Abstract
The chemical transformation of carbon dioxide into useful products becomes increasingly important as CO2 levels in the atmosphere continue to rise as a consequence of human activities. In this article we describe the direct hydrogenation of CO2 into formic acid using a homogeneous ruthenium catalyst, in aqueous solution and in dimethyl sulphoxide (DMSO), without any additives. In water, at 40 °C, 0.2 M formic acid can be obtained under 200 bar, however, in DMSO the same catalyst affords 1.9 M formic acid. In both solvents the catalysts can be reused multiple times without a decrease in activity. Worldwide demand for formic acid continues to grow, especially in the context of a renewable energy hydrogen carrier, and its production from CO2 without base, via the direct catalytic carbon dioxide hydrogenation, is considerably more sustainable than the existing routes.
 The conversion of carbon dioxide into formic acid is attractive for energy storage and chemical production, but the typical use of bases or other additives make isolation of the free acid difficult. Here, the authors report the catalytic conversion of carbon dioxide into formic acid without the need for any additives.
The conversion of carbon dioxide into formic acid is attractive for energy storage and chemical production, but the typical use of bases or other additives make isolation of the free acid difficult. Here, the authors report the catalytic conversion of carbon dioxide into formic acid without the need for any additives.
Concerns over carbon dioxide levels in the atmosphere, which have reached ca. 400 p.p.m.1,2, are leading to political targets and scientific/technological efforts to reduce CO2 emissions and to capture CO2 and store it within porous rock formations3,4,5,6. Converting CO2 into useful feedstock chemicals and fuels represents another important strategy that not only removes CO2 from the atmosphere, but also reduces dependence on petrochemicals7,8. In this context much progress has been made in recent years and several reactions have been commercialized. Examples of products now derived from CO2 include urea, salicylic acid and polyols3. Recently, a company in Iceland started using renewable energy to convert CO2 into methanol, in the region of 1,600 T per year9. However, human activity contributes ~35 GT of CO2 to the atmosphere per year and there is clearly a considerable gap between the amount of CO2 produced and the amount consumed.
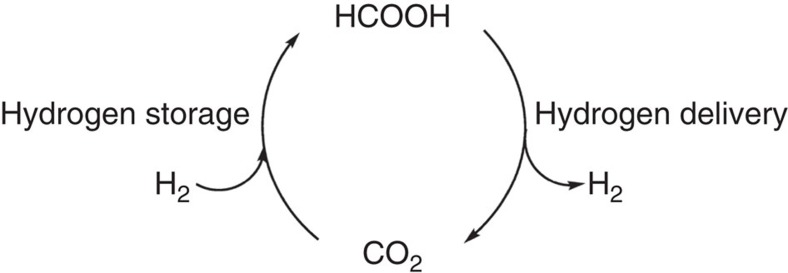
Ideally a multitude of chemical products should be derived from CO2 (ref. 10) and an important one, as it may be employed as both a feedstock chemical and a fuel, is formic acid. Currently worldwide production of formic acid, obtained from the combination of methanol and carbon monoxide with a strong base, is in the order of 800,000 T per year and is used in the textile industry, cleaning and as a preservative, to name a few. Demand could increase considerably due to the development of efficient formic acid dehydrogenation catalysts that could find widespread application within the context of a hydrogen economy11. Today, with the exception of fossil fuels, practical hydrogen storage systems use pressurized bottles or cryogenic conditions12. As formic acid (HCOOH) has a volumetric hydrogen density of 53 g of H2 per liter, a low-toxicity and is a liquid under ambient conditions, it is an ideal hydrogen storage material for certain applications13,14. Hydrogen production from formic acid has been achieved with a variety of homogeneous and heterogeneous catalysts15,16. Although most catalysts are based on noble metals, recent complexes based on iron (a cheap and abundant element) highlight the validity of such systems within the context of a hydrogen economy17. The concept of the hydrogen storage in the form of formic acid would, however, be even more attractive should a viable catalyst for the reverse reaction become available, that is, a catalyst that reduces carbon dioxide with hydrogen and in the absence of a base18,19,20,21,22 (Fig. 1).
Figure 1. The formic acid/carbon dioxide cycle for hydrogen storage.
Several catalysts are available for both reactions, and in general, hydrogen production or consumption is controlled by the pH of the solution23,24,25,26. However, the conversion of carbon dioxide into formic acid is difficult owing to the high kinetic and thermodynamic stability of CO2. The gas phase reaction has a positive ΔG value because of an entropic contribution (ΔG0=+33 KJ mol−1), and the formation of formic acid is more favourable in aqueous solution (ΔG0=−4 KJ mol−1) (ref. 20). For reactions performed in water, the CO2/H2CO3/HCO3− equilibrium, as well as the second deprotonation to CO32−, must be taken into account (Supplementary Fig. 1). From these equilibria and the kinetic studies performed on the hydrogenation of bicarbonates at different pH values27 it is apparent that the reaction is favourable in basic media, conditions where the actual substrate is HCO3− (ΔG0=−35 KJ mol−1) or CO32−.
In contrast to the direct reaction of H2 with CO2, the hydrogenation of carbonate and bicarbonate are well-established reactions28,29,30 that have been achieved in a wide variety of organic solvents, ionic liquids, water and supercritical CO2 (ref. 20). Currently, catalysts that show high activity in the hydrogenation of CO2 require additives such as bases31,32, including amines33 or buffers34,35, producing formate salts. Ogo et al.34,35 were able to produce formic acid (0.06 M) from the direct reaction of H2 with CO2 in an acidic buffer. The presence of base also complicates the separation of formic acid from the reaction mixture although ingenious approaches have been developed to overcome these complications30,36. Herein, we describe a homogeneous ruthenium catalyst that hydrogenates CO2 to formic acid in acidic media, without the need of any bases, amines or buffers. In aqueous solution, 0.2 M formic acid can be obtained, in dimethyl sulphoxide (DMSO) the ruthenium(II) phosphine catalyst provides 1.9 M formic acid.
Results
Direct carbon dioxide hydrogenation in water
In our initial studies the hydrogenation of CO2 was explored in acidic aqueous solutions using ruthenium(II) or rhodium(I) catalysts with water soluble phosphine ligands, that is, 1,3,5-triaza-7-phosphaadamantane (PTA), 3-methyl-1,3,5- triaza-7-phosphaadamantane (MePTA), meta-monosulphonated triphenylphosphine (TPPMS) and meta-trisulphonated triphenylphosphine (TPPTS; Supplementary Table 1). From these studies [RuCl2(PTA)4] was found to be the most active catalyst affording formic acid as the only product under the reaction conditions (at the end of the reaction the pH of the solution is 2.70, Fig. 2 and Supplementary Fig. 2). The ruthenium catalysts were typically 10 times more active than the rhodium analogues and the highest yields were obtained for complexes containing PTA-type ligands, that is, similar activities were observed for the PTA and MePTA ligands, whereas complexes with TPPMS and TPPTS were less active. The reaction conditions were optimized for [RuCl2(PTA)4] to improve performance and the dependence of the catalyst concentration, pressure, P(H2)/P(CO2) ratio (Table 1) and temperature (Supplementary Table 2) on formic acid production was elucidated.
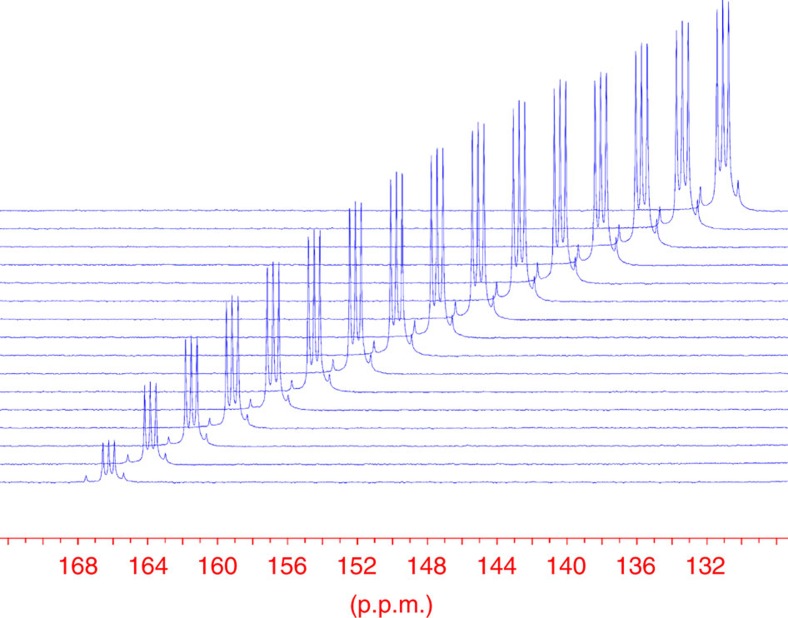
Figure 2. 13C NMR signals of DCOOD in the hydrogenation reactions of CO2 into formic acid in D2O.
[RuCl2(PTA)4] (2.76 mM) was dissolved in D2O (2 ml) under N2 atmosphere in a medium pressure sapphire NMR tube. This solution was pressurized at room temperature to 50 bar with CO2 and completed to 100 bar with H2. The system was heated to 60 °C and the reaction was followed by 13C NMR (100 MHz) spectroscopy. The figure shows the evolution of the 13C NMR signals of DCOOD at 166.3 p.p.m. (time difference between spectra Δt=189 min). The triplet signal of the formic acid is due to the exchange of the hydrogen to deuterium in HCOOH (as D2O was used as solvent). Reaction time: 148 h.
Table 1. Formic acid formation as function of the catalyst concentration (conc.), pressure and P(H2)/P(CO2) ratio*.
| Entry | Catalyst conc. (M) | Pressure (bar) | P(H2)/P(CO2) | HCOOH† (M) | TON‡ |
|---|---|---|---|---|---|
| 1 | 0.00276 | 60 | 1 | 0.030 | 11 |
| 2 | 0.00276 | 70 | 1 | 0.042 | 15 |
| 3 | 0.00276 | 80 | 1 | 0.047 | 17 |
| 4 | 0.00276 | 100 | 1 | 0.083 | 30 |
| 5 | 0.00276 | 100 | 2.3 | 0.086 | 31 |
| 6 | 0.00276 | 100 | 4 | 0.070 | 25 |
| 7 | 0.00276 | 120 | 1.5 | 0.095 | 34 |
| 8 | 0.00276 | 150 | 2 | 0.124 | 45 |
| 9 | 0.00276 | 200 | 3 | 0.204 | 74 |
| 10 | 0.00063 | 100 | 1 | 0.100 | 159 |
| 11 | 0.00546 | 100 | 1 | 0.070 | 13 |
| 12 | 0.00063 | 60 | 1 | 0.035 | 56 |
| 13 | 0.00546 | 60 | 1 | 0.029 | 5 |
*[RuCl2(PTA)4] was dissolved in H2O (2 ml) under N2 atmosphere. This solution was pressurized at room temperature under CO2 followed by H2 to the required pressure. The system was heated at 60 °C and stirred until the equilibrium of the reaction was reached (48–84 h). The final yield of formic acid was determined by 1H NMR spectroscopy with DSS (DSS, 4,4-dimethyl-4-silapentane-1-sulphonic acid) as an internal standard.
†Average values from three to six measurements with a reproducibility of ±15%.
‡Turn over number (TON), that is, the number of moles of CO2 (or H2) that one mole of catalyst converts into HCOOH.
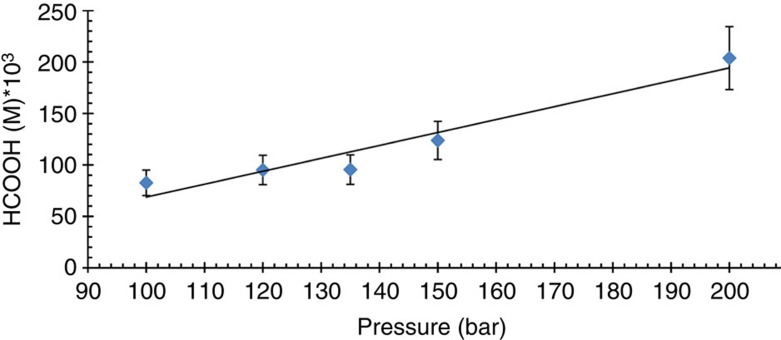
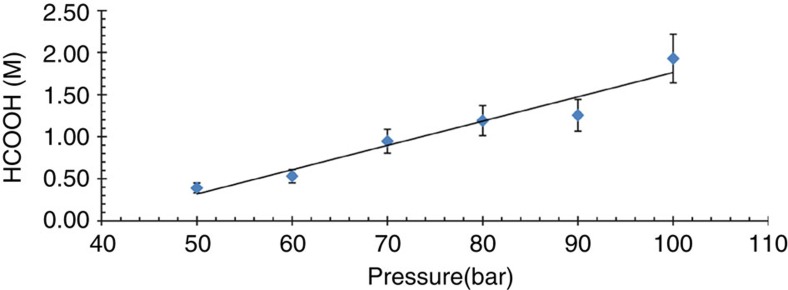
Under relatively mild conditions (60 °C, 30 bar CO2, 30 bar H2, 2.76 mM catalyst), [RuCl2(PTA)4] hydrogenates CO2 to afford a 0.03 M formic acid solution. The concentration of formic acid produced depends on the gas pressure. With a P(H2)/P(CO2) ratio of 1 increasing the total gas pressure from 60 bar to 100 bar affords a 0.083 M formic acid solution (Table 1), and at a total gas pressure of 200 bar the concentration of formic acid obtained is 0.204 M (Fig. 3).
Figure 3. Pressure dependence on the concentration of formic acid obtained in the catalytic hydrogenation of CO2.
Conditions: the catalyst was dissolved in 2 ml H2O ([RuCl2(PTA)4]=2.76 mM) under N2 atmosphere. This solution was pressurized at room temperature with CO2 (50 bar) and completed with H2 to the desired pressure. The system was heated at 60 °C and stirred until the equilibrium of the reaction was reached (72–96 h). The final yield of formic acid was determined by 1H NMR spectroscopy with DSS as an internal standard. The values are averaged from three to six measurements with a reproducibility of ±15%. The trend line is shown as a guide and is not a mathematical fit of the data.
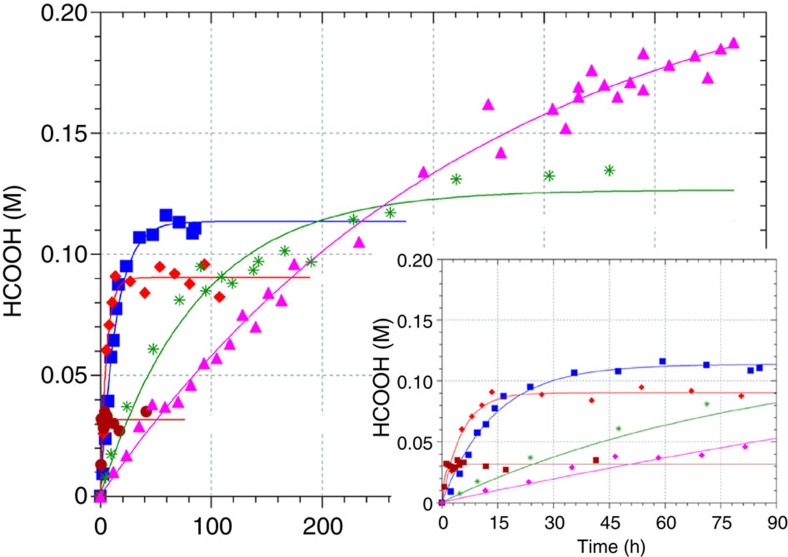
The reaction was also studied at temperatures ranging from 23 to 90 °C under standard conditions ([RuCl2(PTA)4]=2.76 mM, total pressure=100 bar and P(H2)/P(CO2) pressure ratio=1, see Fig. 4 and Supplementary Fig. 3 and Supplementary Table 2). As the hydrogenation of CO2 is exothermic, increasing temperature reduces the final formic acid concentration, which decreases to 0.032 M at 90 °C. The temperature dependence of the reaction rate follows the Arrhenius law from which activation enthalpy of +96 kJ mol−1 was obtained (Supplementary Fig. 4).
Figure 4. Influence of temperature on the hydrogenation of CO2 to formic acid using [RuCl2(PTA)4] as the catalyst: 90 °C (brown circle), 60 °C (red diamond), 50 °C (blue square), 40 °C (green asterisk) and 30 °C (pink triangle).
Conditions: [RuCl2(PTA)4] was dissolved in H2O (2.76 mM, 2 ml), under N2 atmosphere. The solution was pressurized at room temperature with CO2 (50 bar) and completed with H2 to 100 bar. The system was heated to the desired temperature and the reaction was monitored by 1H NMR spectroscopy until the equilibrium of the reaction was reached. The values are averaged from three to six measurements with a reproducibility of ±15%.
Using the optimized catalytic system, recycling experiments were performed and it was found that the catalyst could be reused without the loss of activity (Supplementary Table 3).
Carbon dioxide hydrogenation in other solvents
As [RuCl2(PTA)4] is able to directly hydrogenate carbon dioxide in aqueous acidic solution, that is, at pH=2.70, further studies were performed in water-miscible ionic liquids, that is, 1-butyl-3-methylimidazolium terafluoroborate ([BMIM][BF4]) and 1-butyl-3-methylimidazolium tosylate ([BMIM][tos] where tos=the p-CH3C6H4SO3- anion), and in tetrahydrofuran and DMSO (Table 2).
Table 2. Catalytic hydrogenation of carbon dioxide to formic acid in different solvent mixtures.
| Entry | Solvent | H2O (%) | Solvent (%) | HCOOH (M) | TON |
|---|---|---|---|---|---|
| 1 | H2O | 100 | — | 0.11 | 40 |
| 2 | [BMIM][BF4] | 50 | 50 | 0.15 | 54 |
| 3 | [BMIM][tos] | 52 | 48 | 0.19 | 69 |
| 4 | [BMIM][tos] | 40 | 60 | 0.10 | 36 |
| 5 | [BMIM][BF4] | 20 | 80 | 0.14 | 51 |
| 6 | [BMIM][BF4] | 10 | 90 | 0.12 | 43 |
| 7 | THF | 50 | 50 | 0.095 | 34 |
| 8 | DMSO | 50 | 50 | 0.34 | 123 |
| 9 | DMSO | 20 | 80 | 0.98 | 355 |
| 10 | DMSO | 10 | 90 | 1.31 | 475 |
THF, tetrahydrofuran.
Reaction conditions: [RuCl2(PTA)4] (2.76 mM), P(total)=100 bar, P(H2)/P(CO2) ratio of 1, t=50 °C, reaction time 120 h, solvent (2 ml containing DSS, 0.0130 M), average values of several (two to three) measurements for each cycle, reproducibility is±15%.
High activities were observed in DMSO/H2O with the catalytic activity increasing as the water/solvent ratio was decreased. In pure DMSO at 50 °C, a 1.93 M formic acid solution was obtained at 100 bar with a P(H2)/P(CO2) ratio of 1. Extensive investigations on the activity of [RuCl2(PTA)4] in pure organic solvents, that is, alcohols, acetonitrile, propylene carbonate and toluene (Supplementary Table 4), revealed that the catalyst is active in several solvents, although none were as effective as DMSO. Further screening of other catalysts in DMSO (Supplementary Table 5) confirmed the superiority of [RuCl2(PTA)4] in the hydrogenation of CO2, that is, [RuCl2(PTA)4] is ~20 times more active in DMSO compared with the other catalysts evaluated. Hence, the efficiency of [RuCl2(PTA)4] in DMSO was studied as a function of the total gas pressure (Fig. 5 and Supplementary Table 6) and the P(H2)/P(CO2) partial pressure. Similar trends were observed to those in water.
Figure 5. Pressure effect on the formic acid yield using [RuCl2(PTA)4] catalyst in DMSO.
Reaction conditions: [RuCl2(PTA)4] (2.76 mM), t=50 °C, P(H2)/P(CO2) ratio of 1, reaction time 120 h, DMSO (2 ml), average values of several (two to six) measurements. The trend line is shown as a guide and is not a mathematical fit of the data.
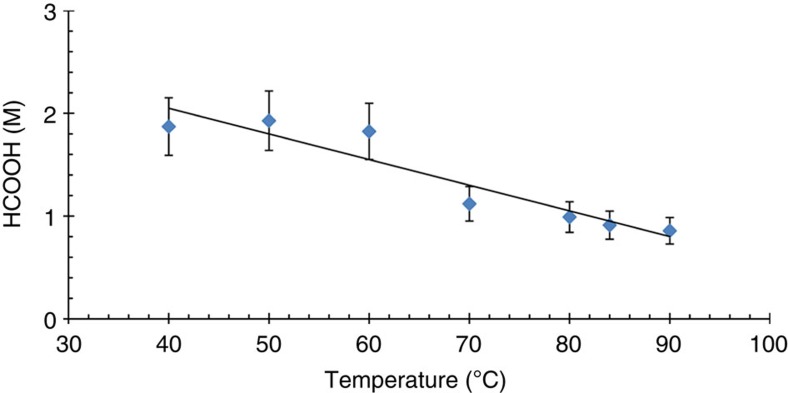
The dependence of the final formic acid concentration and the reaction rate as function of temperature was determined in DMSO (Fig. 6, Supplementary Fig. 5 and Supplementary Table 7). Similar to the aqueous system, the final formic acid concentration increases as the temperature decreases, whereas the reaction rate increases with increasing temperature.
Figure 6. The influence of temperature on the hydrogenation of CO2 to formic acid using [RuCl2(PTA)4] in DMSO.
Conditions: the catalyst was dissolved in 2 ml DMSO ([RuCl2(PTA)4]=2.76 mM) under N2 atmosphere. This solution was pressurized at room temperature with CO2 (50 bar) and completed with H2 to 200 bar (P(H2)/P(CO2)=3). The system was heated to the desired temperature and stirred until the equilibrium of the reaction was reached. The final yield of formic acid was determined by 1H NMR spectroscopy with DSS as an internal standard. The trend line is shown as a guide and is not a mathematical fit of the data. The values are averaged from three to six measurements with a reproducibility of ±15%.
The catalyst, [RuCl2(PTA)4], was recycled and reused several times (Table 3) without any decrease of activity observed, resulting in a total turn over number (TON) of 749 after four recycles and indicating that considerably higher TONs may be achieved.
Table 3. Recyclability of [RuCl2(PTA)4] the carbon dioxide hydrogenation in DMSO.
| Cycle | HCOOH (M) | TON |
|---|---|---|
| 1 | 1.8 | 176 |
| 2 | 2.1 | 206 |
| 3 | 1.8 | 557 |
| 4 | 1.9 | 749 |
[RuCl2(PTA)4] (2.76 mM) was dissolved in DMSO (2 ml) under N2 atmosphere. The system was pressurized at room temperature to 50 bar with CO2 and completed with 50 bar H2. The system was heated at 60 °C and stirred for 120 h until equilibrium of the reaction was reached, average values of several (two to three) measurements are reported with a reproducibility of±15%.
Mechanistic studies with [RuCl2(PTA)4] catalyst
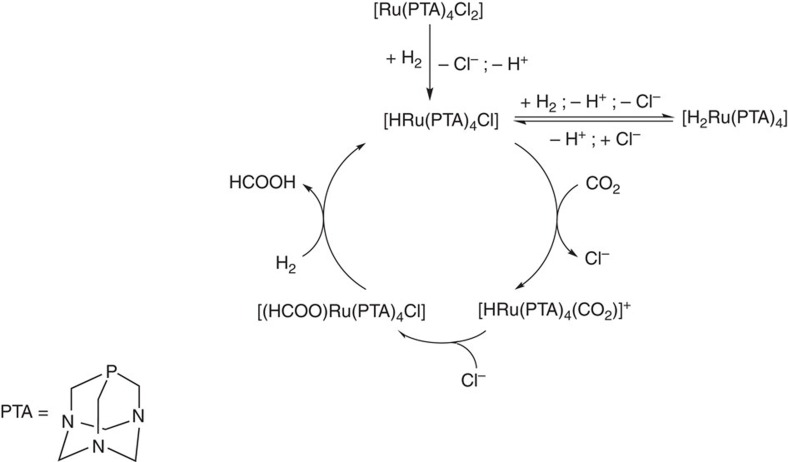
Moreover, possible catalytic intermediates were identified under catalytic conditions using medium pressure sapphire tubes, by nuclear magnetic resonance (NMR) spectroscopy. The [RuCl2(PTA)4] catalyst, both in water and in DMSO-d6, together with DSS (4,4-dimethyl-4-silapentane-1-sulphonic acid) as an internal standard, was pressurized to 100 bar with a P(H2)/P(CO2) pressure ratio of 1 and heated at 50 °C. The hydride region of the resulting 1H NMR spectra reveals the presence of monohydride and dihydride species, that is, [RuH2(PTA)4] and [RuH(PTA)4Cl], assigned from a multiplet at −11.2 p.p.m. and a doublet of quartets at −9.2 p.p.m., previously observed in aqueous solution (Supplementary Fig. 6)37. In the 1H{31P} NMR spectra, the two hydride signals were shown to collapse into singlet resonances (Supplementary Fig. 7). On the basis of these observations and prior literature18,37, a tentative catalytic cycle can be proposed (Fig. 7).
Figure 7. Catalytic cycle.
Proposed catalytic cycle for the hydrogenation of carbon dioxide using [RuCl2(PTA)4] in water or DMSO.
Discussion
The [RuCl2(PTA)4] complex catalyses the direct hydrogenation of CO2 in DMSO to afford formic acid at unprecedentedly high concentrations, that is, 1.9 M (~15% in volume). Importantly, the catalyst operates in the absence of base (or any other additives) and is highly stable and can be recycled and reused without loss of activity. As demand for formic acid continues to grow, notably as a potential hydrogen carrier (HCOOH can selectively decompose into H2 and CO2, Supplementary Figs 8 and 9)38,39,40; viable catalytic processes that directly convert CO2 into formic acid, such as the one described herein may help to propel a hydrogen-based economy.
Methods
General considerations
NaHCO3, NaOH, HCl, HCOOH and sodium 3-(trimethylsilyl)-1-propanesulfonate (DSS) were purchased from Fluka, BASF or Merck. H2 (99.95%) and CO2 (99.9%) were acquired from Carbagas-CH, enriched 13CO2 (99% in 13C) was obtained from Cambridge Isotope Laboratories. Double distilled water, DMSO (Sigma-Aldrich), methanol (Fluka), ethanol (Fluka), acetonitrile (Merck), propylene carbonate (Acros), toluene (Merk), ionic liquids [BMIM][BF4] and [BMIM][tos] (Fluka) were used as solvents. Ligands PTA, TPPTS and TPPMS were purchased from ABCR and Fluka, respectively. The ligand MePTA and the complexes [RuCl2(PTA)4], [RhCl(TPPMS)3], [RuCl2(PTA)([9]aneS3)], [Ru(H2O)4(MePTA)2](tos)4, [RuCl2(TPPMS)2], [RuCl2(TPPTS)2]2 and [RuCl2(p-cymene)]2 were synthesized according to the literature41,42,43,44,45,46,47,48,49,50.
All manipulations were carried out under oxygen-free conditions with degassed solvents, using Schlenk line techniques with N2 as a protective gas. The reactions were carried out in medium pressure sapphire NMR tubes up to 100 bar and were followed by 1H and 13C NMR spectroscopy. At higher pressures Parr autoclaves (25 ml, SS 316) were used. NMR spectra were recorded on a Bruker DRX 400 NMR spectrometer and the spectra were fitted with the program WIN-NMR. Formic acid concentrations were determined by quantitative 1H and 13C NMR, by ion chromatography using an ICS-90 system and by HPLC with an Agilent 1260 Infinity instrument.
Homogeneous catalytic hydrogenation reactions
Preliminary studies with different catalysts were performed in a multi-autoclave (HEL CAT 7) by preparing a 2.76 × 10−3 M (2.76 mM) solution of the corresponding catalyst in degassed water (2 ml). The solutions were then pressurized up to 30 bars of CO2 and then completed up to 60 bars with H2 (partial H2 pressure=30 bar). The system was heated to 60 °C and stirred until the reaction was completed. Formic acid yields were determined by 1H NMR spectroscopy using DSS as an external standard (c=0.0130 M), and were verified by ionic chromatography and by HPLC.
For kinetic measurements, catalysts were dissolved in degassed solvents (2 ml), which were introduced into a sapphire NMR tube or into an autoclave under N2 atmosphere. The solutions were pressurized up to 20–55 bars with CO2 and then completed to 60–200 bar with H2. The system was heated to the required temperature (23–135 °C) and shaken/stirred. In the sapphire NMR tubes, the evolution of [HCOOH] and [CO2] was followed by quantitative 1H or 13C NMR spectroscopy with DSS as an internal standard51. For the autoclaves the yield of formic acid was determined by 1H NMR measurement in the final solution, with DSS as external standard (solution of 0.0130 M), and controlled by ionic chromatography and by HPLC. During the studies using different organic solvents (in which DSS is insoluble), the final formic acid concentrations were determined by 1H NMR using the standard addition method (with a known [HCOOH] solution), and controlled by ionic chromatography and by HPLC.
The dissolved CO2 and H2 concentrations were monitored by 13C and 1H NMR during the catalytic hydrogenation reactions using medium pressure sapphire NMR tubes52,53, and display distinct gas and liquid phases under the reaction conditions.
Recycling experiments
Catalyst recycling experiments were performed using 10 mM [RuCl2(PTA)4] solutions in water or in DMSO, with a pressure P(total)=100 bar, P(H2)/P(CO2) ratio of 1, t=60 °C. Once the reaction reached equilibrium, the systems were depressurized, and the formic acid concentrations were determined. The solvent and the formic acid were removed under vacuum and the remaining solid catalyst was dissolved in the appropriate solvent (H2O or DMSO), pressurized to 100 bar, P(H2)/P(CO2) ratio of 1. After each reaction, the formic acid concentration was determined by 1H NMR spectroscopy and the recycling procedure was repeated. In addition, the resulting formic acid could be dehydrogenated into hydrogen and carbon dioxide using an immobilized catalyst described previously40.
Author contributions
S.M. carried out the syntheses and the kinetic measurements; P.J.D. was involved in scientific discussions; G.L. designed and directed the project, supervised the research work; S.M., P.J.D. and G.L. participated in the preparation of the manuscript.
Additional information
How to cite this article: Moret, S. et al. Direct synthesis of formic acid from carbon dioxide by hydrogenation in acidic media. Nat. Commun. 5:4017 doi: 10.1038/ncomms5017 (2014).
Supplementary Material
Supplementary Figures 1-9, Supplementary Tables 1-7 and Supplementary References
Acknowledgments
Swiss National Science Foundation, EOS Holding and EPFL are thanked for financial support.
Footnotes
A patent has been filed (PCT/IB2014/058883). The authors declare no competing financial interests
References
- Mauna Loa Observatory, Hawaii http://co2now.org/ (2014).
- Tollefson J. Growing agricultural benefits for climate. Nature 462, 966–967 (2009).20033010 [Google Scholar]
- Aresta M. Carbon Dioxide Recovery and Utilization Kluwer Academic Publishers: Dordrecht, (2010). [Google Scholar]
- Metz B., Davidson O., De Coninck H., Loos M. & Meyer L. Carbon Dioxide Capture and Storage Cambridge University Press: Cambridge UK, (2005). [Google Scholar]
- Dzubak A. L. et al. L. Ab initio carbon capture in open-site metal-organic frameworks. Nat. Chem. 4, 810–816 (2012). [DOI] [PubMed] [Google Scholar]
- Lin L.-C. et al. In silico screening of carbon-capture materials. Nat. Mater 11, 633–641 (2012). [DOI] [PubMed] [Google Scholar]
- Balaraman E., Gunanathan C., Zhang J., Shimon L. J. W. & Milstein D. Efficient hydrogenation of organic carbonates, carbamates and formates indicates alternative routes to methanol based on CO2 and CO. Nat. Chem. 3, 609–614 (2011). [DOI] [PubMed] [Google Scholar]
- Centi G. & Perathoner S. Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. Catal. Today 148, 191–205 (2009). [Google Scholar]
- Carbon Recycling International http://cri.is/ (2013).
- Aresta M. & Dibenedetto A. Utilisation of CO2 as a chemical feedstock: opportunities and challenges. Dalton Trans. 2975–2992 (2007). [DOI] [PubMed] [Google Scholar]
- Schlapbach L. & Züttel A. Hydrogen-storage materials for mobile applications. Nature 414, 353–358 (2001). [DOI] [PubMed] [Google Scholar]
- Dalebrook A. F., Gan W., Grasemann M., Moret S. & Laurenczy G. Hydrogen storage: beyond conventional methods. Chem. Commun. 49, 8735–8751 (2013). [DOI] [PubMed] [Google Scholar]
- Enthaler S., von Langermann J. & Schmidt T. Carbon dioxide and formic acid-the couple for environmental-friendly hydrogen storage? Energy Environ. Sci. 3, 1207–1217 (2010). [Google Scholar]
- Joo F. Breakthroughs in Hydrogen Storage-Formic Acid as a Sustainable Storage Material for Hydrogen. ChemSusChem 1, 805–808 (2008). [DOI] [PubMed] [Google Scholar]
- Grasemann M. & Laurenczy G. Formic acid as a hydrogen source -recent developments and future trends. Energy Environ. Sci. 5, 8171–8181 (2012). [Google Scholar]
- Barnard J. H., Wang C., Berry N. G. & Xiao J. L. Long-range metal-ligand bifunctional catalysis: cyclometallated iridium catalysts for the mild and rapid dehydrogenation of formic acid. Chem. Sci. 4, 1234–1244 (2013). [Google Scholar]
- Boddien A. et al. Efficient dehydrogenation of formic acid using an iron catalyst. Science 333, 1733–1736 (2011). [DOI] [PubMed] [Google Scholar]
- Federsel C., Jackstell R. & Beller M. State-of-the-art catalysts for hydrogenation of carbon dioxide. Angew. Chem. Int. Ed. 49, 6254–6257 (2010). [DOI] [PubMed] [Google Scholar]
- Jessop P. G., Joo F. & Tai C. C. Recent advances in the homogeneous hydrogenation of carbon dioxide. Coord. Chem. Rev. 248, 2425–2442 (2004). [Google Scholar]
- Jessop P. G., Ikariya T. & Noyori R. Homogeneous catalytic hydrogenation of supercritical carbon dioxide. Nature 368, 231–233 (1994). [Google Scholar]
- Leitner W. Carbon dioxide as a raw material the synthesis of formic acid and its derivatives from CO2. Angew. Chem. Int. Ed. 34, 2207–2221 (1995). [Google Scholar]
- Jessop P. G. inHandbook of Homogeneous Hydrogenation 489–511Wiley-VCH: Weinheim, (2007). [Google Scholar]
- Tanaka R., Yamashita M. & Nozaki K. Catalytic hydrogenation of carbon dioxide using Ir(III)-pincer complexes. J. Am. Chem. Soc. 131, 14168–14169 (2009). [DOI] [PubMed] [Google Scholar]
- Papp G., Csorba J., Laurenczy G. & Joó F. A charge/discharge device for chemical hydrogen storage and generation. Angew. Chem. Int. Ed. 50, 10433–10435 (2011). [DOI] [PubMed] [Google Scholar]
- Hull J. F. et al. Reversible hydrogen storage using CO2 and a proton-switchable iridium catalyst in aqueous media under mild temperatures and pressures. Nat. Chem. 4, 383–388 (2012). [DOI] [PubMed] [Google Scholar]
- Boddien A. et al. Towards the development of a hydrogen battery. Energy Environ. Sci. 5, 8907–8911 (2012). [Google Scholar]
- Laurenczy G., Joo F. & Nadasdi L. Formation and characterization of water-soluble hydrido-ruthenium(II) complexes of 1,3,5-triaza-7-phosphaadamantane and their catalytic activity in hydrogenation of CO2 and HCO3− in aqueous solution. Inorg. Chem. 39, 5083–5088 (2000). [DOI] [PubMed] [Google Scholar]
- Preti D., Squarcialupi S. & Fachinetti G. Production of HCOOH/NEt3 adducts by CO2/H2 incorporation into neat NEt3. Angew. Chem. Int. Ed. 49, 2581–2584 (2010). [DOI] [PubMed] [Google Scholar]
- Tai C. C., Chang T., Roller B. & Jessop P. G. High-pressure combinatorial screening of homogeneous catalysts: hydrogenation of carbon dioxide. Inorg. Chem. 42, 7340–7341 (2003). [DOI] [PubMed] [Google Scholar]
- Schaub T. & Paciello R. A. A process for the synthesis of formic acid by CO2 hydrogenation: thermodynamic aspects and the role of CO. Angew. Chem. Int. Ed. 50, 7278–7282 (2011). [DOI] [PubMed] [Google Scholar]
- Zhao G. Y. & Joo F. Free formic acid by hydrogenation of carbon dioxide in sodium formate solutions. Catal. Commun. 14, 74–76 (2011). [Google Scholar]
- Joszai I. & Joo F. Hydrogenation of aqueous mixtures of calcium carbonate and carbon dioxide using a water-soluble rhodium(I)–tertiary phosphine complex catalyst. J. Mol. Catal. A Chem. 224, 87–91 (2004). [Google Scholar]
- Graf E. & Leitner W. Direct formation of formic acid from carbon dioxide and dihydrogen using the [{Rh(cod)Cl}2]-Ph2P(CH2)4PPh2 catalyst system. J. Chem. Soc. Chem. Commun. 623–624 (1992). [Google Scholar]
- Ogo S., Hayashi H. & Fukuzumi S. Aqueous hydrogenation of carbon dioxide catalysed by water-soluble ruthenium aqua complexes under acidic conditions. Chem. Commun. 2714–2715 (2004). [DOI] [PubMed] [Google Scholar]
- Ogo S., Kabe R., Hayashi H., Harada R. & Fukuzumi S. Mechanistic investigation of CO2 hydrogenation by Ru(II) and Ir(III) aqua complexes under acidic conditions: two catalytic systems differing in the nature of the rate determining step. Dalton Trans. 4657–4663 (2006). [DOI] [PubMed] [Google Scholar]
- Wesselbaum S., Hintermair U. & Leitner W. Continuous-flow hydrogenation of carbon dioxide to pure formic acid using an integrated scCO2 process with immobilized catalyst and base. Angew. Chem. Int. Ed. 51, 8585–8588 (2012). [DOI] [PubMed] [Google Scholar]
- Laurenczy G., Jedner S., Alessio E. & Dyson P. J. In situ NMR characterisation of an intermediate in the catalytic hydrogenation of CO2 and HCO3− in aqueous solution. Inorg. Chem. Commun. 10, 558–562 (2007). [Google Scholar]
- Fellay C., Dyson P. J. & Laurenczy G. A viable hydrogen-storage system based on selective formic acid decomposition with a ruthenium catalyst. Angew. Chem. Int. Ed. 47, 3966–3968 (2008). [DOI] [PubMed] [Google Scholar]
- Fellay C., Yan N., Dyson P. J. & Laurenczy G. Selective formic acid decomposition for high-pressure hydrogen generation: a mechanistic study. Chem. Eur. J. 15, 3752–3760 (2009). [DOI] [PubMed] [Google Scholar]
- Gan W., Dyson P. J. & Laurenczy G. Heterogeneous silica-supported ruthenium phosphine catalysts for selective formic acid decomposition. ChemCatChem 5, 3124–3130 (2013). [Google Scholar]
- Daigle D. J., Pepperman A. B. & Vail S. L. Synthesis of a monophosphorus analog of hexamethylenetetramine. J. Heterocycl. Chem. 11, 407–408 (1974). [Google Scholar]
- Daigle D. J. & Pepperman A. B. Chemical proof for preferred nitrogen quarternization in 1,3,5-triaza-7-phosphaadamantane. J. Heterocycl. Chem. 12, 579–580 (1975). [Google Scholar]
- Darensbourg D. J. et al. Water-soluble organometallic compounds 4. Catalytic-hydrogenation of aldehydes in an aqueous two-phase solvent system using a 1,3,5-triaza-7-phosphaadamantane complex of ruthenium. Inorg. Chem. 33, 200–208 (1994). [Google Scholar]
- Darensbourg D. J., Joo F., Kannisto M., Katho A. & Reibenspies J. H. Water-soluble organometallic compounds.2. catalytic-hydrogenation of aldehydes and olefins by new water-soluble 1,3,5-triaza-7-phosphaadamantane complexes of ruthenium and rhodium. Organometallics 11, 1991–1993 (1992). [Google Scholar]
- Joó F. et al. (Meta-sulfonatophenyl)diphenylphosphine, sodium salt and its complexes with rhodium(I), ruthenium(II), iridium(I). Inorg. Synth. 32, 1–8 (1998). [Google Scholar]
- Serli B. et al. Is the aromatic fragment of piano-stool ruthenium compounds an essential feature for anticancer activity? The development of new RuII-[9]aneS3 analogues. Eur. J. Inorg. Chem. 3423–3434 (2005). [Google Scholar]
- Bennett M. A. & Smith A. K. Arene ruthenium(II) complexes formed by dehydrogenation of cyclohexadienes with ruthenium(III) trichloride. J. Chem. Soc. Dalton 233–241 (1974). [Google Scholar]
- Gandolfi C., Heckenroth M., Neels A., Laurenczy G. & Albrecht M. Chelating NHC ruthenium(II) complexes as robust homogeneous hydrogenation catalysts. Organometallics 28, 5112–5121 (2009). [Google Scholar]
- Pruchnik F. P., Smoleński P., Galdecka E. & Galdecki Z. Structural, spectroscopic and catalytic properties of water-soluble hydride rhodium complexes [RhH(Rtpa+I−)4]H2O (R=Me, Et). Inorg. Chim. Acta 293, 110–114 (1999). [Google Scholar]
- Kovacs J., Joo F., Benyei A. C. & Laurenczy G. Reactions of [Ru(H2O)6]2+ with water-soluble tertiary phosphines. Dalton Trans. 2336–2340 (2004). [DOI] [PubMed] [Google Scholar]
- Moret S., Dyson P. J. & Laurenczy G. Direct, in situ determination of pH and solute concentrations in formic acid dehydrogenation and CO2 hydrogenation in pressurised aqueous solutions using 1H and 13C NMR spectroscopy. Dalton Trans. 42, 4353–4356 (2013). [DOI] [PubMed] [Google Scholar]
- Symons E. A. Hydrogen gas solubility in the dimethylsulfoxide - water system: a further clue to solvent structure in these media. Can. J. Chem. 49, 3940–3947 (1971). [Google Scholar]
- Dyson P. J., Laurenczy G., Ohlin C. A., Vallance J. & Welton T. Determination of hydrogen concentration in ionic liquids and the effect (or lack of) on rates of hydrogenation. Chem. Commun. 2418–2419 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-9, Supplementary Tables 1-7 and Supplementary References