Abstract
Inflammatory bowel diseases (IBD) are chronic inflammatory disorders of the intestine that result in painful and debilitating complications. Currently no cure exists for IBD, and treatments are primarily aimed at reducing inflammation to alleviate symptoms. Genome-wide linkage studies have identified the Ron receptor tyrosine kinase (TK) and its ligand, hepatocyte growth factor-like protein (HGFL), as genes highly associated with IBD. However, only scant information exists on the role of Ron or HGFL in IBD. Based on the linkage of Ron to IBD, we directly examined the biological role of Ron in colitis. Wild-type mice and mice lacking the TK signaling domain of Ron (TK−/− mice) were utilized in a well-characterized model of chronic colitis induced by cyclic exposure to dextran sulfate sodium. In this model, TK−/− mice were more susceptible to injury as judged by increased mortality compared with control mice and developed more severe colitis. Loss of Ron led to significantly reduced body weights and more aggressive clinical and histopathologies. Ron loss also resulted in a dramatic reduction in colonic epithelial cell proliferation and increased proinflammatory cytokine production, which was associated with alterations in important signaling pathways known to regulate IBD. Examination of human gene expression data further supports the contention that loss of Ron signaling is associated with IBD. In total, our studies point to important functional roles for Ron in IBD by regulating healing of the colonic epithelium and by controlling cytokine secretion.
Keywords: Ron receptor, hepatocyte growth factor-like protein, colitis, inflammatory bowel disease, Met receptor
inflammatory bowel disease (IBD) is a multifactor, chronic inflammatory disorder of the colon and small intestine. Crohn's disease (CD) and ulcerative colitis (UC) are the two main types of IBD and affect over 1.4 million people in the United States (12, 17). Complications from IBD include fistulas, malnutrition, bowel obstruction, and an increased risk of colorectal cancer (30). The development and course of IBD are affected by genetic susceptibility of the host, the intestinal microbial flora, environmental factors, and the status of the host immune system (17). The pathogenesis of IBD is complex and involves intestinal epithelial cell death, immune cell activation, and increased proinflammatory cytokine production. Perturbation of a tightly controlled cytokine network, with abnormal crosstalk between several mucosal cell types within the colon is an important step in progression of chronic IBD. While genome-wide association studies have identified several candidate genes in IBD (17, 27), current treatments for IBD are aimed at the management of symptoms and decreasing inflammation through immunomodulators (12). Key gaps exist in our knowledge of genes that control IBD and in the development of novel targeted therapies to lessen disease pathogenesis.
Genome-wide linkage studies identified a region on chromosome 3 (3p21–22) as an IBD locus (5, 13, 27). This region contains genes including the Ron receptor tyrosine kinase (TK) (MST1R) and its ligand, hepatocyte growth factor-like protein/macrophage-stimulating protein (HGFL, MST1). Furthermore, two single nucleotide polymorphisms (SNPs) in the Ron receptor (R523Q and G133R) and a SNP in HGFL (R689C) have been identified in patients with IBD with strong linkage disequilibrium (5, 13, 27). The Ron receptor is a membrane-spanning protein that belongs to the c-Met family of receptor TKs. Ron is expressed on many cell types (47). Ron mRNA and protein expression has been reported in the intestine and colon as well as in colon epithelial cells in both mice and humans (18, 32, 33). Ron is also expressed in tissue-resident macrophages, including peritoneal macrophages and Kupffer cells, but not in circulating monocytes (16, 43). In epithelial cells, Ron functions to promote proliferation, survival, migration, and differentiation (47). Macrophage Ron expression has been shown to be critical in suppressing M1 activation, limiting inflammation and immunity to pathogens while stimulating M2 activation, which promotes the resolution of inflammation and wound healing (14, 40). HGFL, the sole ligand for Ron, is predominantly produced by the liver and is secreted into the plasma as a pro-protein, which is cleaved by a variety of serum- and membrane-bound proteases to form a biologically active protein (47). Binding of HGFL to Ron leads to the activation of multiple downstream signaling cascades, in a cell type-dependent manner, which induces pleotropic effects. The HGFL and Ron SNPs identified in patients with IBD are predicted to interfere with the ability to activate Ron signaling, and a recent report, while finding no activation differences, showed that the R689C SNP in HGFL reduced serum levels of HGFL in patients with IBD (5, 13, 18). These data suggest that loss or inhibition of Ron signaling may play an important role in IBD.
In this report, the in vivo significance of the HGFL-Ron signaling axes was investigated utilizing a well-established murine model of chronic colitis induced by administration of dextran sodium sulfate (DSS) (22, 51). Following the induction of colitis, mice lacking the Ron signaling exhibited more severe colonic injury characterized by heightened pathologies, diminished colonic epithelial cell proliferation, and increased production of proinflammatory mediators. These studies provide strong evidence of an important role for this signaling cascade in colitis whereby Ron signaling in the colon is required for the healing of the colonic epithelium and for limiting inflammation, which may have wide therapeutic implications for the treatment of IBD.
MATERIALS AND METHODS
Mice and induction of chronic colitis.
Ron TK-deficient mice (TK−/− mice) were generated as previously described and backcrossed into a C57BL/6 background (48). Age-matched 8–10-wk-old male mice were used for all experiments. Chronic colitis was induced by administering 2.5% DSS (molecular weight 36–50 kDa; MP Biomedicals, Solon, OH) in the drinking water for 7 days, followed by 14 days of regular water. This DSS cycle was repeated three times (22, 51). Mice were weighed every other day, and a clinical score was calculated based on well-established criteria including stool score, stool blood score, and mouse appearance. Stool score was scored as 0, normal; 1, moist; 2, soft; 3, diarrhea; stool blood was scored as 0, no blood; 1, blood in stool or around anus; 2, severe bleeding; and mouse appearance was scored as 0, normal; 1, ruffed fur or altered gait; 2 moribund (22). Mice were euthanized 56–62 days after starting treatment. Two hours prior to euthanasia, mice were injected with 5-bromo-2'-deoxyuridine (BrdU). All mice were maintained under specific pathogen-free conditions and were treated and euthanized in accordance with protocols approved by the Institutional Animal Care and Use Committee of University of Cincinnati.
Tissue histology and immunohistochemical analyses.
Colons were isolated, fixed, and embedded in paraffin for histological assessment. The severity of colitis was assessed on stained colonic sections in a blinded fashion using established criteria based on crypt damage and ulceration. Crypt damage was scored as follows: 0, intact crypts; 1, loss of the basal one-third; 2, loss of the basal two-thirds; 3, entire crypt loss; 4, change of epithelial surface with erosion; 5, confluent erosion. Ulceration was scored as follows: 0, absence of ulcer; 1, one or two foci of ulcerations; 2, two to four foci of ulcerations; 3, confluent or extensive ulceration. Values were added to give a maximal histological score of 8 (19). Immunohistochemical (IHC) analyses were performed on colon sections with antigen retrieval performed using citrate buffer or Proteinase K. The sections were incubated overnight with primary antibodies p-STAT1 and p-IKK-α/β (Cell Signaling Technology, Danvers, MA), Ron and HGFL (Santa Cruz Biotechnology, Dallas, TX), plectin, laminin, and F4/80 (Abcam, Cambridge, MA). Biotinylated secondary antibodies (Vector Laboratories, Burlingame, CA) were used to detect the primary antibodies and visualized using a horseradish peroxidase-conjugated ABC system (Vector Laboratories). Diaminobenzidine or Nova red (Vector Laboratories) was used as the chromogen. For the quantification of F4/80, p-STAT1- and p-IKK-α/β-positive cells in colonic sections, positive-staining epithelial cells, or stromal cells were counted from five to seven nonoverlapping (×400 magnification) fields from three to four mice per genotype.
Mitotic and cell death indices were assessed by BrdU incorporation and TUNEL staining according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). A minimum of three animals was assessed per genotype per treatment, with five to eight fields per section and a minimum of 300 epithelial cells assessed per slide. The percentage of crypt epithelial cells that labeled positive was calculated.
Primary colonic cell isolation and immunocytochemistry.
Colon epithelial cells and lamina propria macrophages were isolated based on standard protocols (49). Briefly, colons were cut into small pieces and placed in calcium- and magnesium-free Hanks balanced salt solution containing 5 mM EDTA and 1 mM dithiothreitol, shaken vigorously at 37°C for 30 min, followed by filtration through a 70-μm cell strainer. The filtrate was centrifuged, and the supernatant was removed. The pellet was washed, and the resulting epithelial cells were used for immunofluorescence and RNA isolations. For isolating lamina propria macrophages, colon fragments remaining on the 70-μm strainer were collected and digested with collagenase (Roche Diagnostics, Indianapolis, IN) and DNAse I (Sigma, St. Louis, MO) for 30 min. The solution was filtered and centrifuged, and the pellet was resuspended in a 40% fraction of Percoll solution (GE Healthcare Biosciences, Piscataway, NJ), which was overlaid on an 80% Percoll solution. The Percoll solution was centrifuged, and the cells at the interphase were removed. Colon macrophages were enriched from the immune cell fraction by magnetic beads coated with mouse CD11b according to the manufacturer's instructions (Miltenyi Biotech, Auburn, CA). The resulting population was used for immunofluorescence and RNA isolations. Immunocytochemistry on isolated primary cells was performed as described (43) with antibodies against E-cadherin (BD Biosciences, San Jose, CA), F4/80, and Ron. The isolated epithelial cells and macrophages were 90%-95% pure based on E-cadherin and F4/80 immunostaining, respectively.
Isolation of bone marrow-derived macrophages.
Bone marrow cells were isolated, cultured, and differentiated into macrophages using standard protocols (44). Briefly, femurs and tibia were obtained from 10–12-wk-old C57BL/6 wild-type (TK+/+) and TK−/− mice. Bones epiphyses were removed, and the bones were flushed with DMEM to extrude bone marrow. The marrow was passed through a 40-μm filter, and the cells were plated in cell culture dishes. Cells were differentiated into bone marrow-derived macrophages (BMDMs) using CMG-14–12 cell-conditioned medium for 3 days. To elucidate the changes in chemokine/cytokine secretion, equal numbers of BMDMs from TK+/+ and TK−/− mice were plated and treated with 500 μg/ml lipopolysaccharide (LPS) (Escherichia coli serotype 0111:B4; Sigma) for 1 h for RNA isolation or for the indicated time points for the collection of cell supernatants for cytokine measurements. IL-6 and IL-10 levels were measured by ELISA analyses according to the manufacturer's instructions (R&D Systems, Minneapolis, MN).
RNA isolation and qRT-PCR.
Total RNA was isolated from colon tissues and cells using TriZol reagent (Invitrogen) and converted to cDNA using a high-capacity cDNA kit (Applied Biosystems, Foster City, CA). qRT-PCR was performed using FastStart SYBR Green. The following murine genes and their corresponding primers were chosen: IL-6 (5′-TAGTCCTTCCTACCCCAATTTCC-3′; 5′-TTGGTCCTTAGCCACTCCTTC-3′), Ron (5′-TCCCATTGCAGGTCTGTGTAGA-3′; 5′-CGGAAGCTGTATCGTTGATGTC-3′), TNF-α (5′-CATCTTCTCAAAATTCGAGTGACAA-3′; 5′-TGGGAGTAGACAAGGTACAACCC-3′), IL-17A (5′-TTTAACTCCCTTGGCGCAAAA-3′; 5′-CTTTCCCTCCGCATTGACAC-3′), inducible nitric oxide synthase (iNOS) (5′-TGCCCCTTCAATGGTTGGTA-3′; 5′-ACTGGAGGGACCAGCCAAAT-3′), IL-10 (5′-GGTTGCCAAGCCTTATCGGA-3′; 5′-ACCTGCTCCACTGCCTTGCT-3′), HGFL (5′-TGGTACAGTGTTCAAGGGCTCTT-3′; 5′-GCATGGCTGCTCATG-3′), and 18S (5′-AGTCCCTGCCCTTTGTACACA-3′; 5′-GATCCGAGGGCCTCACTAAAC-3′). Expression levels were normalized to 18S as internal control and relative gene expression reported.
Western blotting.
Protein was isolated from tissues as described previously (43), separated by SDS-PAGE, and transferred to Immobilon-P membranes (BD Biosciences). Membranes were probed with α6-integrin, β4-integrin (Santa Cruz Biotechnology), laminin (Abcam), p-STAT3, total STAT3 (Cell Signaling Technologies), and c4-actin (Seven Hills Bioreagents, Cincinnati, OH). Densitometry was used to quantitate protein band intensities with Image J software (NIH, Bethesda, MD).
Myeloperoxidase activity.
Frozen colons were homogenized in phosphate-buffered saline and centrifuged at 20,000 g. Myeloperoxidase (MPO) activity was performed as previously described (21).
Cytokine measurements.
Sera isolated from mice were analyzed using the Milliplex Map Mouse Cytokine/Chemokine Panel no. MPXMCYTO-70 (Millipore, Billerica, MA) with Luminex Map detection and BD cytometric bead array (552364; BD Biosciences) per manufacturer's instructions.
Statistical analysis.
All data graphs are expressed as means ± SE. Statistical significance was defined as P < 0.05 for all analyses and was determined by Student's t-tests and Kaplan-Meier survival analyses using GraphPad Prism 4.0 software (La Jolla, CA).
RESULTS
Ron and HGFL are expressed in the normal colon and in colitis.
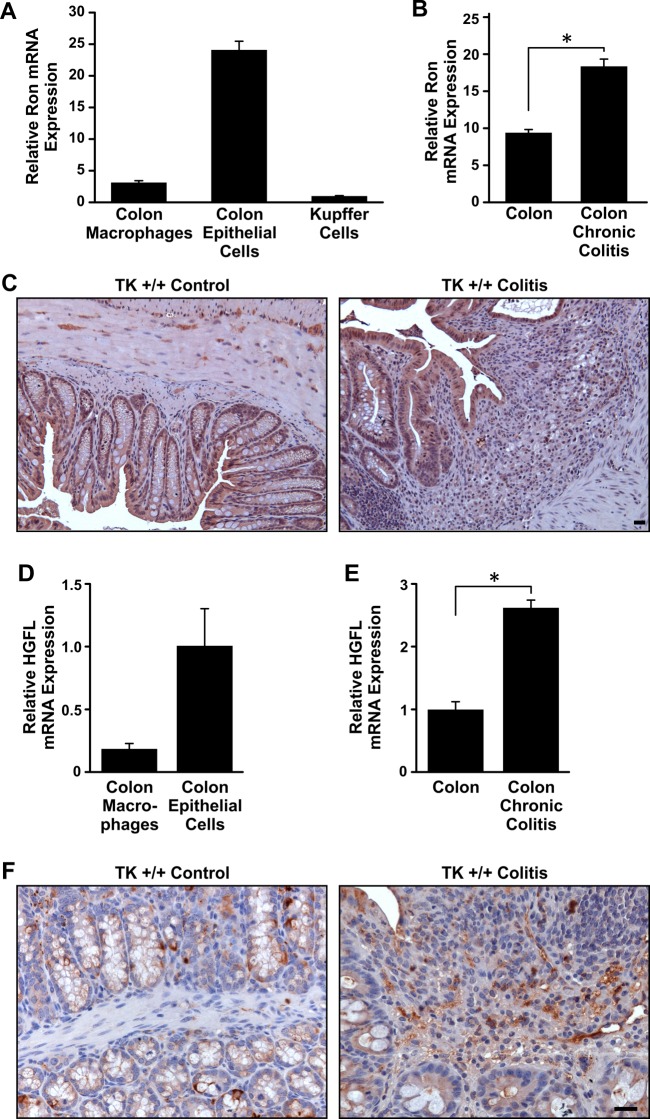
Quantitative data comparing the expression of Ron and HGFL in cell types of normal colon are lacking. To examine Ron and HGFL expression, populations of murine colon epithelial cells and resident macrophages were collected from wild-type (TK+/+) mice (49). Ron mRNA expression was detected in isolated epithelial cells and resident macrophages (Fig. 1A). qRT-PCR analyses on colons from mice before and after DSS-induced chronic colitis showed an increase in Ron mRNA following DSS treatment (Fig. 1B). IHC analyses of normal and DSS-treated colons demonstrated Ron expression on epithelial and stromal cells (Fig. 1C). To examine the expression of HGFL, qRT-PCR on isolated colon epithelial cells and macrophages was performed and showed HGFL expression in both cell types (Fig. 1D). HGFL expression was also present and slightly elevated in the colon following DSS-induced colitis (Fig. 1E). IHC on murine colons showed HGFL expression in crypt epithelial and goblet cells as well as in stromal cells (Fig. 1F), which was maintained following DSS treatment (Fig. 1F). In total, these data demonstrate that Ron and HGFL are expressed locally in multiple cell types of the murine colon and are present during DSS-induced colonic injury.
Fig. 1.
Ron and hepatocyte growth factor-like protein (HGFL) are expressed in the normal colon and during dextran sodium sulfate (DSS)-induced colitis. qRT-PCR showing Ron mRNA expression in intestinal epithelium cells and in resident colonic macrophages from the normal colon (A). Kupffer cells, resident liver macrophages, are used as a positive control. qRT-PCR of whole colon tissue demonstrates that Ron expression is increased in colons subjected to DSS-induced colitis (B). Immunohistochemistry (IHC) depicting Ron expression in normal murine colons and in colons subjected to dextran sodium sulfate (DSS) (C). TK, tyrosine kinase. HGFL mRNA is expressed in normal murine colon epithelial cells and macrophages and is increased during experimental colitis by qRT-PCR (D and E). HGFL protein is detected by IHC analysis in normal murine colons and in colons subjected to DSS-induced colitis (F). Histograms represent the means ± SE of at least 3 isolations/mice per group, *P < 0.05. Scale bar = 50 μm.
Loss of Ron receptor signaling hastens colitis following DSS treatment in mice.
To investigate the functional role of Ron in colitis, TK+/+ mice and mice lacking the TK signaling domain of Ron (TK−/− mice) were subjected to cyclic DSS-induced colitis. During the course of treatment, the TK−/− mice exhibited decreased survival primarily during the first cycle of DSS treatment compared with TK+/+ mice (Fig. 2A). In addition, whereas the surviving mice from both genotypes showed similar weight loss through two cycle of DSS treatment, the weight of TK−/− mice was significantly less than that of TK+/+ mice after the third cycle of treatment (Fig. 2B). The failure to gain weight during the recovery period after DSS treatment was concomitant with TK−/− mice displaying elevated clinical scores (Fig. 2C). At necropsy, colon lengths were measured as a readout for the severity of colitis, and, although DSS treatment shortened colons in both genotypes, TK−/− colon lengths were significantly shorter than TK+/+ colons (Fig. 2D). Whereas colonic histology of TK+/+ and TK−/− colons before colitis did not differ (data not shown), colons of TK−/− exhibited a significant increase in injury compared with TK+/+ colons following DSS treatment characterized by epithelial cell loss, ulceration, and immune cell infiltration (Fig. 2, E and F). To examine the extent of epithelial damage in the TK−/− colons, colons from TK+/+ and TK−/− mice were analyzed for differences in proliferation and death by IHC, BrdU incorporation, and TUNEL staining, respectively. Although no differences in TUNEL staining were observed in the colonic epithelial cells from both genotypes (data not shown), TK−/− colons exhibited significantly less epithelial cell proliferation compared with TK+/+ epithelial cells after DSS treatment (Fig. 2G). Taken together, these data suggest that Ron receptor signaling promotes epithelial cell healing during chronic colitis and that loss of Ron leads to increased lethality and a more severe colitis phenotype compared with mice with intact Ron.
Fig. 2.
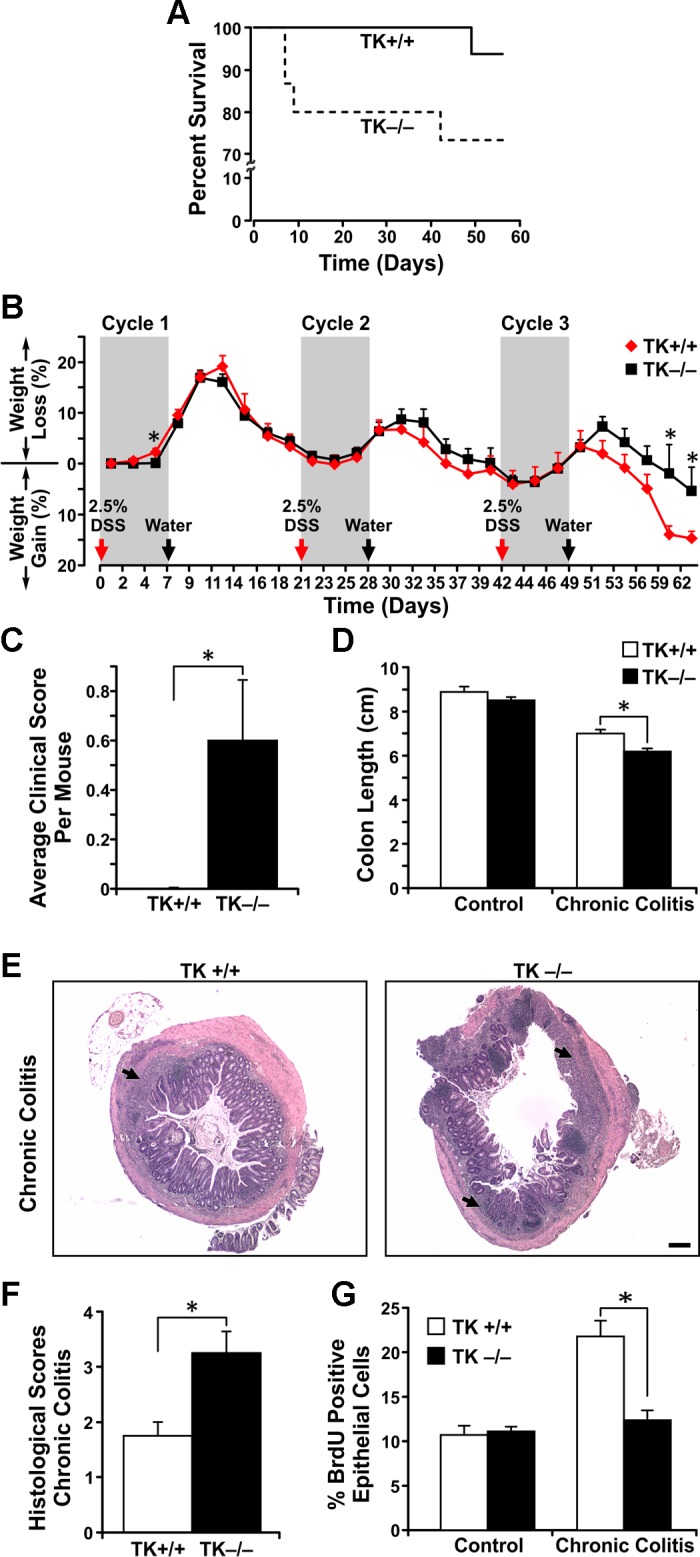
Ron loss heightens the severity of colitis following chronic DSS treatment. Survival curve of TK−/− and TK+/+ mice during chronic colitis is depicted (A). Temporal weight loss in mice following induction of chronic colitis is shown (B). TK−/− mice displayed higher gross clinical pathological scores in the recovery phase after 3 cycles of DSS treatment compared with TK+/+ mice (C). TK−/− colons exhibited more severe colitis and shortening of the colon (D). Representative colon sections from TK+/+ and TK−/− mice were analyzed by hematoxylin and eosin staining for colitis severity (E) with TK−/− colons exhibiting elevated histopathology scores (F). Arrows in E denote regions of ulceration. TK−/− colonic crypts had diminished epithelial cell proliferation following chronic colitis assessed by 5-bromo-2'-deoxyuridine (BrdU) incorporation (G). n = 5–24 mice per group, *P < 0.05, Scale bar = 200 μm.
Cytokine changes in TK−/− colons after chronic colitis.
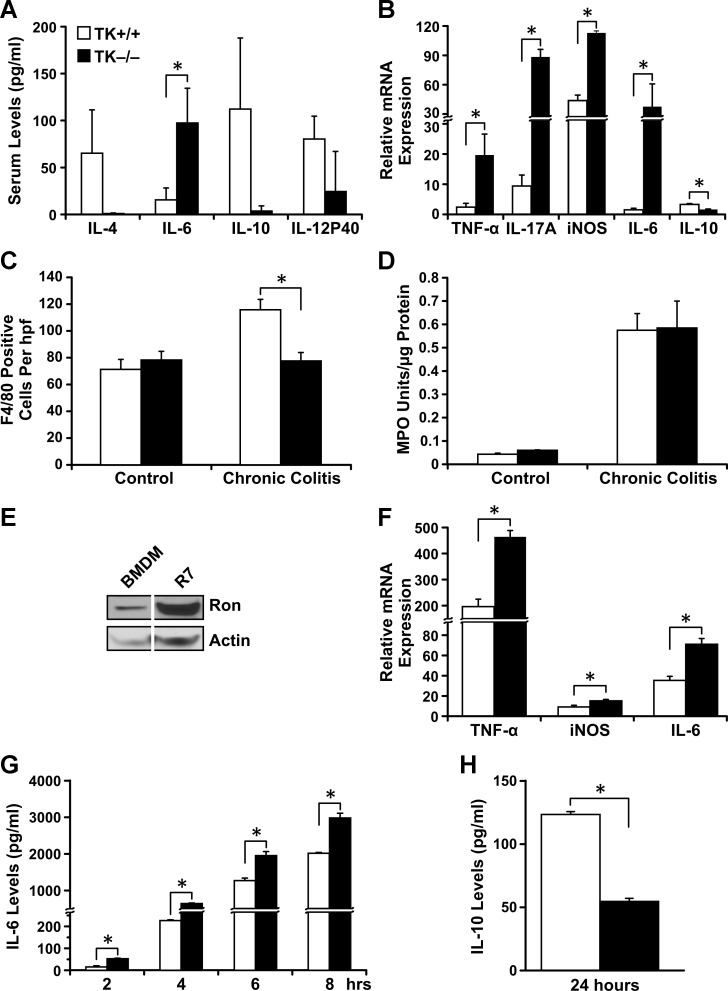
IBD studies in both humans and mice have shown that cytokine production plays a critical role in mediating disease pathogenesis (11, 53). Whereas no differences were observed in resting cytokine levels in sera of TK−/− and TK+/+ mice (data not shown), following cyclic DSS treatment, TK−/− sera exhibited decreased levels of anti-inflammatory cytokines (IL-10 and IL-4) and increased levels of the proinflammatory cytokine, IL-6, compared with control mice (Fig. 3A). qRT-PCR analyses on murine colons after DSS treatment showed that the TK−/− colons had significantly higher IL-6, TNF-α, IL-17A, and iNOS mRNA and lower IL-10 mRNA compared with TK+/+ colons (Fig. 3B). To determine whether changes in cytokines were a result of differential macrophage recruitment or activation, macrophage infiltration was assessed by F4/80 IHC. Interestingly, TK−/− colons contained fewer macrophages after chronic colitis compared with TK+/+ colons (Fig. 3C). Colonic neutrophil activity was assessed by MPO activity, and, although it increased following colitis, no changes were apparent between genotypes (Fig. 3D). To examine functional differences in macrophages and because inflammatory cells of the gut are recruited from the bone marrow (54), bone BMDMs from TK+/+ and TK−/− mice were isolated and examined ex vivo. Western analyses on BMDM from TK+/+ mice showed that these macrophages express Ron (Fig. 3E). BMDM from each genotype were stimulated with bacterial LPS and examined by qRT-PCR. TK−/− BMDM expressed significantly more mRNA for the proinflammatory cytokines TNF-α, IL-6, and iNOS mRNA compared with TK+/+ BMDM (Fig. 3F). Expression was similar to that observed in colon tissues of DSS-treated mice. No differences in the expression of these cytokines were observed in TK−/− and TK+/+ BMDM before LPS treatment (data not shown). Consistent with mRNA expression and serum cytokine levels, TK−/− BMDMs produced significantly higher levels of IL-6 (Fig. 3G) and lower levels of IL-10 (Fig. 3H) following LPS stimulation compared with TK+/+ BMDMs. These data show that Ron signaling affects the balance of pro- and anti-inflammatory cytokines during colitis.
Fig. 3.
Ron loss leads to altered cytokine expression and immune cell recruitment following the induction of chronic colitis. Analyses of sera from TK+/+ and TK−/− mice after DSS-induced chronic colitis over controls (A). qRT-PCR analyses of whole colons from TK+/+ and TK−/− mice after DSS-induced chronic colitis for TNF-α, IL-17A, inducible nitric oxide synthase (iNOS), IL-6, and IL-10 mRNA (B). Macrophage counts based on F4/80 IHC of colons from control and DSS-treated mice (C). Myeloperoxidase (MPO) assays revealed no significant difference in neutrophil activity between TK+/+ and TK−/− colons after DSS-induced chronic colitis (D). Western blot analyses for Ron expression in bone marrow-derived macrophages (BMDM) from TK+/+ mice. R7 cells [a murine breast cancer cell line (46)] are used as positive control (E). qRT-PCR analyses of LPS-treated BMDM from TK+/+ and TK−/− mice for TNF-α, IL-6, and iNOS mRNA (F). Cell culture supernatants of BMDMs from TK+/+ and TK−/− mice treated with LPS for the indicated times were examined for IL-6 (G) and IL-10 (H) production by ELISA. Data represent the means ± SE of n = 3–6 per group, *P < 0.05.
Ron loss alters the activation of downstream signaling pathways in the epithelium and stroma after chronic colitis.
Activation of the NF-κB and STAT signaling pathways is a critical component in IBD. Upregulation of NF-κB activity has been shown to promote epithelial barrier dysfunction and inflammation (7). Activation of STAT1 has been shown to worsen colitis in experimental mice (52), whereas STAT3 activation is important for efficient regeneration of the epithelium and in diminishing inflammation in experimental colitis (26, 50). To determine the effect of Ron loss on the NF-κB and STAT signaling pathways, IHC analysis for the activation of IKK-α/β, an upstream marker for NF-κB activation, and STAT1 phosphorylation was performed on colon sections from DSS-treated TK+/+ and TK−/− mice. TK−/− mice displayed increased nuclear STAT1 phosphorylation in colonic epithelial cells compared with TK+/+ mice (Fig. 4, A and B). In addition, significantly more STAT1 and IKK-α/β phosphorylation was observed in the stroma of the TK−/− colons compared with that of TK+/+ colons (Fig. 4, A–D). Western analyses of colons from DSS-treated TK+/+ and TK−/− mice showed a decrease in STAT3 phosphorylation in the TK−/− compared with TK+/+ colons (Fig. 4, E and F). These experiments suggest an important role for Ron in regulating the balance of critical signaling pathways needed to support the resolution of colitis in vivo.
Fig. 4.
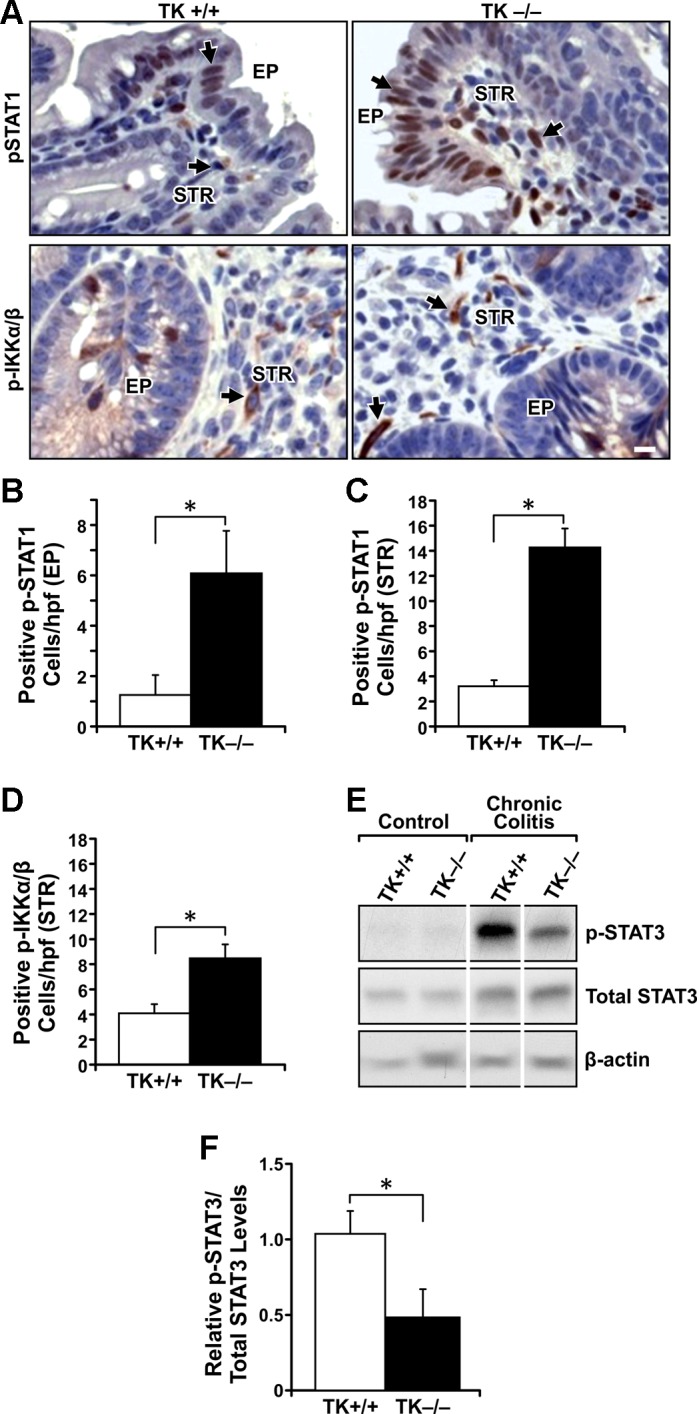
Signaling differences between TK+/+ and TK−/− colons after the induction of chronic colitis. IHC analyses for phosphorylated (p)-STAT1 and p-IKK-α/β (A) in colons of TK+/+ and TK−/− mice after DSS-induced colitis. Quantification of p-STAT1-positive cells in the colon epithelium (EP) (B) shows more p-STAT1-positive cells in TK−/− mice. Quantification of p-STAT1 (C) and p-IKK-α/β (D) cells in the colonic stroma (STR) shows more positive cells in TK−/− colons after DSS treatment. Western analyses of colons from TK+/+ and TK−/− mice before (Control) and after DSS treatments show more p-STAT3 in TK+/+ mice after colitis (E). Densitometry analysis for p-STAT3 expression normalized to total STAT3 is shown (F). *P < 0.05, scale bar = 50 μm. Images are representative of n = 5–6 mice per group with histograms representing the mean ± SE of n = 5–6 per group, *P < 0.05.
TK−/− mice have reduced expression of cell-matrix interaction molecules after chronic colitis.
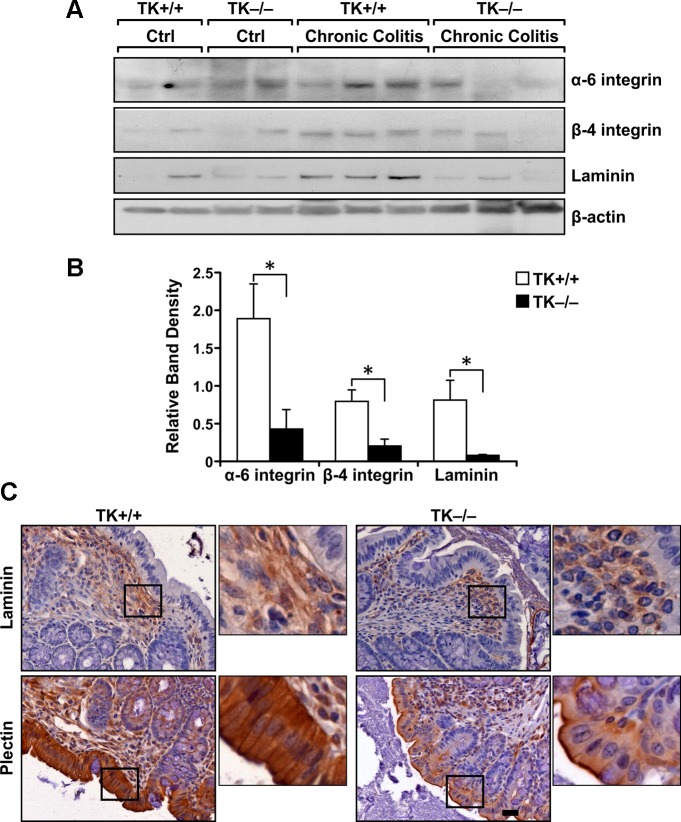
Cell adhesion molecules and cell-matrix interaction molecules play a vital role in maintaining biological activities of the intestinal epithelium including cell cycle progression as well as barrier function and are required for healing after injury (4). Western blot analyses and IHC were used to examine the expression of various cell-matrix interaction molecules in colons from TK+/+ and TK−/− mice after DSS treatment. Western analyses showed an increase in α6-integrin, β4-integrin, and laminin expression in TK+/+ colons in the recovery phase following DSS-induced colitis compared with colons from untreated mice. In contrast, this increase was not observed in DSS-treated TK−/− colons (Fig. 5, A and B). IHC analyses on colons from TK+/+ and TK−/− mice after chronic colitis showed that the TK−/− colons expressed less laminin and plectin compared with TK+/+ colons (Fig. 5C). These experiments suggest that Ron signaling plays a role in the expression of cell-matrix interacting proteins that are required for healing of colon after injury in colitis.
Fig. 5.
Ron mediated changes in cell-matrix molecules following the induction of colitis. Western analyses of colon tissue from TK+/+ and TK−/− mice before (Ctrl) and after DSS-induced chronic colitis showing changes in the expression of various cell-matrix interacting molecules (A). Each lane represents an independent colon sample. Densitometry analyses normalized to β-actin are shown (B). IHC analyses on colons for laminin and plectin (C) showing diminished expression of these molecules in colons of TK−/− mice following the induction of chronic colitis. Scale bar = 50 μm. Images are representative of n = 5–6 mice per group with histograms representing the mean ± SE of n = 5–6 per group. *P < 0.05.
Ron signaling is diminished in human patients with IBD.
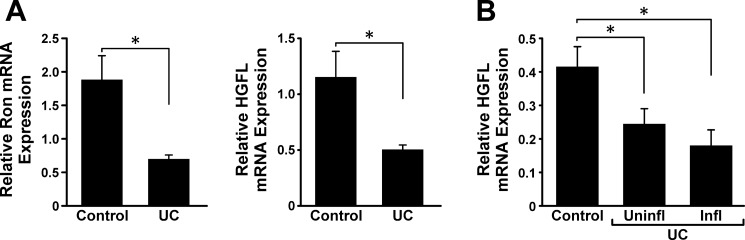
To examine the expression of Ron and HGFL in settings of IBD, GEO datasets were examined. Analyses of the GEO/GSE9686 database, which includes pediatric patient biopsies with active IBD (6), showed diminished Ron and HGFL in patients with UC (Fig. 6A). Analyses of the GEO/GSE11223, which includes biopsies of adult patients with active as well as quiescent IBD (31), showed diminished expression of HGFL in the colons of patients with UC with and without associated inflammation, compared with control colon samples (Fig. 6B). These data provide additional support that diminished HGFL-Ron signaling is associated with IBD and provide further evidence on colonic HGFL expression.
Fig. 6.
Colonic Ron and HGFL mRNA expression is reduced in patients with ulcerative colitis (UC). Analyses of GEO dataset GSE9686 shows significantly lower expression of Ron and HGFL mRNA in colons from pediatric patients with UC with active disease compared with controls (A). Analyses of GEO/GSE11223 dataset documents significantly lower expression of HGFL mRNA in colons from adult patients with UC with uninflamed (uninfl) and inflamed (infl) colons compared with controls (B). *P < 0.05.
DISCUSSION
In the present study, we provide the first direct in vivo evidence for a functional role for the Ron receptor TK in colitis. Similar to previous findings, Ron expression was observed in normal and colitis-affected colons as well as in resident colonic macrophages (15, 18). Furthermore, we have established HGFL expression in the colonic epithelium and macrophages. To our knowledge, this is the first report to document HGFL expression in the colon, although the significance of this localized HGFL production is not known. This novel finding is corroborated by human patient data documenting diminished HGFL and Ron mRNA expression in colon tissue of patients with UC (Fig. 6). Combined, these data suggest a possible requirement for local HGFL-mediated Ron activation in the colon for the resolution of intestinal injury. Using mice lacking Ron signaling (48), we demonstrated an exacerbated colitis phenotype after cyclic DSS treatment. We show that Ron loss leads to a decrease in ability of the colonic epithelium to proliferate and heal and is associated with an increased inflammatory environment following injury. These studies suggest that loss or blunting of this signaling cascade, consistent with the proposed role of the SNPs identified in Ron and HGFL through genome-wide association studies, may contribute to the pathogenesis of IBD (5, 13, 27).
Colonic epithelial cell turnover is increased in patients with UC and is required for replenishing the lost epithelium following injury (8). Ron signaling has been shown to promote wound healing by stimulating epithelial cell migration, proliferation, and survival at the wound site (25, 36). The decreased epithelial cell proliferation and increased ulceration observed in TK−/− colons following colitis suggest that Ron signaling is required for the proliferation of colon epithelium contributing to the resolution of injury in IBD. Furthermore, as the epithelial barrier remains lost or ulcerated in TK−/− mice, there may be continued interaction between colon immune cells and microbial flora, leading to increased inflammation. Interestingly, studies examining the 689C SNP in HGFL showed that this alteration did not alter binding to Ron but lowered HGFL concentration in the serum of human patients with IBD (18). It is interesting to speculate that the local colonic production of HGFL is also reduced in 689C-expressing patients and may decrease the extent of Ron activation in the colon and lessen proliferation and migration needed for healing.
Experimental colitis induced by DSS treatment is characterized by cytokine changes (9). In the TK−/− mice, significant increases were observed in TNF-α, iNOS, and IL-6 expression, whereas decreases were found in IL-4 and IL-10 compared with TK+/+ mice (Fig. 3) following DSS treatment. Consistent with these in vivo data, ex vivo LPS treatment of TK−/− BMDMs led to significant increases in TNF-α, iNOS, and IL-6 expression compared with TK+/+ cells. These studies are in accord with numerous publications citing the macrophage-specific regulation of cytokines and iNOS expression by Ron in vivo and in vitro (23, 28, 29, 34, 43). Macrophages play a vital role in inflammation, and their activation status determines the repertoire of proinflammatory or anti-inflammatory cytokine production. M1-activated macrophages produce proinflammatory mediators and can worsen the pathology of colitis, whereas M2-activated or alternately activated macrophages promote angiogenesis, tissue remodeling, and wound healing in chronic inflammatory conditions such as colitis (42). Data in TK−/− BMDM and in TK−/− mice subjected to DSS support prior studies showing Ron signaling as an important determinate in mediating an M2 phenotype with Ron loss associating with the polarization of macrophages to an M1 phenotype (40, 41, 48). Thus, during IBD, Ron may control the polarization status of macrophages during injury with Ron loss expected to contribute to a more severe colitis phenotype. A detailed characterization of the role of Ron in macrophage polarization during colitis is warranted and may suggest opportunities to intervene in IBD pathogenesis through Ron immune cell modulation.
NF-κB is expressed on colon epithelial cells and macrophages in normal and IBD colons in humans and mice. The amount of activated NF-κB correlates significantly with the severity of inflammation in colitis, and upregulation of NF-κB activity promotes epithelial barrier dysfunction (1–3, 7, 39). Furthermore, myeloid but not epithelial NF-κB activity has been shown to promote inflammation in a murine model of chronic colitis with deletion of IKK-β, a molecule upstream of NF-κB activation, in myeloid cells diminishing colon inflammation (10). Based on our previous findings that Ron signaling in macrophages suppresses NF-κB activity (43), we postulate that Ron loss in colon macrophages leads to increased NF-κB activity and ensuing proinflammatory cytokine secretion in TK−/− mice. STAT transcription factors play a role in colitis, and the expression of STAT1 and STAT3 increases in human and murine colitis (24). The balance of STAT1 and STAT3, especially increased STAT3 over STAT1, controls cell survival, inflammation, and drives healing responses in various clinical settings (35, 37). STAT1 activation has been shown to worsen colitis in mice (52), whereas STAT3 activation aids in the regeneration of epithelium and diminish inflammation in models of experimental colitis (26, 50). In TK−/− colons, decreases in p-STAT3 and increases in p-STAT1 were observed after DSS treatment compared with TK+/+ colons (Fig. 4). Ron has been shown to activate STAT3, and our data suggest that Ron-mediated STAT3 activation in TK+/+ colons promotes healing following colitis (14). Ron receptor activation has been shown to induce the activation of a number of downstream signaling cascades including MAPK, AKT, phosphatidylinositol 3-kinase, and β-catenin (47). The role of these signaling molecules as mediators of Ron activation in colitis remains to be determined.
Cell-matrix interactions are important to maintain the normal barrier function of the colon epithelium and for renewal of epithelial cells in the normal colon and after injury. Analyses of patient biopsies have shown that expression of laminin in the colon extracellular matrix (ECM) is diminished in active UC and is correlated with clinical severity (38), an observation similar to DSS-induced colitis in TK−/− mice. The decrease in laminin is hypothesized to affect epithelial cell migration, differentiation, and regeneration during colitis leading to more severe disease (38). α6- and β4-integrins are expressed on colon epithelial cells. α6/β4-integrin together with plectin are involved in hemidesmosome formation, structures that anchor cells to the ECM by interacting with laminin (4, 45). In a wound-healing model of colon epithelial cells, hemidesmosome formation is the first step, followed by lamellipodia formation, in which the α6/β4-integrin complexes relocalize from hemidesmosomes to help cells migrate into denuded regions (20). Ron activation by HGFL has been shown to disrupt plectin-α6/β4-integrin interactions in hemidesmosomes and relocalizes α6/β4-integrin to lamellipodia promoting keratinocyte migration to promote wound healing (36). The higher amount of plectin and α6/β4-integrin in Ron-expressing colons may help facilitate healing following DSS-induced injury. Further studies are needed to elucidate the mechanisms associated with diminished expression of cell-matrix interacting proteins in TK−/− mice.
GEO database analyses showed diminished HGFL and Ron expression in human patients with UC, which correlates well with our findings suggesting a need for local HGFL-mediated Ron receptor activation in the colon. Based on the data presented here and the established role for Ron in inflammation and wound healing, our data suggest a model wherein Ron expression in the colon epithelium promotes proliferation and modulates cell-matrix interactions required for epithelial cell migration. Moreover, in macrophages, the Ron receptor suppresses key proinflammatory cytokine secretion by diminishing NF-κB activation during colitis. The net effect of Ron signaling allows for the resolution of colitis, and alterations that inhibit or block Ron action, such as SNPs, which effect Ron activity, may have deleterious effects. Taken together, these studies have identified a critical role for the Ron receptor TK in chronic colitis although the cell type-specific roles for this receptor remain to be fully characterized. Further investigation of the Ron signaling pathway is warranted and may be an important new target for patients with IBD.
GRANTS
This work was supported in part by the Public Health Services Grants CA125379 (S. Waltz) and NIH P30 DK078392 from the National Institutes of Health, Veteran's Administration VA1001BX000803 (S. Waltz) grant, and 12POST12040055 (R. Kulkarni) from the American Heart Association Great Rivers Affiliate.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.M.K., W.D.S., and D.G. performed experiments; R.M.K., W.D.S., and S.E.W. analyzed data; R.M.K., W.D.S., and S.E.W. interpreted results of experiments; R.M.K., W.D.S., and S.E.W. prepared figures; R.M.K. and S.E.W. drafted manuscript; R.M.K., W.D.S., and S.E.W. edited and revised manuscript; R.M.K., W.D.S., D.G., and S.E.W. approved final version of manuscript; S.E.W. conception and design of research.
ACKNOWLEDGMENTS
The authors thank Dr. Nancy M. Benight for critical review of the manuscript.
REFERENCES
- 1.Ardite E, Panes J, Miranda M, Salas A, Elizalde JI, Sans M, Arce Y, Bordas JM, Fernandez-Checa JC, Pique JM. Effects of steroid treatment on activation of nuclear factor kappaB in patients with inflammatory bowel disease. Br J Pharmacol 124: 431–433, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Int Med Res 263: 591–596, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Bantel H, Berg C, Vieth M, Stolte M, Kruis W, Schulze-Osthoff K. Mesalazine inhibits activation of transcription factor NF-kappaB in inflamed mucosa of patients with ulcerative colitis. Am J Gastroenterol 95: 3452–3457, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Beaulieu JF. Integrin alpha6beta4 in colorectal cancer. World J Gastrointest Pathophysiol 1: 3–11, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckly JB, Hancock L, Geremia A, Cummings JR, Morris A, Cooney R, Pathan S, Guo C, Jewell DP. Two-stage candidate gene study of chromosome 3p demonstrates an association between nonsynonymous variants in the MST1R gene and Crohn's disease. Inflamm Bowel Dis 14: 500–507, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Carey R, Jurickova I, Ballard E, Bonkowski E, Han X, Xu H, Denson LA. Activation of an IL-6:STAT3-dependent transcriptome in pediatric-onset inflammatory bowel disease. Inflamm Bowel Dis 14: 446–457, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catalioto RM, Maggi CA, Giuliani S. Intestinal epithelial barrier dysfunction in disease and possible therapeutical interventions. Curr Med Chem 18: 398–426, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Danese S. Immune and nonimmune components orchestrate the pathogenesis of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 300: G716–G722, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol 114: 385–391, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckmann L, Nebelsiek T, Fingerle AA, Dann SM, Mages J, Lang R, Robine S, Kagnoff MF, Schmid RM, Karin M, Arkan MC, Greten FR. Opposing functions of IKKbeta during acute and chronic intestinal inflammation. Proc Natl Acad Sci USA 105: 15058–15063, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, Eysselein VE, Buchler MW. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion 62: 240–248, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Engel MA, Neurath MF. New pathophysiological insights and modern treatment of IBD. J Gastroenterol 45: 571–583, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Goyette P, Lefebvre C, Ng A, Brant SR, Cho JH, Duerr RH, Silverberg MS, Taylor KD, Latiano A, Aumais G, Deslandres C, Jobin G, Annese V, Daly MJ, Xavier RJ, Rioux JD. Gene-centric association mapping of chromosome 3p implicates MST1 in IBD pathogenesis. Mucosal Immunol 1: 131–138, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gurusamy D, Gray JK, Pathrose P, Kulkarni RM, Finkleman FD, Waltz SE. Myeloid-specific expression of Ron receptor kinase promotes prostate tumor growth. Cancer Res 73: 1752–1763, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirayama I, Ide M, Asao T, Kuwano H. Receptor protein tyrosine kinase Ron is highly expressed in colorectal mucosa of ulcerative colitis patients. Hepatogastroenterology 54: 1672–1675, 2007 [PubMed] [Google Scholar]
- 16.Iwama A, Wang MH, Yamaguchi N, Ohno N, Okano K, Sudo T, Takeya M, Gervais F, Morissette C, Leonard EJ, Suda T. Terminal differentiation of murine resident peritoneal macrophages is characterized by expression of the STK protein tyrosine kinase, a receptor for macrophage-stimulating protein. Blood 86: 3394–3403, 1995 [PubMed] [Google Scholar]
- 17.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol 28: 573–621, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kauder SE, Santell L, Mai E, Wright LY, Luis E, N'Diaye EN, Lutman J, Ratti N, Sa SM, Maun HR, Stefanich E, Gonzalez LC, Graham RR, Diehl L, Faubion WA, Jr, Keir ME, Young J, Chaudhuri A, Lazarus RA, Egen JG. Functional consequences of the macrophage stimulating protein 689C inflammatory bowel disease risk allele. PLoS One 8: e83958, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laroui H, Ingersoll SA, Liu HC, Baker MT, Ayyadurai S, Charania MA, Laroui F, Yan Y, Sitaraman SV, Merlin D. Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS One 7: e32084, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lotz MM, Rabinovitz I, Mercurio AM. Intestinal restitution: progression of actin cytoskeleton rearrangements and integrin function in a model of epithelial wound healing. Am J Pathol 156: 985–996, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mallakin A, Kutcher LW, McDowell SA, Kong S, Schuster R, Lentsch AB, Aronow BJ, Leikauf GD, Waltz SE. Gene expression profiles of Mst1r-deficient mice during nickel-induced acute lung injury. Am J Respir Cell Mol Biol 34: 15–27, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maxwell JR, Brown WA, Smith CL, Byrne FR, Viney JL. Methods of inducing inflammatory bowel disease in mice. Current Protocols in Pharmacology, edited by Enna SJ. Hoboken, NJ: Wiley, Chapter 5-Unit 5, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Morrison AC, Wilson CB, Ray M, Correll PH. Macrophage-stimulating protein, the ligand for the stem cell-derived tyrosine kinase/RON receptor tyrosine kinase, inhibits IL-12 production by primary peritoneal macrophages stimulated with IFN-gamma and lipopolysaccharide. J Immunol 172: 1825–1832, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Mudter J, Weigmann B, Bartsch B, Kiesslich R, Strand D, Galle PR, Lehr HA, Schmidt J, Neurath MF. Activation pattern of signal transducers and activators of transcription (STAT) factors in inflammatory bowel diseases. Am J Gastroenterol 100: 64–72, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Nanney LB, Skeel A, Luan J, Polis S, Richmond A, Wang MH, Leonard EJ. Proteolytic cleavage and activation of pro-macrophage-stimulating protein and upregulation of its receptor in tissue injury. J Invest Dermatol 111: 573–581, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Neufert C, Pickert G, Zheng Y, Wittkopf N, Warntjen M, Nikolaev A, Ouyang W, Neurath MF, Becker C. Activation of epithelial STAT3 regulates intestinal homeostasis. Cell Cycle 9: 652–655, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Neuman MG, Nanau RM. Single-nucleotide polymorphisms in inflammatory bowel disease. Transl Res 160: 45–64, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Nikolaidis NM, Gray JK, Gurusamy D, Fox W, Stuart WD, Huber N, Waltz SE. Ron receptor tyrosine kinase negatively regulates TNFalpha production in alveolar macrophages by inhibiting NF-kappaB activity and Adam17 production. Shock 33: 197–204, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikolaidis NM, Kulkarni RM, Gray JK, Collins MH, Waltz SE. Ron receptor deficient alveolar myeloid cells exacerbate LPS-induced acute lung injury in the murine lung. Innate Immun 17: 499–507, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikolaus S, Schreiber S. Diagnostics of inflammatory bowel disease. Gastroenterology 133: 1670–1689, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Noble CL, Abbas AR, Cornelius J, Lees CW, Ho GT, Toy K, Modrusan Z, Pal N, Zhong F, Chalasani S, Clark H, Arnott ID, Penman ID, Satsangi J, Diehl L. Regional variation in gene expression in the healthy colon is dysregulated in ulcerative colitis. Gut 57: 1398–1405, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Okino T, Egami H, Ohmachi H, Takai E, Tamori Y, Nakagawa A, Nakano S, Sakamoto O, Suda T, Ogawa M. Immunohistochemical analysis of distribution of RON receptor tyrosine kinase in human digestive organs. Dig Dis Sci 46: 424–429, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Quantin B, Schuhbaur B, Gesnel MC, Doll'e P, Breathnach R. Restricted expression of the ron gene encoding the macrophage stimulating protein receptor during mouse development. Dev Dyn 204: 383–390, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Ray M, Yu S, Sharda DR, Wilson CB, Liu Q, Kaushal N, Prabhu KS, Hankey PA. Inhibition of TLR4-induced IkappaB kinase activity by the RON receptor tyrosine kinase and its ligand, macrophage-stimulating protein. J Immunol 185: 7309–7316, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Regis G, Pensa S, Boselli D, Novelli F, Poli V. Ups and downs: the STAT1:STAT3 seesaw of Interferon and gp130 receptor signalling. Semin Cell Dev Biol 19: 351–359, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Santoro MM, Gaudino G, Marchisio PC. The MSP receptor regulates alpha6beta4 and alpha3beta1 integrins via 14-3-3 proteins in keratinocyte migration. Dev Cell 5: 257–271, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem 282: 20059–20063, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Schmehl K, Florian S, Jacobasch G, Salomon A, Korber J. Deficiency of epithelial basement membrane laminin in ulcerative colitis affected human colonic mucosa. Int J Colorectal Dis 15: 39–48, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut 42: 477–484, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharda DR, Yu S, Ray M, Squadrito ML, De Palma M, Wynn TA, Morris SM, Jr, Hankey PA. Regulation of macrophage arginase expression and tumor growth by the Ron receptor tyrosine kinase. J Immunol 187: 2181–2192, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122: 787–795, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith PD, Smythies LE, Shen R, Greenwell-Wild T, Gliozzi M, Wahl SM. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol 4: 31–42, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stuart WD, Kulkarni RM, Gray JK, Vasiliauskas J, Leonis MA, Waltz SE. Ron receptor regulates Kupffer cell-dependent cytokine production and hepatocyte survival following endotoxin exposure in mice. Hepatology 53: 1618–1628, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takeshita S, Kaji K, Kudo A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J Bone Miner Res 15: 1477–1488, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Teller IC, Beaulieu JF. Interactions between laminin and epithelial cells in intestinal health and disease. Exp Rev Mol Med 3: 1–18, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Wagh PK, Gray JK, Zinser GM, Vasiliauskas J, James L, Monga SP, Waltz SE. beta-Catenin is required for Ron receptor-induced mammary tumorigenesis. Oncogene 30: 3694–3704, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagh PK, Peace BE, Waltz SE. Met-related receptor tyrosine kinase Ron in tumor growth and metastasis. Adv Cancer Res 100: 1–33, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waltz SE, Eaton L, Toney-Earley K, Hess KA, Peace BE, Ihlendorf JR, Wang MH, Kaestner KH, Degen SJ. Ron-mediated cytoplasmic signaling is dispensable for viability but is required to limit inflammatory responses. J Clin Invest 108: 567–576, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath MF. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat Protocol 2: 2307–2311, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Willson TA, Jurickova I, Collins M, Denson LA. Deletion of intestinal epithelial cell STAT3 promotes T-lymphocyte STAT3 activation and chronic colitis following acute dextran sodium sulfate injury in mice. Inflamm Bowel Dis 19: 512–525, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protocol 2: 541–546, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Wu X, Guo W, Wu L, Gu Y, Gu L, Xu S, Shen Y, Ke Y, Tan R, Sun Y, Xu Q. Selective sequestration of STAT1 in the cytoplasm via phosphorylated SHP-2 ameliorates murine experimental colitis. J Immunol 189: 3497–3507, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Zahn A, Giese T, Karner M, Braun A, Hinz U, Stremmel W, Ehehalt R. Transcript levels of different cytokines and chemokines correlate with clinical and endoscopic activity in ulcerative colitis. BMC Gastroenterol 9: 13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zigmond E, Jung S. Intestinal macrophages: well educated exceptions from the rule. Trends Immunol 34: 162–168, 2013 [DOI] [PubMed] [Google Scholar]