Abstract
Chylothorax is a rare complication of neck dissection, and bilateral chylothorax is even rarer. However, both are potentially serious and sometimes life-threatening, especially those that are associated with left neck dissection for head and neck neoplasms. We report one case of bilateral chylothorax following left supraclavicular dissection for breast cancer. This case was treated successfully with a new conservative management approach.
Keywords: Bilateral chylothorax, treatment, neck dissection, supraclavicular, breast cancer
Isolated regional nodal recurrences after curative treatment of breast cancer are rare, especially in supraclavicular nodes[1],[2]. Clinical outcomes are similar for patients with ipsilateral supraclavicular lymph node metastases at first presentation and for patients with recurrent ipsilateral supraclavicular lymph node metastases[3],[4]. Per the last accepted staging system for breast cancer[5], ipsilateral supraclavicular lymph node metastases is classified as regional disease (stage IIIC); but the survival rate was lower in patients with ipsilateral supraclavicular lymph node metastases than in patients with lower axillary or subclavian nodal involvement[6]. Nevertheless, patients with ipsilateral supraclavicular lymph node metastases who were treated with surgery or radiotherapy and achieved good neck control were reported to achieve better survival than those for whom surgical treatment or irradiation did not result in good local control[6]–[8]. Thus, neck dissection is sometimes necessary for breast cancer patients with ipsilateral supraclavicular lymph node metastases.
Chylous leakage is a common complication following neck dissection. However, postoperative chylothorax is uncommon, and bilateral chylothorax is even rarer. This condition may cause severe respiratory, metabolic, and immunologic dysfunctions and can even be fatal. Currently, there are no reported cases of bilateral chylothorax following neck dissection for breast cancer in the literature. Herein, we report a case of bilateral chylothorax following left supraclavicular dissection for breast cancer, which was treated successfully using a novel conservative management strategy.
Case Report
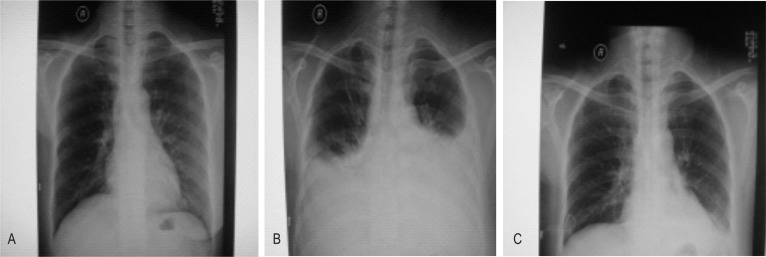
A 43-year-old woman presented with a 3-year history of a left-sided palpable breast mass. Physical examination revealed a 15 cm × 15 cm lump, fixed, invading the skin, accompanied with left supraclavicular lymphadenectasis. Results of chest X-ray, ventral ultrasonography, radioisotope bony scanning, and nuclear magnetic resonance imaging (MRI) of the head did not show distant metastasis. The pathologic diagnosis via core biopsy was adenocarcinoma, and clinical stage was IIIC (T4N3M0). The patient underwent 4 cycles of neoadjuvant chemotherapy, and therefore the mass was reduced to 25% of its original size (partial response). Radical mastectomy and left supraclavicular lymph node dissection were then performed. Chylous fluid output in the neck drainage tube was approximately 500 mL on the first day postoperation, but increased to 1,600 mL on the second day. An emergency operation was performed to ligate the fistula, and the leakage was ceased. Nevertheless, on the fourth day postoperation, the patient experienced dyspnea and chest discomfort. Physical examination revealed no neck edema or swelling, but bilateral breath sounds were diminished on auscultation. Chest X-ray, which did not show obvious abnormalities preoperatively (Figure 1A), revealed medium, bilateral pleural effusions (Figure 1B). Diagnostic thoracentesis was performed and milky fluid was aspirated from both sides. Biochemical analysis confirmed bilateral chylothorax.
Figure 1. Bilateral chylothorax occured after left supraclavicular dissection.
A, no obvious abnormalities are present on the preoperative chest X-ray. B, on the fourth day postoperation, chest X-ray shows medium, bilateral pleural effusions, accompanied with dyspnea and chest discomfort. Bilateral breath sounds were diminished upon auscultation. C, after conservative management, a repeated chest X-ray shows no evidence of residual effusions. These results suggest that conservative treatment of chylous leakage is effective.
We implemented a conservative management approach, which involved insertion of bilateral pigtail catheters into the thoracic cavity, total parenteral nutrition with total enteric rest, and administration of somatostatin (250 µg/h) by continuous intravenous drip on the basis of the review of literature. On the 12th day postoperation, the patient achieved complete remission of dyspnea and chest discomfort; 970 mL of chyle-like liquid was aspirated intermittently from bilateral pleural spaces. A repeated chest X-ray showed no evidence of residual effusions (Figure 1C). Thus, the drainage tubes were removed, and the patient was started on a low fat and protein diet for 3 days before resuming a regular diet. On the 15th day post-operation, the patient had recovered completely.
Discussion
Bilateral chylothorax is a rare complication that results from injury to the thoracic duct or one of its branches following neck dissection. We searched the English-language literature through PubMed and found 26 cases of bilateral chylothorax reported between 1951 and 2011[9]–[29]. We analyzed the primary diseases, treatment, and prognosis of bilateral chylothorax. The results showed that the majority of bilateral chylothorax cases followed neck dissection associated with head and neck cancers, such as thyroid cancer, laryngocarcinoma, and tongue cancer (Table 1). However, none of the reported cases described breast cancer as a cause for bilateral chylothorax.
Table 1. Cases of bilateral chylothorax following neck dissection reported in the literature.
| Year | Authors (reference) | Disease | Neck dissection | Treatment | Prognosis |
| 1951 | Frazell et al.[9] | Carcinomacutaneumfaciale | Left | Conservative | Recovery |
| 1976 | Coates et al.[10] | Laryngocarcinoma | Left | Conservative | Recovery |
| 1981 | Saraceno et al.[11] | Carcinoma of the floor of the mouth | Left | Conservative | Recovery |
| 1985 | Har-EI et al.[12] | Thyroid cancer | Left | Conservative | Recovery |
| 1985 | Ng et al.[13] | Carcinoma of the floor of the mouth | Left | Conservative | Recovery |
| 1988 | Pace-Balzet et al.[14] | Adenolymphoma | Left | Conservative | Recovery |
| 1989 | Oi et al.[15] | Maxillary sinus carcinoma | Left | Conservative | Recovery |
| 1992 | Biurrun et al.[16] | Unclear | Unclear | Conservative | Recovery |
| 1995 | Jabbar et al.[17] | Thyroid cancer | Bilateral | Conservative | Recovery |
| 2000 | Gregor et al.[18] | Nasopharyngeal carcinoma | Right | Conservative | Recovery |
| 2001 | Al-Sebeih et al.[19] | Laryngocarcinoma | Bilateral | Conservative | Recovery |
| 2001 | Jortay et al.[20] | Unclear | Left | Conservative | Recovery |
| 2003 | Kamasaki et al.[21] | Tongue cancer | Bilateral | Conservative | Recovery |
| 2004 | Busquets et al.[22] | Adenolymphoma | Left | Surgery + conservative | Recovery |
| 2006 | Srikumar et al.[23] | Nasopharyngeal carcinoma | Left | Surgery + conservative | Recovery |
| 2007 | Tsukahara et al.[24] | Thyroid cancer | Left | Conservative | Recovery |
| Tongue cancer | Left | Conservative | Recovery | ||
| Laryngocarcinoma | Bilateral | Conservative | Recovery | ||
| 2007 | Bae et al.[25] | Thyroid cancer | Left | Conservative | Recovery |
| Thyroid cancer | Left | Conservative | Recovery | ||
| 2009 | Han et al.[26] | Thyroid cancer | Left | Conservative | Recovery |
| 2010 | AL-Khudaris et al.[27] | Laryngocarcinoma | Bilateral | Conservative | Recovery |
| 2011 | Rodier et al.[28] | Thyroid cancer | Left | Conservative | Recovery |
| 2011 | Tian W et al.[29] | Thyroid cancer | Bilateral | Conservative | Recovery |
| Thyroid cancer | Bilateral | Conservative | Recovery |
The majority of injuries to the thoracic duct cause merely chylous leakage; chylothorax, especially bilateral, is rare. Milky fluid suggests a chylous effusion, but the absence of a milky appearance does not preclude chylothorax, especially if the patient is fasting or on a low-fat diet. Biochemical analysis of the fluid should be the initial diagnostic test, with a triglyceride concentration greater than 1,100 mg/L confirming the diagnosis[30]. When the triglyceride level is between 550 and 1,100 mg/L, a lipoprotein analysis is indicated to detect chylomicrons, whereas a triglyceride level less than 500 mg/L has no more than a 5% chance of being chylous[30]. Clinically, low-output chylothorax (<500 mL/day) could be treated using a conservative approach, such as drainage and a low-fat diet. High-output chylothorax (>500 mL/day) could cause a fluid and electrolyte imbalance and a loss of nutrients, and it can even be fatal. Thus, high-output chylothorax requires surgical intervention[31]. However, from the literature we reviewed, only 2 cases were treated with surgery[22],[23], whereas the remaining 24 cases were treated conservatively and recovered at length.
In this case, we performed an emergency operation to ligate the fistula on the second day postoperation, when the chylous leakage reached 1,600 mL in volume. Based on our clinical experience, postoperative adhesions develop by 3 days postoperation, making surgical intervention for chylous leakage a challenge. Although the surgery ceased the neck leakage, bilateral chylothorax was confirmed 4 days after operation. To treat this condition, we prefer to follow a conservative management plan rather than perform additional surgeries, unless the conservative approach is insufficient. Here, we chose nonsurgical treatments for the patient, including drainage, total parenteral nutrition with total enteric rest, continuous intravenous infusion of somatostatin (250 µg/h), and intermittent thoracocentesis for aspirating effusion, and the patient was cured.
Our report also supports that the use of somatostatin may be a new conservative therapeutic approach. The exact mechanism of action of this neurohormonal and panacrine agent remains unknown. It may reduce gastrointestinal chyle production by decreasing splanchnic blood flow and decreasing gastric, biliary, pancreatic, and intestinal secretions[28].
Taken together, our results and the results described in the previous literature suggest that conservative management is an effective treatment for bilateral chylothorax following neck dissection. Our report shows that the use of somatostatin may be a new conservative therapeutic approach.
References
- 1.Recht A, Pierce SM, Abner A, et al. Regional nodal failure after conservative surgery and radiotherapy for early-stage breast carcinoma. J Clin Oncol. 1991;9:988–996. doi: 10.1200/JCO.1991.9.6.988. [DOI] [PubMed] [Google Scholar]
- 2.Fentiman IS, Lavelle MA, Caplan D, et al. The significance of supraclavicular fossa node recurrence after radical mastectomy. Cancer. 1986;57:908–910. doi: 10.1002/1097-0142(19860301)57:5<908::aid-cncr2820570504>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 3.Kiricuta IC, Willner J, Kolbl O, et al. The prognostic significance of the supraclavicular lymph node metastases in breast cancer patients. Int J Radiat Oncol Biol Phys. 1994;28:387–393. doi: 10.1016/0360-3016(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 4.van der Sangen MJ, Coebergh JW, Roumen RM, et al. Detection, treatment, and outcome of isolated supraclavicular recurrence in 42 patients with invasive breast carcinoma. Cancer. 2003;98:11–17. doi: 10.1002/cncr.11469. [DOI] [PubMed] [Google Scholar]
- 5.Singletary SE, Allred C, Ashley P, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002;20:3628–3636. doi: 10.1200/JCO.2002.02.026. [DOI] [PubMed] [Google Scholar]
- 6.Chen SC, CHang HK, Lin YC, et al. Prognosis of breast cancer after supraclavicular lymph node metastasis: not a distant metastasis. Ann Surg Oncol. 2006;13:1457–1465. doi: 10.1245/s10434-006-9012-1. [DOI] [PubMed] [Google Scholar]
- 7.Huang EH, Tucker SL, Strom EA, et al. Predictors of locoregional recurrence in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy, mastectomy, and radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:351–357. doi: 10.1016/j.ijrobp.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 8.Park HJ, Shin KH, Cho KH, et al. Outcomes of positron emission tomography-staged clinical N3 breast cancer treated with neoadjuvant chemotherapy, surgery, and radiotherapy. Int J Radiat Oncol Biol Phys. 2011;81:689–695. doi: 10.1016/j.ijrobp.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 9.Frazell EL, Harrold CC, Rasmussen L. Bilateral Chyalothorax: an unusual complication of radical neck dissection with recovery. Ann Surg. 1951;134:135–137. doi: 10.1097/00000658-195107000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coates HL, Desanto LW. Bilateral chylothorax as a complication of radical neck dissection. J Laryngol Otol. 1976;90:967–970. doi: 10.1017/s0022215100082980. [DOI] [PubMed] [Google Scholar]
- 11.Saraceno CA, Farrior RT. Bilateral chylothorax. Rare complication of neck dissection. Arch Otolaryngol. 1981;107:497–499. doi: 10.1001/archotol.1981.00790440037011. [DOI] [PubMed] [Google Scholar]
- 12.Har-EI G, Segal K, Sidi J. Bilateral chylothorax complicating radical neck dissection: report of a case with no concurrent external chylous leakage. Head Neck Surg. 1985;7:225–230. doi: 10.1002/hed.2890070307. [DOI] [PubMed] [Google Scholar]
- 13.Ng RS, Kerbavaz RJ, Hilsinger RL. Bilateral chylothorax from radical neck dissection. Otolaryngol Head Neck Surg. 1985;93:814–817. doi: 10.1177/019459988509300624. [DOI] [PubMed] [Google Scholar]
- 14.Pace-Balzan A, Moriarty B. Bilateral chylothorax following left radical neck dissection (case report) J Laryngol Otol. 1988;102:288–290. doi: 10.1017/s002221510010475x. [DOI] [PubMed] [Google Scholar]
- 15.Oi K, Haraguchi N, Machida S. Dyspnea resulting from accumulation of pleural effusion after radical neck dissection. A case report. Oral Surg Oral Med Oral Pathol. 1989;67:258–261. doi: 10.1016/0030-4220(89)90348-4. [DOI] [PubMed] [Google Scholar]
- 16.Biurrun O, Sabater F, Traserra J. Bilateral chylothorax after radical neck dissection. Apropos of a case. Rev Laryngol Otol Rhinol (Bord) 1992;113:111–113. [in French] [PubMed] [Google Scholar]
- 17.Jabbar AS, al-Abduckareem A. Bilateral chylothorax following neck dissection. Head Neck. 1995;17:69–72. doi: 10.1002/hed.2880170115. [DOI] [PubMed] [Google Scholar]
- 18.Gregor RT. Management of chyle fistulization in association with neck dissection. Otolaryngol Head Neck Surg. 2000;122:434–439. doi: 10.1016/S0194-5998(00)70061-1. [DOI] [PubMed] [Google Scholar]
- 19.Al-Sebein H, Sadeghi N, Al-Dhahri S Bilateral chylothorax following neck dissection: a new method of treatment. Ann Otol Rhinol Laryngol. 2001;110:381–384. doi: 10.1177/000348940111000416. [DOI] [PubMed] [Google Scholar]
- 20.Jortay A, Bisschop P. Bilateral chylothorax after left radical neck dissection. Acta Otorhinolaryngol Belg. 2001;55:285–289. [PubMed] [Google Scholar]
- 21.Kamasaki N, Ikeda H, Wang ZL, et al. Bilateral chylothorax following radical neck dissection. Int J Oral Maxillo Surg. 2003;32:91–93. doi: 10.1054/ijom.2002.0312. [DOI] [PubMed] [Google Scholar]
- 22.Busquets JM, Rullan PJ, Trinidad-Pinedo J. Bilateral chylothorax after neck dissection. Otolaryngol Head Neck Surg. 2004;130:492–495. doi: 10.1016/j.otohns.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Srikumar S, Newton JR, Westin TA. Bilateral chylothorax following left-sided radical neck dissection. J Laryngol Otol. 2006;120:705–707. doi: 10.1017/S0022215106001344. [DOI] [PubMed] [Google Scholar]
- 24.Tsukahara K, Kawabata K, Mitani H, et al. Three cases of bilateral chylothorax developing after neck dissection. Auris Nasus Largnx. 2007;34:573–576. doi: 10.1016/j.anl.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Bae JS, Song BJ, Kim MR, et al. Bilateral chylothoraces without chyle leakage after left-sided neck dissection for thyroid cancer: report of two cases. Surg Today. 2007;37:652–655. doi: 10.1007/s00595-006-3449-5. [DOI] [PubMed] [Google Scholar]
- 26.Han C, Guo L, Wang KJ, et al. Bilateral chylothorax following neck dissection for thyroid cancer. Int J Oral Maxillofac Surg. 2009;38:1119–1122. doi: 10.1016/j.ijom.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 27.AL-Khudaris, Vitale L, Ghanem T, et al. Recurrent high output chyle fistula post neck dissection resolution with conservative management. Laryngoscope. 2010;120:S141. doi: 10.1002/lary.21605. [DOI] [PubMed] [Google Scholar]
- 28.Rodier JF, Volkmar PP, Bodin F, et al. Thoracic duct fistula after thyroid cancer surgery: towards a new treatment? Case Rep Oncol. 2011;4:255–259. doi: 10.1159/000328801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian W, Li ZY, Wang P, et al. Chylothorax after neck dissection for thyroid carcinomas: report of three cases. Surg Today. 2012;42:89–92. doi: 10.1007/s00595-011-0015-6. [DOI] [PubMed] [Google Scholar]
- 30.Valentine VG, Raffin TA. The management of chylothorax. Chest. 1992;102:586–591. doi: 10.1378/chest.102.2.586. [DOI] [PubMed] [Google Scholar]
- 31.Binkert CA, Yucel EK, Davison BD, et al. Percutaneous treatment of high-output chylothorax with embolization or needle disruption technique. J Vasc Interv Radiol. 2005;16:1257–1262. doi: 10.1097/01.rvi.0000167869.36093.43. [DOI] [PubMed] [Google Scholar]