Abstract
Background
Recent studies have demonstrated that morphology of the first polar body (1stPB) is related to oocyte viability, which can be used as a prognostic tool to predict oocyte performance and pregnancy outcomes in an intracytoplasmic sperm injection (ICSI) program. According to some studies, there is a correlation between oocyte performance and 1stPB morphology, while others have not reported any correlation. The objective of this study is to evaluate the role of 1stPB morphology on rates of fertilization and embryo development in ICSI cases.
Materials and Methods
In this prospective study morphological characteristics of 470 metaphase II (MII) oocytes were assessed in 80 ICSI cycles. The women were ages 21-42 years (mean 32.6 ± 0.2). Their oocytes were retrieved after a hyperstimulation protocol. After denudation, all oocytes were evaluated for 1stPB morphology. The oocytes were divided into two groups of A (normal 1st
Results
Twenty-seven percent of oocytes had fragmented 1stPB, while the remainder was associated with other morphological abnormalities. A total of 46.1% and 26.9% of oocytes showed double and multiple defects, respectively. RF was the most common abnormality observed in group B. No significant differences in women’s’ ages between groups A and B were noted (p=0.3). A total of 179 and 107 oocytes (61.5% vs. 59.8%) were fertilized in groups A and B, respectively (p=0.7). The rates of good embryo formation for A and B groups were 66.5% and 55.6% (p=0.07), and cleavage rates were 77.7% and 68.5%, respectively (p=0.09).
Conclusion
The data demonstrated that 1stPB morphology does not appear to be a prognostic factor for rates of fertilization and embryo development in ICSI cycles.
Keywords: First Polar Body, Oocyte Morphology, ICSI, Fertilization Rate, Embryo Development
Introduction
One of the most important factors that determine success in assisted reproductive technology (ART) is the oocyte. It is clear that the quality of oocytes can affect fertilization and embryo development (1). By introduction of intracytoplasmic sperm injection (ICSI), many couples with male factor infertility have taken this opportunity to overcome their infertility. One of the capabilities of ICSI is the evaluation of oocyte morphology and maturity after denudation of cumulus cells for microinjection. The majority of all metaphase II (MII) oocytes (60%-70%) have at least one morphological abnormality (2). Many studies have reported the effect of morphological characteristics of oocytes on fertilization rate and embryo development. The outcome of ART is dependent on both patient parameters and embryo variables (3). Evaluation of first polar body (1st PB) morphology is useful for distinguishing the post-ovulatory age of the oocyte (4). Correlation between oocyte morphology and ICSI outcome is still a matter of controversy (5-9).
Ebner et al. (10) have reported that the 1st PB shape can affect fertilization rate and embryo quality in ICSI cycles. Recent studies have also demonstrated the relationship of 1st PB morphology to mature oocyte viability, which may be used as a prognostic factor to predict oocyte performance and pregnancy achievement after an ICSI treatment (11, 12). Some studies have shown a correlation between oocyte performance and 1st PB morphology during ICSI treatment cycles (9,10,13-15). However, others did not show any correlation between 1st PB and ICSI outcomes (16-19).
Additionally, the correlation between blastocyst formation, implantation rate and 1st PB morphology has been reported by Ebner and colleagues in 2002 (14). Germinal vesicle breakdown (GVBD) and simultaneous extrusion of the 1st PB shows completion of the first meiotic division in human oocytes. As a result, the 1st PB is a marker which indicates that the oocyte is ready to undergo the fertilization process. This event is synchronized with nuclear and cytoplasmic maturation (20) The main goal of this prospective study is to evaluate the correlation of 1st PB morphological characteristics with rates of fertilization and embryo development in ICSI cycles.
Materials and Methods
Patient selection
In this prospective study, we evaluated morphological characteristics of 470 MII oocytes from 80 ICSI cycles. Maternal age was between 21-42 years. All patients underwent ICSI treatment at Yazd Research and Clinical Center for Infertility between April 2010 and August 2010. This study was approved by our Center’s Ethics Committee. Patients signed informed consents.
Controlled ovarian hyperstimulation
In most patients, controlled ovarian hyperstimulation was undertaken with GnRH agonist downregulation, followed by rec FSH. An antagonist protocol was also used. Next, 10,000 IU of human chorionic gonadotrophin (hCG, i.m. DRG Co., Germany) was administered. The ovarian response was controlled by transvaginal ultrasound and serum estradiol concentration. Oocyte retrieval was done approximately 36 hours after hCG injection under transvaginal ultrasound-guidance.
Semen analysis and sperm preparation
Semen analysis was done according to a WHO laboratory manual (21). Sperm specimens were obtained by ejaculation or testicular biopsy in azoospermic patients. We used a Makler chamber and light microscopy at ×200 magnification to determine sperm counts and motility evaluation. Progressive and nonprogressive spermatozoa were reported as percentages. Sperm morphology was evaluated using Giemsa staining. All sperm preparations were performed using the swim-up or density gradient techniques (22). For swim-up, 1 ml of semen was mixed with 3 ml of Ham’s F10 medium (Seromed Co., Germany) supplemented with 10% human serum albumin (HSA). After gentle mixing, the sample was centrifuged twice (2000 rpm for 10 minutes, followed by 5 minutes). Following the removal of the supernatant, 0.2-1.0 ml of the culture medium was added, dependent upon the size of the pellet and the quality of the original sample. The suspension was then incubated at 37°C in 5% CO2 until use.
ICSI procedure
After oocyte aspiration, the oocytes were incubated for about 4 hours, then denudation from cumulus cells occurred with the use of 80 IU hyaluronidase/ ml (Sigma Chemical Co., USA) along with the mechanical aid of appropriate Pasture pipettes. Each of the MII oocytes were washed in culture media and before microinjection, their morphological characteristics were evaluated. For sperm injection, the motile spermatozoon were aspirated by Pasture pipette and then transferred to a 10% PVP droplet. The best morphologically well-shaped spermatozoa were selected for the microinjection procedure. Each spermatozoa was immobilized by touching its tail near the mid-piece with an injecting pipette, and then aspirated from the tail. The injected oocytes were washed twice, then individually placed in fresh droplets of G1 covered with mineral oil.
Oocyte evaluation
The morphological characteristics of the MII oocytes were evaluated by inverted microscope just prior to microinjection. The characteristics employed for the assessment of oocyte morphology were: a. normal oocytes had clear cytoplasms with homogenous fine granularity; b. granular oocytes, dark with granularity either homogenous in the whole cytoplasm or concentrated in the central portion of the oocyte; c. cytoplasmic inclusions comprised of vacuoles presumed to be of endocytotic origin; d. anomalies of zona pellucida (ZP); e. fragmented polar body; f. non-spherical shaped oocyte; g. wide previtelline space (wPVS); h. refractile bodies (RF); i. bull’s eye; j. debris in the PVS; and k. smooth endoplasmic reticulum cluster (SERc) (8).
First polar body evaluation
After denudation, we evaluated all oocytes for 1st PB morphology. The oocytes according to their polar bodies were divided into two groups of A (normal intact 1st PB) and B (abnormal fragmented 1st PB) (Fig 1). Other abnormalities, such as RF, wPVS, central and general granulation, bull’s eye, vacuoles, SERc, debris in the PVS, as well as oocyte shape and color were noted.
Fig 1.
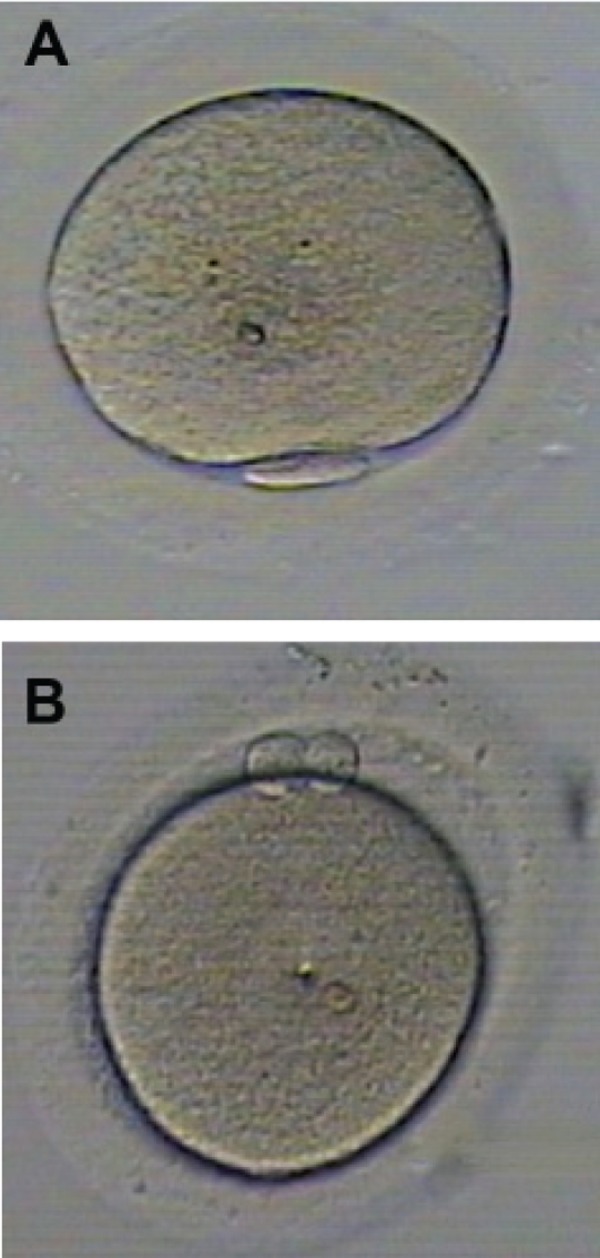
MII oocytes after denudation of cumulus cell before microinjection. a: normal 1st PB and b: fragmented 1st PB.
Fertilization evaluation
The injected oocytes were incubated followed by fertilization evaluation 18-19 hours after injection by visualizing the oocytes under a microscope and determining the presence of 2PN.
Embryo evaluation and transfer
About 48 hours post-injection, we evaluated the embryos according to the procedure of Hill et al. (23). Briefly, grading was as follows: grade A, equal size blastomeres without fragmentation; grade B, slightly unequal blastomeres, up to 10% cytoplasmic fragments; grade C, unequal sized blastomeres up to 50% fragments and large granules; and grade D, unequal blastomeres with significant fragmentation and large black granules. We considered grades A and B to be good quality embryos, whereas grades C and D were poor quality embryos.
Inclusion and exclusion criteria
All retrieved oocytes were included in the study. No oocytes were cryopreserved or discarded. Egg donation, natural cycles and degenerated oocytes after microinjections due to mechanical error were excluded from the study.
Statistical analysis
Data was presented as mean ± SE. Statistical analysis chi-square and Fisher`s exact tests were chosen. Data were presented as odds ratio (OR), 95% confidence interval (95% CI) and p value. The ORs referred to fertilization rate and good quality or early cleaved embryos. Independent sample ttests were used wherever appropriate. P<0.05 was considered significant. Statistical analysis was done with the Statistical Program for Social Science (SPSS 16.0, Chicago, IL) software.
Results
A total of 286 oocytes were normally fertilized, of which 179 were normal and 107 had fragmented 1st PBs. Additionally, 287 embryos were formed of which 179 were good embryos (119 normal oocytes and 60 fragmented 1st PB). From the total of 287 embryos, 213 had early cleavage that resulted from 139 normal oocytes and 74 fragmented 1st PB (Table 1).
Table 1.
Frequencies in groups A and B
| Normal oocytes(group A) | Fragmented 1st PB oocytes(group B) | |
|---|---|---|
| MII oocytes | 291 (61.9%) | 179 (38.1%) |
| Fertilized oocytes | 179 (62.6%) | 107 (37.4%) |
| Good quality embryos | 119 (66.5%) | 60 (33.5%) |
| Early cleavage embryos | 139 (65.2%) | 74 (34.8%) |
The data showed that 27% of the oocytes had fragmented 1st PB with no other morphological abnormalities, while the remainder were associated with other abnormalities. There were 46.1% of the oocytes that had double defects and 26.9% of the oocytes had multiple defects. In group B, RF (19%) and granulation (9%) were double defects; wPVS with RF (10%) were the most common abnormalities observed for multiple defects. Overall, other abnormalities with fragmentation of 1st PB for both double and multiple defect oocytes was less than 10%. The least anomaly combined with 1st PB fragmentation was darkness in the oocyte cytoplasm (0.6%). For oocytes with multiple defects, the least common anomaly was bull’s eye with debris in the PVS (0.6%).
There was no significant relationship between groups A and B in terms of oocyte fertilization rate; 1st PB morphology did not predict fertilization rate. The rates of good embryo formation for groups A was 66.5%, whereas it was 55.6% for group B. Cleavage rates for group A was 77.7% and 68.5% for group B. The embryo development rates were not statistically significant in the two groups (Table 2).
Table 2.
Comparison of fertilization and embryo development rates between both groups
| Normal intact 1st PB (group A) | Abnormal fragmented 1st PB (group B) | Odds ratio (95% CI) | p value | |
|---|---|---|---|---|
| Fertilization rate | 61.5% | 59.8% | 1.07 (0.73-1.57) | NS |
| Good quality embryos | 66.5% | 55.6% | 1.58 (0.97-2.59) | NS |
| Embryo cleavage rate | 77.7% | 68.5% | 1.59 (0.93-2.73) | NS |
CI: confidence interval
Chi-square test was used for analysis
Women with group A had a mean age of 32.3 ± 0.3 years, whereas those with group B were 32.8 ± 0.4 years, which was not statistically significant. However, we found a significant difference between maternal age in groups A and B (p=0.016) with regard to the formation of good embryos (Table 3).
Table 3.
Maternal age comparison for fertilization rates and embryo development
| 1st PB morphology | p | Fertilization | p | Embryo quality | p | Cleavage rate | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal | A | B | NS | yes | no | NS | good | poor | 0.016 | early | late | NS |
| age | 32.3 ± 0.3 | 32.8 ± 0.4 | 32.3 ± 0.3 | 32.9 ± 0.4 | 31.8 ± 0.3 | 33.3 ± 0.5 | 32.2 ± 0.3 | 32.8 ± 0.6 | ||||
Independent sample t test was used for analysis
Discussion
Because of different nuclear maturity, the retrieved oocytes post ovarian hyper-stimulation show different grades of 1st PB morphology. The presence of 1st PB and its observation by an embryologist before ICSI is very important because extrusion of the 1st PB reflects MII oocyte maturity. One of the attractive issues in ART is finding criteria(s) to predict which oocytes will fertilize and which oocyte characteristic(s) may affect embryo development during the ART procedure.
Our results demonstrate no correlation between 1st PB morphology and fertilization rate, embryo quality or even cleavage rate in women undergoing ICSI treatments. There is no significant difference between maternal age in groups A and B. Also, patients’ ages were not related to fertilization and cleavage rates, similar to the findings of Ciotti et al. (16) but is related to embryo quality. In the literature, the prognostic value of 1st PB morphology for fertilization rate, embryo quality and cleavage rate is controversial. Our findings are similar to previous studies reported by reproductive scientists (11, 14-17, 19, 24, 25), but conflict with reports from others (9, 10, 13). One reason for this contradiction may be related to the methodological variation in oocyte evaluation. Xia (9) and Mikkelsen and Collouge (24) have reported that the 1st PB morphology combined with perivitelline space and cytoplasmic inclusions can be used as prognostic factors for fertilization rate and cleavaged embryo quality (9, 24), however we only evaluated 1st PB morphology. The other reason for this discrepancy may be related to the use of a different 1st PB grading system. Some studies divide 1st PB morphology simply into two groups of normal and fragmented, as we did. However, others may grade 1st PB according to criteria such as surface, size and maturity (9, 10, 13).
Considering the positive relationship between 1st PB morphology and time elapsed in culture, therefore, PB morphology may alter after a few hours, and it can change according to the timing of the observation. Ciotti et al. have noted that 1st PB fragmentation is related to the time elapse between retrieval, denudation and ICSI performance (16). They checked a subgroup of oocytes twice (at the moment of denudation and injection, respectively) for 1st PB fragmentation and have observed different degrees of fragmentation at the first and second efforts (11.1% and 22.8%, respectively). However, as they increased the time elapsed for denudation to >3.5 hours, the fragmentation rate of the oocytes was 26.7%. Another dispensary may be related to the procedure that occurs for the time of the 1st PB evaluation and performing the ICSI. In different studies, distinct ICSI procedures were applied, but timing for cumulus cell denudation and ultimately the time in which the 1st PB was evaluated may not be the same. It is well known that, because of controlled ovarian hyperstimulation, not all retrieved oocytes have the same quality, and different ovarian hyperstimulation protocols may be applied in studies. Therefore, oocyte quality may be influenced by the aforementioned protocols.
Moreover, Verlinsky and his colleagues in 2003 have shown that PB morphology is not related to the genotype analyzed for aneuploidy in patients who underwent preimplantation genetic diagnosis (PGD). They noticed no correlation between polar body shape and genetic constitution of the oocyte (19). In addition, they detected 1st PB morphology grading changes in terms of fragmentation in over one-third of the oocytes studied. Hence, 1st PB morphology assessment may not serve as a reliable marker for assessment of oocyte quality and competence.
Conclusion
The data demonstrated that 1st PB morphology does not appear to be a prognostic factor for oocyte competence in the process of fertilization and early embryo development in ICSI cycles.
Acknowledgments
The authors would like to thank Ms. Farimah Shamsi for statistical consultation. The technical assistance of Ms. Motamedzadeh is also appreciated. There is no conflict of interest in this study.
References
- 1.Lasienë K, Vitkus A, Valanciūte A, Lasys V. Morphological criteria of oocyte quality. Medicina(Kaunas) 2009;45(7):509–515. [PubMed] [Google Scholar]
- 2.Balaban B, Urman B. Effect of oocyte morphology on embryo development and implantation. Reprod BioMed Online. 2006;12(5):608–615. doi: 10.1016/s1472-6483(10)61187-x. [DOI] [PubMed] [Google Scholar]
- 3.Serhal PF, Ranieri DM, kinis A, Marchant S, Davies M, Khadum IM. Oocyte morphology predicts outcome of intracytoplasmic sperm injection. Hum Reprod. 1997;12(6):1267–1270. doi: 10.1093/humrep/12.6.1267. [DOI] [PubMed] [Google Scholar]
- 4.Eichenlaub-Ritter U, Schmiady H, Kentenich H, Soewarto D. Recurrent failure in polar body formation and premature chromosome condensation in oocytes from a human patient: Indicators of asynchrony in nuclear and cytoplasmic maturation. Hum Reprod. 1995;10(9):2343–2349. doi: 10.1093/oxfordjournals.humrep.a136297. [DOI] [PubMed] [Google Scholar]
- 5.Balaban B, Urman B, Sertac C, Alatas C, Aksoy S, Mercan R. Oocyte morphology does not affect fertilization rate, embryo quality and implantation rate after intracytoplasmic sperm injection. Hum Reprod. 1998;13(12):3431–3433. doi: 10.1093/humrep/13.12.3431. [DOI] [PubMed] [Google Scholar]
- 6.De Sutter P, Dozortsev D, Qian C, Dhont M. Oocyte morphology does not correlate with fertilization rate and embryo quality after intracytoplasmic sperm injection. Hum Reprod. 1996;11(3):595–597. doi: 10.1093/humrep/11.3.595. [DOI] [PubMed] [Google Scholar]
- 7.Loutradis D, Drakakis P, Kallianidis K, Milingos S, Dendrinos S, Michalas S. Oocyte morphology correlates with embryo quality and pregnancy rate after intracytoplasmic sperm injection. Fertil Steril. 1999;72(2):240–244. doi: 10.1016/s0015-0282(99)00233-2. [DOI] [PubMed] [Google Scholar]
- 8.Khalili MA, Sultan AM, Mojibian M. Role of oocyte morphology on fertilization and embryo formation in assisted reproductive techniques. Middle East Fertil Soc. 2005;10(1):72–77. [Google Scholar]
- 9.Xia P. lntracytoplasmic sperm injection: correlation of oocyte grade based on polar body, perivitelline space and cytoplasmic inclusion with fertilization rate and embryo quality. Hum Reprod. 1997;12(8):1750–1755. doi: 10.1093/humrep/12.8.1750. [DOI] [PubMed] [Google Scholar]
- 10.Ebner T, Yaman C, Moser M, Sommergruber M, Feichtinger O, tews G. Prognostic value of first polar body morphology on fertilization rate and embryo quality in intracytoplasmic sperm injection. Hum Reprod. 2000;15(2):427–430. doi: 10.1093/humrep/15.2.427. [DOI] [PubMed] [Google Scholar]
- 11.Younis JS, Radin O, Izhaki I, Ben-Ami M. Does first polar body morphology predict oocyte performance during ICSI treatment? J Assist Reprod Genet. 2009;26(11-12):561–567. doi: 10.1007/s10815-009-9368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Younis JS, Radin O, Mirsky N, Izhaki I, Majara T, Bar-Ami S, et al. First polar body and nucleolar precursor body morphology is related to the ovarian reserve of infertile women. Reprod BioMed Online. 2008;16(6):851–858. doi: 10.1016/s1472-6483(10)60152-6. [DOI] [PubMed] [Google Scholar]
- 13.Balaban B, Urman B, Isiklar A, Alatas C, Aksoy S, Mercan R. The effect of polar body (PB) morphology on embryo quality, implantation and pregnancy rates. Ferti Steril. 2001;76(3):S7–S7. [Google Scholar]
- 14.Ebner T, Moser M, Sommergruber M, Yaman C, Pfleger U, Tews G. First polar body morphology and blastocyst formation rate in ICSI patients. Hum Reprod. 2002;17(9):2415–2418. doi: 10.1093/humrep/17.9.2415. [DOI] [PubMed] [Google Scholar]
- 15.Ebner T, Moser M, Yaman C, Feichtinger O, Hartl J, Tews G. Elective transfer of embryos selected on the basis of first polar body morphology is associated with increased rates of implantation and pregnancy. Fertil Steril. 1999;72(4):599–603. doi: 10.1016/s0015-0282(99)00315-5. [DOI] [PubMed] [Google Scholar]
- 16.Ciotti PM, Notarangelo L, Morselli-Labate AM, Felletti V, Porcu E, Venturoli S. First polar body morphology before ICSI is not related to embryo quality or pregnancy rate. Hum Reprod. 2004;19(10):2334–2439. doi: 10.1093/humrep/deh433. [DOI] [PubMed] [Google Scholar]
- 17.De Santis L, Cino I, Rabellotti E, Calzi F, Persico P, Borini A, et al. Polar body morphology and spindle imaging as predictors of oocyte quality. Reprod BioMed Online. 2005;11(1):36–42. doi: 10.1016/s1472-6483(10)61296-5. [DOI] [PubMed] [Google Scholar]
- 18.Scott L, Finn A, O'Leary T, McLellan S, Hill J. Morphologic parameters of early cleavage-stage embryos that correlate with fetal development and delivery: Prospective and applied data for increased pregnancy rates. Hum Reprod. 2007;22(1):230–240. doi: 10.1093/humrep/del358. [DOI] [PubMed] [Google Scholar]
- 19.Verlinsky Y, Lerner S, Illkevitch N, Kuznetsov V, Kuznetsov I, Cieslak J, et al. Is there any predictive value of first polar body morphology for embryo genotype or developmental potential? Reprod BioMed Online. 2003;7(3):336–341. doi: 10.1016/s1472-6483(10)61874-3. [DOI] [PubMed] [Google Scholar]
- 20.Scott L. The biological basis of non-invasive strategies for selection of human oocytes and embryos. Hum Reprod Update. 2003;9(3):237–249. doi: 10.1093/humupd/dmg023. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. laboratory manual for the examination of human semen and semen-cervical mucus interaction. Cambridge: Cambridge University Press; 1999. pp. 232–232. [Google Scholar]
- 22.Khalili MA, Mir-Rokni F, Kalantar SM. Application of vitality tests on asthenozoospermic semen from infertile men. Iran Biomed J. 1999;3(2):77–81. [Google Scholar]
- 23.Hill GA, Freeman M, Bastias MC, Rogers BJ, Herbert CM, Osteen KG, et al. The influence of oocyte maturity and embryo quality on pregnancy rate in a program for in vitro fertilization-embryo transfer. Fertil Steril. 1989;52(5):801–806. doi: 10.1016/s0015-0282(16)61034-8. [DOI] [PubMed] [Google Scholar]
- 24.Mikkelsen AL, Lindenberg S. Morphology of in-vitro matured oocytes: impact on fertility potential and embryo quality. Hum Reprod. 2001;16(8):1714–1718. doi: 10.1093/humrep/16.8.1714. [DOI] [PubMed] [Google Scholar]
- 25.Miller KF, Sinoway C, Fly KL, Flacone T. Fragmentation of the polar body at the time of ICSI does not predict fertilization or early embryo developmental but may be associated with improved pregnancy and implantation. Fertil Steril. 2001;76(3):S201–S201. [Google Scholar]


