Abstract
Plasma levels of the orexigenic hormone ghrelin are suppressed by meals with an efficacy dependent on their macronutrient composition. We hypothesized that heterogeneity in osmolarity among macronutrient classes contributes to these differences. In three studies, the impact of small intestinal hyperosmolarity was examined in Sprague-Dawley rats. In study 1, isotonic, 2.5×, and 5× hypertonic solutions of several agents with diverse absorption and metabolism properties were infused duodenally at a physiological rate (3 ml/10 min). Jugular vein blood was sampled before and at 30, 60, 90, 120, 180, 240, and 300 min after infusion. Plasma ghrelin was suppressed dose dependently and most strongly by glucose. Hyperosmolar infusions of lactulose, which transits the small intestine unabsorbed, and 3-O-methylglucose (3-O-MG), which is absorbed like glucose but remains unmetabolized, also suppressed ghrelin. Glucose, but not lactulose or 3-O-MG, infusions increased plasma insulin. In study 2, intestinal infusions of hyperosmolar NaCl suppressed ghrelin, a response that was not attenuated by coinfusion with the neural blocker lidocaine. In study 3, we reconfirmed that the low-osmolar lipid emulsion Intralipid suppresses ghrelin more weakly than isocaloric (but hypertonic) glucose. Importantly, raising Intralipid's osmolarity to that of the glucose solution by nonabsorbable lactulose supplementation enhanced ghrelin suppression to that seen after glucose. Hyperosmolar ghrelin occurred particularly during the initial 3 postinfusion hours. We conclude that small intestinal hyperosmolarity 1) is sufficient to suppress ghrelin, 2) may combine with other postprandial mechanisms to suppress ghrelin, 3) might contribute to altered ghrelin regulation after gastric bypass surgery, and 4) may inform dietary modifications for metabolic health.
Keywords: ghrelin, intestinal osmolarity, 3-O-methylglucose, lactulose, satiation
ghrelin is the only known appetite-stimulating gastrointestinal hormone in humans and other vertebrates. Exogenous administration of this 28-amino-acid peptide increases short-term hunger, food intake, and motivation to eat, as well as body weight (14, 16, 17, 67). Circulating ghrelin levels correlate with energy balance, i.e., they increase with weight loss or during meal anticipation and decrease with weight gain or after food intake. Ghrelin's orexigenic effects lend practical importance to a detailed understanding of its regulation by diet.
The mechanisms underlying postmeal ghrelin suppression are incompletely understood, although some factors have been elucidated. First, the magnitude of ghrelin suppression correlates with the total caloric content of ingested food (8). Second, the nutrient sensors that initiate postprandial ghrelin suppression are not located in the stomach, i.e., the site of most ghrelin-producing cells, but more distally in the gastrointestinal tract and/or at postabsorptive sites (69, 91). Third, the magnitude of ghrelin suppression depends on the macronutrient composition of meals; e.g., ingested carbohydrates and proteins both suppress ghrelin more effectively than isocaloric/isovolumic lipids do (26, 69). This difference could conceivably result from the greater ability of carbohydrates and proteins to stimulate insulin secretion, since exogenous insulin infusions suppress ghrelin (62), and postprandial ghrelin suppression usually coincides with a rise of insulin. However, a postprandial rise in plasma insulin levels is not required for meal-related ghrelin suppression, since humans and rats lacking insulin entirely still display ghrelin suppression after meals (28, 62). Thus macronutrient-related differences in ghrelin suppression cannot be fully explained by the differential ability of various macronutrient types to induce insulin secretion.
The present studies were undertaken to assess whether small intestinal hyperosmolarity (as occurs after meal ingestion) affects ghrelin levels and could partially explain differential ghrelin suppression by various food types. This effort was prompted in part by our earlier observation that intestinal lipid infusions suppress ghrelin poorly and have low osmolarity, whereas isocaloric/isovolumic but hyperosmolar carbohydrates or amino acids are strong suppressants of ghrelin (69). Moreover, a considerable, decades-old literature reports multiple effects of hyperosmolar foods that are theoretically compatible with suppressed ghrelin levels, i.e., inhibition of gastric emptying, decreased food intake, and increased metabolic rate (11, 19, 21, 29, 36, 38, 39, 66, 89).
Small intestinal hyperosmolarity, which naturally accompanies food digestion, reflects the intestinal presence of water-soluble, unabsorbed meal constituents, including salts, nutrients, and soluble fibers (33). Upon leaving the stomach, chyme may have a higher osmolarity than that of surrounding body fluids and tissues, which are generally maintained at ∼285 mosmol/l in blood and interstitial fluids and within the 400–700 mosmol/l range in small intestinal villi (34). Although compensatory mechanisms operate to restore osmotic equilibrium between hyperosmolar intestinal chyme and its surroundings (24), various mixed meals and hypertonic agents are capable of inducing a more sustained hyperosmolar milieu in the gastric and intestinal lumen (5, 25, 37, 39, 43, 48, 66, 82, 83).
To assess directly the role of intestinal hyperosmolarity in postprandial ghrelin suppression, three studies were conducted in rats. In the first study, animals with duodenal cannulas received infusions, on different occasions, of agents (glucose, 3-O-methylglucose, or lactulose) selected to induce intestinal hyperosmolarity, but differing in route of digestion, absorption kinetics, caloric value, and insulin-stimulating effects (see materials and methods). For each ingredient, infusions were administered at osmolarities (isotonic, 2.5× hypertonic, and 5× hypertonic) that were similar to those of body fluids, nondiet soda, and sherbet ice cream, respectively (38, 90), and infusion rates were within the physiological range of gastric emptying. Blood was serially sampled via jugular vein catheters to assess ghrelin suppression over the 5-h period following infusions. In study 2, we examined the role of intestinal afferent neural activity in ghrelin responses to 5-fold hypertonic infusion of sodium chloride, using intestinal pre- and coinfusion with lidocaine, a local neural blocker. In the third study, we sought to confirm that osmolarity contributes to the earlier-mentioned differences in ghrelin suppression by intestinal lipids vs. carbohydrates (26, 69), which could not be attributed to different insulin responses alone (28, 62). Carbohydrate solutions are of higher osmolarity than isocaloric/isovolumic lipid emulsions, in part because lipids contain more calories per molecule than do carbohydrates. Hence, we monitored ghrelin responses following three small intestinal infusions: 25 wt/vol% glucose, isocaloric Intralipid, and the same dose of Intralipid rendered equally hypertonic as the 25 wt/vol% glucose infusion by supplementation with nonabsorbable lactulose.
MATERIALS AND METHODS
Animals.
Experiments were performed on male Sprague-Dawley rats (ATL, Kent, WA), aged 16–25 wk and weighing 300–450 g. Animals were housed individually at the Veterans Affairs Puget Sound Health Care System (VAPSHCS), Seattle Division, an AAALAC-accredited facility. Rats had ad libitum access to pelleted chow and water between experimental sessions. They were maintained on a 12:12-h light-dark cycle, with lights on at 0600. All procedures were approved by the VAPSHCS Institutional Animal Care and Use Committee.
Rat surgery.
Each rat was fitted with a duodenal catheter for infusions of test substances, and also with a jugular vein catheter for undisturbed serial blood sampling, according to procedures described previously in greater detail (69).
Duodenal feeding catheter placement and testing.
Rats were anesthetized with a mixture of 60 mg/kg ketamine and 3 mg/kg xylazine (Phoenix, St. Joseph, MO), administered intraperitoneally. A duodenal catheter was inserted 2 cm distal to the pylorus through a puncture hole made with an 18-gauge needle. Silastic tubing (outer diameter 0.047 in., inner diameter 0.025 in., Braintree Scientific, Braintree, MA) was bonded to a small piece of Bard surgical mesh (Davol, Cranston, RI). After insertion, the catheter-mesh ensemble was tethered to the intestine with a three-loop purse-string of 6-0 silk suture (US Surgical, Norwalk, CT).
Jugular vein catheter placement.
The right jugular vein was cannulated with Silastic tubing (outer diameter 0.037 in., inner diameter 0.02 in., VWR), inserted through a 2-cm skin incision. The proximal end of the tubing was tunneled subcutaneously to the head, on which it was secured, along with the intestinal catheter, by using an acrylic skull cap (Lang Dental, Wheeling, IL) (73).
Experimental protocol.
Rats recovered from surgery for at least 2 wk before being tested. They were habituated to residence in Plexiglas test cages (30 × 25 × 35 cm) for two nights before the start of each experiment. At 1700 on the day before infusions, animals were placed individually in the experimental cages, allowing intestinal infusions and repeated blood sampling to be performed without disturbing the rats. To establish high baseline ghrelin levels, food was withheld for 18 h before each experimental session. One hour before infusions, each animal's head cap was connected with separate lines (PE-100, VWR) for blood sampling and administration of infusates, respectively. Infusions were initiated at 1100, and blood was sampled repeatedly during the ensuing 5-h period. Infusates were given in random order, spaced at least 6 days apart from one another.
Blood sampling and analysis.
Five minutes before the start of each nutrient infusion, a baseline blood sample (volume: 300 μl) was drawn from the jugular vein catheter, and additional samples were collected at 30, 60, 90, 120, 180, 240, and 300 min after the onset of infusion. Blood glucose levels were immediately analyzed with a portable glucose meter (Accu-Check, Roche, Indianapolis, IN). The remaining blood was transferred to 0.5-ml microcentrifuge tubes containing 10 μl of 7.5% EDTA and placed immediately on ice. Between 2 and 3 h after collection, blood samples were centrifuged, and the plasma was withdrawn and stored at −80°C. Plasma levels of total ghrelin were measured by radioimmunoassay using a primary antibody against rat ghrelin and 131I-labeled ghrelin as the tracer (kit RK-031-31, Phoenix Pharmaceuticals, Belmont, CA) (17). This assay detects both acylated and des-acyl ghrelin. Although acylated ghrelin is the primary bioactive form, levels of the two forms correlate closely with one another across a wide variety of physiological manipulations that affect ghrelin concentrations (4, 26, 61, 63). Plasma insulin was measured by a commercial radioimmunoassay (kit SRI-13K Linco Research, St. Charles, MO).
Infusions in study 1: ghrelin suppression by duodenal infusions of agents with different absorption kinetics and metabolism at three osmolarity levels.
Selected for their diversity in absorption kinetics and metabolism, four ingredients were used to test effects of intestinal osmolarity on circulating ghrelin levels: glucose, 3-O-methylglucose (3-O-MG), lactulose (all purchased from Sigma-Aldrich, St. Louis, MO), and isotonic saline (0.9% NaCl). On separate occasions, these solutions were infused into the duodenum, each at the same volume (3 ml) but varying osmolarities. Glucose is a caloric, absorbable monosaccharide [molecular weight (MW): 180] that suppresses ghrelin levels very effectively following ingestion or intestinal infusion (69). 3-O-MG is a modified, noncaloric form of glucose (MW: 194) that is likewise absorbed via intestinal transporters SGLT-1 and SGLT-3 but remains unmetabolized and is excreted unchanged in urine (13, 85). Small intestinal infusions of 3-O-MG increase satiation in rats (56). Because of their common route of absorption, intestinal glucose and 3-O-MG represent similar, transient osmotic stimuli. Lactulose is a synthetic disaccharide (MW: 342) consisting of one fructose and one lactose unit linked by a β-1,4 glucosidic bond that resists digestive breakdown by small intestinal glucosidases and therefore transits the full length of the small intestine unabsorbed (77). The prolonged presence of nonabsorbable substances like lactulose in the small intestinal lumen causes more durable hyperosmotic and anorexigenic effects than do absorbable substances (19). Upon entry into the cecum and colon, lactulose is fermented by resident bacteria into lactate and short-chain fatty acids, which contribute their own osmotic activity (77). Isotonic saline (0.9% wt/vol NaCl) was used as a control. All test solutions were infused at 0.3 ml/min over 10 min. This infusion rate is equal to the estimated physiological gastric emptying rate of 25 wt/vol% glucose solution (44), i.e., the most energy-dense and hypertonic test solution in the present studies. Consequently, the other isovolumic infusions in our studies also represented caloric and osmotic stimuli within the physiological range.
Infusions in study 2: ghrelin suppression by duodenal infusions of iso- and hyperosmolar saline.
Isotonic (0.9 wt/vol% NaCl) and hypertonic (4.5 wt/vol % NaCl) saline solutions were duodenally infused at 0.3 ml/min over 10 min to deliver a noncaloric, nonglycemic, noninsulinotropic, nonfermentable stimulus. Hypertonic saline is known to have anorexic effects (11, 38). The role of neural signaling in ghrelin suppression by hypertonic saline was evaluated by intestinal infusion of lidocaine, which blocks intestinal-afferent and submucosal neuronal activity (50, 93). Related, locally acting compounds are known to attenuate hyperosmolarity-induced anorexic effects in pigs and cattle (3, 38). Mucosal application of lidocaine has a rapid onset of action (<5 min; Refs. 78, 93) and a duration of action of up to 30 min (78, 86). We administered lidocaine as a combined pre- and coinfusion. The preinfusion (0.5 wt/vol% lidocaine in 1 ml isotonic saline; duration 1 min) was timed 5 min prior to the hypertonic saline infusion, whereas a second dose of lidocaine (0.5% wt/vol) was coinfused with the hypertonic saline (3 ml). We had previously observed that lidocaine administered in this fashion attenuated ghrelin suppression by 2.5-fold hypertonic glucose infusions (t-tests P < 0.02 for ghrelin nadir, and P < 0.005 for decremental area under the ghrelin curve) and that lidocaine alone did not raise ghrelin levels (68).
Infusions in study 3: ghrelin suppression by intestinal infusions of isocaloric lipids, glucose, and lipids rendered iso-osmotic to the glucose by supplementation with nonabsorbable lactulose.
The purpose of the third study was to test whether hyperosmolarity contributed to the stronger ghrelin suppression by glucose than by isocaloric/isovolumic Intralipid that we observed previously (69). Intralipid (Baxter Healthcare, Deerfield, IL) is a near-isotonically formulated, broad-spectrum mixture of primarily long-chain triglycerides, with small quantities of phospholipids, glycerol, and ions. The osmolarity of Intralipid infusions should be considerably less than that of the isocaloric glucose solution because 1) lipids contain more calories per molecule than does glucose and 2) in contrast to glucose, lipids and their hydrolysates poorly mix with the aqueous, osmolarity-carrying phase of the intestinal chyme. In the present study, a 3-kcal Intralipid infusate was mixed with distilled water to a 3-ml volume. The isocaloric glucose infusion (3-ml, 25% wt/vol glucose in water) had an estimated osmolarity of 1,390 mosmol/l, i.e., considerably larger than that of the Intralipid infusion (osmolarity between 105 and 210 mosmol/l, depending on the intestinal hydrolysis of the infusate). The third infusion contained the Intralipid infusate supplemented with 1.27 g lactulose to equalize osmolarity, volume, and small intestinally bioavailable calories with the 25% wt/vol glucose solution.
Data analysis.
Data were analyzed by use of SYSTAT software (Systat Software, San Jose, CA). To limit confounding by interanimal differences, ghrelin levels were expressed as percentages of preinfusion baseline values. Three parameters were calculated to describe the dynamics of ghrelin responses to gastrointestinal infusions. Ghrelin nadir (GN) is the lowest ghrelin level (expressed as a percentage of baseline) reached within the 5-h observation period following infusions. Time to ghrelin nadir (TGN) is the time required for ghrelin levels to reach their nadir. Decremental area under the curve (D-AUCtotal) was calculated as an overall index of the ghrelin response over the entire 5-h monitoring period and defined as 100% minus the area under the ghrelin curve, determined by the trapezoidal rule. Following our earlier approach of meal-induced ghrelin responses in humans (26) and post hoc inspection of our present data, we found that distinct and interpretable between-condition differences in ghrelin curves occurred mainly during the initial 3 h after infusion. To capture this pattern of responses, we calculated and analyzed the corresponding response parameter, the decremental area under the curve during the first 3 h (D-AUC3h). Effects of infusion type or dose on ghrelin-response parameters were tested by one-way ANOVA, followed by post hoc Bonferroni-corrected t-tests (departing from P value of 0.05 as significance threshold) to confirm pairwise, between-condition differences.
RESULTS
Study 1. Ghrelin suppression by duodenal infusions of agents with different absorption kinetics and metabolism at three osmolarity levels.
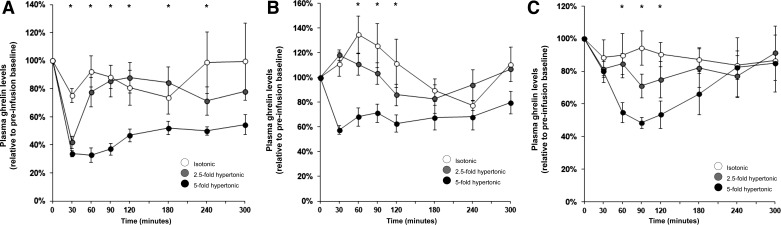
Dose-response effects per substance. Intestinal glucose infusions suppressed plasma ghrelin levels dose dependently, as reflected by main effects of glucose dose on GN, D-AUC3h, and D-AUCtotal (Table 1; Fig. 1A). Post hoc analyses confirmed different GN between the two hyperosmolar glucose doses vs. isotonic saline (see Fig. 3 for ghrelin responses to isotonic saline infusions) and different D-AUC3h across all glucose doses. D-AUCtotal was larger after the highest glucose dose vs. the lowest glucose dose and saline. Glucose dose did not affect TGN. These results confirm that duodenal glucose infusions, which represent an absorbable/metabolizable caloric load, insulinotropic stimulus, and intestinal osmotic stimulus, suppress ghrelin in a dose-dependent fashion.
Table 1.
Results of ANOVA statistical tests for studies 1, 2 and 3 showing F-statistics and significant differences (P < 0.05) for differences across conditions
| Ghrelin Nadir |
D-AUC3h |
D-AUCtotal |
||||
|---|---|---|---|---|---|---|
| Study 1: Infusions of glucose, 3-O-MG, and lactulose | ||||||
| Dose effects | ||||||
| Glucose | F(3,27) = 12 | P < 0.001 | F(3,27) = 18 | P < 0.001 | F(3,27) = 5.8 | P = 0.003 |
| 3-O-MG | F(3,25) = 3.5 | P = 0.03 | F(3,25) = 5.8 | P = 0.004 | NS | |
| Lactulose | F(3,30) = 3.4 | P = 0.03 | F(3,30) = 3.4 | P < 0.03 | NS | |
| Substance effects | ||||||
| Isotonic | NS | NS | NS | |||
| 2.5-fold hypertonic | F(2,18) = 4.5 | P = 0.02 | NS | NS | ||
| 5-fold hypertonic | F(3,23) = 6.3 | P = 0.003 | F(3,23) = 20 | P < 0.001 | F(3,23) = 3.5 | P = 0.03 |
| Study 2: Infusions of isotonic saline, 5-fold hypertonic saline, and 5-fold hypertonic saline + lidocaine | ||||||
| Between-condition effect | F(2,20) = 7.6 | P = 0.003 | F(2,20) = 4.3 | P = 0.03 | NS | |
| Study 3: Infusions of Intralipid, isocaloric glucose, and iso-osmotic Intralipid + lactulose | ||||||
| Between-condition effect | F(2,13) = 10 | P = 0.002 | F(2,13) = 34 | P < 0.001 | F(2,13) = 6.6 | P = 0.01 |
Data are shown for 3 parameters of plasma ghrelin responses to infusions: ghrelin nadir, i.e., ghrelin levels at maximum suppression and D-AUC3 h and D-AUCtot, i.e., the decremental area under the ghrelin curve for the 3-h and 5-h postinfusion time intervals, respectively. Post hoc test results with Bonferroni correction are described in the main text. Results for a fourth parameter, time to ghrelin nadir (TGN) were all nonsignificant and were omitted from the table. 3-O-MG, 3-O-methylglucose. NS, nonsignificance (P > 0.05).
Fig. 1.
Plasma ghrelin responses to duodenal infusions of isotonic, 2.5-fold, and 5-fold hypertonic solutions of the following different saccharides: glucose (intestinally absorbable and metabolizable) (A); 3-O-methylglucose (3-O-MG; intestinally absorbable, but unmetabolizable) (B); lactulose (intestinally unabsorbable) (C). All infusions started at t = 0 min and lasted for 10 min, thus delivering a 3-ml volume at a physiological rate. Analyses of variance showed that all substances suppressed ghrelin levels dose dependently in terms of ghrelin nadir (GN) and 3-h decremental area under the curve (D-AUC3h). At equivalent osmolarity, glucose suppressed ghrelin more strongly than did lactulose and 3-O-MG, as judged by GN and D-AUC3h. *Significant between-condition differences (ANOVA P < 0.05) for individual time points.
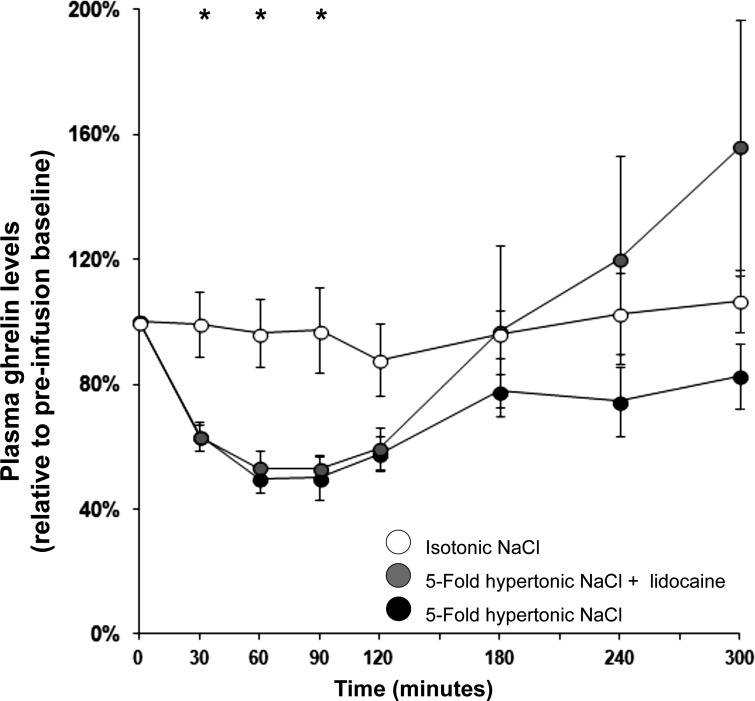
Fig. 3.
Plasma ghrelin responses to duodenal infusions of isotonic saline, 5-fold hypertonic saline, and 5-fold hypertonic saline pre- and coinfused with the local neural blocker lidocaine. Ghrelin was suppressed by the small intestinal presence of hypertonic saline, i.e., a nonglycemic, noninsulinotropic, noncaloric, nonfermentable hyperosmolar substance. Pre- and coinfusion of the neural blocker lidocaine did not attenuate ghrelin suppression. *Significant between-condition differences (ANOVA P < 0.05) for individual time points.
Hypertonic 3-O-MG infusions suppressed plasma ghrelin levels, as shown by main effects of dose on GN, D-AUC3h, and D-AUCtotal (Table 1; Fig. 1B). The highest-dose and saline condition significantly differed in post hoc tests. These results show that ghrelin can be suppressed by hyperosmolar 3-O-MG, i.e., a noncaloric, nonmetabolizable stimulus with similar intestinal absorption as glucose, but without stimulatory effect on insulin secretion.
Intestinal lactulose infusions suppressed ghrelin as well. There was a significant dose-effect on GN, D-AUC3h, but not D-AUCtotal, which underscores that ghrelin suppression occurred primarily during the initial hours after infusion (Table 1; Fig. 1C). Effects on D-AUC3h were further supported by a statistically significant difference between the highest lactulose dose and saline. No statistically significant effects were found for TGN. Thus ghrelin can be suppressed by hyperosmolar solutions of lactulose, which remains undigested and unabsorbed from the small intestine and which does not significantly stimulate blood glucose or insulin levels.
Furthermore, effects of infusions of glucose, 3-O-MG, and lactulose were compared at three osmotic levels: isotonic, 2.5-fold hypertonic, and 5-fold hypertonic. Also, hypertonic saline (of study 2) was included in this analysis.
At the 5-fold hyperosmolar level, solutions with different substances exerted different effects on GN, D-AUC3h, and D-AUCtotal (Table 1), reflecting more effective ghrelin suppression by hyperosmolar glucose than by hyperosmolar saline, lactulose, and 3-O-MG solutions. No differences were seen in TGN.
Across 2.5-fold hyperosmolar-infusion conditions, a main effect for infusion type was found for GN (Table 1), supported by a significantly larger ghrelin suppression after glucose than after 3-O-MG infusions. At this osmotic level, no infusion-type effects were found for D-AUC3h, D-AUCtotal, or TGN.
The lowest, isotonic dose of all three infusates suppressed ghrelin levels least effectively. Also, at this level of osmolarity, no differences among agents were found regarding GN, D-AUC3h, D-AUCtotal, or TGN.
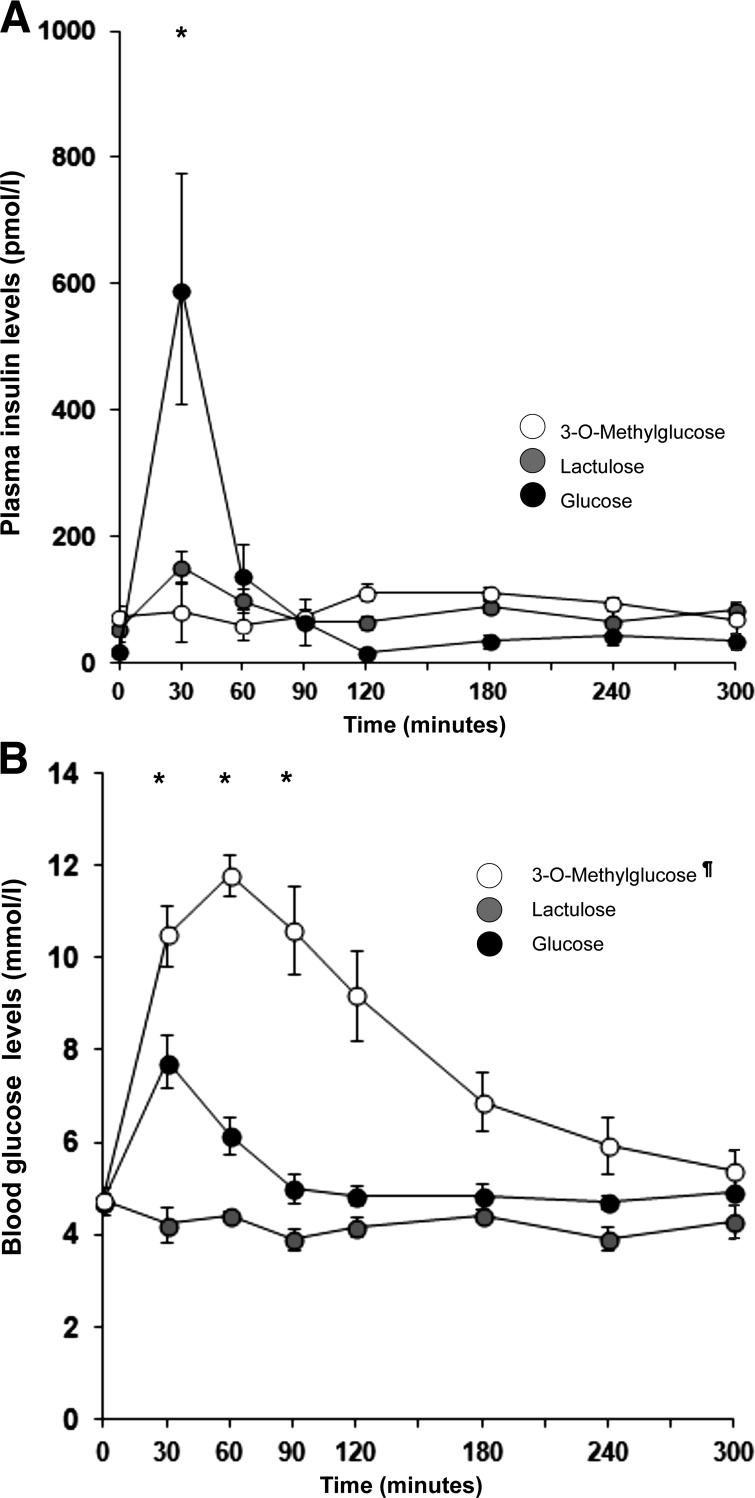
Plasma insulin and blood glucose responses were analyzed for the highest (5-fold hypertonic) doses of glucose, lactulose, and 3-O-MG. A main effect of infusion type [peak level; F(2,13) = 7.9, P = 0.006] revealed a much larger insulin response to glucose than to lactulose or 3-O-MG infusions (Fig. 2A). Glucose infusions raised insulin levels significantly (10-fold), whereas lactulose caused a minimal, transient rise in insulin, and 3-O-MG infusions caused no rise at all. Blood glucose levels increased more after glucose infusions than after lactulose infusions (peak rises of 3.1 and 0.2 mmol/l, respectively; P < 0.001; Fig. 2B). The measured, durable rise in blood glucose readings after 3-O-MG infusions is presumably an artifact caused by a combination of 1) cross-reactivity of circulating 3-O-MG with the glucose sensor of the measurement device and 2) an absent insulin response, so that the removal of 3-O-MG from the circulation is not supported by insulin-stimulated transport, as it is for glucose. Indeed, earlier studies using more specific assays for 3-O-MG showed unaltered blood glucose levels and near-complete recovery in urine of 3-O-MG after oral infusions (9, 13). The glucometer readings in our study do, however, imply that the small intestinal absorption of 3-O-MG is rapid and extensive and that hyperosmolarity-related ghrelin suppression does not require increases in plasma insulin or blood glucose.
Fig. 2.
Plasma-insulin (INS; A) and blood-glucose (BLG; B) responses to 3-ml duodenal infusions of hyperosmolar (5-fold hypertonic) glucose, lactulose, and 3-O-MG solutions. Only glucose infusions induced a distinct rise of INS and BLG, which demonstrates that significant ghrelin suppression by hypertonic substances does not require a rise in INS or BLG. ¶The measured rise in BLG after 3-O-MG infusion is presumed to be artifactual, caused by cross reactivity of 3-O-MG with the glucometer sensor. Previous studies with different assays indicate that oral doses of 3-O-MG do not raise BLG levels (9, 13). *Significant differences (P < 0.05) across conditions (Fig. 2A) or between lactulose vs. glucose (Fig. 2B) conditions for individual time points.
Study 2. Ghrelin suppression by duodenal infusions of iso- and hyperosmolar saline.
As shown in Fig. 3, hyperosmolar infusions of NaCl (4.5 wt/vol%) suppressed ghrelin more deeply than did isotonic (0.9 wt/vol%) NaCl, as reflected by main effects on GN and D-AUC3h (Table 1) and between-condition post hoc tests (P < 0.05). Effects on D-AUCtotal and TGN did not reach significance. Contrary to our expectation, ghrelin suppression by hyperosmolar NaCl was unattenuated by pre- and coinfusion of lidocaine.
Study 3. Ghrelin suppression by intestinal infusions of isocaloric lipids, glucose, and lipids rendered iso-osmotic to the glucose by supplementation with nonabsorbable lactulose.
To test whether the osmolarity of infusions could explain differences in ghrelin suppression by intralipid vs. glucose, we compared effects of three isovolumic (3-ml) intestinal infusions: 1) Intralipid (low-osmolar), 2) isocaloric glucose (25 wt/vol%; hyperosmolar), and 3) Intralipid rendered iso-osmotic to the 25% glucose solution by addition of the nonabsorbable osmotic agent lactulose.
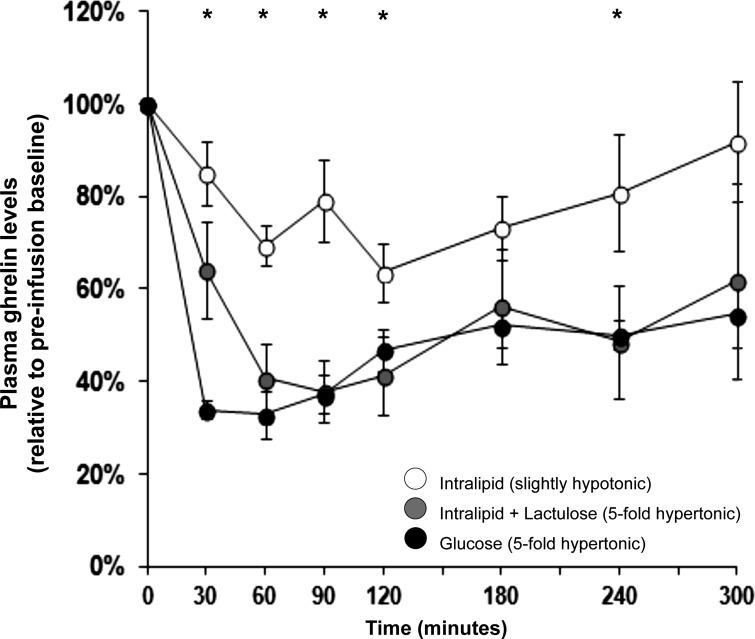
One-way ANOVAs over three conditions showed significance on GN, D-AUC3h, and D-AUCtotal (Table 1). Post hoc tests of these ghrelin-response parameters showed that the rank order of efficacy by which the three infusions suppressed ghrelin was Intralipid < Intralipid + lactulose = glucose (Fig. 4). Thus, although the weaker ghrelin suppression by Intralipid compared with isocaloric glucose infusions replicated our previously data (69), Intralipid could be made to suppress ghrelin as strongly as glucose by osmotic supplementation with lactulose.
Fig. 4.
Plasma ghrelin responses to duodenal infusions of mixed lipids (Intralipid; slightly hypotonic), isocaloric glucose (5-fold hypertonic), and Intralipid rendered iso-osmotic with the glucose infusion by supplementation with nonabsorbable lactulose. Ghrelin suppression as reflected in GN and D-AUC3h covaried with the osmolarity of the infusions. *Significant between-condition differences (ANOVA P < 0.05) for individual time points.
DISCUSSION
Here we demonstrate for the first time that circulating ghrelin levels are suppressed by increases in small intestinal osmolarity, even in the absence of nutrient absorption and metabolism, or insulin stimulation.
We examined the role of small intestinal osmolarity as a contributing factor in ghrelin suppression in three studies. Ghrelin levels were monitored in rats receiving duodenal infusions of various osmotically active agents at isotonic, 2.5-fold, and 5-fold hyperosmolar doses, thus covering osmolarities of a wide range of common foods (38, 90). Plasma ghrelin was suppressed dose dependently by glucose, as well as by hyperosmolar infusions of lactulose, 3-O-MG, and saline, substances that in contrast to glucose are noninsulinotropic and either noncaloric or unabsorbable by the small intestine. Small intestinal pre- and coinfusion of lidocaine did not abolish ghrelin suppression by hypertonic saline. Finally, supplementation of lipids, the weakest macronutrient ghrelin suppressors (26, 69), with nonabsorbable lactulose to make the infusate iso-osmotic with a glucose infusion, rendered the two solutions equivalent in capacity to suppress ghrelin.
Attention was paid to the physiological relevance of the infusions. The volume (3 ml) and caloric loads (≤3 kcal) of the infusions are at the lower end of the range of regular spontaneous liquid meals in rats (6), and the infusion rate (0.3 ml/min) was within the physiological range of gastric emptying after oral intake of liquid food (44). The osmolarities of the hyperosmolar infusions reflected those of sucrose-containing consumer foods, e.g., nondiet soda drinks and sherbet ice cream (36, 90). Although gastrointestinal mechanisms such as influx of fluids and gradual nutrient absorption probably moderated the postinfusion hyperosmolarity within the intestinal lumen, it is reasonable to assume that sustained small intestinal hyperosmolarity was achieved (37–39, 66, 82).
Because of the intestinal infusion site (2 cm distal to the pylorus) and the forward direction of peristaltic flow, contact of the infusates with gastric ghrelin cells probably played a negligible role in ghrelin suppression. Intestinal actions, e.g., via mechanosensitive, neurocrine, or hormonal pathways were likely involved, as discussed below (69, 92). This is underscored by the observation that for all infusions, osmolarity-related differences in ghrelin suppression occurred primarily during the first 3 h, i.e., the time window in which infused substances were present in the small intestine. Remarkably, in spite of their transient gastrointestinal presence, quickly absorbed substances (glucose, 3-O-MG, and hypertonic NaCl) suppressed ghrelin as effectively as did the unabsorbed disaccharide lactulose, which might also have additional action in the colon (see below).
Six decades ago, Hunt (40) postulated the existence of small intestinal osmoreceptors responsive to ingested food. Several physiological mechanisms described since then have likely contributed to ghrelin suppression in the present study. First, osmotically induced, early intracellular responses and longer-lasting gene transcription effects occur in enterocytes (2, 12, 51). Second, intestinally absorbed, ghrelin-suppressing agents, e.g., glucose and 3-O-MG are known to trigger the secretion of neurocrine and hormonal factors, e.g., CCK, GLP-1, and 5-HT, from enteroendocrine cells (30, 45, 72, 93). Third, osmotic influx of fluid into the intestinal lumen stimulates mechanosensors and activate enteric, vagal, and spinal neural pathways (19, 27, 32). We hypothesized partial neural mediation of hyperosmolarity-induced ghrelin suppression based on neurophysiological, neurosurgical, and pharmacological studies (e.g., Refs. 21, 39, 54, 93) and our prior observations that coinfusion with lidocaine attenuated ghrelin suppression by 2.5-fold hypertonic glucose for at least 1 h (68). However, unexpectedly, hypertonic-saline-induced ghrelin suppression was not attenuated by lidocaine. This may indicate that hyperosmolar-saline-induced neuronal signals originate predominantly from outer, serosal layers of the intestinal wall, beyond reach of luminally infused lidocaine (35, 50, 93). It should be noted that lidocaine's efficacy may have been weakened because of dilution by osmotically attracted fluid in the intestinal lumen and proceeding systemic absorption of the drug. Unfortunately, the use of still higher doses of lidocaine was unattainable, given the acute cardiorespiratory toxicity of lidocaine in rats (55). Selective blockade of a considerable number of neurochemical (e.g., gut-peptide and neurotransmitter) as well as neuroanatomical (vagal and nonvagal) pathways would be required to identify the key mechanisms in hyperosmolar ghrelin suppression. Such interventions, however, were beyond the scope of the present studies.
A notable implication of the present results is that noncaloric hyperosmolar substances may reduce plasma levels of ghrelin, thereby controlling a physiological signal of short-term hunger, long-term negative energy balance, and motivation to eat (14, 16, 67). This statement should be qualified for lactulose. Although lactulose does not provide detectable calories while in the small intestine, it can be fermented by resident cecal and colonic microbiota (77). A role of colonic fermentation is possible but probably not crucial in lactulose-induced ghrelin suppression because of the early onset of ghrelin suppression after duodenal infusion and the observed equal efficacy of hyperosmolar lactulose and unfermented agents NaCl and 3-O-MG to suppress ghrelin. Also, the literature is inconclusive as to whether colonically fermented, “prebiotic” saccharides enhance ghrelin suppression (compare Ref. 10 vs. Refs. 70 and 74).
Another implication of the present results is that neither meal-induced insulin secretion nor postprandial rises in blood glucose levels are required for ghrelin suppression. Although, in general, meal-induced ghrelin suppression coincides with the postmeal rise in insulin (17, 69), suppression of ghrelin by hypertonic lactulose and 3-O-MG in our rats occurred without a distinct rise in insulin, confirming results in diabetic humans and animal models (28, 62).
Overall, our present results indicate that either nutrient- or hyperosmolarity-related mechanisms are sufficient to suppress ghrelin, but that they may also act additively, or synergistically. Additive effects of nutrient and osmotic factors are illustrated by study 1, in which ghrelin was suppressed more strongly by osmotic and caloric glucose than by equally osmotic but noncaloric or lower-caloric infusions of 3-O-MG, sodium chloride, and lactulose. Another illustration, from study 3, is the enhancement of lipid-induced ghrelin suppression by osmotically active lactulose. Mechanistically, lactulose-induced osmotic flow into the intestinal lumen might have increased the volume of the coinfused lipid bolus and accelerated the spread of nutrients over a lengthier segment of the small intestine (1, 7), thus enhancing ghrelin suppression (52) analogously to mechanisms underlying satiation (56, 57).
The finding of ghrelin suppression by small intestinal hyperosmolarity may help explain a number of older observations and inform the design of dietary interventions.
First, our data indicate that the larger ghrelin suppression by intestinal glucose or amino acids compared with isocaloric/isovolumic lipids may be related to the greater osmolarity of the former two (smaller-sized and more hydrophilic) substances for any given number of calories.
Second, ghrelin suppression might be one of the mechanisms by which hyperosmolar foods reduce food intake in humans, rats, monkeys, sheep, cows and pigs (19, 29, 38, 49, 89) and increase thermogenesis in rats (66). Presumably, single hypertonic meals have a transient anorexic effect. The possibility, however, that sustained or repeated hyperosmolar intestinal stimuli could suppress food intake durably deserves more scrutiny. Some studies found that hyperosmolar diets acutely reduced energy intake without evoking a compensatory increase in meal frequency (19, 38). This would suggest an effect on meal-to-meal regulation, in keeping with ghrelin's role as a signal in meal initiation and motivation to eat (15, 67). However, other studies showed increases in meal frequency after initial hyperosmolarity-induced suppression of food intake (3, 59). Another intriguing long-term effect on feeding reported by Davis and Perez (20) is that intestinal hyperosmolarity may limit meal size on subsequent occasions through a flavor-conditioning process.
Third, hyperosmolarity-induced ghrelin suppression could play a role in patients who have undergone Roux-en-Y gastric bypass surgery (RYGB) as a treatment for severe obesity and/or Type 2 diabetes (79, 84). In the surgically rearranged gastrointestinal tract, ingested food can enter the proximal small intestine at an accelerated rate (87) because the pylorus, which naturally regulates gastric emptying, is bypassed. The ingested food remains segregated from bile and pancreatic digestive enzymes until it reaches the common intestinal limb downstream. The flow of undigested food, which has pleiotropic effects on the structure and metabolism of the intestinal wall (60, 76) and may support postprandial ghrelin suppression (47, 75, 79), can trigger hyperosmotic episodes, as evidenced by the increased prevalence of discomfort and dumping syndrome after RYGB (58, 80, 87). Dietary recommendations for RYGB-treated patients are therefore to moderate consumption of high-sugar foods (22, 71, 81). Hyperosmolarity-related ghrelin suppression might be a novel, contributing factor to reduced appetite after RYGB, as well as a compromise of the normal compensatory increase in ghrelin levels that occurs with diverse mechanisms of weight loss, but typically not after RYGB (18, 84).
Finally, enhancing small intestinal osmolarity might be used as a dietary strategy to limit ghrelin levels, e.g., to support attempts to lose weight. Prima facie, a sensible approach would be to increase dietary content of fibers, which by definition are poorly absorbable or nonabsorbable from the intestine. However, available data on fiber-rich diets are inconclusive, with several studies demonstrating ghrelin reduction (31, 42, 49, 64), but others not (46). A plausible reason for this is that ingredients classed as “fiber” have diverse gastrointestinal effects (viscosity enhancement, fermentation, increased water binding), that may or may not increase small intestinal osmolarity (53). In fact, two common properties of fibers may even weaken meal-related ghrelin suppression: 1) the sequestering of luminal nutrients by viscous fibers may blunt gastrointestinal hormonal responses (42), and 2) the large molecular weights of many dietary fibers [typically between 10 kDa and 5,000 kDa (41)] may impede a significant contribution to osmolarity. Note that, in the present study, the ghrelin-suppressant hyperosmotic agents (saccharides and sodium chloride) were of low molecular weight, i.e., below 1 kDa, with low viscosity and, except lactulose, nonfermentable. Further research should determine whether and how various fibers could be formulated to enhance small intestinal hyperosmolarity and ghrelin suppression, without causing discomfort.
A second dietary approach to stimulate small intestinal hyperosmolarity could be to enhance levels of macronutrient polymers (i.e., proteins and carbohydrates), which during digestion produce many transiently osmotically active oligo- and monomers (23, 65). One observation supporting this concept is that weight loss after a low-fat, high-carbohydrate diet is not associated with the usually observed compensatory rise in ghrelin (88).
In conclusion, we propose small intestinal hyperosmolarity as a novel factor contributing to postprandial ghrelin suppression. In support of this notion, plasma ghrelin levels in rats are suppressed by hyperosmolar duodenal infusions, delivered at physiological rate, of ingredients with different absorption routes, insulinotropic efficacies, metabolic fates, and calorie contents. Osmotically active agents may also interact with nutrients to enhance ghrelin suppression, as shown by combinations of lactulose and lipids. Hyperosmolarity-related ghrelin suppression may help explain several previously reported phenomena, including different macronutrient efficacies to suppress ghrelin, anorexigenic effects of hypertonic foods in various species, and altered ghrelin regulation after RYGB-induced weight loss.
Future analyses should identify the contribution of different neural and hormonal pathways and determine whether intestinal hyperosmolarity could serve as a dietary target to influence ghrelin levels, energy intake, and, ultimately, metabolic health.
GRANTS
The studies in this paper were supported by National Institute of Health grant NIDDK RO1 DK61516 awarded to D. E. Cummings.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.O. and D.E.C. conceived and designed the research; J.O., T.S.T., and R.S.F. performed experiments; J.O., T.S.T., R.S.F., and D.E.C. analyzed data; J.O. and D.E.C. interpreted results of experiments; J.O. and D.E.C. drafted manuscript; J.O., T.S.T., R.S.F., and D.E.C. edited and revised manuscript; J.O., T.S.T., R.S.F., and D.E.C. approved final version of manuscript.
REFERENCES
- 1.Adkin DA, Davis SS, Sparrow RA, Huckle PD, Phillips AJ, Wilding IR. The effects of pharmaceutical excipients on small intestinal transit. Br J Clin Pharmacol 39: 381–387, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfieri RR, Petronini PG. Hyperosmotic stress response: comparison with other cellular stresses. Pflügers Arch 454: 173–185, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Allen MS. Effects of diet on short-term regulation of feed intake by lactating dairy cattle. J Dairy Sci 83: 1598–1624, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Ariyasu H, Takaya K, Hosoda H, Iwakura H, Ebihara K, Mori K, Ogawa Y, Hosoda K, Akamizu T, Kojima M, Kangawa K, Nakao K. Delayed short-term secretory regulation of ghrelin in obese animals: evidenced by a specific RIA for the active form of ghrelin. Endocrinology 143: 3341–3350, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Billeaud C, Senterre J, Rigo J. Osmolality of the gastric and duodenal contents in low birth weight infants fed human milk or various formulae. Acta Paediatr Scand 71: 799–803, 1982 [DOI] [PubMed] [Google Scholar]
- 6.Blevins JE, Overduin J, Fuller JM, Cummings DE, Matsumoto K, Moralejo DH. Normal feeding and body weight in Fischer 344 rats lacking the cholecystokinin-1 receptor gene. Brain Res 1255: 98–112, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown NJ, Rumsey RD, Read NW. Adaptation of hydrogen analysis to measure stomach to caecum transit time in the rat. Gut 28: 849–854, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callahan HS, Cummings DE, Pepe MS, Breen PA, Matthys CC, Weigle DS. Postprandial suppression of plasma ghrelin level is proportional to ingested caloric load but does not predict intermeal interval in humans. J Clin Endocrinol Metab 89: 1319–1324, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Campbell PN, Young FG. Metabolic studies with 3-methyl glucose. I. Its fate in the animal body. Biochem J 52: 439–444, 1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cani PD, Dewever C, Delzenne NM. Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. Br J Nutr 92: 521–526, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Carter RR, Grovum WL. Factors affecting the voluntary intake of food by sheep. 5. The inhibitory effect of hypertonicity in the rumen. Br J Nutr 64: 285–299, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Chen M, Sastry SK, O'Connor KL. Src kinase pathway is involved in NFAT5-mediated S100A4 induction by hyperosmotic stress in colon cancer cells. Am J Physiol Cell Physiol 300: C1155–C1163, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Csáky TZ, Wilson JE. The fate of 3-O-14CH3-glucose in the rat. Biochim Biophys Acta 22: 185–186, 1956 [DOI] [PubMed] [Google Scholar]
- 14.Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav 89: 71–84, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am J Physiol Endocrinol Metab 287: E297–E304, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest 117: 13–23, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50: 1714–1719, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346: 1623–1630, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Davis JD, Collins BJ. Distention of the small intestine, satiety, and the control of food intake. Am J Clin Nutr 31: S255–S258, 1978 [DOI] [PubMed] [Google Scholar]
- 20.Davis JD, Perez MC. The acquired control of ingestive behavior in the rat by flavor-associated postingestional stimulation. Physiol Behav 54: 1221–1226, 1993 [DOI] [PubMed] [Google Scholar]
- 21.Davis JD, Smith GP, Kung TM. Abdominal vagotomy attenuates the inhibiting effect of mannitol on the ingestive behavior of rats. Behav Neurosci 109: 161–167, 1995 [PubMed] [Google Scholar]
- 22.Decker GA, Swain JM, Crowell MD, Scolapio JS. Gastrointestinal and nutritional complications after bariatric surgery. Am J Gastroenterol 102: 2571–2580; quiz 2581, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Ehrlein H, Stockmann A. Absorption of nutrients is only slightly reduced by supplementing enteral formulas with viscous fiber in miniature pigs. J Nutr 128: 2446–2455, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Ferraris RP, Yasharpour S, Lloyd KC, Mirzayan R, Diamond JM. Luminal glucose concentrations in the gut under normal conditions. Am J Physiol Gastrointest Liver Physiol 259: G822–G837, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Forbes JM, Barrio JP. Abdominal chemo- and mechanosensitivity in ruminants and its role in the control of food intake. Exp Physiol 77: 27–50, 1992 [DOI] [PubMed] [Google Scholar]
- 26.Foster-Schubert KE, Overduin J, Prudom CE, Liu J, Callahan HS, Gaylinn BD, Thorner MO, Cummings DE. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J Clin Endocrinol Metab 93: 1971–1979, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furness JB, Kunze WA, Clerc N. Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Physiol Gastrointest Liver Physiol 277: G922–G928, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Gelling RW, Overduin J, Morrison CD, Morton GJ, Frayo RS, Cummings DE, Schwartz MW. Effect of uncontrolled diabetes on plasma ghrelin concentrations and ghrelin-induced feeding. Endocrinology 145: 4575–4582, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Greenberg D. Intestinal satiety. In: Satiation: From Gut to Brain, edited by Smith GP. New York, Oxford: Oxford University Press, 1998, p. 40–70 [Google Scholar]
- 30.Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 52: 1147–1154, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Gruendel S, Garcia AL, Otto B, Mueller C, Steiniger J, Weickert MO, Speth M, Katz N, Koebnick C. Carob pulp preparation rich in insoluble dietary fiber and polyphenols enhances lipid oxidation and lowers postprandial acylated ghrelin in humans. J Nutr 136: 1533–1538, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Grundy D. What activates visceral afferents? Gut 53, Suppl 2: ii5–ii8, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guyton AC, Hall JE. Textbook of Medical Physiology (11th ed.). Philadelphia, PA: Saunders, 2006 [Google Scholar]
- 34.Hallback DA, Jodal M, Mannischeff M, Lundgren O. Tissue osmolality in intestinal villi of four mammals in vivo and in vitro. Acta Physiol Scand 143: 271–277, 1991 [DOI] [PubMed] [Google Scholar]
- 35.Hillsley K, Grundy D. Sensitivity to 5-hydroxytryptamine in different afferent subpopulations within mesenteric nerves supplying the rat jejunum. J Physiol 509: 717–727, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houpt KA. Gastrointestinal factors in hunger and satiety. Neurosci Biobehav Rev 6: 145–164, 1982 [DOI] [PubMed] [Google Scholar]
- 37.Houpt TR. Patterns of duodenal osmolality in young pigs fed solid food. Am J Physiol Regul Integr Comp Physiol 261: R569–R575, 1991 [DOI] [PubMed] [Google Scholar]
- 38.Houpt TR, Baldwin BA, Houpt KA. Effects of duodenal osmotic loads on spontaneous meals in pigs. Physiol Behav 30: 787–795, 1983 [DOI] [PubMed] [Google Scholar]
- 39.Houpt TR, Houpt KA, Swan AA. Duodenal osmoconcentration and food intake in pigs after ingestion of hypertonic nutrients. Am J Physiol Regul Integr Comp Physiol 245: R181–R189, 1983 [DOI] [PubMed] [Google Scholar]
- 40.Hunt JN. Some properties of an alimentary osmoreceptor mechanism. J Physiol 132: 267–288, 1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Izydorczyk M, Cui S, Wang Q. Polysaccharide gums: structures, functional properties and applications. In: Food Carbohydrates: Chemistry, Physical Properties, and Applications, edited by Cui S. Boca Raton, FL: Taylor & Francis, 2005, p. 263–307 [Google Scholar]
- 42.Juvonen KR, Purhonen AK, Salmenkallio-Marttila M, Lahteenmaki L, Laaksonen DE, Herzig KH, Uusitupa MI, Poutanen KS, Karhunen LJ. Viscosity of oat bran-enriched beverages influences gastrointestinal hormonal responses in healthy humans. J Nutr 139: 461–466, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Kalantzi L, Goumas K, Kalioras V, Abrahamsson B, Dressman JB, Reppas C. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm Res 23: 165–176, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Kaplan JM, Spector AC, Grill HJ. Dynamics of gastric emptying during and after stomach fill. Am J Physiol Regul Integr Comp Physiol 263: R813–R819, 1992 [DOI] [PubMed] [Google Scholar]
- 45.Kim M, Cooke HJ, Javed NH, Carey HV, Christofi F, Raybould HE. d-glucose releases 5-hydroxytryptamine from human BON cells as a model of enterochromaffin cells. Gastroenterology 121: 1400–1406, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Klosterbuer A, Greaves K, Slavin J. Fiber intake inconsistently alters gut hormone levels in humans following acute of chronic intake. J Food Res 1: 255–273, 2012 [Google Scholar]
- 47.Korner J, Bessler M, Cirilo LJ, Conwell IM, Daud A, Restuccia NL, Wardlaw SL. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab 90: 359–365, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Ladas SD, Isaacs PE, Sladen GE. Post-prandial changes of osmolality and electrolyte concentration in the upper jejunum of normal man. Digestion 26: 218–223, 1983 [DOI] [PubMed] [Google Scholar]
- 49.Lee YP, Mori TA, Sipsas S, Barden A, Puddey IB, Burke V, Hall RS, Hodgson JM. Lupin-enriched bread increases satiety and reduces energy intake acutely. Am J Clin Nutr 84: 975–980, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Li Y, Owyang C. Peptone stimulates CCK-releasing peptide secretion by activating intestinal submucosal cholinergic neurons. J Clin Invest 97: 1463–1470, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim CH, Bot AG, de Jonge HR, Tilly BC. Osmosignaling and volume regulation in intestinal epithelial cells. Methods Enzymol 428: 325–342, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Little TJ, Doran S, Meyer JH, Smout AJ, O'Donovan DG, Wu KL, Jones KL, Wishart J, Rayner CK, Horowitz M, Feinle-Bisset C. The release of GLP-1 and ghrelin, but not GIP and CCK, by glucose is dependent upon the length of small intestine exposed. Am J Physiol Endocrinol Metab 291: E647–E655, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Lyon MR, Kacinik V. Is there a place for dietary fiber supplements in weight management? Curr Obes Rep 1: 59–67, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mei N. Intestinal chemosensitivity. Physiol Rev 65: 211–237, 1985 [DOI] [PubMed] [Google Scholar]
- 55.Mets B, Janicki PK, James MF, Erskine R, Sasman B. Lidocaine and bupivacaine cardiorespiratory toxicity is additive: a study in rats. Anesth Analg 75: 611–614, 1992 [DOI] [PubMed] [Google Scholar]
- 56.Meyer JH, Hlinka M, Tabrizi Y, DiMaso N, Raybould HE. Chemical specificities and intestinal distributions of nutrient-driven satiety. Am J Physiol Regul Integr Comp Physiol 275: R1293–R1307, 1998 [DOI] [PubMed] [Google Scholar]
- 57.Meyer JH, Tabrizi Y, DiMaso N, Hlinka M, Raybould HE. Length of intestinal contact on nutrient-driven satiety. Am J Physiol Regul Integr Comp Physiol 275: R1308–R1319, 1998 [DOI] [PubMed] [Google Scholar]
- 58.Monteforte MJ, Turkelson CM. Bariatric surgery for morbid obesity. Obes Surg 10: 391–401, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Moran TH, McHugh PR. Distinctions among three sugars in their effects on gastric emptying and satiety. Am J Physiol Regul Integr Comp Physiol 241: R25–R30, 1981 [DOI] [PubMed] [Google Scholar]
- 60.Mumphrey MB, Patterson LM, Zheng H, Berthoud HR. Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterol Motil 25: e70–e79, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murakami N, Hayashida T, Kuroiwa T, Nakahara K, Ida T, Mondal MS, Nakazato M, Kojima M, Kangawa K. Role for central ghrelin in food intake and secretion profile of stomach ghrelin in rats. J Endocrinol 174: 283–288, 2002 [DOI] [PubMed] [Google Scholar]
- 62.Murdolo G, Lucidi P, Di Loreto C, Parlanti N, De Cicco A, Fatone C, Fanelli CG, Bolli GB, Santeusanio F, De Feo P. Insulin is required for prandial ghrelin suppression in humans. Diabetes 52: 2923–2927, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Nakai Y, Hosoda H, Nin K, Ooya C, Hayashi H, Akamizu T, Kangawa K. Plasma levels of active form of ghrelin during oral glucose tolerance test in patients with anorexia nervosa. Eur J Endocrinol 149: R1–R3, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Nedvidkova J, Krykorkova I, Bartak V, Papezova H, Gold PW, Alesci S, Pacak K. Loss of meal-induced decrease in plasma ghrelin levels in patients with anorexia nervosa. J Clin Endocrinol Metab 88: 1678–1682, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Noah L, Lecannu G, David A, Kozlowski F, Champ M. Digestion of starch and glycaemic response to mixed meals in pigs. Reprod Nutr Dev 39: 245–254, 1999 [DOI] [PubMed] [Google Scholar]
- 66.Osaka T, Kobayashi A, Inoue S. Thermogenesis induced by osmotic stimulation of the intestines in the rat. J Physiol 532: 261–269, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Overduin J, Figlewicz DP, Bennett-Jay J, Kittleson S, Cummings DE. Ghrelin increases the motivation to eat, but does not alter food palatability. Am J Physiol Regul Integr Comp Physiol 303: R259–R269, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Overduin J, Frayo R, Baskin D, Cummings D. Peripheral nervous contribution to nutrient-induced ghrelin regulation in rats. 34th Annual Meeting of the Society for Neuroscience, San Diego, CA, 2004 [Google Scholar]
- 69.Overduin J, Frayo RS, Grill HJ, Kaplan JM, Cummings DE. Role of the duodenum and macronutrient type in ghrelin regulation. Endocrinology 146: 845–850, 2005 [DOI] [PubMed] [Google Scholar]
- 70.Overduin J, Schoterman MH, Calame W, Schonewille AJ, Ten Bruggencate SJ. Dietary galacto-oligosaccharides and calcium: effects on energy intake, fat-pad weight and satiety-related, gastrointestinal hormones in rats. Br J Nutr 109: 1338–1348, 2013 [DOI] [PubMed] [Google Scholar]
- 71.Ponsky TA, Brody F, Pucci E. Alterations in gastrointestinal physiology after Roux-en-Y gastric bypass. J Am Coll Surg 201: 125–131, 2005 [DOI] [PubMed] [Google Scholar]
- 72.Reimann F, Gribble FM. Glucose-sensing in glucagon-like peptide-1-secreting cells. Diabetes 51: 2757–2763, 2002 [DOI] [PubMed] [Google Scholar]
- 73.Remie R, van Dongen JJ, Rensema JW, van Wunnik GHJ. General techniques. In: Manual of Microsurgery on the Laboratory Rat, edited by Remie R, van D, ongen JJ, Rensema JW, van W, unnik GHJ. Amsterdam: Elsevier, 1990, p. 81–156 [Google Scholar]
- 74.Robertson MD, Bickerton AS, Dennis AL, Vidal H, Frayn KN. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr 82: 559–567, 2005 [DOI] [PubMed] [Google Scholar]
- 75.Rodieux F, Giusti V, D'Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 16: 298–305, 2008 [DOI] [PubMed] [Google Scholar]
- 76.Saeidi N, Meoli L, Nestoridi E, Gupta NK, Kvas S, Kucharczyk J, Bonab AA, Fischman AJ, Yarmush ML, Stylopoulos N. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science 341: 406–410, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schiller LR. Review article: the therapy of constipation. Aliment Pharmacol Ther 15: 749–763, 2001 [DOI] [PubMed] [Google Scholar]
- 78.Schonemann NK, van der Burght M, Arendt-Nielsen L, Bjerring P. Onset and duration of hypoalgesia of lidocaine spray applied to oral mucosa—a dose response study. Acta Anaesthesiol Scand 36: 733–735, 1992 [DOI] [PubMed] [Google Scholar]
- 79.Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology 151: 1588–1597, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shuster MH, Vazquez JA. Nutritional concerns related to Roux-en-Y gastric bypass: what every clinician needs to know. Crit Care Nurs Q 28: 227–260; quiz 261–222, 2005 [DOI] [PubMed] [Google Scholar]
- 81.Tack J, Arts J, Caenepeel P, De Wulf D, Bisschops R. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat Rev Gastroenterol Hepatol 6: 583–590, 2009 [DOI] [PubMed] [Google Scholar]
- 82.Teichberg S, Lifshitz F, Pergolizzi R, Wapnir RA. Response of rat intestine to a hyperosmotic feeding. Pediatr Res 12: 720–725, 1978 [DOI] [PubMed] [Google Scholar]
- 83.Temple JG, Birch A, Shields R. Postprandial osmotic and fluid changes in the upper jejunum after truncal vagotomy and drainage in man. Gut 16: 957–960, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thaler JP, Cummings DE. Minireview: Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology 150: 2518–2525, 2009 [DOI] [PubMed] [Google Scholar]
- 85.Uhing MR, Kimura RE. Active transport of 3-O-methyl-glucose by the small intestine in chronically catheterized rats. J Clin Invest 95: 2799–2805, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van der Burght M, Schonemann NK, Laursen JK, Arendt-Nielsen L, Bjerring P. Onset and duration of hypoalgesia following application of lidocaine spray on genital mucosa. Acta Obstet Gynecol Scand 73: 809–811, 1994 [DOI] [PubMed] [Google Scholar]
- 87.Wang G, Agenor K, Pizot J, Kotler DP, Harel Y, Van Der Schueren BJ, Quercia I, McGinty J, Laferrere B. Accelerated gastric emptying but no carbohydrate malabsorption 1 year after gastric bypass surgery (GBP). Obes Surg 22: 1263–1267, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weigle DS, Cummings DE, Newby PD, Breen PA, Frayo RS, Matthys CC, Callahan HS, Purnell JQ. Roles of leptin and ghrelin in the loss of body weight caused by a low fat, high carbohydrate diet. J Clin Endocrinol Metab 88: 1577–1586, 2003 [DOI] [PubMed] [Google Scholar]
- 89.Weller A, Tsitolovskya L, Gispan IH, Smith GP. Ontogeny of hypertonic preabsorptive inhibitory control of intake in neonatal rats. Am J Physiol Regul Integr Comp Physiol 278: R44–R49, 2000 [DOI] [PubMed] [Google Scholar]
- 90.Wendland BE, Arbus GS. Oral fluid therapy: sodium and potassium content and osmolality of some commercial “clear” soups, juices and beverages. Can Med Assoc J 121: 564–566, 568, 571, 1979 [PMC free article] [PubMed] [Google Scholar]
- 91.Williams DL, Cummings DE, Grill HJ, Kaplan JM. Meal-related ghrelin suppression requires postgastric feedback. Endocrinology 144: 2765–2767, 2003 [DOI] [PubMed] [Google Scholar]
- 92.Williams DL, Grill HJ, Cummings DE, Kaplan JM. Vagotomy dissociates short- and long-term controls of circulating ghrelin. Endocrinology 144: 5184–5187, 2003 [DOI] [PubMed] [Google Scholar]
- 93.Zhu JX, Zhu XY, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol 530: 431–442, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]