Abstract
αvβ8-Integrin is most abundantly expressed in the kidney, brain, and female reproductive organs, and its cognate ligand is latent transforming growth factor (LTGF)-β. Kidney αvβ8-integrin localizes to mesangial cells, and global β8-integrin gene (Itgb8) deletion results in embryonic lethality due to impaired placentation and cerebral hemorrhage. To circumvent the lethality and better define kidney αvβ8-integrin function, Cre-lox technology was used to generate mesangial-specific Itgb8-null mice. Platelet-derived growth factor-β receptor (PDGFBR)-Cre mice crossed with a reporter strain revealed functional Cre recombinase activity in a predicted mesangial pattern. However, mating between two different PDGFBR-Cre or Ren1d-Cre strains with Itgb8 flox/− mice consistently resulted in incomplete recombination, with no renal phenotype in mosaic offspring. Induction of a renal phenotype with Habu snake venom, a reversible mesangiolytic agent, caused exaggerated glomerular capillary microaneurysms and delayed recovery in Cre+/− PDGFRB flox/− mice compared with Cre+/− PDGFRB flox/+ control mice. To establish the mechanism, in vitro experiments were conducted in Itgb8-null versus Itgb8-expressing mesangial cells and fibroblasts, which revealed β8-integrin-regulated adhesion to Arg-Gly-Asp (RGD) peptides within a mesangial-conditioned matrix as well as β8-integrin-dependent migration on RGD-containing LTGF-β or vitronectin matrices. We speculate that kidney αvβ8-integrin indirectly controls glomerular capillary integrity through mechanical tension generated by binding RGD peptides in the mesangial matrix, and healing after glomerular injury may be facilitated by mesangial cell migration, which is guided by transient β8-integrin interactions with RGD ligands.
Keywords: cell migration, extracellular matrix, glomerular endothelial cell, glomerular injury
integrins are extracellular matrix receptors that regulate vital functions, including cell adhesion and migration, and are composed of α- and β-glycoprotein subunits, which heterodimerize through disulfide bridges between extracellular domains. Eighteen α-subunits and eight β-subunits combine to form twenty-four heterodimers. The β8-subunit partners with the αv-subunit, and expression is restricted primarily to the kidney, brain, and female reproductive organs (30). Global β8-integrin gene (Itgb8) or alpha-v integrin gene (Itgav) deletion is embryonic lethal due to placenta and brain angiogenesis defects (28, 29, 32, 50). We previously found that kidney β8-integrin is localized to mesangial cells (22, 25). Itgb8−/− mice bred to a genetic background that permitted postnatal survival developed a renal phenotype that included modest albuminuria and azotemia, and the mechanism appeared to be due to indirect effects of mesangial cell β8-integrin on neighboring glomerular endothelial cells (22). However, few Itgb8−/− mice lived beyond 6 wk, and the kidney phenotype therefore remained incompletely characterized.
The mesangium is the central region of the glomerulus, which is composed of mesangial cells and the extracellular mesangial matrix. Mesangial cells are modified pericytes, which contact the glomerular basement membrane at the base of the glomerular capillary loops to counteract hemodynamic forces associated with capillary loop distention (24). The most dramatic evidence of this function is derived from platelet-derived growth factor (PDGF)-B or PDGF-β receptor (PDGFBR) knockout mice, which develop glomerular capillary microaneurysms due to the absence of mesangial cells (27, 41). In response to developmental cues or glomerular injury, mesangial cells participate in glomerular basement membrane and extracellular matrix remodeling, phagocytosis, cytokine secretion, migration, and regulation of glomerular circulation and ultrafiltration (11, 18, 37); many of these functions are regulated by integrins (11).
To circumvent the lethality associated with global Itgb8 deletion and better define kidney αvβ8-integrin function, Cre-lox technology was used to generate mesangial-specific Itgb8-null mice. Complete recombination was not achieved, using three different Cre mouse strains. However, induction of mesangial injury in β8-integrin mosaic mice unmasked a glomerular capillary phenotype. When combined with clarifying in vitro experiments, we conclude that mesangial cell αvβ8-integrin binds to mesangial matrix ligands and may indirectly regulate glomerular capillary structure and integrity through the generation of mechanotransduction signals and migration to sites of glomerular capillary repair (33).
MATERIALS AND METHODS
Mice.
Itgb8 flox/− mice were a gift from Dr. Louis Reichardt (University of California, San Francisco, CA) (32). PDGFRB-Cre mice [PDGFRB-Cre(RA) mice] were a gift from Dr. Ralf Adams (Cancer Research UK London Research Institute) (10). A second strain of PDGFRB-Cre mice, which contain a longer promoter with the transgene [PDGFRB-Cre(VL) mice], was a gift from Dr. Volkhard Lindner (Maine Medical Center Research Institute) (8). Ren1d-Cre mice were a gift from Dr. Ariel Gomez (University of Virginia) (40). All transgenic mice were backcrossed with C57BL/6 wild-type mice for at least 12 generations to create congenic strains. Cre/+ mice were crossed with Itgb8 flox/− mice to generate Cre/+ Itgb8 flox/− and Cre/+ Itgb8 +/− progeny, which were then intercrossed to produce Cre/+ Itgb8 flox/+ and Cre/+ Itgb8 flox/− mice for phenotyping. Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J reporter mice were purchased from Jackson Laboratories (Bar Harbor, ME) (9). All protocols were approved by the Institutional Animal Care and Use Committees of Case Western Reserve University.
Genotyping.
All mice were genotyped by PCR analysis using tail DNA according to previously published methods (32). Primers used to identify each allele were as follows: Itgb8 wild-type (250 bp) and floxed allele (370 bp), 5′-GAGATGCAAGAGTGTTTACC-3′ and 5′-CACTTTAGTATGCTAATGATGG-3′; Itgb8-null allele (450 bp), 5′-AGAGGCCACTTGTGTAGCGCCAAG-3′ and 5′-GAGGCATACAGTCTAAATTGT-3′; and Cre (400 bp), 5′-CTGGCAATTTCGGCTATACGTAACAGGGTG-3′ and 5′-GCCTGCATTACCGGTCGATGCAAC-3′.
Immunoblot analysis.
Methods for the immunoblot analysis have been previously described (1). Briefly, cell monolayers were lysed and denatured in boiling buffer [125 mM Tris (pH 6.8), 2% SDS, 5% glycerol, 1% β-mercaptoethanol, and 0.003% bromphenol blue] for 5 min. Samples (20 μg protein/lane) were resolved by SDS-PAGE and transferred to polyvinylidine difluoride membranes. Blots were blocked in 5% dried milk and probed with anti-β8-integrin IgG (Santa Cruz Biotechnology, Santa Cruz, CA; 1:1,000 in 5% BSA, overnight, 4°C) and then horseradish peroxidase-conjugated IgG (1:10,000, 1 h, room temperature). Band intensity was detected by enhanced chemiluminescence. Blots were exposed to stripping buffer (Thermo Scientific, Waltham, MA; 10 min, room temperature) and then reprobed with anti-α-tubulin IgG (Santa Cruz Biotechnology; 1:3,000, 1 h, room temperature) followed by horseradish peroxidase-conjugated IgG (1:10,000, 1 h, room temperature).
Quantitative RT-PCR.
Functional Itgb8 deletion was verified by measuring β8-integrin mRNA content by quantitative RT-PCR from microdissected mouse glomeruli, which was normalized to GAPDH expression, according to previously described methods (22, 25). Oligonucleotide primer sequences were as follows: β8-integrin (66 bp), 5′-ACTTCTCCTGTCCCTATCTCCA-3′ and 5′-ATCTGCCACCTTCACACTCC-3′; and GAPDH (625 bp), 5′-GGAGCCAAACGGGTCATC-3′ and 5′-TGTTGCTGTAGCCGTATTCAT-3′.
Cre reporter mouse.
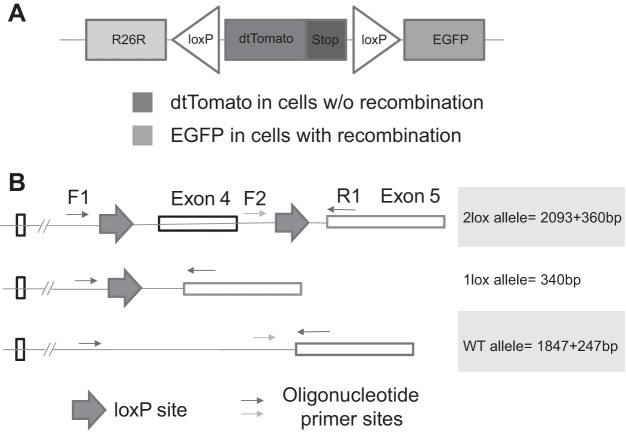
Cre recombinase activity was screened in double-fluorescent Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J mice (9), which contain a membrane-targeted tdTomato transgene and a stop codon flanked by loxP sites (Fig. 1A). Mice constitutively fluoresce red in all tissues, but in cells expressing active Cre, the dtTomato transgene is deleted, and removal of the stop codon allows expression of downstream, membrane-targeted enhanced green fluorescence protein (EGFP).
Fig. 1.
Cre-lox recombination efficiency assays. A: reporter gene map. B: multiplex PCR strategy to identify one lox, two lox, and wild-type (WT) β8-integrin alleles. EGFP, enhanced green fluorescent protein.
Recombination efficiency assay.
A multiplex PCR strategy was used to identify the extent of recombination after crosses between the Cre strains and Itgb8-floxed mice using DNA extracted from tails and whole kidneys, according to published methods (48). Upstream primer sequences were 5′-GTGGTTAAGAGCACCGATTG-3′ (F1) and 5′-GAGATGCAAGAGTGTTTACC-3′ (F2); the downstream primer sequence was 5′-CACTTTAGTATGCTAATGATG-3′ (R1). The cycling conditions were as follows: preincubation at 95°C for 10 min and then 45 cycles at 95°C for 10 s (denaturation), 58°C for 45 s (annealing), and 72°C for 1 s (extension). The amplicons from wild-type DNA were predicted to be 1,847 and 247 bp in size; recombined DNA with only one loxP site was predicted to yield a single 340-bp band; unrecombined DNA with both loxP sites intact yielded 2,093- and 360-bp bands (Fig. 1B).
Renal phenotype induction.
Tails of anesthetized mice were warmed with cotton balls immersed in hot water to stimulate vasodilation before an intravenous injection with Habu toxin (Sigma, St. Louis, MO, 2–10 μg/g) or saline vehicle control (3, 4). Mice were monitored twice daily for the ensuing 72 h for signs of distress and then daily thereafter. Mice were euthanized at 2, 7, and 14 days for phenotype evaluation.
Kidney histology.
Kidneys were fixed in formaldehyde, embedded in paraffin, sectioned, deparaffinized, and then stained with periodic acid-Schiff reagent or Masson's trichrome stain. Ki-67 and TUNEL staining experiments were conducted as previously described (22, 34). Glomerular capillary dilation was defined as the percentage of glomeruli (from a total of 50 glomeruli/kidney, 5 kidneys/genotype) containing a capillary that exceeded 25 μm in diameter. Mesangial hypercellularity was defined as clusters of greater than four mesangial cells (16).
Renal function assays.
Albuminuria was measured using the Mouse Albumin ELISA Quantitation Kit (ab108792, Abcam, Cambridge, MA) as previously described (23). Serum and urine creatinine were measured by an enzymatic assay (ab65340, Abcam), which is superior to colorimetric and comparable with HPLC methods in mice (21).
Cell culture.
Mouse mesangial cells and glomerular endothelial cells [gift from Dr. Michael Madaio (Medical College of Georgia) (2)] were harvested and maintained in cell cultures according to previously described methods (22). Temperature-sensitive, conditionally immortalized mouse podocytes were maintained in the undifferentiated, proliferating state at 33°C and induced to differentiate as previously described (42). Mesangial cells derived from Itgb8−/− mice have previously been characterized (25). CHO-B2/v7 fibroblasts, which are devoid of β-integrins and stably express αv-integrin, were transiently transfected with β8-integrin cDNA or empty vector and maintained in cell cultures as previously described (25).
Cell adhesion assays.
The mesangial matrix was isolated using the methods of Langhofer et al. (26). Briefly, mouse mesangial cells in primary culture were grown to confluence, washed with PBS, and then lysed in NH4OH (20 mM, 15 min, room temperature). The residual extracellular matrix, which remains coated on wells, was washed with sterile water followed by PBS. Adhesion was then assayed in wild-type and Itgb8−/− mesangial cells and in β8-integrin-expressing (CHO-B2/v7-β8) and β8-integrin-null (CHO-B2/v7) fibroblasts. All cell lines were maintained in serum-free media overnight, washed in PBS, and then seeded in 96-well plates (2 × 104 cells/well, 30 min) with or without GRGDSP [Arg-Gly-Asp (RDG)] or GRGESP [Arg-Gly-Glu (RGE)] peptides (100 μg/ml) on the mesangial matrix or poly-l-lysine (PLL; 5 μg/ml). Background was established in wells coated with 0.2% BSA and subtracted from total binding. Adherent cells were fixed in paraformaldehyde, and nuclei were stained with crystal violet, which was eluted with sodium acetate and ethanol and quantitated by spectrophotometry (absorbance wavelength: 550 nm), as previously described (19).
Cell migration assays.
Cell migration in wells coated with 5 μg/ml LTGF-β, latency-associated peptide-β1 (LAP), vitronectin, or PLL was determined by scratch wound assays, whereby a sterile 20-μl pipette tip was used to create a wound of ∼300 μm width in confluent cell culture monolayers. Images were captured at 0 and 14 h with a Nikon epifluorescence microscope (Tokyo, Japan) in phase-contrast mode at ×10 magnification, and the extent of migration was calculated by counting the number of cells migrating into the wound gap, which was normalized to scratch length. For experiments with mouse mesangial cells, which had a tendency to migrate in sheets, a second measure of migration was also performed. The baseline gap created by the scratch was measured at 10–15 locations over a 1.5- to 2.0-mm distance using Metamorph software (Molecular Devices, Sunnyvale, CA). The distance across the wound was then remeasured at the same locations after 15 h of migration.
Immunocytochemistry.
Cells were evaluated by previously described methods (25). Briefly, CHO-B2/v7 and CHO-B2/v7-β8 fibroblasts were plated on glass coverslips with or without LAP (5 μg/ml). After 2 h, cells were fixed in paraformaldehyde (4%, 10 min, room temperature), blocked, and then permeabilized with 5% BSA in 0.2% Triton X-100. Actin was labeled with Alexa 568-conjugated phalloidin (1:40, room temperature). Coverslips were mounted in 4′,6-diamidino-2-phenylindole-containing Vectashield medium (Vector Laboratories, Burlingame, CA) and viewed with a Nikon epifluorescence microscope. Images were generated with a Spot Digital System camera (Diagnostic Instruments, Sterling Heights, MI) and Image Pro software (Media Cybernetics, Silver Spring, MD).
Statistics.
All experiments were conducted a minimum of three times. Results are expressed as means ± SE. Two-tailed paired t-tests were used for statistical analysis between two groups. Two-way ANOVA was used for comparisons between more than two groups. Statistical significance was defined as P < 0.05.
RESULTS
Efficiency of PDGFRB-Cre activity.
We (22, 25) have previously shown that αvβ8-integrin is expressed in a glomerular mesangial pattern by in situ hybridization and immunohistochemistry. We verified by immunoblot analysis that β8-integrin is not expressed in cell lines derived from the other major resident glomerular cells, podocytes and glomerular endothelial cells (Fig. 2A). To understand the role of αvβ8-integrin in kidney mesangial cell function, we used transgenic mice harboring floxed alleles of Itgb8. Floxed mice were then crossed with the PDGFRB-Cre strain because the PDGF-β receptor is abundantly expressed in mesangial cells and pericytes but not in cells of placental and neuroepithelial origin, which are responsible for global Itgb8−/− embryonic lethality (50). Cre recombinase activity was screened in double-fluorescent Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J mice (9), as described in materials and methods. Figure 2, B–D, shows a glomerulus from the Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J reporter mouse expressing the PDGFRB-Cre(RA) transgene, which showed tdTomato fluorescence in all nonmesangial glomerular cells, whereas EGFP fluorescence was only observed in a mesangial distribution.
Fig. 2.
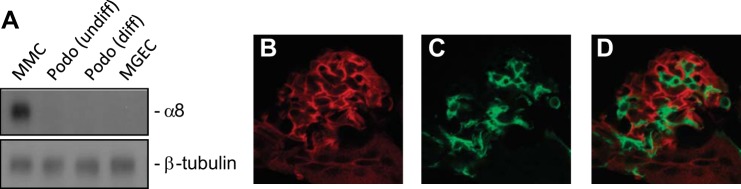
Platelet-derived growth factor-β receptor (PDGFBR)-Cre activity in a mesangial pattern. A: β8-integrin immunoblot of cell culture lysates from mouse mesangial cells (MMC), undifferentiated and differentiated mouse podocytes (Podo), and mouse glomerular endothelial cells (MGEC). PDGFBR-Cre mice were crossed with floxed reporter mice. B: the red tag marks nonrecombined cells. C: the green tag marks cells where recombination has occurred. D: merged image.
Itgb8 deletion in only a fraction of mesangial cells.
Because previous studies indicated that mesangial Cre activity was robust, it was surprising to consistently detect β8-integrin mRNA in isolated glomeruli from PDGFRB-Cre(RA)Itgb8 flox/− (Fig. 3A) as well as PDGFRB-Cre(RA)Itgb8 flox/flox mice (not shown). To determine whether these results were specific to crosses involving PDGFRB-Cre(RA) mice, we tested two different Cre strains, each driven by a mesangial-selective promoter [PDGFRB-Cre(VL) and Ren1d-Cre mice], and noted similar residual β8-integrin mRNA levels (Fig. 3A). Since mice with the Itgb8 flox/− genotype have only one functional Itgb8 gene, we hypothesized that the source of β8-integrin mRNA in Cre-expressing animals is unrecombined, floxed, Itgb8. To investigate recombination efficiency, we used a PCR-based method, which determines the proportion of functional (two loxP sites preserved) versus truncated (collapsed to one loxP site) alleles in Cre/+ mice (48). Figure 3B, top, shows amplicons corresponding to both one lox and two lox alleles in tails from PDGFRB-Cre(RA)Itgb8flox/− mice. Figure 3B, bottom, shows the persistence of the unrecombined floxed β8-integrin allele in isolated glomeruli from PDGFRB-Cre(RA)Itgb8flox/− mice. Since each diploid Itgb8 flox/− cell contains a null allele paired with either a floxed or recombined β8-integrin allele, tissue showing three PCR bands would be composed of two cell types: β8-integrin floxed/null or β8-integrin recombined/null, thus generating mosaic tissue in these animals.
Fig. 3.
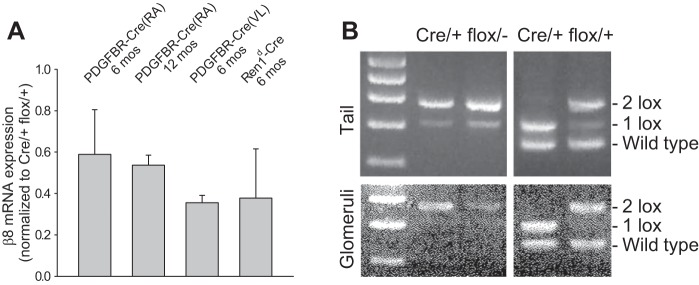
Floxed β8-integrin is incompletely deleted by Cre recombinase. A: β8-integrin mRNA expression was determined by quantitative RT-PCR in isolated glomeruli derived from crosses between floxed β8-integrin and three different Cre mouse strains. B: to investigate incomplete recombination as a possible mechanism, a PCR-based strategy was used, whereby complete recombination collapses the target gene to one loxP site (1 lox), whereas the unrecombined target gene retains both loxP sites (2 lox). Results from two mice per genotype revealed incomplete recombination in PDGFRB(VL)-Cre/+ flox/− mice and variable, but sometimes complete, recombination in PDGFRB(VL)-Cre/+ flox/+ mice.
Embryonic lethality in Cre-lox mice.
Of the crosses between floxed mice and the three Cre strains, PDGFRB-Cre(RA)Itgb8flox/− and Ren1d-Cre Itgb8 flox/− progeny were observed in normal Mendelian ratios, whereas PDGFRB-Cre(VL)Itgb8 flox/− progeny were born at less than the expected frequency (Table 1). Cre(VL)/+ flox/− survivors demonstrated incomplete recombination of the floxed allele, and, interestingly, complete recombination was achieved in some Cre(VL)/+ mice, but only if the floxed allele was accompanied by a wild-type copy of β8-integrin providing functional mRNA, independent of the Cre-lox system. The data shown in Table 1 and Fig. 3 suggest that absence of β8-integrin function in a PDGFBR-Cre-expressing cell lineage leads to embryonic lethality. We proceeded by determining if there was a phenotype difference in PDGFRB-Cre(VL) flox/− versus PDGFRB-Cre(VL) flox/+ mice, hereafter designated as Cre/+ flox/− and Cre/+ flox/+, respectively.
Table 1.
Genotypes of progeny from crosses between floxed β8-integrin gene and three different Cre strains
|
PDGFRB(RA)-Cre Mice |
Ren1d-Cre Mice |
PDGFRB(VL)-Cre Mice |
||||
|---|---|---|---|---|---|---|
| Cre/+ flox/+ | Cre/+ flox/− | Cre/+ flox/+ | Cre/+ flox/− | Cre/+ flox/+ | Cre/+ flox/− | |
| Anticipated | 84 | 84 | 44 | 44 | 65 | 65 |
| Observed | 74 | 94 | 50 | 38 | 98 | 31* |
| Complete recombination | 0 | 0 | 0 | 0 | 67 | 0* |
PDGFBR, platelet-derived growth factor-β receptor.
P < 0.001 by χ2-test.
Glomerular phenotype in Cre/+ flox/− mice.
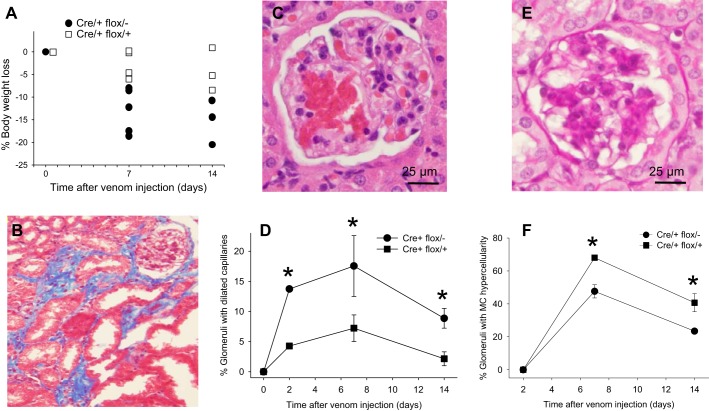
Significant differences in baseline renal function were not observed between Cre/+ flox/− and Cre/+ flox/+ mice, as determined by serum creatinine (Fig. 5B), albuminuria (Fig. 5C), or histology (not shown). Because kidney αvβ8-integrin expression is restricted to mesangial cells, Cre/+ flox/− and Cre/+ flox/+ mice were treated with Habu snake venom to induce mesangiolysis and a renal phenotype (3, 4). Dose finding experiments in wild-type mice revealed that 10 μg/g was lethal within 24 h, whereas concentrations of 2–6 μg/g yielded modest but discernible glomerular lesions (Fig. 4). Mice were therefore treated with 2 μg/g Habu toxin to allow phenotype accentuation in conditional mutants. All mice lost weight after they received Habu toxin, but weight loss was more pronounced in Cre/+ flox/− mice compared with Cre/+ flox/+ mice (Fig. 4A). Interstitial fibrosis was rarely observed in Cre/+ flox/− kidneys (Fig. 4B), and the major lesion was glomerular capillary dilation (Fig. 4C), which was observed significantly more frequently in Cre/+ flox/− mice and persisted for up to 2 wk after Habu administration (Fig. 4D). During the recovery phase, mesangial hypercellularity was less in Cre/+ flox/− kidneys compared with Cre/+ flox/+ kidneys (Fig. 4, E and F).
Fig. 5.
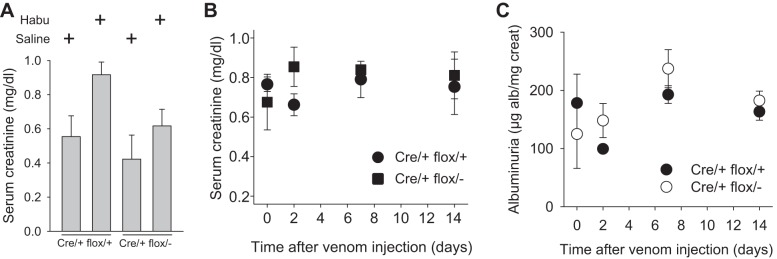
Renal function in Habu-treated mice. Renal function was determined by serum creatinine (A and B) or by the urine albumin-to-creatinine ratio (C). Data in A were generated 2 days after Habu venom or saline control injection in Cre/+ flox/+ and Cre/+ flox/− mice. Data in B and C were generated at the indicated time points after Habu toxin or saline injection.
Fig. 4.
Phenotypes in Habu venom-injected mice. A: body weights in Cre/+ flox/+ and Cre/+ flox/− mice after Habu injection. B: a Masson's trichrome-stained Cre/+ flox/− kidney revealed interstitial fibrosis 14 days after Habu injection. C: glomerular capillary dilation in a Cre/+ flox/− kidney 7 days after Habu injection. D and E: glomerular capillary dilation was quantitated in Cre/+ flox/+ and Cre/+ flox/− mice at the indicated time points. E: mesangial hypercellularity in a Cre/+ flox/− kidney 7 days after Habu injection. F: mesangial hypercellularity was quantitated in Cre/+ flox/+ and Cre/+ flox/− mice at the indicated time points. Results are expressed as means ± SE. *P < 0.05 compared with Cre/+ flox/+ kidneys at the same time point.
Renal function in Habu toxin-treated mice.
Habu toxin administration caused a modest rise in serum creatinine compared with saline control (Fig. 5A), but the difference between Habu-treated Cre/+ flox/− and Cre/+ flox/+ mice was not significant at any time point (Fig. 5B). A surrogate marker of glomerular function, albuminuria, also did not differ between Habu-treated Cre/+ flox/− and Cre/+ flox/+ mice (Fig. 5C).
β8-Integrin effects on apoptosis and proliferation.
The mechanism of healing after Habu-induced glomerular injury involves initial mesangial cell proliferation, which is followed by mesangial cell apoptosis, to prevent persistent hypercellularity (15). To investigate the mechanism for impaired recovery from Habu toxin-induced glomerular capillary dilation in Cre/+ flox/− mice, we determined whether diminished mesangial hypercellularity is attributed to decreased hyperplasia or increased apoptosis by Ki-67 and TUNEL staining, respectively. Figure 6 shows that there were no differences in proliferation or apoptosis between Cre/+ flox/+ and Cre/+ flox/− glomeruli at 2 or 7 days after Habu toxin injection.
Fig. 6.
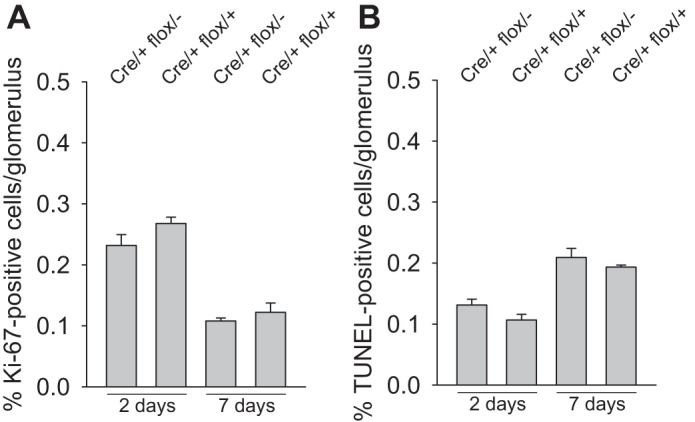
β8-Integrin effects on apoptosis and proliferation. Mice were evaluated for mesangial cell proliferation by Ki-67 labeling (A) or apoptosis by TUNEL (B) 2 and 7 days after Habu injection.
β8-Integrin regulates adhesion to the mesangial matrix.
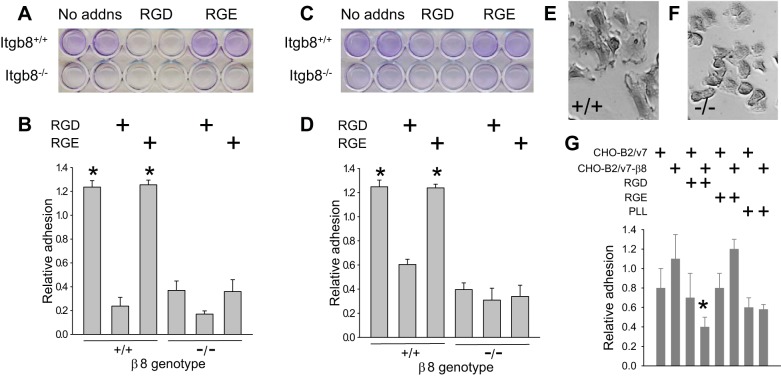
To test whether cell migration, a previously described β8-integrin function (19) and a process by which mesangial cells populate glomeruli during development and repair (18, 27), could be impaired and therefore responsible for delayed glomerular healing in Habu-treated Cre/+ flox/− mice, we first established that αvβ8-integrin-expressing cells bind canonical RGD peptide ligands within the mesangial matrix. Mesangial cells derived from Itgb8+/+ or Itgb8−/− mice were cultured on plastic, grown to confluence, and then removed, leaving the deposited extracellular matrix behind (see materials and methods). Itgb8+/+ or Itgb8−/− mesangial cells were then replated, and adhesion to the residual matrix was assayed in the presence or absence of RGD or control RGE peptides. Figure 7, A and B, shows the increased adherence of wild-type compared with Itgb8−/− mesangial cells. Adhesion was significantly inhibited by RGD but not by RGE peptide preincubation. Similar results were observed for adhesion to the matrix deposited by Itgb8−/− mesangial cells (Fig. 7, C and D), suggesting that β8-integrin is not required for synthesis or deposition of the mesangial matrix. Of note, Itgb8+/+ mesangial cells assumed a more spread morphology compared with Itgb8−/− mesangial cells (Fig. 7, E and F).
Fig. 7.
β8-Integrin regulates adhesion to the mesangial matrix. A–F: mesangial cells derived from Itgb8+/+ and Itgb8−/− kidneys were seeded in 24-well plates coated with an extracellular matrix deposited by Itgb8+/+ (A, B, E, and F) or Itgb8−/− (C and D) mesangial cells with or without GRGDSP [Arg-Gly-Asp (RDG)] or GRGESP [Arg-Gly-Glu (RGE)] peptides. Adherent cells were fixed in paraformaldehyde, and nuclei were stained with crystal violet, which was eluted and quantitated, as described in materials and methods. A and C: representative plates with samples in duplicate. B and D: quantification of adhesion. E and F: representative phase-contrast microscopy fields for Itgb8+/+ (+/+; E) and Itgb8−/− (−/−; F) mouse mesangial cells at ×40 magnification. G: CHO-B2/v7 and CHO-B2/v7-β8 cells were maintained in serum-free media overnight, washed in PBS, and then seeded in 96-well plates with or without RGD or RGE peptides on a matrix deposited by wild-type mesangial cells or poly-l-lysine (PLL). Adhesion was assayed as described in A–D. * P < 0.05 compared with cells on a mesangial matrix without RGD peptide.
Mesangial cells express other αvβ8-integrin partners, which recognize RGD sequences, consistent with incomplete inhibition of adhesion with RGD peptide preincubation. To confirm that αvβ8-integrin mediates adhesion to the mesangial matrix, adhesion assays were repeated using CHO-B2v7 fibroblasts, which are devoid of β-integrin subunits. Figure 7G shows that CHO-B2v7 cells transfected to express β8-integrin (CHO-B2/v7-β8) adhered better than β8-integrin-null CHO-B2/v7 cells to the wild-type, mesangial-conditioned matrix, implying the presence of a β8-integrin ligand within the mesangium. Figure 7G also shows that CHO-B2/v7-β8 binding was inhibited by RGD peptide preincubation to levels observed with PLL, which supports adhesion through integrin-independent pathways. CHO-B2/v7 adhesion was not inhibited by RGD, and neither cell line was affected by RGE peptide competition.
β8-Integrin regulates migration on RGD-containing matrix.
To simulate the impact of αvβ8-integrin on recovery from Habu injury, cell migration was measured in Itgb8+/+ and Itgb8−/− mesangial cells as well as CHO-B2/v7 and CHO-B2/v7-β8 fibroblasts plated in wells coated with the αvβ8-integrin ligand LTGF-β. Figure 8, A and B, shows the enhanced migration in wild-type compared with Itgb8−/− cells. Similar disparities were observed between Itgb8+/+ and Itgb8−/− mesangial cell migration on vitronectin (Fig. 8, C and D), an alternative RGD ligand for αvβ8-integrin that is expressed exclusively in diseased glomeruli (36, 39). Cells migrated less well on vitronectin compared with LAP, perhaps reflecting the relatively weaker affinity of vitronectin for β8-integrin (31). Figure 8, E and F, shows the negligible migration on PLL. Mesangial cell migration was quantitated by two different methods. Figure 8G shows the number of cell migrating into the scratch over 15 h. Because mesangial cells tended to migrate in sheets, data are also expressed as mean distances of migration into the wound (Fig. 8H).
Fig. 8.
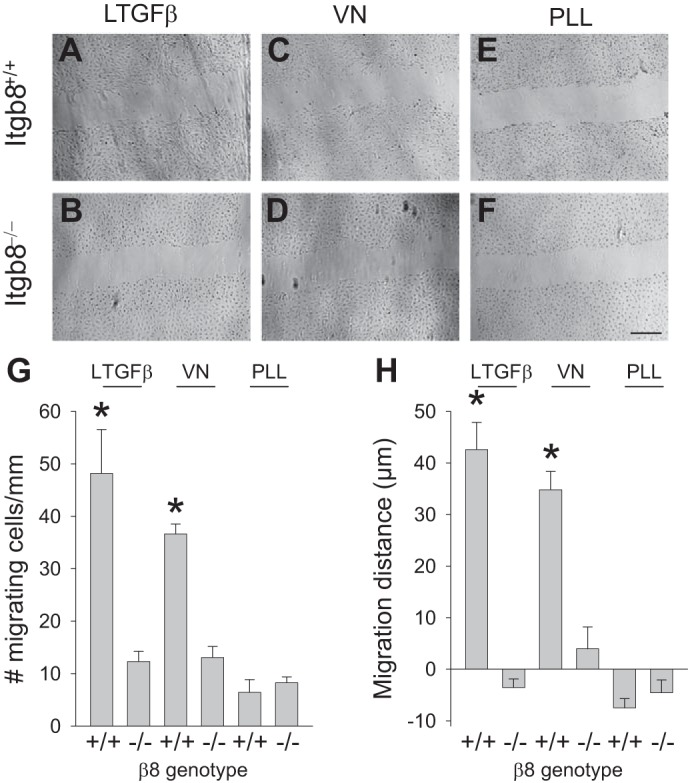
Mesangial cell β8-integrin regulates migration on a RGD-containing matrix. A–F: scratch migration assays for wild-type (Itgb8+/+; A, C, and E) and Itgb−/− (B, D, and F) mesangial cells plated on latent transforming growth factor (LTGF)-β (A and B), vitronectin (VN; C and D), or PLL (E and F), all at 5 μg/ml, for 15 h. G and H: quantification of the scratch migration data. Results are representative of 5 scratches/well. *P < 0.05 compared with the similarly treated Itgb8−/− group. Bar = 300 μm.
Mesangial cell data were confirmed in CHO-B2/v7-β8 and CHO-B2/v7 fibroblasts (Fig. 9, A–F) and quantitated (Fig. 9G). Of note, CHO cells migrated much more robustly compared with mesangial cells and more commonly as individual cells rather than in sheets. LAP-stimulated CHO-B2/v7-β8 cells exhibited prominent lamellipodia (Fig. 9H), which was lacking in cells maintained on glass (Fig. 9I). These morphological changes are consistent with β8-integrin-regulated Rac1 signaling (25), which is associated with lamellipodia formation in motile cells (14).
Fig. 9.
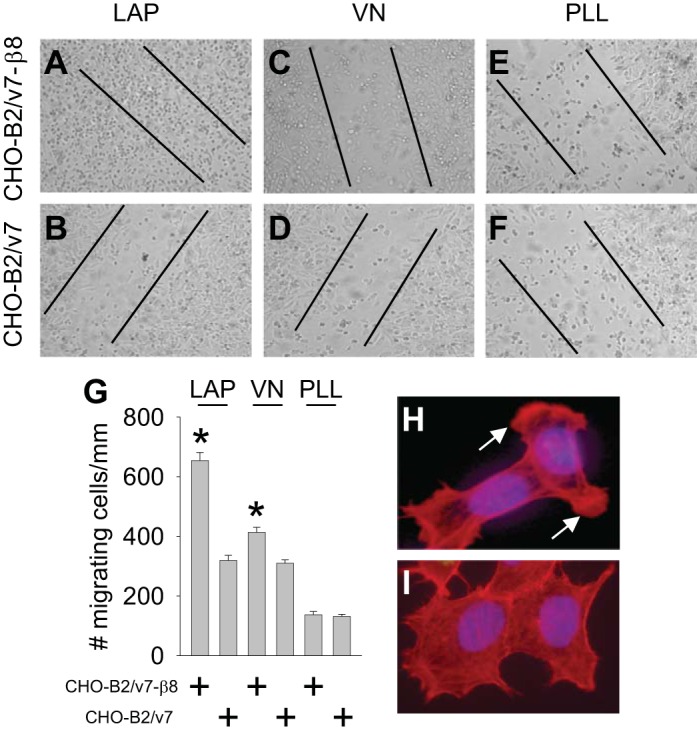
β8-Integrin regulates migration on a RGD-containing matrix. A–F: scratch migration assays for fibroblasts expressing β8-integrin (CHO-B2/v7-β8; A, C, and E) and devoid of β8-integrin (CHO-B2/v7; B, D, and F) plated on latency-associated peptide-β1 (LAP; A and B), VN (C and D), or PLL (E and F), all at 5 μg/ml, for 14 h. Lines represent the width of the scratch at time 0. G: quantification of the scratch migration data. Results are representative of 3 scratches/well. *P < 0.05 compared with CHO-B2/v7 cells. H and I: morphology of CHO-B2/v7-β8 cells on LAP (5 μg/ml, 2 h; H) or glass coverslips (I). Actin was stained in red with phalloidin. Arrows mark lamellipodia.
DISCUSSION
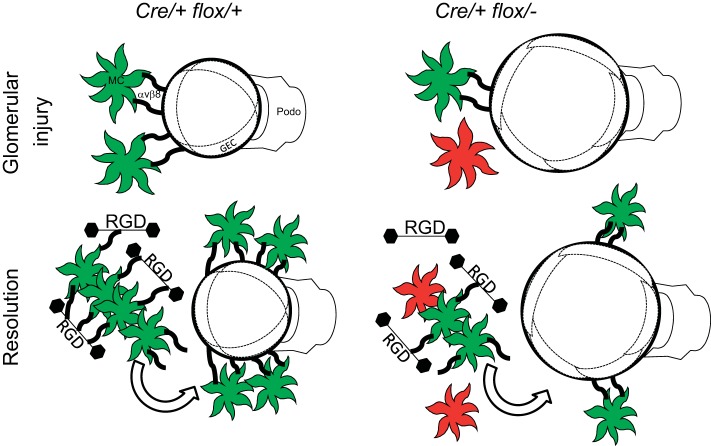
In this report, we evaluated conditional knockout mice to determine the role of β8-integrin in mesangial cell function. The major finding is that β8-integrin influences glomerular capillary morphology and repair after mesangial injury. Using in vitro approaches with β8-integrin-expressing and null cells, we confirmed the preference of β8-integrin for RGD peptide ligands, including within the mesangial matrix. To reconcile the in vivo and in vitro results, we demonstrated that mesangial cell β8-integrin binds ligands within mesangial matrix and that this process is likely to regulate glomerular capillary architecture in a homeostatic fashion. This paradigm extends the interpretation of our previous study (22) implicating mesangial β8-integrin as a governor of TGF-β release to juxtaposed glomerular endothelial cells. In addition, our data support the notion that during recovery from glomerular injury, β8-integrin-expressing mesangial cells migrate to areas of capillary repair, using paths provided by mesangial matrix ligands (Fig. 10).
Fig. 10.
Schematic diagram of the β8-integrin influence on glomerular capillary injury and repair. The reduced Cre-lox recombination in Cre/+ flox/− mice, resulting in mosaicism, is shown by the greater number of mesangial cells, which do not express β8-integrin (shown in red). The reduced number of β8-integrin-expressing mesangial cells (shown in green), causes enhanced capillary dilation with injury and delayed mesangial cell migration during the recovery phase in Cre/+ flox/− glomeruli. GEC, glomerular endothelial cell.
Cre/+ flox/− mice expressed 40–60% as much glomerular β8-integrin mRNA as Cre/+ flox/+ mice, which was sufficient to prevent a renal phenotype. However, spontaneous and induced phenotypes are influenced by genetic background, and congenic C57BL/6 background mice, which were used for all experiments, tend to be disease resistant (12, 20). Rather than breeding mice to a more permissive background, we opted to induce a phenotype after mesangial injury. Habu toxin was chosen to stimulate mesangiolysis, which reliably causes widespread glomerular capillary microaneurysms in mice with prior unilateral nephrectomy (4, 15). In our experiments, the nephrectomy step was omitted to cause a milder baseline lesion, which permitted evaluation of disease accentuation. Using this model, β8-integrin-deficient mice developed more severe glomerular capillary microaneurysms and delayed repair of these lesions. This β8-integrin function in recovery may be vital, since the aftermath of mesangiolysis and glomerular capillary injury has been implicated in glomerulosclerosis (43) and diabetic nodular sclerosis (47). A similar role has been proposed for α8β1-integrin (13, 15), suggesting that there could be cooperation and/or redundancy among mesangial integrins.
αvβ8-Integrin has been assigned a variety of biological functions, which could be relevant to glomerular capillary repair, including cell migration (19), proliferation (7), angiogenesis (6, 50), adhesion to the extracellular matrix (45), and regulation of cytokines (22, 31). Our data demonstrated that adherence to the mesangial matrix was dependent on ligation with RGD peptide-containing proteins, and, although RGD ligation is a feature of all αv-integrins, our results are specific to αvβ8-integrin, since CHO-B2v7 cells do not express β-integrin subunits that partner with αv-integrin subunits (49). β8-Integrin regulated adhesion to the mesangial matrix, in agreement with mesangial cell migration as the predominant mechanism in glomerular capillary remodeling after Habu-induced injury (4). Specific ligands tested for β8-integrin-dependent migration were LAP and vitronectin, both of which localize to injured glomeruli (22, 36). The in vitro migration on LTGF-β or LAP had not previously been described and suggests that LTGF-β imbedded within the extracellular matrix may provide guidance cues for migrating mesangial cells during recovery from glomerular injury.
Cre-lox technology represents a valuable technique to delineate specific gene functions, but there are limitations that must be considered. A frequent issue is incomplete recombination, resulting in mosaic mice. This was first suspected in our experiments when β8-integrin transcript persistence was observed in PDGFRB-Cre(RA)Itgb8 flox/− mice and then confirmed using PCR-based strategies to assess recombination efficiency. The most common explanation for deficient recombination is weak Cre activity (35). However, in experiments with the Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J reporter mouse, Cre activity, driven by a PDGFRB promoter, corresponded to a mesangial pattern. That mosaic mice resulted from crosses between floxed Itgb8 and three different Cre strains suggests that the target allele is resistant to recombination. Indeed, significantly different recombination frequencies have been described between the same Cre and different floxed alleles as well as with the same Cre-lox combination in different tissues (46). Because recombination was routinely better when the accompanying allele was wild type rather than null (Fig. 1E), we propose that the mechanism could be due to an altered local chromatin structure (46).
The observation that Itgb8 flox/− progeny resulting from breeding with PDGFRB-Cre(VL) mice were born in reduced numbers implies that a minimum Itgb8 dose in PDGFBR-expressing cells is required to sustain survival. We speculate that cells capable of bypassing PDGFBR-Cre-mediated recombination of the floxed β8-integrin allele gain a competitive advantage over cells in which floxed β8-integrin recombination is achieved, a mechanism for which there is precedence (5). Since we did not observe a kidney development phenotype in global β8-integrin knockout mice (22) and renal dysfunction does not generally cause embryonic lethality, we reason that the relevant, vital PDGFBR-expressing cells are located in nonrenal tissues.
The alternative possibility that persistent glomerular β8-integrin expression stems from nonmesangial β8-integrin expression seems unlikely, since β8-integrin mRNA and protein expression correlated with a mesangial pattern in vivo (22, 25), and neither β8-integrin mRNA (unpublished observations) nor protein (Fig. 1A) expression was detected in podocytes or glomerular endothelial cells, the other major resident cells of the glomerulus. However, αvβ8-integrin is expressed in dendritic cells (44), which may be rarely present in the kidney, though much more commonly in the tubulointerstitium than glomerulus (17, 22, 38).
In this study, which combines in vivo and in vitro techniques to define kidney αvβ8-integrin function, we conclude that the β8-integrin indirectly controls glomerular capillary integrity, which we speculate occurs through mechanical tension generated by binding RGD peptides in the mesangial matrix. Healing after glomerular injury may be facilitated by mesangial cell migration, which is guided by transient β8-integrin interactions with RGD ligands.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-067528 and R01-DK-072348.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.L.-R., S.K., and J.R.S. conception and design of research; S.L.-R., V.L., T.D.A., and S.K. performed experiments; S.L.-R., T.D.A., and J.R.S. analyzed data; S.L.-R., T.D.A., and J.R.S. interpreted results of experiments; S.L.-R. and J.R.S. prepared figures; S.L.-R. and J.R.S. drafted manuscript; S.L.-R., S.K., and J.R.S. edited and revised manuscript; S.L.-R., V.L., T.D.A., S.K., and J.R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Louis Reichardt (University of California-San Francisco), who supplied the Itgb8-floxed mice, to Dr. Ralf Adams (Cancer Research UK London Research Institute), who supplied the PDGFBR-Cre(RA) mice, to Dr. Volkhard Lindner (Maine Medical Center), who supplied the PDGFBR-Cre(VL) mice, to Dr. Ariel Gomez (University of Virginia), who supplied the Ren1d-Cre mice, to Dr. Michael Madaio (Medical College of Georgia), who supplied glomerular endothelial cells, and to Dr. Bingcheng Wang (Case Western Reserve University), who supplied CHO-B2/v7 cells and Metamorph software.
REFERENCES
- 1.Abu Jawdeh BG, Khan S, Deschênes I, Hoshi M, Goel M, Lock JT, Shinlapawittayatorn K, Babcock G, Lakhe-Reddy S, DeCaro G, Yadav SP, Mohan ML, Naga Prasad SV, Schilling WP, Ficker E, Schelling JR. Phosphoinositide binding differentially regulates NHE1 Na+/H+ exchanger-dependent proximal tubule cell survival. J Biol Chem 286: 42435–42445, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akis N, Madaio MP. Isolation, culture, and characterization of endothelial cells from mouse glomeruli. Kidney Int 65: 2223–2227, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Barnes JL, Abboud HE. Temporal expression of autocrine growth factors corresponds to morphological features of mesangial proliferation in Habu snake venom-induced glomerulonephritis. Am J Pathol 143: 1366–1376, 1993 [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes JL, Hevey KA, Hastings RR, Bocanegra RA. Mesangial cell migration precedes proliferation in Habu snake venom-induced glomerular injury. Lab Invest 70: 460–467, 1994 [PubMed] [Google Scholar]
- 5.Betz UA, Vosshenrich CA, Rajewsky K, Muller W. Bypass of lethality with mosaic mice generated by Cre-loxP-mediated recombination. Curr Biol 6: 1307–1316, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, Einheber S, Boudreau N, Nishimura SL. Integrin αvβ8-mediated activation of transforming growth factor-β by perivascular astrocytes: an angiogenic control switch. Am J Pathol 166: 1883–1894, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cambier S, Mu DZ, O'Connell D, Boylen K, Travis W, Liu WH, Broaddus VC, Nishimura SL. A role for the integrin αvβ8 in the negative regulation of epithelial cell growth. Cancer Res 60: 7084–7093, 2000 [PubMed] [Google Scholar]
- 8.Cuttler AS, LeClair RJ, Stohn JP, Wang Q, Sorensson J, Liaw L, Lindner V. Characterization of Pdgfrb-Cre transgenic mice reveals reduction of ROSA26 reporter activity in remodeling arteries. Genesis 49: 673–680, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Gasperi R, Rocher AB, Sosa MA, Wearne SL, Perez GM, Friedrich VL, Jr, Hof PR, Elder GA. The IRG mouse: a two-color fluorescent reporter for assessing Cre-mediated recombination and imaging complex cellular relationships in situ. Genesis 46: 308–317, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 124: 161–173, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Gauer S, Yao J, Schoecklmann HO, Sterzel RB. Adhesion molecules in the glomerular mesangium. Kidney Int 51: 1447–1453, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM. Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol 290: F214–F222, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Haas CS, Amann K, Schittny J, Blaser B, Müller U, Hartner A. Glomerular and renal vascular structural changes in α8 integrin-deficient mice. J Am Soc Nephrol 14: 2288–2296, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Hall A. Rho family GTPases. Biochem Soc Trans 40: 1378–1382, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Hartner A, Marek I, Cordasic N, Haas C, Schocklmann H, Hulsmann-Volkert G, Plasa I, Rascher W, Hilgers KF, Amann K. Glomerular regeneration is delayed in nephritic α8-integrin-deficient mice: contribution of α8-integrin to the regulation of mesangial cell apoptosis. Am J Nephrol 28: 168–178, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Heptinstall RH. Classification of glomerulonephritis; focal and proliferative forms. In: Pathology of the Kidney. Boston, MA: Little, Brown, and Company, 1992, p. 261–296 [Google Scholar]
- 17.Heymann F, Meyer-Schwesinger C, Hamilton-Williams EE, Hammerich L, Panzer U, Kaden S, Quaggin SE, Floege J, Grone HJ, Kurts C. Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J Clin Invest 119: 1286–1297, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hugo C, Shankland SJ, Bowen-Pope DF, Couser WG, Johnson RJ. Extraglomerular origin of the mesangial cell after injury. A new role of the juxtaglomerular apparatus. J Clin Invest 100: 786–794, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarad G, Wang B, Khan S, DeVore J, Miao H, Wu K, Nishimura SL, Wible BA, Konieczkowski M, Sedor JR, Schelling JR. Fas activation induces renal tubular epithelial cell β8 integrin expression and function in the absence of apoptosis. J Biol Chem 277: 47826–47833, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Johnstone DB, Ikizler O, Zhang J, Holzman LB. Background strain and the differential susceptibility of podocyte-specific deletion of Myh9 on murine models of experimental glomerulosclerosis and HIV nephropathy. PLOS ONE 8: e67839, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keppler A, Gretz N, Schmidt R, Kloetzer HM, Groene HJ, Lelongt B, Meyer M, Sadick M, Pill J. Plasma creatinine determination in mice and rats: an enzymatic method compares favorably with a high-performance liquid chromatography assay. Kidney Int 71: 74–78, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Khan S, Lakhe-Reddy S, McCarty JH, Sorenson CM, Sheibani N, Reichardt LF, Kim JH, Wang B, Sedor JR, Schelling JR. Mesangial cell integrin αvβ8 provides glomerular endothelial cell cytoprotection by sequestering TGF-β and regulating PECAM-1. Am J Pathol 178: 609–620, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan S, Wu KL, Sedor JR, Abu Jawdeh BG, Schelling JR. The NHE1 Na+/H+ exchanger regulates cell survival by activating and targeting ezrin to specific plasma membrane domains. Cell Mol Biol 52: 115–121, 2006 [PubMed] [Google Scholar]
- 24.Kikkawa Y, Virtanen I, Miner JH. Mesangial cells organize the glomerular capillaries by adhering to the G domain of laminin α5 in the glomerular basement membrane. J Cell Biol 161: 187–196, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakhe-Reddy S, Khan S, Konieczkowski M, Jarad G, Wu KL, Reichardt LF, Takai Y, Bruggeman LA, Wang B, Sedor JR, Schelling JR. β8 integrin binds Rho GDP dissociation inhibitor-1 and activates Rac1 to inhibit mesangial cell myofibroblast differentiation. J Biol Chem 281: 19688–19699, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langhofer M, Hopkinson SB, Jones JC. The matrix secreted by 804G cells contains laminin-related components that participate in hemidesmosome assembly in vitro. J Cell Sci 105: 753–764, 1993 [DOI] [PubMed] [Google Scholar]
- 27.Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF-B show renal, cardiovascular, and hematological abnormalities. Genes Dev 8: 1875–1887, 1994 [DOI] [PubMed] [Google Scholar]
- 28.McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, Savill J, Roes J, Hynes RO. Selective ablation of αv integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development 132: 165–176, 2005 [DOI] [PubMed] [Google Scholar]
- 29.McCarty JH, Monahan-Earley RA, Brown LF, Keller M, Gerhardt H, Rubin K, Shani M, Dvorak HF, Wolburg H, Bader BL, Dvorak AM, Hynes RO. Defective associations between blood vessels and brain parenchyma lead to cerebral hemorrhage in mice lacking αv integrins. Mol Cell Biol 22: 7667–7677, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moyle M, Napier MA, McLean JW. Cloning and expression of a divergent integrin subunit β8. J Biol Chem 266: 19650–19658, 1991 [PubMed] [Google Scholar]
- 31.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J Cell Biol 157: 493–507, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Proctor JM, Zang K, Wang D, Wang R, Reichardt LF. Vascular development of the brain requires β8 integrin expression in the neuroepithelium. J Neurosci 25: 9940–9948, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roca-Cusachs P, Iskratsch T, Sheetz MP. Finding the weakest link: exploring integrin-mediated mechanical molecular pathways. J Cell Sci 125: 3025–3038, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schelling JR, Nkemere N, Kopp JB, Cleveland RP. Fas-dependent fratricidal apoptosis is a mechanism of tubular epithelial cell deletion in chronic renal failure. Lab Invest 78: 813–824, 1998 [PubMed] [Google Scholar]
- 35.Schulz TJ, Glaubitz M, Kuhlow D, Thierbach R, Birringer M, Steinberg P, Pfeiffer AF, Ristow M. Variable expression of Cre recombinase transgenes precludes reliable prediction of tissue-specific gene disruption by tail-biopsy genotyping. PLOS ONE 2: e1013, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schvartz I, Seger D, Shaltiel S. Vitronectin. Int J Biochem Cell Biol 31: 539–544, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Sedor JR. Cytokines, kidney disease, and therapy: a molecular characterization provides insights into mechanism. J Lab Clin Med 124: 470–472, 1994 [PubMed] [Google Scholar]
- 38.Segerer S, Heller F, Lindenmeyer MT, Schmid H, Cohen CD, Draganovici D, Mandelbaum J, Nelson PJ, Grone HJ, Grone EF, Figel AM, Nossner E, Schlondorff D. Compartment specific expression of dendritic cell markers in human glomerulonephritis. Kidney Int 74: 37–46, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Seiffert D, Crain K, Wagner NV, Loskutoff DJ. Vitronectin gene expression in vivo. Evidence for extrahepatic synthesis and acute phase regulation. J Biol Chem 269: 19836–19842, 1994 [PubMed] [Google Scholar]
- 40.Sequeira Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell 6: 719–728, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Soriano P. Abnormal kidney development and hematological disorders in PDGFβ-receptor mutant mice. Genes Dev 8: 1888–1896, 1994 [DOI] [PubMed] [Google Scholar]
- 42.Srichai MB, Konieczkowski M, Padiyar A, Konieczkowski DJ, Mukherjee A, Hayden PS, Kamat S, El Meanawy MA, Khan S, Mundel P, Lee SB, Bruggeman LA, Schelling JR, Sedor JR. A WT1 co-regulator controls podocyte phenotype by shuttling between adhesion structures and nucleus. J Biol Chem 279: 14398–14408, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Stahl RA, Thaiss F, Wenzel U, Helmchen U. Morphologic and functional consequences of immune-mediated mesangiolysis: development of chronic glomerular sclerosis. J Am Soc Nephrol 2: S144–S148, 1992 [DOI] [PubMed] [Google Scholar]
- 44.Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, Bluestone JA, Sheppard D. Loss of integrin αvβ8 on dendritic cells causes autoimmunity and colitis in mice. Nature 449: 361–365, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venstrom K, Reichardt L. β8 integrins mediate interactions of chick sensory neurons with laminin-1, collagen IV, and fibronectin. Mol Biol Cell 6: 419–431, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vooijs M, Jonkers J, Berns A. A highly efficient ligand-regulated Cre recombinase mouse line shows that LoxP recombination is position dependent. EMBO Rep 2: 292–297, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wada T, Shimizu M, Yokoyama H, Iwata Y, Sakai Y, Kaneko S, Furuichi K. Nodular lesions and mesangiolysis in diabetic nephropathy. Clin Exp Nephrol 17: 3–9, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Weis B, Schmidt J, Lyko F, Linhart HG. Analysis of conditional gene deletion using probe based real-time PCR. BMC Biotechnol 10: 75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Z, Morla AO, Vuori K, Bauer JS, Juliano RL, Ruoslahti E. The αvβ1 integrin functions as a fibronectin receptor but does not support fibronectin matrix assembly and cell migration on fibronectin. J Cell Biol 122: 235–242, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu JW, Motejlek K, Wang DN, Zang KL, Schmidt A, Reichardt LF. β8 integrins are required for vascular morphogenesis in mouse embryos. Development 129: 2891–2903, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]