Abstract
Previous studies suggested that smoking and passive smoking could increase the risk of breast cancer, but the results were inconsistent, especially for Chinese females. Thus, we systematically searched cohort and case-control studies investigating the associations of active and passive smoking with breast cancer risk among Chinese females in four English databases (PubMed, Embase, ScienceDirect, and Wiley) and three Chinese databases (CNKI, WanFang, and VIP). Fifty-one articles (3 cohort studies and 48 case-control studies) covering 17 provinces of China were finally included in this systematic review. Among Chinese females, there was significant association between passive smoking and this risk of breast cancer [odds ratio (OR): 1.62; 95% confidence interval (CI): 1.39–1.85; I2 = 75.8%, P < 0.001; n = 26] but no significant association between active smoking and the risk of breast cancer (OR: 1.04; 95% CI: 0.89–1.20; I2 = 13.9%, P = 0.248; n = 31). The OR of exposure to husband's smoking and to smoke in the workplace was 1.27 (95% CI: 1.07–1.50) and 1.66 (95% CI: 1.07–2.59), respectively. The OR of light and heavy passive smoking was 1.11 and 1.41, respectively, for women exposed to their husband's smoke (< 20 and ≥ 20 cigarettes per day), and 1.07 and 1.87, respectively, for those exposed to smoke in the workplace (< 300 and ≥ 300 min of exposure per day). These results imply that passive smoking is associated with an increased risk of breast cancer, and the risk seems to increase as the level of passive exposure to smoke increases among Chinese females. Women with passive exposure to smoke in the workplace have a higher risk of breast cancer than those exposed to their husband's smoking.
Keywords: Systematic review, meta-analysis, active smoking, passive smoking, breast cancer, Chinese females
Chinese females have a lower incidence of breast cancer compared with their counterparts in Western countries. However, the incidence of breast cancer has increased steadily at an alarming rate over the past two decades (from 29.9/100,000 in 1989–1993 to 50.1/100,000 in 2004–2008 in Chinese urban areas, and from 6.5/100,000 to 17.3/100,000 in Chinese rural areas), making breast cancer the most common and fifth most common cancer for Chinese urban and rural females, respectively[1].
As a country with one of the highest rates of tobacco consumption[2],[3], China is one of the most seriously affected countries by tobacco. Nearly 700,000 Chinese died as a result of active smoking in 2005, and another 100,000 deaths were attributable to passive smoking in 2002[2],[3]. Although the prevalence of active smoking among Chinese females has been low (3.8% in 1996 and 2.4% in 2010), the prevalence of passive smoking in this population has remained high for nearly two decades (approximately 60% between 1996 and 2010)[4],[5]. Women also bear nearly 80% of the total cancer burden from passive smoking[3].
Although there is no way to completely prevent breast cancer[6], tobacco is considered the most preventable cause of cancer worldwide. Several studies suggest that women who consume tobacco or who were exposed to passive smoking may have an increased risk of breast cancer. However, a collaborative re-analysis of the 53 worldwide epidemiologic studies found that active smoking had little or no independent effect on breast cancer incidence[7]. Another three large American cohort studies revealed little association between passive smoking and breast cancer risk[8]–[10]. The unclear associations between active or passive smoking and breast cancer risk also exist in China. Until now, there has been no systematic review summarizing current studies on active smoking and breast cancer risk in China. The only systematic review focusing on the associations between passive smoking and breast cancer risk among Chinese females[11] missed more than 10 important studies, which would inevitably incur bias in the final conclusion. And this review did not include subgroup analyses (quality of study, sample size, region of China, etc) or any further analysis to explore the different effects of exposure to husband's smoking and to smoke in the workplace.
To shed light on the potential roles of active and passive smoking on the risk of breast cancer among Chinese females, we performed this systematic review and meta-analysis to help resolve these uncertainties.
Materials and Methods
We conducted this systematic review according to the guideline of meta-analysis of observational studies in epidemiology (MOOSE)[12].
Searching strategy
Two reviewers independently searched the literature published in four English databases (PubMed, Embase, ScienceDirect, and Wiley) and three Chinese databases (CNKI, WanFang, and VIP) up to June 2013. These searches were complemented by manual searches. Authors of potential literature were contacted when more information or clarification was needed. Three groups of keywords were used in the Chinese databases: (1) case-control study, cohort study, and prospective study; (2) breast cancer, breast carcinoma, breast tumor, breast neoplasm, mammary cancer, mammary carcinoma, mammary tumor, mammary neoplasm; and (3) smoking, tobacco, risk factor, etiology, polymorphism, and susceptibility. Other groups of keywords were also used in the English databases: Chinese, China, and the Han population. In the PubMed database, all keywords were used with Medical Subject Headings (MeSH).
Eligibility criteria
Cohort studies and case-control studies investigating the associations between active or passive smoking and breast cancer risk among Chinese females were initially reviewed. Studies that reported risk estimates [odds ratios (ORs) or relative risks (RRs)] and 95% confidence intervals (CIs) or cross-table data were included.
The following studies were excluded: (1) case-control study with benign breast disease selected as controls, (2) case-control study with sample size less than 100 in each arm, (3) study with incomplete data of interest, and (4) duplicate publications.
Study selection and data extraction
Two review authors, working independently and in parallel, scanned the abstracts for information concerning the association between active or passive smoking and breast cancer risk, and obtained the full texts of the studies when necessary. After obtaining the full texts, the review authors independently assessed the eligibility of the studies. In the case of multiple publications or overlapping data sets, only studies with the largest or the most updated results were included.
Information on the baseline characteristics (type of study, year of publication, first author, sample size in each arm, and region of China), the methodologic quality of study, and the risk estimates (ORs or RRs) and their 95% CIs or cross-table data were collected. ORs calculated from both the univariate and multivariate logistic regression models were used in the final analysis.
Any disagreement in study selection and data collection was adjudicated by a third reviewer.
Assessment of the methodologic quality of study
The methodologic quality of observational study was independently assessed by two reviewers according to Newcastle-Ottawa Scale (NOS) based on three broad perspectives[13]: (1) the selection of the study groups, (2) the comparability of the groups, and (3) the ascertainment of exposure or outcome of interest. All studies were finally divided into three groups based on NOS scores: scores of 8–9, 5–7, and 0–4. To minimize the bias due to the judgment of NOS, any disagreement in this assessment was adjudicated by a third reviewer.
Statistical analysis
First, for studies with cross-table data, the ORs and 95% CIs were calculated based on these cross tables. Second, the ORs and 95% CIs calculated from the cross tables were combined with ORs and 95% CIs calculated from univariate logistic regression, which was performed for studies only reporting these data but not cross-table data. Finally, overall ORs and 95% CIs were calculated from all of these ORs and 95% CIs using a random-effect model weighted with the inverse of the variance.
The I2 statistic was calculated to determine the size of heterogeneity[14]. Potential publication bias was assessed with the Egger tests and represented graphically with funnel plots of the OR versus its standard error[15]. Pre-specified subgroup meta-analyses were used to explore potential sources of heterogeneity according to type of study, NOS scores, sample size (≥ 400 vs. < 400), year of publication (≥ 2007 vs. < 2007), and regions of China.
Sensitivity analyses on studies reporting multivariate adjusted ORs were conducted to explore the effect of the potential confounding factors. Sensitivity analyses were also conducted to test whether the primary results were affected by the studies that fell outside of the funnel plot.
Additional analyses were conducted to explore whether (1) breast cancer risk differed between exposure to husband's smoking and to smoke in the workplace and (2) the risk of breast cancer increased as the exposure of passive smoking increased. After summarizing the definition of light and heavy passive smoking in the included studies, light and heavy passive smoking as a result of husband's smoking were defined here as < 20 cigarettes per day and ≥ 20 cigarettes per day, respectively, and light and heavy passive smoking as a result of exposure in the workplace were defined here as < 300 min per day and ≥ 300 min per day, respectively.
All the statistical analyses were performed with STATA 12.0. P values < 0.05 were considered significant in all tests except the heterogeneity test (P < 0.10).
Results
A total of 56 articles were initially identified as case-control studies or cohort studies reporting association between active or passive smoking and breast cancer risk among Chinese females[16]–[71]. After discarding five duplicate publications[67]–[71], 51 articles covering 17 provinces of China were finally included in this systematic review, including 3 cohort studies[35],[42],[54] and 48 case-control studies. There were 21 articles focusing on active smoking only, 19 articles on passive smoking only, and 11 articles on both active and passive smoking (Table 1).
Table 1. Characteristics of included studies on the relationship between active or passive smoking and breast cancer.
| Reference | Authors | Year of publication | Region of China | Study design | Number of cases | Number of controls | NOSa | Includedb |
| [16] | Lu et al. | 1992 | Shanghai | Case-control | 552 | 552 | B | 2 |
| [17] | Liu et al. | 1994 | Guangdong | Case-control | 125 | 250 | B | 3 |
| [18] | Ye et al. | 1995 | Anhui | Case-control | 100 | 100 | B | 3 |
| [19] | Lai et al. | 1996 | Taiwan | Case-control | 114 | 228 | B | 1 |
| [20] | Xu et al. | 1997 | Hebei | Case-control | 101 | 101 | B | 1 |
| [21] | Yang et al | 1997 | Taiwan | Case-control | 244 | 450 | B | 1 |
| [22] | Liu et al. | 1998 | Chongqing | Case-control | 155 | 155 | B | 3 |
| [23] | Tan et al. | 1998 | Hunan | Case-control | 146 | 146 | B | 3 |
| [24] | Wei et al. | 1998 | Heilongjiang | Case-control | 160 | 320 | B | 1 |
| [25] | Huang et al. | 1999 | Taiwan | Case-control | 150 | 150 | A | 1 |
| [26] | Zhao et al. | 1999 | Sichuan | Case-control | 265 | 265 | B | 2 |
| [27] | Liu et al. | 2000 | Chongqing | Case-control | 186 | 186 | B | 3 |
| [28] | Zhu et al. | 2000 | Jiangsu | Case-control | 116 | 116 | B | 3 |
| [29] | Cao et al. | 2001 | Guangdong | Case-control | 348 | 348 | B | 3 |
| [30] | Lin et al. | 2001 | Shandong | Case-control | 186 | 186 | B | 3 |
| [31] | Zha et al. | 2001 | Guangdong | Case-control | 352 | 352 | B | 3 |
| [32] | Zou et al. | 2002 | Hubei | Case-control | 112 | 112 | B | 2 |
| [33] | Shrubsole et al. | 2004 | Shanghai | Case-control | 1,459 | 1,556 | A | 3 |
| [34] | Louis et al. | 2005 | Hongkong | Case-control | 198 | 358 | B | 1 |
| [35] | Shannon et al. | 2005 | Shanghai | Cohort | 378 | 1,070 | A | 3 |
| [36] | Chou et al. | 2006 | Taiwan | Case-control | 146 | 285 | B | 1 |
| [37] | Huang et al. | 2006 | Guangdong | Case-control | 133 | 133 | B | 2 |
| [38] | Li et al. | 2006 | Sichuan | Case-control | 104 | 154 | B | 1 |
| [39] | Li et al. | 2006 | Sichuan | Case-control | 121 | 211 | B | 1 |
| [40] | Li et al. | 2006 | Liaoning | Case-control | 449 | 363 | B | 1 |
| [41] | Wang et al. | 2006 | Zhejiang | Case-control | 101 | 101 | B | 3 |
| [42] | Wang et al. | 2006 | Zhejiang | Cohort | 84 | 269 | A | 2 |
| [43] | Jin et al. | 2007 | Jiangsu | Case-control | 206 | 214 | B | 2 |
| [44] | Li et al. | 2007 | Hebei | Case-control | 175 | 175 | B | 3 |
| [45] | Ma et al. | 2007 | Shandong | Case-control | 105 | 100 | B | 1 |
| [46] | Lin et al. | 2008 | Zhejiang | Case-control | 237 | 237 | B | 3 |
| [47] | Ren et al. | 2008 | Liaoning | Case-control | 200 | 200 | B | 3 |
| [48] | Nie et al. | 2009 | Yunnan | Case-control | 200 | 200 | B | 1 |
| [49] | Wang et al. | 2009 | Chongqing | Case-control | 367 | 367 | B | 1 |
| [50] | Zhang et al. | 2009 | Guangdong | Case-control | 438 | 438 | B | 2 |
| [51] | Zhang et al. | 2009 | Zhejiang | Case-control | 1,009 | 1,009 | B | 3 |
| [52] | Qian et al. | 2010 | Jiangsu | Case-control | 698 | 813 | B | 1 |
| [53] | Shi et al. | 2010 | Jiangsu | Case-control | 223 | 223 | B | 3 |
| [54] | Shrubsole et al. | 2011 | Shanghai | Cohort | 718 | 72,519 | A | 1 |
| [55] | Wang et al. | 2011 | Shandong | Case-control | 150 | 150 | B | 3 |
| [56] | Wang et al. | 2011 | Sichuan | Case-control | 400 | 400 | A | 1 |
| [57] | Zang et al. | 2011 | Shandong | Case-control | 348 | 1,044 | B | 2 |
| [58] | Zhang et al. | 2011 | Zhejiang | Case-control | 1,009 | 1,009 | B | 1 |
| [59] | Zheng et al. | 2011 | Tianjin | Case-control | 1,541 | 1,598 | A | 1 |
| [60] | Liao et al. | 2012 | Guangdong | Case-control | 285 | 285 | B | 1 |
| [61] | Xu et al. | 2012 | Multi-center | Case-control | 416 | 1,156 | B | 1 |
| [62] | Yu et al. | 2012 | Shandong | Case-control | 103 | 309 | B | 2 |
| [63] | Zhang et al. | 2012 | Zhejiang | Case-control | 252 | 248 | B | 1 |
| [64] | Gao et al. | 2013 | Jiangsu | Case-control | 669 | 682 | A | 2 |
| [65] | Hu et al. | 2013 | Hubei | Case-control | 196 | 211 | B | 2 |
| [66] | Tang et al. | 2013 | Guangdong | Case-control | 839 | 863 | B | 3 |
aNOS, Newcastle-Ottawa scale. A, NOS scores 8-9; B, NOS scores 5-7; C, NOS scores 1-4.
b1, only included in the meta-analysis of active smoking with breast cancer; 2, included in the meta-analysis of both active and passive smoking with breast cancer; 3, only included in the meta-analysis of passive smoking with breast cancer
Overall association of active and passive smoking with breast cancer risk
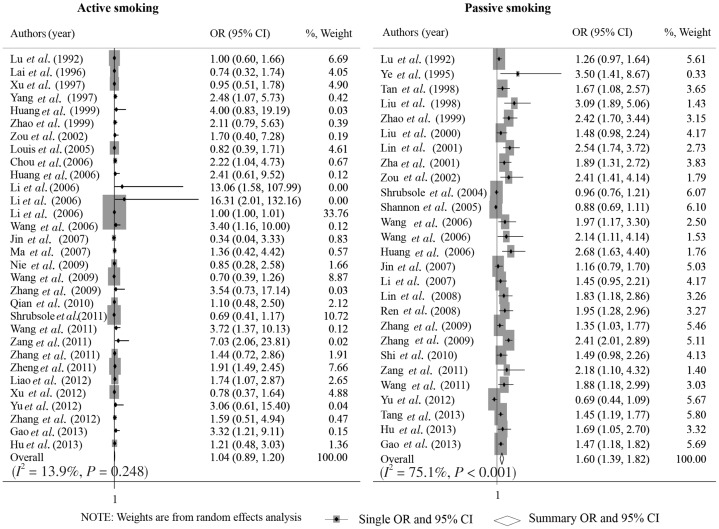
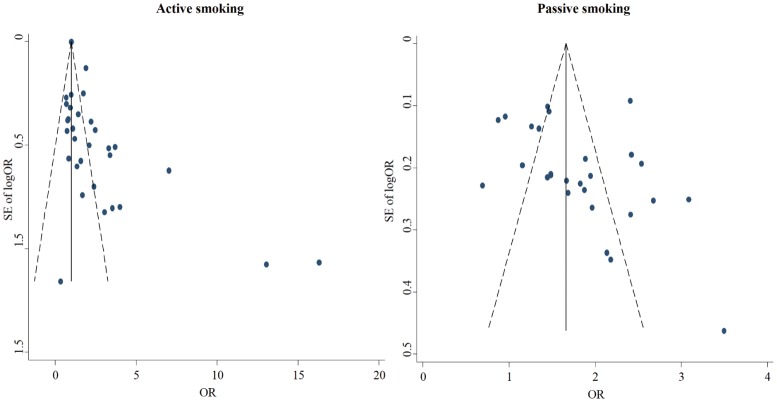
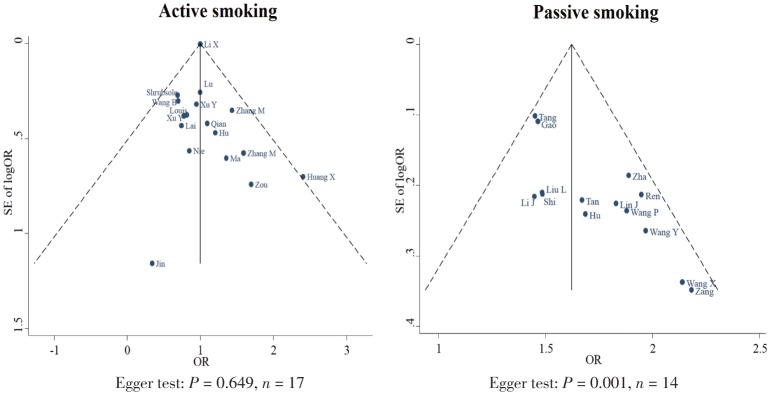
As shown in Figure 1, the overall OR of active and passive smoking with breast cancer risk was 1.04 (95% CI: 0.89–1.20; I2 = 13.9%, P = 0.248; n = 31) and 1.62 (95% CI: 1.39–1.85; I2 = 75.8%, P < 0.001; n = 26), respectively. The funnel plots showed no evidence of publication bias among the included studies on passive smoking (Egger test, P = 0.166), but there might be publication bias among those on active smoking (Egger test, P = 0.001; Figure 2).
Figure 1. Forest graph on the association of active and passive smoking with breast cancer risk.
Each row in the forest graph represents the original odds ratio (OR) with 95% confidence interval (CI) reported in one study. And the last row represents the overall association of active and passive smoking with breast cancer risk.
Figure 2. Funnel plots on the association of active and passive smoking with breast cancer risk.
Each plot represents the original OR against its standard error of OR reported in one included study. The full line in the middle and the imaginary lines in the two sides represent the overall OR with 95% CI.
Association of active and passive smoking with breast cancer risk for different subgroups
As shown in Figure 3, there was a significant association between passive smoking and risk of breast cancer in case-control studies (OR: 1.66; 95% CI: 1.42–1.90) but not in cohort studies, and in studies with NOS score of 5–7 (OR: 1.75; 95% CI: 1.47–2.02) but not in studies with a higher NOS score. Both the studies with sample size larger or equal to and less than 400 revealed a significant association of passive smoking with breast cancer risk, with an OR of 1.44 (95% CI: 1.17–1.70) and 1.87 (95% CI: 1.59–2.14), respectively. And studies either published in and after 2007 or before 2007 also observed a significant association of passive smoking with breast cancer risk, with an OR of 1.55 (95% CI: 1.23–1.88) and 1.71 (95% CI: 1.37–2.04), respectively. Although there was no significant association of passive smoking with breast cancer risk in studies conducted in Shandong or Shanghai, the significant association was observed in studies conducted in Jiangsu, Zhejiang, and other regions of China, with an OR of 1.38 (95% CI: 1.14–1.62), 2.24 (95% CI: 1.89–2.60), and 1.76 (95% CI: 1.49–2.03), respectively. No significant associations between active smoking and breast cancer risk were found in subgroup analyses.
Figure 3. Association of active and passive smoking with breast cancer risk for different subgroups.
Each diamond represents the overall OR with 95% CI for the specific subgroups studies.
Association of active and passive smoking with breast cancer risk after adjusting for potential confounding factors
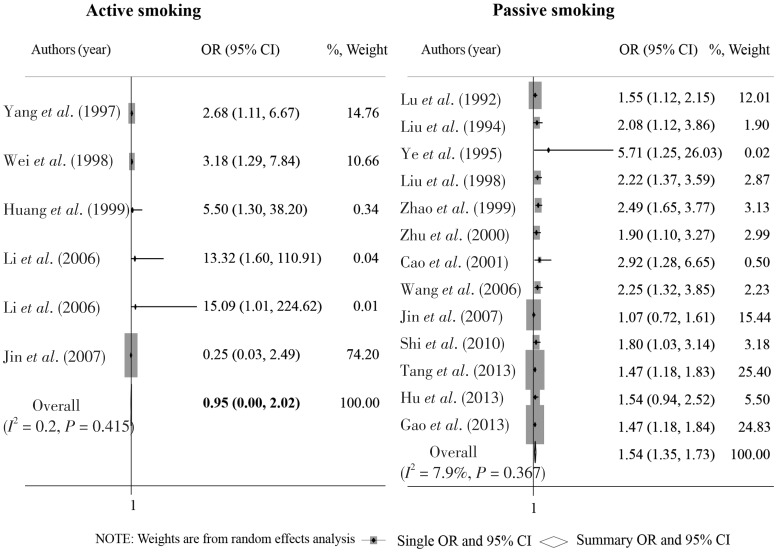
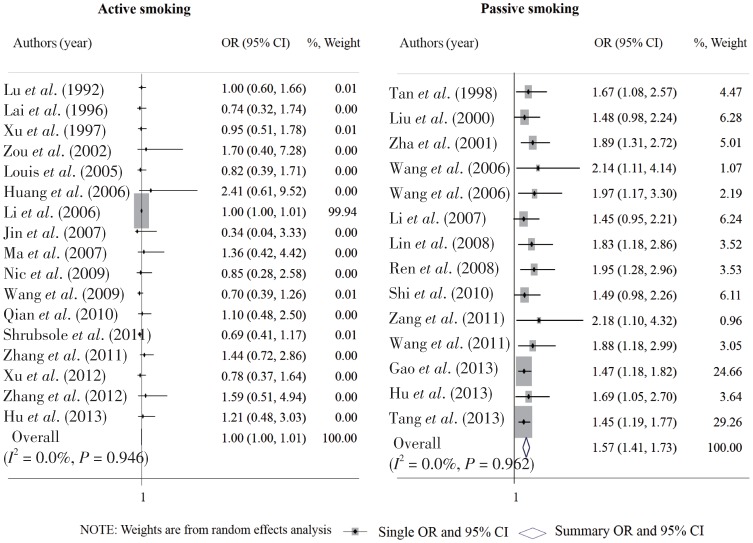
A total of 6 and 12 studies reported adjusted ORs of breast cancer risk with active and passive smoking, respectively. The overall OR based on these adjusted ORs was 0.95 (95% CI: 0.00–2.02) for active smoking and 1.59 (95% CI: 1.34–1.83) for passive smoking (Figure 4). After excluding studies that fell outside of the funnel plot in the primary analyses, the overall OR was 1.00 (95% CI: 1.00–1.01) for active smoking and 1.62 (95% CI: 1.43–1.80) for passive smoking (Figures 5 and 6). No significant heterogeneity was found among these sensitivity analyses.
Figure 4. Forest graph on the association of active and passive smoking with breast cancer risk after adjusting for potential confounding factors.
Each row in the forest graph represents the original odds ratio (OR) with 95% confidence interval (CI) reported in one study. The diamond in the last row represents the overall OR with 95% CI.
Figure 5. Sensitivity analysis after excluding studies that fell outside of the funnel plots in the primary meta-analysis on the association of active and passive smoking with breast cancer risk.
Each row in the forest graph represents the original OR with 95% CI reported in one study. The diamond in the last row represents the overall OR with 95% CI.
Figure 6. Funnel plots based on sensitivity analysis after excluding studies that fell outside of the funnel plots in the primary meta-analysis on the association of active and passive smoking with breast cancer risk.
Each plot represents the original OR against its standard error of OR reported in one included study. The full line in the middle and the imaginary lines in the two sides represent the overall OR with 95% CI.
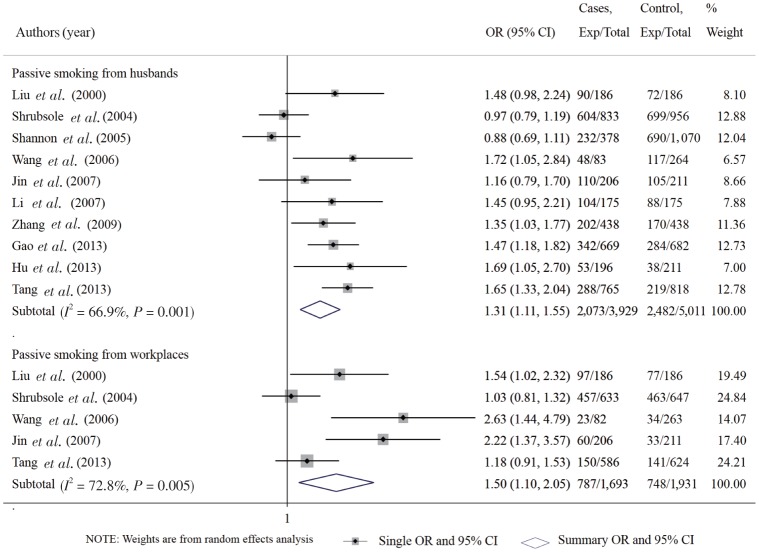
Association between passive smoking and breast cancer risk for different sources and levels of exposure
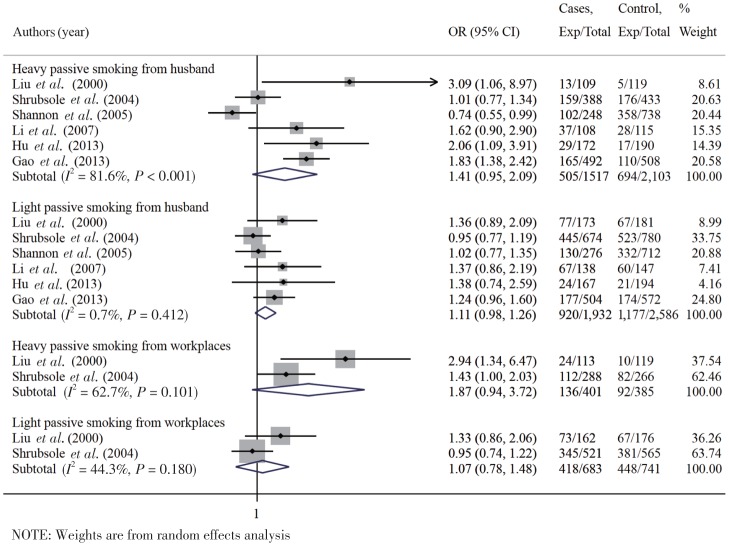
Additional analyses showed that breast cancer risk was significantly associated with passive exposure to husband's smoking (n = 9 studies) and to smoke in the workplace (n = 4 studies), with overall OR of 1.27 (95% CI: 1.07–1.50) and 1.66 (95% CI: 1.07–2.59), respectively (Figure 7). Further additional analysis showed that the OR of light and heavy passive smoking was 1.11 (95% CI: 0.98–1.25) and 1.41 (95% CI: 0.95–2.09) for women exposed to their husband's smoking (< 20 and≥20 cigarettes per day), and 1.07 (95% CI: 0.78–1.48) and 1.87 (95% CI: 0.94–3.72) for those exposed to smoke in the workplace (< 300 and≥300 min of exposure per day), respectively (Figure 8).
Figure 7. Forest graph on the association of passive smoking with breast cancer risk according to exposure to husband's smoking or to the smoke of workplace.
Each row in the forest graph represents the original OR with 95% CI reported in one study. The diamond in the last row represents the overall OR with 95% CI.
Figure 8. Forest graph on the association of passive smoking with breast cancer risk for different sources and levels of exposure.
Each row in the forest graph represents the original OR with 95% CI reported in one study. The diamond in the last row represents the overall OR with 95% CI.
Discussion
The present study suggests that Chinese females exposed to secondhand smoke have an increased risk of breast cancer, and the risk seems to increase as the level of passive exposure to smoke increases. Furthermore, women passively exposed to smoke in the workplace had a higher risk of breast cancer than those exposed to their husband's smoking in the home.
Our main findings were consistent with those in several previous studies, but three large American cohort studies revealed a negative association between passive smoking and breast cancer risk[8]–[10]. Although cohort studies were generally considered superior to case-control studies because they avoided recall bias, they have serious limitations[72]. The potential primary cause for the non-significant associations in the three cohort studies was substantial exposure misclassification: women with regular passive exposure to smoke may have been categorized in the unexposed group[72]. This misclassification induced bias and underestimated the association of passive smoking with breast cancer risk. Specifically, in two of the three American cohort studies, passive exposure to smoke was either based exclusively on the husband's smoking history[8] or household exposure[10], and thus women who were exposed to others' smoking or had occupational exposure to smoke were misclassified as unexposed. In the third cohort study[9], the exposure measure was based on women's self-reported passive exposure to smoke and ignored historical exposure (including exposure from the 32.6% of husbands who were former smokers, historic workplace exposure, and all childhood exposure).
This misclassification bias may also be the most important reason why we did not observe significant association between passive smoking and breast cancer risk based on cohort studies. In Shannon's cohort (from Shanghai)[35] and Gao's study[64], passive smoking was based exclusively on the husband's smoking history. In Wang's cohort[42] and Shrubsole's study (from Shanghai)[33], sources of passive exposure to smoke only included the husband's smoking within the household but ignored exposure to smoke from other sources and exposure during childhood. As these four studies had NOS scores of 8–9 and two were studies conducted in Shanghai, the additional and subgroup analyses did not produce significant results among studies with NOS scores of 8–9 and studies conducted in Shanghai.
Additionally, high passive exposure to smoke in the control group might be another important determinant of the observed non-significant associations in the subgroup analyses. In fact, in several studies, more than 50% of females in the control group were passively exposed to smoke, including studies reported by Shrubsole et al.[33] (80%), Zha et al.[31] (72%), Lu et al.[16] (69%), Shannon et al.[35] (64%), Yu et al.[62] (59%), Ren et al.[47] (58%), and Wang et al.[42] (51%). As argued by Brind for studies on induced abortion in China, once the prevalence of a given exposure rises to a level of predominance in the control group, statistical adjustment cannot remove all confounding effects caused by the adjustment terms.
Consistent with the collaborative re-analysis of the worldwide evidence from 53 epidemiological studies[7], we found active smoking had no or little effects on the risk of breast cancer. There might be several reasons for the non-significant association of active smoking with breast cancer risk. First, breast cancer caused by smoking was theoretically associated with long latency period of exposure, nearly 30 years or more[66],[74]. However, in most included studies, the observation intervals were not long enough to ascertain breast cancer, which underestimated the real effects of smoking on breast cancer risk. Second, potential confounders, especially alcohol drinking, greatly attenuated the real association between active smoking and breast cancer risk. As we knew, smokers generally drink more alcohol than non-smokers, and alcohol intake may also be associated with breast cancer risk[7]. Although we conducted sensitivity analyses based on the adjusted OR, it is possible that residual confounding effects could still attenuate the association between active smoking and breast cancer risk. Third, the small prevalence of female smokers in China, such as 0.46% in Zhang et al.[50], 0.65% in Li et al.[38], 0.73% in Gao et al.[64], and 0.97% in Yu et al.[62], may also limit the power of finding a significant association between active smoking and breast cancer risk. Finally, active smoking in most studies was self-reported, which might also attenuate the possible association between active smoking and breast cancer risk.
Although the present study revealed non-significant association between active smoking and breast cancer risk, active smoking still warrants attention. Tobacco smoke contains over 7,000 chemicals including 69 established carcinogens, 20 of which are known mammary carcinogens[47]. There is also strong evidence that many of these carcinogens can reach mammary tissue[63]. Therefore, we still emphasize the importance of smoking cessation and tobacco control.
Our study had several strengths. First, we extended our search strategy to include all potential studies with information on smoking among Chinese females, rather than focusing solely on smoking. Thus, we included more than 10 studies that were excluded in Chen's study[11]. Second, we performed several subgroup and sensitivity analyses and found these analyses confirmed the reliability of our primary results. Third, additional analyses helped us to better elucidate the role of active and passive smoking on breast cancer risk. However, because the studies analyzed here did not include passive exposure to smoke from other members within the household, therefore, based on the current evidences, we could only conclude that passive smoking in the workplace poses a greater risk for breast cancer than passive exposure to husband's smoking but not passive smoking in the household.
In addition, there are also several potential limitations to our meta-analysis. First, moderate heterogeneity was observed in the primary analysis for passive smoking, and the heterogeneity was not significantly improved in subgroup analysis. Thus, factors in addition to those listed in the subgroup analysis may influence our results. Second, the current systematic review cannot overcome the limitations of the original studies. Though a detailed protocol with explicit criteria for study selection and strict strategies for data extraction were developed before the study, the limitations in exposure definitions and population selection in the original will still affect the current results. Third, due to lack of individual information as in other systematic reviews, the current systematic review cannot control the potential confounding bias caused by other genetic and environmental factors of breast cancer, even if the maximum adjusted ORs were used for sensitivity analysis. Fourth, due to inadequate overall power, we observed a borderline but not significant dose-response relationship between the level of passive exposure to smoke and breast cancer risk. Therefore, the current results should be interpreted carefully.
In summary, this study suggests that passive smoking is associated with an increased risk of breast cancer among Chinese females, and the risk seems to increase as passive exposure to smoke increases. Women passively exposed to smoke in the workplace have a higher risk of breast cancer than those passively exposed to their husbands' smoking. If passive smoking were to be confirmed as a risk factor for breast cancer, high rates of passive smoking in China may contribute to increasing breast cancer incidence. Tobacco control, especially in the public places, is urgently needed in China in the future.
Acknowledgments
We would like to thank all the staff in the Department of Epidemiology and Biostatistics, Tianjin Medical University Cancer Institute and Hospital. Our work was supported partly by grants from the National Natural Science Foundation of China (No. 81172762), program for Changjiang Scholars and Innovation Research Team in University in China (No. IRT1076), National Scientific and Technological Project (No. 2011ZX09307-001-04), Tianjin Science Committee Foundation (No. 09ZCZDSF04800 and No. 09ZCZDSF04700), Tianjin Science and Technology Committee Foundation (No. 12ZCDZSY16000 and No. 11ZCGYSY02200), and Major State Basic Research Program for China (973 Program; No. 2009CB918903).
References
- 1.Chen W, Zheng R, Zeng H, et al. Trend analysis and projection of cancer incidence in China between 1989 and 2008. Zhonghua Zhong Liu Za Zhi. 2012;7:517–524. doi: 10.3760/cma.j.issn.0253-3766.2012.07.010. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 2.Gu D, Kelly T, Wu X, et al. Mortality attributable to smoking in China. N Engl J Med. 2009;360:150–159. doi: 10.1056/NEJMsa0802902. [DOI] [PubMed] [Google Scholar]
- 3.Gan Q, Smith K, Hammond S, et al. Disease burden of adult lung cancer and ischaemic heart disease from passive tobacco smoking in China. Tobacco control. 2007;16:417–422. doi: 10.1136/tc.2007.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang G, Fan L, Tan J, et al. Smoking in China: findings of the 1996 national prevalence survey. JAMA. 1999;282:1247–1253. doi: 10.1001/jama.282.13.1247. [DOI] [PubMed] [Google Scholar]
- 5.Li Q, Hsia J, Yang G. Prevalence of smoking in China in 2010. N Engl J Med. 2011;364:2469–2470. doi: 10.1056/NEJMc1102459. [DOI] [PubMed] [Google Scholar]
- 6.WHO . WHO report on the global tobacco epidemic, 2008: The MPOWER package. Geneva: World Health Organization; 2008. [Google Scholar]
- 7.Hamajima N, Hirose K, Tajima K, et al. Alcohol, tobacco and breast cancer—collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87:1234–1245. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wartenberg D, Calle EE, Thun MJ, et al. Passive smoking exposure and female breast cancer mortality. J Natl Cancer Inst. 2000;92:1666–1673. doi: 10.1093/jnci/92.20.1666. [DOI] [PubMed] [Google Scholar]
- 9.Egan KM, Stampfer MJ, Hunter D, et al. Active and passive smoking in breast cancer: prospective results from the nurses' health study. Epidemiology. 2002;13:138–145. doi: 10.1097/00001648-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds P, Hurley S, Goldberg DE, et al. Active smoking, household passive smoking, and breast cancer: evidence from the California teachers study. J Natl Cancer Inst. 2004;96:29–37. doi: 10.1093/jnci/djh002. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z, Shao J, Gao X, et al. Effect of passive smoking on female breast cancer in China: a meta-analysis. Asia Pca J Public Health. 2013 Apr 9; doi: 10.1177/1010539513481493. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observa-tional studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 13.Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. 2013 Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed on June 5, 2013. [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsis-tency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu R, Cai H, Xu X, et al. A case-control study for breast cancer of women in Shanghai. Zhong Liu. 1992;12:65–69; 78. [in Chinese] [Google Scholar]
- 17.Liu R, Huang W, Guo G. Logistic regression analysis on risk factors of female breast cancer. Jinan Da Xue Xue Bao. 1994;21:102–104. [in Chinese] [Google Scholar]
- 18.Ye D, Zhang L, Ling D, et al. Study on the risk factors of female breast cancer in Hefei. Anhui Yi Ke Da Xue Xue Bao. 1995;30:16–18. [in Chinese] [Google Scholar]
- 19.Lai FM, Chen P, Ku HC, et al. A case-control study of parity, age at first full-term pregnancy, breast feeding and breast cancer in Taiwanese women. Zhong Guo Ke Xue Yuan Yuan Kan. 1996;20:71–77. [in Chinese] [PubMed] [Google Scholar]
- 20.Xu Y, Meng Y, Zhang W. A case-control study of female breast cancer in Tangshan rural area. Shi Yong Ai Zheng Za Zhi. 1997;12:54–57. [in Chinese] [Google Scholar]
- 21.Yang PS, Yang TL, Liu CL, et al. A case-control study of breast cancer in Taiwan—a low-incidence area. Br J Cancer. 1997;75:752–756. doi: 10.1038/bjc.1997.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, Wu K, Lin X, et al. A case control study on risk factors of female breast cancer in Chongqing. Zhong Guo Zhong Liu. 1998;7:12–14. [in Chinese] [Google Scholar]
- 23.Tan H, Liu A, Wang J, et al. A case control study on multiple risk factors of female breast cancer. Hunan Yi Ke Da Xue Xue Bao. 1998;23:38–41. [in Chinese] [PubMed] [Google Scholar]
- 24.Wei Q, Li H, Liu R, et al. Research on the etiology of breast cancer in Harbin. Ji Bing Kong Zhi Za Zhi. 1998;1:15–17. [in Chinese] [Google Scholar]
- 25.Huang CS, Shen CY, Chang KJ, et al. Cytochrome p4501a1 polymorphism as a susceptibility factor for breast cancer in postmenopausal Chinese women in Taiwan. Br J Cancer. 1999;80:1838–1843. doi: 10.1038/sj.bjc.6690608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, Shi Z, Liu L, et al. Matched case-control study for detecting risk factors of breast cancer in women living in Chengdu. Zhonghua Liu Xing Bing Xue Za Zhi. 1999;20:91–94. [in Chinese] [PubMed] [Google Scholar]
- 27.Liu L, Wu K, Lin X, et al. Passive smoking and other factors at different periods of life and breast cancer risk in Chinese women who have never smoked—a case-control study in Chongqing, People's Republic of China. APJCP. 2000;1:131–137. [PubMed] [Google Scholar]
- 28.Zhu M, Tong J, Zhang Z, et al. Case-control study on risk factors of female breast cancer. Zhong Guo Gong Gong Wei Sheng. 2000;16:251. [in Chinese] [Google Scholar]
- 29.Cao K, Wu Y, Ma G, et al. A case control study on risk factors of female breast cancer in Guangzhou. Zhong Guo Zhong Liu. 2001;10:702–704. [in Chinese] [Google Scholar]
- 30.Lin X, Xu G, Xu H, et al. Case-control study on the risk factors of breast cancer among women in Jinan. Shandong Da Xue Xue Bao. 2001;39:552–553. [in Chinese] [Google Scholar]
- 31.Zha Y. Case-control study on the risk factors of female breast cancer. Shi Yong Yi Xue Za Zhi. 2001:646. [in Chinese] [Google Scholar]
- 32.Zou L, Tian J, Wu H. Case-control study on the risk factors of female breast cancer in Qingshan district of Wuhan city. Zhong Guo Zhong Liu Hu Li Xue Hui Jiao Liu Hui Yi Lun Wen Hui Bian. Dalian, Liaoning, China. 2003:56–59. [in Chinese] [Google Scholar]
- 33.Shrubsole MJ, Gao YT, Dai Q, et al. Passive smoking and breast cancer risk among non-smoking Chinese women. Int J Cancer. 2004;110:605–609. doi: 10.1002/ijc.20168. [DOI] [PubMed] [Google Scholar]
- 34.Chow LWC, Lui KL, Chan JCY, et al. Association between body mass index and risk of formation of breast cancer in Chinese women. Asian J Surg. 2005;28:179–184. doi: 10.1016/S1015-9584(09)60338-9. [DOI] [PubMed] [Google Scholar]
- 35.Shannon J, Ray R, Wu C, et al. Food and botanical groupings and risk of breast cancer: a case-control study in shanghai, China. Cancer Epidemiol Biomarkers Prev. 2005;14:81–90. [PubMed] [Google Scholar]
- 36.Chou YC, Wu MH, Yu JC, et al. Genetic polymorphisms of the methylenetetrahydrofolate reductase gene, plasma folate levels and breast cancer susceptibility: a case-control study in Taiwan. Carcinogenesis. 2006;27:2295–2300. doi: 10.1093/carcin/bgl108. [DOI] [PubMed] [Google Scholar]
- 37.Huang X, Wang C, Zhou Y, et al. A elementary study on risk factors of breast cancer of women in Shenzhen Baoan area. Zhong Yuan Yi Kan. 2006:37–39. [in Chinese] [Google Scholar]
- 38.Li J, Sheng W, Yang F, et al. Study on serum organ chlorines pesticides (DDTs) level, CYP1a1 genetic polymorphism and risk of breast cancer: A case control study. Zhonghua Liu Xing Bing Xue Za Zhi. 2006;27:217–222. [in Chinese] [PubMed] [Google Scholar]
- 39.Li S, Li J, Lei F, et al. A case-control study on risk factors of breast cancer. Xian Dai Yi Xue Za Zhi. 2006:2233–2235. [in Chinese] [Google Scholar]
- 40.Li X, He M, Xu Z, et al. A case-control study on risk factors of female breast cancer. Ji Bing Kong Zhi Za Zhi. 2006;10:8–11. [in Chinese] [Google Scholar]
- 41.Wang X, Chen W, Lei T, et al. A case-control study on risk factors of female breast cancer in rural area of Cixi city, Zhejiang province. Zhong Guo Zhong Liu. 2006;15:294–297. [in Chinese] [Google Scholar]
- 42.Wang Y. Case-control study on the risk factors of breast cancer based on a cohort population in Jiashan country [dissertation] Hangzhou, Zhejiang, China: Zhejiang University; 2006. [in Chinese] [Google Scholar]
- 43.Ji Y. Case-control study on the associations of genetic polymorphisms of ER and PR with the risk of breast cancer [dissertation] Suzhou, Jiangsu, China: Suzhou University; 2007. [in Chinese] [Google Scholar]
- 44.Li J, Liu H, Rong S, et al. A case-control study on environmental risk factors of female breast cancer in Tangshan. Zhong Guo Gong Gong Wei Sheng. 2007;23:312–314. [in Chinese] [Google Scholar]
- 45.Ma R. The association of serum hormones levels with both risk and prognosis of female breast cancer [dissertation] Jinan, Shandong, China: Shandong University; 2007. [in Chinese] [Google Scholar]
- 46.Lin J, Yu J. A case-control study on risk factors of breast cancer among women in Cixi. Zheijiang Yu Fang Yi Xue. 2008;20:3–5. [in Chinese] [Google Scholar]
- 47.Ren X. A 1:1 case-control study on risk factors of breast cancer [dissertation] Dalian, Liaoning, China: : Dalian Medical University; 2008. [in Chinese] [Google Scholar]
- 48.Nie JY, Jin CG, Tang YY, et al. Study on behavior risk factors of female breast cancer in Yunnan province China. Xian Dai Zhong Liu Yi Xue. 2009;17:656–657. [in Chinese] [Google Scholar]
- 49.Wang B, Mi M, Wang J, et al. Does the increase of endogenous steroid hormone levels also affect breast cancer risk in Chinese women? A case-control study in Chongqing, China. Int J Cancer. 2009;124:1892–1899. doi: 10.1002/ijc.24132. [DOI] [PubMed] [Google Scholar]
- 50.Zhang CX, Ho SC, Chen YM, et al. Meat and egg consumption and risk of breast cancer among Chinese women. Cancer Causes Control. 2009;20:1845–1853. doi: 10.1007/s10552-009-9377-0. [DOI] [PubMed] [Google Scholar]
- 51.Zhang M, Huang J, Xie X, et al. Dietary intakes of mushrooms and green tea combine to reduce the risk of breast cancer in Chinese women. Int J Cancer. 2009;124:1404–1408. doi: 10.1002/ijc.24047. [DOI] [PubMed] [Google Scholar]
- 52.Qian Y, Zhang J, Dong J, et al. Relationship between polymor-phisms of X-ray repair cross-complementing group 1 gene Arg194Trp, Arg399Gln and susceptibility of breast cancer. Zhonghua Yu Fang Yi Xue Za Zhi. 2010;44:242–246. [in Chinese] [PubMed] [Google Scholar]
- 53.Shi P, Xu M, Qian Y, et al. Matched case—control study for detecting risk factors of breast cancer in women living in Wuxi. Xian Dai Yi Xue Za Zhi. 2010;37:2428–2431. [in Chinese] [PubMed] [Google Scholar]
- 54.Shrubsole MJ, Shu XO, Li HL, et al. Dietary b vitamin and methionine intakes and breast cancer risk among Chinese women. Am J Epidemiol. 2011;173:1171–1182. doi: 10.1093/aje/kwq491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang P, Xie S, Zhang C, et al. Relationship of passive smoking and N-acetyltransferase 2 polymorphisms with breast cancer susceptibility. Zhong Guo Xian Dai Yi Xue Za Zhi. 2011;21:3271–3275. [in Chinese] [Google Scholar]
- 56.Wang Q, Li H, Tao P, et al. Soy isoflavones, CYP1A1, CYP1B1, and COMT polymorphisms, and breast cancer: a case-control study in southwestern China. DNA Cell Biol. 2011;30:585–595. doi: 10.1089/dna.2010.1195. [DOI] [PubMed] [Google Scholar]
- 57.Zang S, Zhang Y. Analysis of 312 cases of breast cancer risk factors. Shi Yong Yi Yao Za Zhi. 2011:1078–1079. [in Chinese] [Google Scholar]
- 58.Zhang M, Holman CD. Low-to-moderate alcohol intake and breast cancer risk in Chinese women. Br J Cancer. 2011;105:1089–1095. doi: 10.1038/bjc.2011.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng H, Song F, Zhang L, et al. Genetic variants at the miR-124 binding site on the cytoskeleton-organizing IQGAP1 gene confer differential predisposition to breast cancer. Int J Oncol. 2011;38:1153–1161. doi: 10.3892/ijo.2011.940. [DOI] [PubMed] [Google Scholar]
- 60.Liao Y, Weng Q, You W. A conditional logistic 1:1 matched case-control study on the risk factors of female breast cancer in Huizhou region. Zhong Liu Yao Xue. 2012;2:229–231. [in Chinese] [Google Scholar]
- 61.Xu YL, Sun Q, Shan GL, et al. A case-control study on risk factors of breast cancer in China. Arch Med Sci. 2012;8:303–309. doi: 10.5114/aoms.2012.28558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu ZG, Jia CX, Geng CZ, et al. Risk factors related to female breast cancer in regions of northeast China: a 1:3 matched case-control population-based study. Chin Med J (Engl) 2012;125:733–740. [PubMed] [Google Scholar]
- 63.Zhang M, Jin M, Yu Y, et al. Associations of MiRNA polymorphisms and female physiological characteristics with breast cancer risk in Chinese population. Eur J Cancer Care (Engl) 2012;21:274–280. doi: 10.1111/j.1365-2354.2011.01308.x. [DOI] [PubMed] [Google Scholar]
- 64.Gao CM, Ding JH, Li SP, et al. Active and passive smoking, and alcohol drinking and breast cancer risk in Chinese women. APJCP. 2013;14:993–996. doi: 10.7314/apjcp.2013.14.2.993. [DOI] [PubMed] [Google Scholar]
- 65.Hu M, Han D, Sun S, et al. Bleomycin-induced mutagen sensitivity, passive smoking, and risk of breast cancer in Chinese women: a case-control study. Cancer Causes Control. 2013;24:629–636. doi: 10.1007/s10552-012-0137-1. [DOI] [PubMed] [Google Scholar]
- 66.Tang LY, Chen LJ, Qi ML, et al. Effects of passive smoking on breast cancer risk in pre/post-menopausal women as modified by polymorphisms of PARP1 and ESR1. Gene. 2013;524:84–89. doi: 10.1016/j.gene.2013.04.064. [DOI] [PubMed] [Google Scholar]
- 67.Zhang J, Xie Z, Zai S, et al. Matched case-control study for detecting risk factors of breast cancer in women living in Xinxiang, Henan province. Henan Zhong Liu Xue Za Zhi. 2003;16:201–203. [in Chinese] [Google Scholar]
- 68.Gao CM, Tajima K, Ding JH, et al. Body size, physical activity and risk of breast cancer-a case control study in Jiangsu province of China. APJCP. 2009;10:877–881. [PubMed] [Google Scholar]
- 69.Gao CM, Tang JH, Cao HX, et al. MTHFR polymorphisms, dietary folate intake and breast cancer risk in Chinese women. J Hum Genet. 2009;54:414–418. doi: 10.1038/jhg.2009.57. [DOI] [PubMed] [Google Scholar]
- 70.Murff HJ, Shu XO, Li H, et al. Dietary polyunsaturated fatty acids and breast cancer risk in Chinese women: a prospective cohort study. Int J Cancer. 2011;128:1434–1441. doi: 10.1002/ijc.25703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou L, He T, Jin Y, et al. A case-control study on risk factors of breast cancer. Zhong Guo Zhong Liu. 2011;18:27–30. [in Chinese] [Google Scholar]
- 72.Johnson KC. Accumulating evidence on passive and active smoking and breast cancer risk. Int J Cancer. 2005;117:619–628. doi: 10.1002/ijc.21150. [DOI] [PubMed] [Google Scholar]
- 73.Brind J, Chinchilli VM. Breast cancer and induced abortions in China. Br J Cancer. 2004;90:2244–2245. doi: 10.1038/sj.bjc.6601853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Terry PD, Miller AB, Rohan TE. Cigarette smoking and breast cancer risk: a long latency period? Int J Cancer. 2002;100:723–728. doi: 10.1002/ijc.10536. [DOI] [PubMed] [Google Scholar]