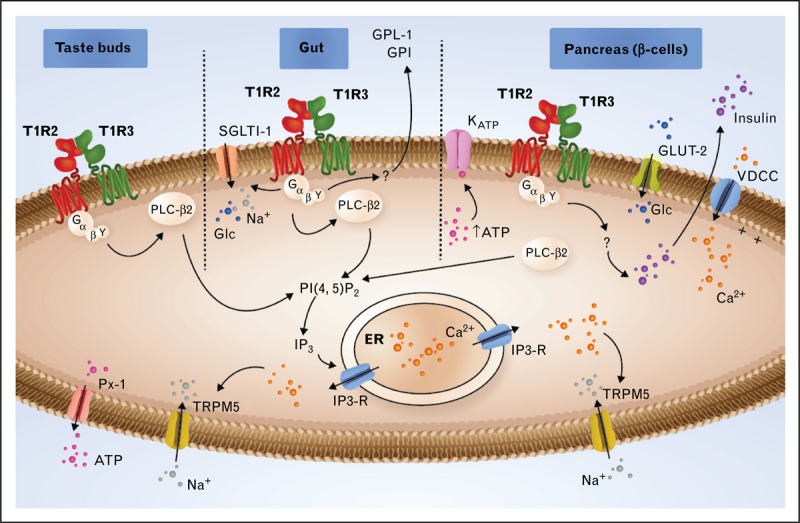
Signaling through T1R2/T1R3 in type II cells of taste buds, in the gut and in the β cells of the pancreas. In the taste buds, activated T1R2/T1R3 interacts with heterotrimeric G proteins comprising α-gustducin, Gβ3, and Gγ13. After the dissociation of the G protein subunits, the Gβγ subunit interacts with phospholipase C-β2 (PLC-β2), which in turn cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-triphosphate (IP3), producing diacylglycerol (DAG). IP3 stimulates Ca2+ release from the endoplasmic reticulum (ER) via type III IP3 receptor activation (IP3-R). The elevated intracellular Ca2+ activates the TRPM5 channel, leading to membrane depolarization, which enables the ATP channel Pannexin-1 (PX1) to open and release ATP, which stimulates efferent nerve fibers. In the gut, the signal transduction pathway is similar to that described in taste buds. The glucose (Glc) stimulation of the T1R2/T1R3 receptor triggers the secretion of two incretins, GLP-1 and GIP, and increases the expression of sodium-glucose cotransporter-1 (SGLT-1) to the plasma membrane. In the β cells of the pancreas, Glc is transported by glucose transporter-2 (GLUT-2). Glycolysis leads to an ATP increase, leading to KATP channel closure, which causes depolarization. This depolarization, in turn, activates the voltage-dependent calcium channel (VDCC), leading to the accumulation of Ca2+ in the cytoplasm and to insulin secretion. The T1R2/T1R3 receptor has been proposed to be implicated in the regulation of insulin secretion. ATP, adenosine triphosphate; GIP, glucose-dependent insulinotropic peptide; GLP-1, glucagon-like peptide-1; TRPM5, transient receptor potential cation channel subfamily M member 5.
Some specific sweet taste inhibitors have been used as pharmacological tools in the studies of signal transduction cascades or to investigate the physiological functions of the sweet taste receptor in oral and extraoral tissues. The most studied inhibitors include lactisole, a selective competitive inhibitor of human sweet taste perception and gurmarin, a potent inhibitor of the rodent sweet taste receptor [6].
ROLE OF THE SWEET TASTE RECEPTOR IN METABOLIC REGULATION
In the past several years, much new information on the expression and function of the sweet taste receptor in different sections of the gastrointestinal tract has emerged (Fig. 3). In the stomach, T1R3 and α-gustducin are expressed in the enteroendocrine cells and brush cells. Enteroendocrine cells are known to secrete ghrelin, a hunger hormone. After a rise in the blood concentration of glucose or amino acids, ghrelin release is suppressed. Because T1R3 is expressed in ghrelin-producing cells in the stomach, it has been proposed that T1R3 is involved in adjusting ghrelin release after glucose intake [7,8]. Although the effect of sweet molecules on ghrelin release has not yet been studied, experiments conducted on α-gustducin knockout mice have demonstrated that after the administration of a bitter taste mixture, no suppression of ghrelin release was observed, revealing a more complex role of taste perception in nutrient sensing [7,8]. The function of brush cells is currently unknown, although there is some speculation as to whether these cells might function as chemosensory detectors because of their morphological similarities with type II taste cells from taste buds and the fact that they express taste transduction molecules (Fig. 2), such as TRPM5 and PLC-β2 [4].
FIGURE 3.
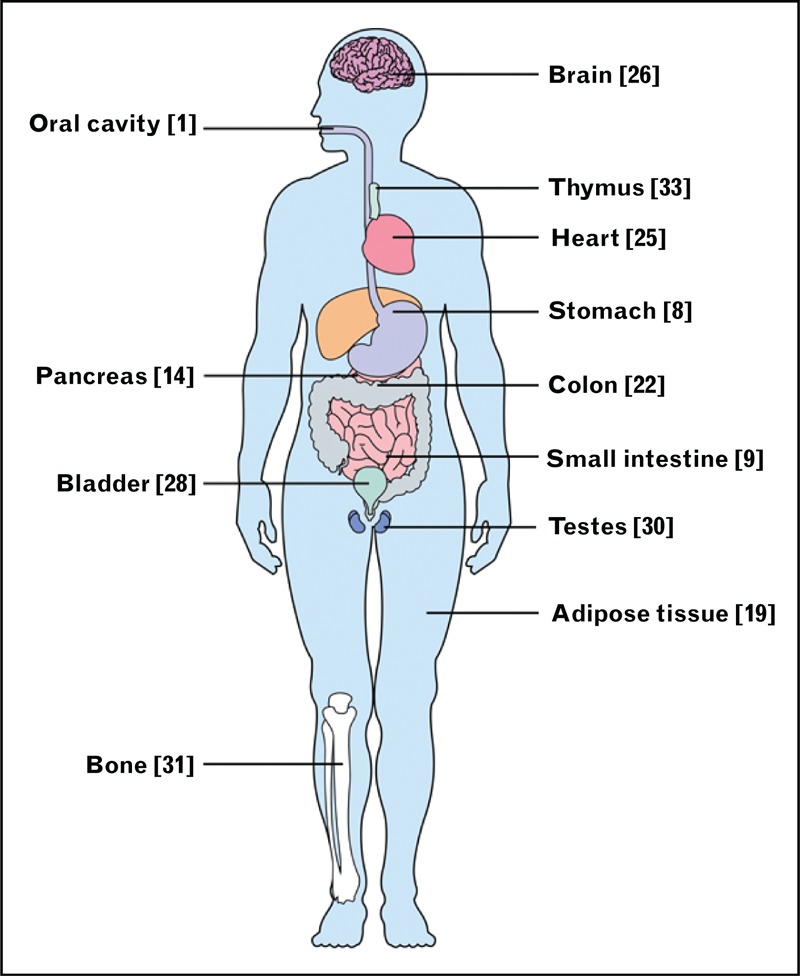
Oral and extraoral tissues where T1R2/T1R3 or α-gustducin have been described as being expressed. The reference numbers are indicated in brackets.
T1R2 and T1R3 are expressed throughout the small intestine in enteroendocrine cells (Fig. 2) that secrete two satiety hormones: glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) [9,10]. Jang et al.[10] showed that α-gustducin knockout mice did not secrete increased GLP-1 in response to direct glucose administration. Using a human enteroendocrine cell line, the authors confirmed that after stimulation with sucrose or glucose, these cells released GLP-1. Using murine enteroendocrine cell lines, when the T1R2/T1R3 receptor was inhibited by gurmarin, a sweet taste inhibitor in mice [6], GLP-1 and GIP secretion was further blocked, confirming the involvement of the sweet taste receptor in regulating the secretion of these incretins. Importantly, the knockout of either α-gustducin or T1R3 in mice resulted in the inability to increase sodium-glucose cotransporter-1 (SGLT-1) expression after an increase in carbohydrate intake [11]. Interestingly, in the Felidae family and chickens, which naturally lack T1R2 expression, an inability to increase SGLT-1 expression following carbohydrate intake has been observed [12]. Finally, in the duodenum, the expression level of the sweet taste receptor was shown to be regulated by luminal and systemic glucose levels and to be disordered in type 2 diabetes patients [13▪▪]. Taken together, all these data suggest that both the sweet taste receptor heterodimer and α-gustducin play key roles in the gut carbohydrate-sensing process (Fig. 2).
In the pancreas, β cells located in the islets of Langerhans are involved in the regulation of the blood glucose level via insulin secretion (Fig. 2). When the glucose concentration in the bloodstream increases, the amount of glucose actively transported into β cells also increases. Glucose is transported by a glucose transporter (GLUT2); subsequently, glycolysis leads to ATP production. The ATP increase is then responsible for the closure of ATP-sensitive K+ channels, which generates an intracellular Ca2+ increase via the activation of the voltage-dependent calcium channel, leading to membrane depolarization. Many important lines of evidence support the existence of a second synergistic pathway for the regulation of insulin release: all the components necessary for the detection of sweet-tasting compounds, including α-gustducin, T1R2, and T1R3, are present on the human β cell surface [14]; an increase in insulin release in the presence of a physiological level of glucose is induced by stimulation with fructose or artificial sweeteners, such as saccharin, cyclamate, or acesulfame-K [15]; and the insulin release induced by fructose stimulation is rapid compared with the response expected for a metabolic pathway, and no metabolic effect of fructose on β cells has been reported. Importantly, the fructose-induced insulin release was abolished by lactisole, a known T1R3 inhibitor, and in the β cells of T1R2−/− mice [16▪▪]. It has been shown that the nonmetabolic pathway of insulin release regulation generates a complex response depending on the identity of the sweet-tasting compounds. Indeed, after cell stimulation, sweeteners, such as acesulfame-K, sucralose, saccharin, and glycyrrhizin, all evoked different patterns of intracellular signals in MIN6 clonal mouse β cells [17]. T1R3 has been suggested as a potential candidate for the cellular detection of these artificial sweeteners, as demonstrated by T1R3 knockdown using siRNA. The last observation raises the question of the functional role of T1R2 in insulin release after fructose stimulation in mouse β cells. Some physiological differences between clonal MIN6 and pancreatic β cells after fructose and artificial sweetener stimulation have been observed. These differences may be explained by the lower T1R2 expression level observed in MIN6 mouse β cells [16▪▪]. The role of the nonmetabolic pathway in glucose sensing is still subject to debate. Kyriazis et al.[16▪▪] did not observe any change in insulin secretion after glucose stimulation in T1R2−/− mice compared with wild-type mice. This result does not exclude an eventual role of T1R2 in glucose sensing, which may be masked by the metabolic effect of glucose on insulin release. More recently, an effect of a glucose-sensing receptor was demonstrated in MIN6 clonal β cells. A nonmetabolizable analogue of glucose, 3-O-methylglucose, induced an elevation of ATP despite the inability of glucokinase to metabolize it or to inhibit it. These data suggest an activation of the metabolic pathway via T1Rs. T1R3/T1R3 has been proposed to be the more likely candidate involved in glucose sensing in MIN6 cells because of the low expression of T1R2 in these cells [18]. The role of the sweet receptor in insulin regulation in the pancreas raises the question of whether the sweet taste receptor has more extensive roles in hormone release and energy metabolism regulation.
Adipose tissue, mainly composed of adipocytes, is important for lipid storage but is also a major endocrine ‘organ’. T1R2 and T1R3 are functionally expressed in mouse adipose tissue, with significantly higher expression of T1R3 compared with T1R2; this suggests that a high percentage of T1R3 is present as a homomer [19], most likely in a homodimeric form [20]. The stimulation of adipogenesis and the suppression of lipolysis have been observed after the stimulation of adipocytes with saccharin and acesulfame-K. Interestingly, these effects were still observed in the absence of T1R2 and T1R3, suggesting the existence of an unknown additional receptor for these sweeteners [21▪].
Finally, the expression of both the T1R2 and T1R3 subunits has been demonstrated in enteroendocrine cells from the human colon, although their function remains unknown [22,23▪]. It has been proposed that T1R2 and T1R3, along with bitter taste receptors, may play roles in gastric emptying and intestinal motility by regulating the secretion of other actors, such as hormones [24]. Interestingly, α-gustducin was observed to be expressed in L cells, which secrete GLP-1 and peptide tyrosine tyrosine (peptide YY) [22]; the latter is known to play an important role in energy homeostasis by balancing food intake.
ROLE OF SWEET TASTE RECEPTORS IN OTHER ORGANS
Sweet taste receptors have been identified in many other different organs (heart, brain, bladder, nasal respiratory epithelium, kidney, and in some specific cells, such as B lymphocytes), wherein their function appears less obvious and remains to be deciphered in most cases (Fig. 3). The T1R3 subunit has been detected in the whole human heart by the neonatal stage, and its expression level is constant throughout life. Surprisingly, 24 h of glucose deprivation was observed to not modify the expression level of T1R3 in myocytes [25].
In the mouse brain, the T1R2 and T1R3 subunits have been demonstrated to be expressed in the hippocampus, the brain Cornu ammonis fields (CA fields), and the dentate gyrus neurons. It has been proposed that the sweet receptor located in the hypothalamus may be directly involved in glucose homeostasis, which is supported by a report that T1R2 expression patterns in mouse hypothalamic cells varied according to the glycemic index of the medium in which the cells were cultured [26]. A quantitative PCR analysis demonstrated both that the nutritional state of the animal regulated the sweet taste receptor in neurons and that ischemia caused by blood restriction in the brain induced sweet taste receptor expression [27].
Both the T1R2 and T1R3 subunits have been shown to be expressed in the bladder urothelium. It has been proposed that the activation of the receptor by artificial sweeteners, such as acesulfame-K or saccharin, could enhance bladder contraction [28].
The expression of T1R3 and α-gustducin, but not T1R2, was recently described in human spermatozoa [29] and male reproductive organs [30▪▪]. Mosinger et al.[30▪▪], using double-knockout mice for T1R3−/− and α-gustducin (Gnat−/−) expressing transgenic human T1R3, showed that when human T1R3 was blocked by a lactisole analogue (clofibrate), male mice exhibited sterility and pathological changes in their reproductive organs [30▪▪]. Many molecules commonly used as pesticides or drugs are able to block human T1R3; these molecules could have dramatic effects on the fertility of males exposed to these substances.
Simon et al.[31▪▪] recently suggested a potential regulatory role of T1R2 in repressing osteogenesis and stimulating bone marrow adipogenesis. T1R2−/− knockout mice showed significantly reduced numbers of adipocytes in the bone marrow microenvironment. Because adipogenesis and osteogenesis are closely related, the authors then studied the bone mass in both T1R2 and T1R3 knockout mice; both modified mice showed an increase in bone density.
In-situ hybridization has revealed the presence of swine T1R3 in the kidney and follicular B lymphocytes [32]. The latter localization was confirmed by the additional detection of a T1R3 mRNA transcript in human lymphocytes, in agreement with the previous detection of a low level of T1R3 mRNA in the human thymus [33]. This observation raises the possibility of a novel role for this sweet taste receptor in immunity.
CONCLUSION
On the basis of the recent findings presented here, the roles of the sweet taste receptor beyond the oral cavity are important for providing input on the caloric and macronutrient contents of ingested food. These newly identified roles of the sweet taste receptor in the regulation of metabolic processes highlight the question of the potential health risks of sugar substitutes. These recently described functions of the sweet taste receptor will set the stage for the development of translational research aimed at identifying new therapeutic drugs to treat certain related metabolic dysfunctions, including obesity and type 2 diabetes. Human genetic studies have identified single-nucleotide polymorphisms in the human T1R2 and T1R3 genes [34]. A genetic variation in the T1R3 promoter was associated with differences in sucrose taste sensitivity. In addition, an allelic variation in the gene for α-gustducin, which affects its mRNA expression, was recently correlated with individual differences in sweet sensitivity [35]. Further studies will be necessary to elucidate the impacts of these polymorphisms on human physiology.
Acknowledgements
This work was supported by grants from the Institut National de la Recherche Agronomique and the Burgundy council (PARI 2010-2011-2012, AGRALE1 Project), as well as a fellowship from the Burgundy council (A.L.).
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Behrens M, Meyerhof W. Gustatory and extragustatory functions of mammalian taste receptors. Physiol Behav 2011; 105:4–13 [DOI] [PubMed] [Google Scholar]
- 2.Pin JP, Kniazeff J, Goudet C, et al. The activation mechanism of class-C G-protein coupled receptors. Biol Cell 2004; 96:335–342 [DOI] [PubMed] [Google Scholar]
- 3.Damak S, Rong M, Yasumatsu K, et al. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science (New York, NY) 2003; 301:850–853 [DOI] [PubMed] [Google Scholar]
- 4.Iwatsuki K, Uneyama H. Sense of taste in the gastrointestinal tract. J Pharmacol Sci 2012; 118:123–128 [DOI] [PubMed] [Google Scholar]
- 5.Tomonari H, Miura H, Nakayama A, et al. Galpha-gustducin is extensively coexpressed with sweet and bitter taste receptors in both the soft palate and fungiform papillae but has a different functional significance. Chem Senses 2012; 37:241–251 [DOI] [PubMed] [Google Scholar]
- 6.Sigoillot M, Brockhoff A, Meyerhof W, et al. Sweet-taste-suppressing compounds: current knowledge and perspectives of application. Appl Microbiol Biotechnol 2012; 96:619–630 [DOI] [PubMed] [Google Scholar]
- 7.Janssen S, Laermans J, Verhulst PJ, et al. Bitter taste receptors and alpha-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc Natl Acad Sci U S A 2011; 108:2094–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hass N, Schwarzenbacher K, Breer H. T1R3 is expressed in brush cells and ghrelin-producing cells of murine stomach. Cell Tissue Res 2010; 339:493–504 [DOI] [PubMed] [Google Scholar]
- 9.Dyer J, Salmon KS, Zibrik L, et al. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans 2005; 33 (Pt 1):302–305 [DOI] [PubMed] [Google Scholar]
- 10.Jang HJ, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A 2007; 104:15069–15074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margolskee RF, Dyer J, Kokrashvili Z, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A 2007; 104:15075–15080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shirazi-Beechey SP, Daly K, Al-Rammahi M, et al. Role of nutrient-sensing taste 1 receptor (T1R) family members in gastrointestinal chemosensing. Br J Nutr 2014; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13▪▪.Young RL, Chia B, Isaacs NJ, et al. Disordered control of intestinal sweet taste receptor expression and glucose absorption in type 2 diabetes. Diabetes 2013; 62:3532–3541 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that intestinal T1R2 expression is regulated by the level of luminal glucose, and it is disordered in type 2 diabetes patients during acute hyperglycemia.
- 14.Henquin JC. Do pancreatic beta cells ‘taste’ nutrients to secrete insulin? Sci Signal 2012; 5:e36. [DOI] [PubMed] [Google Scholar]
- 15.Malaisse WJ, Vanonderbergen A, Louchami K, et al. Effects of artificial sweeteners on insulin release and cationic fluxes in rat pancreatic islets. Cell Signal 1998; 10:727–733 [DOI] [PubMed] [Google Scholar]
- 16▪▪.Kyriazis GA, Soundarapandian MM, Tyrberg B. Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc Natl Acad Sci U S Am 2012; 109:E524–E532 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated the role of the sweet taste receptor in insulin release. This key study revealed that fructose is able to amplify insulin release in human and mouse islet cells through the activation of the sweet taste receptor and that knocking out the T1R2 subunit obliterates this response.
- 17.Nakagawa Y, Nagasawa M, Mogami H, et al. Multimodal function of the sweet taste receptor expressed in pancreatic beta-cells: generation of diverse patterns of intracellular signals by sweet agonists. Endocr J 2013; 60:1191–1206 [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa Y, Ohtsu Y, Nagasawa M, et al. Glucose promotes its own metabolism by acting on the cell-surface glucose-sensing receptor T1R3. Endocr J 2014; 61:119–131 [DOI] [PubMed] [Google Scholar]
- 19.Masubuchi Y, Nakagawa Y, Ma J, et al. A novel regulatory function of sweet taste-sensing receptor in adipogenic differentiation of 3T3-L1 cells. PloS One 2013; 8:e54500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maitrepierre E, Sigoillot M, Le Pessot L, et al. Recombinant expression, in vitro refolding, and biophysical characterization of the N-terminal domain of T1R3 taste receptor. Protein Expr Purif 2012; 83:75–83 [DOI] [PubMed] [Google Scholar]
- 21▪.Simon BR, Parlee SD, Learman BS, et al. Artificial sweeteners stimulate adipogenesis and suppress lipolysis independently of sweet taste receptors. J Biol Chem 2013; 288:32475–32489 [DOI] [PMC free article] [PubMed] [Google Scholar]; An important study showing that artificial sweeteners are able to regulate adipocyte differentiation and metabolism through a mechanism that is apparently independent of the sweet taste receptor.
- 22.Rozengurt N, Wu SV, Chen MC, et al. Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol 2006; 291:G792–802 [DOI] [PubMed] [Google Scholar]
- 23▪.Foster SR, Roura E, Thomas WG. Extrasensory perception: odorant and taste receptors beyond the nose and mouth. Pharmacol Ther 2014; 142:41–61 [DOI] [PubMed] [Google Scholar]; This study demonstrated that T1R3, one of the sweet taste receptor subunits, is expressed in the human heart.
- 24.Rozengurt E. Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and alpha-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol 2006; 291:G171–G177 [DOI] [PubMed] [Google Scholar]
- 25.Foster SR, Porrello ER, Purdue B, et al. Expression, regulation and putative nutrient-sensing function of taste GPCRs in the heart. PloS One 2013; 8:e64579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren X, Zhou L, Terwilliger R, et al. Sweet taste signaling functions as a hypothalamic glucose sensor. Front Integr Neurosci 2009; 3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin YJ, Park JH, Choi JS, et al. Enhanced expression of the sweet taste receptors and alpha-gustducin in reactive astrocytes of the rat hippocampus following ischemic injury. Neurochem Res 2010; 35:1628–1634 [DOI] [PubMed] [Google Scholar]
- 28.Elliott RA, Kapoor S, Tincello DG. Expression and distribution of the sweet taste receptor isoforms T1R2 and T1R3 in human and rat bladders. J Urol 2011; 186:2455–2462 [DOI] [PubMed] [Google Scholar]
- 29.Meyer D, Voigt A, Widmayer P, et al. Expression of Tas1 taste receptors in mammalian spermatozoa: functional role of Tas1r1 in regulating basal Ca(2)(+) and cAMP concentrations in spermatozoa. PloS One 2012; 7:e32354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30▪▪.Mosinger B, Redding KM, Parker MR, et al. Genetic loss or pharmacological blockade of testes-expressed taste genes causes male sterility. Proc Natl Acad Sci U S A 2013; 110:12319–12324 [DOI] [PMC free article] [PubMed] [Google Scholar]; An important article that investigated the role of the T1R3 subunit in male fertility and revealed the effects of blocking the sweet taste receptor with pharmaceutical agents.
- 31▪▪.Simon BR, Learman BS, Parlee SD, et al. Sweet taste receptor deficient mice have decreased adiposity and increased bone mass. PloS One 2014; 9:e86454. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed a potential role for the T1R2/T1R3 receptor in the stimulation of adipogenesis. The authors also investigated the role of the receptor in osteogenesis and revealed a potential repressive role of T1R2 in osteogenesis.
- 32.Iwatsuki K, Nomura M, Shibata A, et al. Generation and characterization of T1R2-LacZ knock-in mouse. Biochem Biophys Res Commun 2010; 402:495–499 [DOI] [PubMed] [Google Scholar]
- 33.Max M, Shanker YG, Huang L, et al. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet 2001; 28:58–63 [DOI] [PubMed] [Google Scholar]
- 34.Kim UK, Wooding S, Riaz N, et al. Variation in the human TAS1R taste receptor genes. Chem Senses 2006; 31:599–611 [DOI] [PubMed] [Google Scholar]
- 35.Fushan AA, Simons CT, Slack JP, et al. Association between common variation in genes encoding sweet taste signaling components and human sucrose perception. Chem Senses 2010; 35:579–592 [DOI] [PMC free article] [PubMed] [Google Scholar]


