Abstract
Bone marrow-derived mesenchymal stem cells (MSCs) sustain cancer cells by creating a microenvironment favorable for tumor growth. In particular, MSCs have been implicated in gastric cancer development. There is extensive evidence suggesting that Hedgehog signaling regulates tumor growth. However, very little is known regarding the precise roles of Hedgehog signaling and MSCs in tumor development within the stomach. The current study tests that hypothesis that Sonic Hedgehog (Shh), secreted from MSCs, provides a proliferative stimulus for the gastric epithelium in the presence of inflammation. Red fluorescent protein-expressing MSCs transformed in vitro (stMSCs) were transduced with lentiviral constructs containing a vector control (stMSCvect) or short hairpin RNA (shRNA) targeting the Shh gene (stMSCShhKO). Gastric submucosal transplantation of wild-type MSCs (wtMSCs), wild-type MSCs overexpressing Shh (wtMSCShh), stMSCvect, or stMSCShhKO cells in C57BL/6 control (BL/6) or gastrin-deficient (GKO) mice was performed and mice analyzed 30 and 60 days posttransplantation. Compared with BL/6 mice transplanted with wtMSCShh and stMSCvect cells, inflamed GKO mice developed aggressive gastric tumors. Tumor development was not observed in mouse stomachs transplanted with wtMSC or stMSCShhKO cells. Compared with stMSCShhKO-transplanted mice, within the inflamed GKO mouse stomach, Shh-expressing stMSCvect- and wtMSCShh-induced proliferation of CD44-positive cells. CD44-positive cells clustered in gland-like structures within the tumor stroma and were positive for Patched (Ptch) expression. We conclude that Shh, secreted from MSCs, provides a proliferative stimulus for the gastric epithelium that is associated with tumor development, a response that is sustained by chronic inflammation.
Keywords: inflammation, cancer stem cells, Sonic Hedgehog, CD44
bone marrow-derived mesenchymal stem cells (BM-MSCs) are known to sustain cancer cells by creating a microenvironment favorable for tumor growth and immunosuppression of the host immune response (13). Normally BM-MSCs do not exhibit malignant properties, but with long-term culture BM-MSCs “spontaneously transform” (stMSCs), can be propagated in vitro for extended periods, and develop a cancer-promoting phenotype (18). In vivo, in the inflamed mammary tissue and also in response to chronic gastritis BM-MSCs undergo mutations and acquire a cancer-promoting phenotype (9, 10, 13, 25). Bone marrow-derived cells, in particular cancer-associated fibroblasts generated from MSCs, are recruited to the gastric epithelium in response to chronic gastritis (14, 25). In vivo recruitment of bone marrow-derived cells occurs over several months with chronic inflammation (14, 25). In addition, the mechanism by which these cells contribute to tumor development is unclear. Therefore, the current study uses transplantation of an in vitro malignantly transformed mesenchymal stem cell line (stMSC) to accelerate cancer progression in a murine model that in vivo would occur over the course of 1 yr with chronic inflammation.
With recruitment to the gastric mucosa, BM-MSCs may sustain cancer cells by creating a microenvironment favorable for tumor growth and suppression of the host immune response (10, 13, 25). Our laboratory has demonstrated that gastritis promotes the in vivo activation of BM-MSCs to a phenotype reminiscent of a cancer-promoting cell (9, 10). In particular, this cancer-promoting phenotype is characterized by an IFNγ and transforming growth factor-β (TGF-β)-dependent induction of increased levels of MSC-derived Sonic Hedgehog (Shh) expression (9). There is a well-established role for ligand-dependent Shh signaling in the development of cancer in a number of tissues (3, 33). Earlier studies demonstrated that growth of epithelial digestive tract tumors that included esophagus, biliary tract, pancreas, and stomach is regulated by endogenous expression of Hedgehog (Hh) ligands such as Shh in an autocrine-juxtacrine manner (3). Since then, Yauch et al. (33) have shown that Hh ligands fail to activate signaling in tumor epithelial cells but rather promote signaling in the tumor stroma. The source and target of Shh ligand in the stomach during gastric cancer development are not well defined. The presence of MSCs in the tumor stroma could facilitate tumorigenesis via the secretion of a number of cancer promoting factors including Shh.
Biomarkers are comprised of cluster-of-differentiation (CD) genes and in particular CD44, a cell surface receptor for hyaluronate (1), is a known β-catenin target gene and is a cell surface adhesion molecule expressed on a variety of cells including gastric epithelial cells. Notably, CD44 has been identified as a gastric cancer stem cell marker and is expressed by resident tissue stem cells along the gastrointestinal tract (16, 27, 30). Typically, BM-MSCs physiologically lack expression of CD44 and acquire CD44 expression only after in vitro culture (24). However, in vivo within the tumor microenvironment, it is also possible that CD44 expression contributes to the differentiation of MSCs into an activated fibroblast phenotype (26). Thus it is unknown whether increased CD44 expression associated with gastric tumors is attributed directly to engraftment of recruited MSCs or the expansion of a resident epithelial stem cell population.
We hypothesize that cancer-promoting BM-MSCs promote the aberrant proliferation of the epithelium via induction of the Shh signaling pathway. To test this hypothesis, the current study uses the gastric submucosal transplantation of in vitro malignantly transformed stMSCs and wild-type (wt)MSCs, which overexpress Shh ligand, to accelerate cancer progression in a murine model. The current studies demonstrate that Shh, derived from MSCs, provides a proliferative stimulus for the gastric epithelium, a response that is sustained by chronic inflammation.
MATERIALS AND METHODS
Animals.
C57BL/6 (strain no. 000664, BL/6) and IRG (insulator/red/green, strain no. 008705) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Gastrin-deficient (GKO) mice backcrossed onto a C57BL/6 background were the gift of Dr. Linda Samuelson (University of Michigan) (12). All mouse studies were approved by the University of Cincinnati Institutional Animal Care and Use Committee that maintains an American Association of Assessment and Accreditation of Laboratory Animal Care Facility.
BM-MSC culture.
stMSCs used were isolated as previously described and transfected with a pDsRed-Hyg-C1 plasmid expressing red fluorescent protein (RFP) before being maintained in culture for 12 mo (17). wtMSCs were isolated by flushing 6- to 8-wk-old IRG femur and tibia and subsequent culture and passage of the adherent cell population. All cells were cultured using HyClone DMEM culture media supplemented with 15% fetal calf serum and 1% penicillin-streptomycin under normal conditions.
Shh knockdown by lentivirus-mediated short hairpin RNA using stMSCs.
Five MISSION lentiviral transduction particle clones containing different sequences of short hairpin RNA (shRNA) for Shh and a pLKO.1-puro component conferring puromycin resistance were used to transduce the RFP-tagged stMSCs according to the provided protocol (Sigma-Aldrich). Briefly, RFP-labeled stMSCs were incubated with lentiviral particles with ExpressMag magnetic beads (Sigma-Aldrich) on a magnet (Oz Biosciences Super Magnetic Plate, MF10000) for 15 min. Cells were removed from the magnet and incubated for a further 16 h after which time media were changed and replaced with puromycin selection culture media (DMEM culture media containing 10% fetal calf serum, 1% penicillin/streptomycin, and 10 μg/ml puromycin) for 7–10 days. The effective puromycin dose needed to eliminate nontransduced cells was determined previously through a puromycin kill curve that tested concentrations from 0 to 50 μg/ml (data not shown). The lowest concentration that killed all nontransduced cells at 3–4 days post treatment was established as 10 μg/ml. Transduced cells were denoted by a unique clone ID number with cell lines transduced with shRNA for Shh (stMSCShhKO) numbered 59–63. A pLKO.1-puro vector transduced RFP-MSC cell line (stMSCvect) served as the control for the endogenous level of Shh gene expression. Immunoprecipitation and Western blot analysis were used to confirm Shh knockdown and RFP expression.
Transfection of MSCs to overexpress Shh.
The plasmid (Origene CW101340) was combined with the Origene Turbofectin reagent at ratios of 0:1, 1:1, 2:1, 3:1, and 4:1 and incubated for 15 min at room temperature (RT). The transfection reagent was then added drop-wise to wtMSCs at 70% confluence and allowed to incubate for 24 h before cells were put under selection in normal media with puromycin. The effective puromycin dose needed to eliminate nontransfected cells was determined previously through a puromycin kill curve testing concentrations from 0 to 10 μg/ml (data not shown). The lowest concentration that killed all nontransfected cells at 3–4 days post treatment was established as 2 μg/ml.
Flow cytometry.
The Mouse Multipotent Mesenchymal Stromal Cell Marker Antibody Panel was used to label all MSC cell lines for Sca-1, CD106, CD105, CD73, CD29, and CD44 and the hematopoietic markers CD45 and CD11b (R&D Systems, SC018). Cells were suspended at a concentration of 1 × 106 cells/ml and 90 μl transferred to eppendorf tubes for staining. Cells were incubated with 10 μl of primary antibody for 30 min at 4°C, washed, and then incubated with a 1:100 dilution of Alexa Fluor anti-rat 488 for 30 min at 4°C. Cells were washed, suspended in fixation media (Invitrogen GAS001S5) for 15 min at RT, and then washed and resuspended in 5% BSA/PBS for analysis. To measure proliferation, the cells were stained with propidium iodide for 45 min for measurement of DNA content using the FACSCalibur system (Becton Dickinson). The measured values of peak fluorescence per total number of cells were obtained using the program CellQuest Pro (Becton Dickson). The percentage of cells from the population in each phase of the cell cycle was calculated from the peak fluorescence measurements through analysis with ModFit LT software. All analyses were performed on a BD FACSCalibur flow cytometer and analyzed using FlowJo Software, Version 9.
Gastric wall injection of bone marrow and stMSCs.
Injection of MSCs into the serosa of the gastric wall in C57BL/6 and GKO mice was performed according to a previously established protocol (22). Briefly, mice were anesthetized and prepared aseptically before the stomach was exteriorized. Cultured wtMSC, wtMSCShh, stMSCvect, or stMSCShhKO cells were trypsinized at 70–80% confluence and washed, and 2 × 106 cells were resuspended in 1 ml of sterile PBS. With the use of BD U-100 insulin syringes, 10-μl volumes of the cell suspension were injected at five locations along the greater curvature of the gastric wall, with sites within both the fundus and antrum of the stomach. The stomach was placed back inside the abdominal cavity, and the abdominal muscle layer and cutaneous layers were sutured using 4–0 Ethicon Vicryl sutures. Mice were injected with 200 μl of bromodeoxyuridine (BrdU) labeling reagent at least 24 h before euthanasia at 30 and 60 days postsurgery.
Epithelial-mesenchymal separation of gastric tissue.
A gastric biopsy was collected from each experimental group after the stomach was removed, opened along the greater curvature, and rinsed in PBS with forestomach discarded. Biopsies were collected in 10 ml HBSS without Ca2+ Mg2+ on ice and then transferred to 10 ml of 10 mM EDTA/HBSS, without Ca2+ and Mg2+ for 3 min at RT. After being washed with HBSS without Ca2+ and Mg2+, the biopsies were placed at 37°C in 10 ml of 1 mg/ml dispase/HBSS without Ca2+ and Mg2+ with agitation for 30 min. Mesenchymal and epithelial layers were separated manually using forceps and a dissecting scope. Tissue was harvested and homogenized in either Trizol or protein lysis buffer with protease inhibitor before further analysis.
Laser capture microdissection using paraffin-embedded gastric tissue.
Eight micron paraffin-embedded sections were stained using the ARCTURUS Paradise PLUS FFPE Laser Capture Microdissection (LCM) Staining Kit (Life Technologies) and dehydrated (30-s ethanol submersions followed by a 5-min submersion in xylene and a 5-min drying period) according to the manufacturer's protocol. Cells specific to each region were isolated using the Molecuar Devices/Arcturus VERITAS LCM System (model 704). The ultraviolet cutting laser was used to ablate the cells within the morpholgically distinct gland-like structures within the tumor stroma. These areas are representative of those staining positive for CD44 by immunofluorescence. An infrared laser power of 80 mW and pulse of 2,500 ms was then used to capture the cells. RNA was extracted using the Arcturus Paradise Plus RNA Extraction and Isolation Kit (Life Technologies) and analyzed by quantitative (q)RT-PCR.
RNA isolation and qRT-PCR.
Total RNA was isolated from cells or stomach tissue using Trizol reagent according to the manufacturers protocol (TriReagent; Molecular Research Center). For qRT-PCR using Taqman Reagents and the Applied Biosystems StepOne Real Time PCR System, the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) was used for cDNA synthesis following the recommended protocol. Analysis of Shh Pathway component expression was performed using the following TaqMan Gene Expression Assays according to the recommended protocol: Shh, 00436528_m1; Patched (Ptch), Mm00970977_m1; Gli1, Mm00494645_m1; Gli2, Mm01293111_m1; Gli3, Mm00492345_m1; Hedgehog interacting protein (Hhip), Mm00469580_m1; and hypoxanthine guanine phosphoribosyl transferase (HPRT), Mm00446968_m1. Fold change of gene expression was determined relative to wtMSCs for all cell lines using the method of Livak and Schmittgen (19a). For qRT-PCR of epithelial and mesenchymal gastric layers, RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit according to the manufacturer's protocol. Analysis of epithelium and mesenchyme was then performed using the following TaqMan Gene Expression Assays according to the recommended protocol: vimentin, Mm01333430_m1; Actg2a, Mm00725412_s1; and cytokeratin 20, Mm00508106_m1. All samples were run on a 1.5% agarose gel and bands visualized and quantified using the Bio-Rad Quantity One 1-D Analysis Software.
Western blot analysis.
Epithelial or mesenchymal gastric samples were loaded onto 4–20% Tris-Glycine Gradient Gels (Invitrogen) and run at 80 V, 3.5 h before transfer to nitrocellulose membranes (Whatman Protran, 0.45 μM) at 105 V, 1.5 h. Membranes were blocked for 1 h at RT using prepared KPL Detector Block Solution (Kirkegaard & Perry Laboratories). Membranes were incubated overnight at 4°C with a 1:200 dilution of Shh antibody (N-19, sc-1194; Santa Cruz Biotechnology) followed by a 1-h incubation with a 1:1000 dilution Alexa Fluor anti-goat 680 (Invitrogen). An antibody against GAPDH (Millipore, MAB374) and Alexa Fluor anti-mouse 680 were used at 1:1,000 for 1 h at RT. Blots were imaged using a scanning densitometer along with analysis software (Odyssey Infrared Imaging Software System).
Immunohistochemical and immunofluorescent staining.
Stomach sections spanning both the fundic and antral regions collected from experimental mice were fixed for 16 h in either 4% paraformaldehyde or Carnoy's Fixative (for BrdU-labeled samples), paraffin embedded, and sectioned at 5 μM with H&E staining. Prepared slides were deparaffinized with antigen retrieval performed by submerging in boiling solution (1:100 dilution, Antigen Unmasking Solution in dH2O; Vector Laboratories H-3300) for 10 min followed by 20 min at RT. For immunohistochemistry, endogenous peroxidase activity was blocked by incubating slides in 3% hydrogen peroxide/ethanol for an additional 30 min. Sections were then blocked with 5% BSA/PBS (for BrdU-labeled samples) or 20% normal serum for 20 min, RT. BrdU color development was performed according to manufacturer's protocol (Roche cat. no. 11 296 736 001).
The following primary antibodies were used: anti-BrdU (1:20, 30 min, 37°C; Roche cat. no. 11 296 736 001), anti-CD44 (1:100, 1 h, RT; Abcam ab25340), anti-CD44v6 (1:100, 16 h, RT; AbD Serotec MCA1967), anti-Patched G-19 (1:200, 16 h, 4°C; Santa Cruz sc-6149), and anti-mouse Ki67 (prediluted Ki67 from Ventana Medical Systems, Tucson, AZ, catalog no. 790–4286). Secondary antibodies were anti-mouse IgG (1:20, 30 min, 37°C; Roche cat. no. 11 296 736 001), biotin-conjugated rabbit anti-goat according to the manufacturer's protocol (VECTASTAIN Elite ABC Kit; Vector Laboratories), or Alexa Fluors at 1:100 for 1 h at RT (Invitrogen). For immunohistochemistry, sections were first incubated in ABC Reagent then DAB substrate for 5 min at RT (Vector Laboratories SK-4100). Immunohistochemical slides were dehydrated and mounted using Permount and images viewed and captured under light microscopy (Olympus BX60 with Diagnostic Instruments “Spot” Camera). Immunofluorescent slides were mounted using Vectashield HardSet Mounting Medium (Vectors Laboratories H-1400). Images were captured using a Zeiss LSM510 META confocal microscope.
In situ hybridization for the Y chromosome.
In situ hybridization for the Y chromosome was performed using a digoxigenin-labeled Y chromosome probe (Empire Genomics Buffalo, NY) on the Discovery XT machine (Ventana Medical Systems) using manufacturer's instructions. Briefly, deparaffinized sections were pretreated with proteinase and incubated with the digoxigenin-labeled Y chromosome probe at 37°C for 4 h in RiboHyb solution. After the incubation, slide were washed in SSC using manufacturer's recommendations. The slides were then incubated with Anti-DIG rabbit antibody (Sigma) for 12 min with detection using iVIEW Blue Plus Detection Kit (Ventana Medical Systems). Images were captured using an Olympus BX60 with Diagnostic Instruments “Spot” Camera.
Histology and histological scoring.
All tissue processing and staining were performed by McClinchey Histology Laboratories (Stockbridge, MI). Histological score was graded on parietal cell loss (atrophy), foveolar hyperplasia, and neutrophil and lymphocytic infiltration as previously performed (31). A score of 1, 2, 3, or 4 corresponds to 25, 26–50, 51–75, or 76–100% of the total mucosa affected, respectively.
Luminex-based multiplex assay.
Tissue was homogenized in PBS saline supplemented with protease inhibitor (Roche) and centrifuged for 30 min at 13,000 rpm at 4°C. Cytokine concentrations (IFNγ, IL-1β, TGF-β, and IL-6) in the tissue sample supernatants were determined by ELISA using Milliplex Multiplex kits (Millipore, Billerica, MA) according to manufacturer's protocol. Briefly, in a 96-well multiscreen filter plate, 25-μl sample in duplicate was incubated with 25 μl antibody-coated beads overnight at 4°C on a plate shaker. Plates were then washed two times on a vacuum apparatus and 25 μl of secondary antibody were added and incubated at RT for 1 h, shaking. Finally, 25 μl of streptavidin-RPE were added directly to the secondary antibody and incubated for 30 min at RT with shaking. Plates were then washed two more times, and 100 μl of sheath fluid were added. Plates were shaken for 5 min and then read using luminex technology on the Bio-Plex (Bio-Rad, Hercules, CA). Concentrations were calculated from standard curves using recombinant proteins and expressed in picograms per milliliters. The cytokine analysis was conducted by the Cytokine and Mediator Measurement Core Laboratory run by Dr. Marsha Wills-Karp (Cincinnati Children's Digestive Health Center).
Statistical analysis.
Results were analyzed by unpaired t-test or two-way ANOVA as appropriate using commercially available software (GraphPad Prism; GraphPad Software, San Diego, CA). A P value <0.05 was considered significant.
RESULTS
Loss of Shh within the gastric mucosa of GKO mice by 4 mo of age correlates with severe gastritis, parietal cell atrophy, and elevated inflammatory cytokines.
Mice that lack the gastrin gene (gastrin-deficient mice, GKO) develop severe inflammation and mucous gland metaplasia due to hypochlorhydria or loss of gastric acidity (38). As a consequence of the hypochlorhydria, bacterial overgrowth develops and initiates chronic gastritis (38). The subsequent morphological changes in the GKO gastric mucosa from the initiation of this inflammatory response to tumor development mimic Helicobacter pylori-infected human mucosa (5, 35, 38). Shh expression and processing occurs via a mechanism that is dependent on acid secretion (36, 37). Because GKO mice are hypochlorhydric from birth (12), it is not surprising that Shh expression and processing is lost in the stomach of these animals (36). This made the GKO model ideal for the study of the role of Shh derived from exogenous or recruited cell types in gastric pathology.
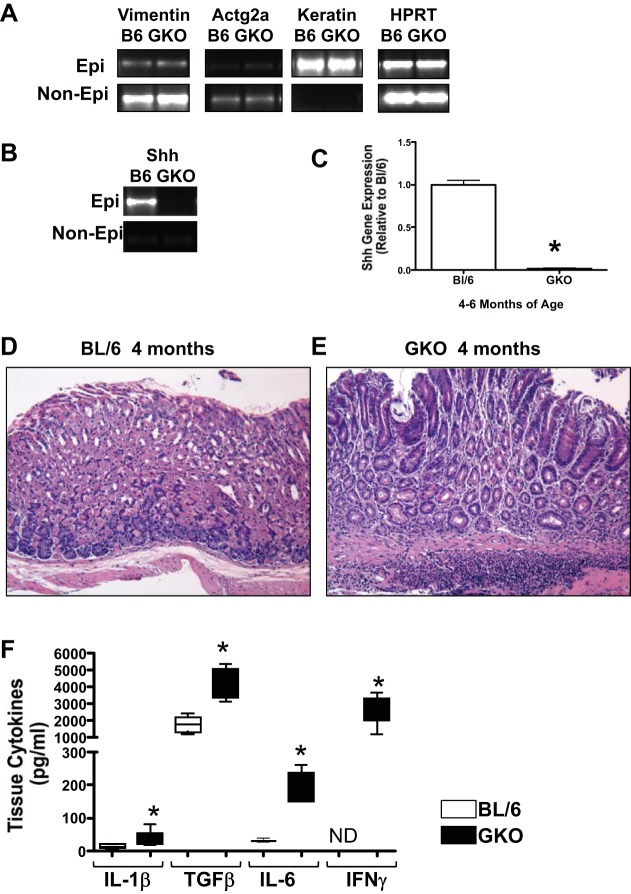
To quantify the expression of Shh within the stomachs of control (BL/6) and GKO mice, we used an enzymatic dissociation to separate the epithelium from the mesenchymal (nonepithelium) tissue. Purity of the epithelium and mesenchymal compartments were assessed based on the expression of mesenchymal (vimentin, Actg2a) and epithelial (cytokeratin 20, keratin) markers (Fig. 1A). Consistent with published findings, we observed a significant loss of Shh within the epithelium of the GKO mouse stomachs compared with the noninflamed BL/6 controls and undetectable levels of Shh expression within the mesenchymal compartment in both experimental groups (Fig. 1, B and C). Histological evaluation revealed significant atrophic gastritis within the stomachs of GKO (Fig. 1E) mice compared with BL/6 (Fig. 1D) control animals by 4 mo of age, coincident with the loss in Shh gene expression (Fig. 1C). Quantification of cytokine expression by Luminex-based multiplex assay revealed significantly elevated IL-1β, TGF-β, IL-6, and IFNγ concentrations within the stomach tissues collected from GKO mice compared with the BL/6 group (Fig. 1F). Collectively, these findings demonstrate that GKO mice exhibit significant gastric inflammation that is accompanied by the loss of Shh within the epithelium.
Fig. 1.
Sonic Hedgehog (Shh) and inflammatory cytokine expression in gastrin-deficient (GKO) mice. A: separated epithelium and nonepithelium from C57BL/6 (BL/6) and GKO mouse stomachs. Quantitative (q)RT-PCR analysis of the mesenchymal markers vimentin and Actg2 and the epithelial marker cytokeratin 20 (keratin). Representative of 5 independently dissociated stomachs. B: quantitative RT-PCR was performed on RNA prepared from stomachs dissociated from BL/6 and GKO mice. C: shown is the expression of Shh mRNA relative to the BL/6 group in epithelium. Data are expressed as the means ± SE. *P < 0.05 vs. BL/6 mice; n = 4 mice/group. Hematoxylin and eosin (H&E) stains of stomach sections collected from 4-mo-old BL/6 (D) and GKO mice (E). F: quantification of IL-1β, transforming growth factor-β (TGF-β), IL-6, and IFNγ concentrations by Luminex-based multiplex assay using stomach tissues collected from GKO mice compared with the BL/6 mice. Data are expressed as the means ± SE. *P < 0.05 vs. BL/6 mice; n = 4 mice/group.
Overexpression and silencing of Shh within BM-MSCs.
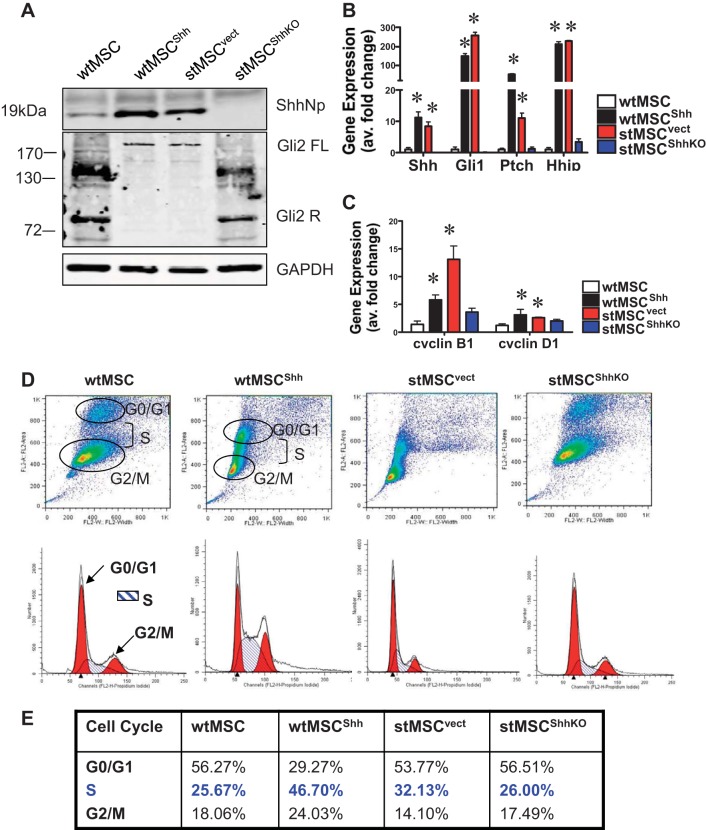
To study the effect of MSC-derived Hh signaling on the gastric epithelium, several MSC cell lines were established. As a model of malignant MSCs, an in vitro spontaneously transformed MSC cell line (stMSC) was used that express elevated levels of Shh as shown by immunoblot (Fig. 2A) (13, 17). A pLKO.1-puro base lentiviral vector expressing a short hairpin sequence against the transcript of the Shh gene was used. As a control the pLKO.1-puro base lentiviral vector was transduced into stMSCs (stMSCvect). Five lentiviral clones, each clone expressing a different short hairpin sequence, were analyzed (stMSCShhKO59-stMSCShhKO63). Knockdown of Shh was confirmed by Western blot analysis that showed undetectable levels of Shh protein expression in all clones compared with the untransduced cells. Clone stMSCShhKO59 was used for subsequent experiments. Expression of pDsRed-Hyg-C1 plasmid expressing RFP was confirmed by immunoblot using cell lysate collected from transduced stMSCs (data not shown).
Fig. 2.
Hh signaling and expression, and changes in proliferation in wild-type and spontaneously transformed mesenchymal stem cells (MSCs). A: immunoblot of Shh and Gli2 protein expression in wild-type MSCs (wtMSC), wild-type MSCs overexpressing Shh (wtMSCShh), spontaneously transformed MSCs (stMSCvect), and spontaneously transformed MSCs with Shh knockdown (stMSCShhKO) demonstrating elevated Hedgehog (Hh) signaling and expression. B: quantitative RT-PCR was performed on RNA prepared from wtMSC, wtMSCShh, stMSCvect, and stMSCShhKO cells. Shown is the expression of Shh, Gli1, Patched (Ptch), and Hedgehog interacting protein (Hhip) mRNA relative to the wtMSC group showing elevated Hh signaling. C: quantitative RT-PCR was performed on RNA prepared from wtMSC, wtMSCShh, stMSCvect, and stMSCShhKO cells. Shown is the elevated expression of cyclin B1 and cyclin D1 mRNA relative to the wtMSC group. *P < 0.05, compared with wtMSC group. D: cells were stained with propidium iodide and cell cycle analyzed by flow cytometry. The data were calculated from the cell cycle phase analysis by MODFIT software showing changes in distribution of G0/G1, S, and G2/M phases in wtMSC, wtMSCShh, stMSCvect, and stMSCShhKO cells. E: mean values from 3 individual experiments of wtMSC, wtMSCShh, stMSCvect, and stMSCShhKO cells.
The spontaneous transformation of the stMSC cell line in vitro produced a mutation in the p53 gene leading to altered expression (17). To control for changes in oncogene expression in the cell line that could affect interpretation of the role of Shh, MSCs were isolated and cultured using the IRG mouse model expressing RFP under the chicken β-actin promoter (wtMSC) (8). Freshly isolated and culture expanded MSCs maintained at low passage number do not exhibit malignant properties. These cells were then transfected to overexpress Shh at levels comparable to the stMSCvect cell line so any morphological changes or tumor development observed could be attributed to variation in the level of MSC-derived Shh (Fig. 2A). Figure 2A shows that there was a significant increase in Shh protein expression in the wtMSCShh and stMSCvect cells. In the presence of Shh and activation of Smoothened, Gli2 and Gli3 processing to the repressor form is blocked (7, 23, 29). Consistent with activation of Shh signaling in the wtMSCShh and stMSCvect cells, we observed a significant increase in the full-length Gli2 protein and a decrease in the repressor fragment by immunoblot (Fig. 2A).
The role of autocrine Hh signaling within each MSC cell line was also examined by qRT-PCR using primers specific for Shh, Ptch, Gli 1, and Hhip. With activation of the Hh signaling pathway the transcription of these components is also elevated (21, 28). Relative to the wtMSC, the gene expression of Shh, Gli1, Ptch, and Hhip were significantly increased in the wtMSCShh and stMSCvect cell lines while the stMSCShhKO cell line displayed no apparent Shh expression or pathway activation based on transcript levels. The expression of Ptch was increased in both the wtMSCShh and stMSCvect cell lines, with a 50-fold increase in gene transcript levels observed in the wtMSCShh and a two- to threefold increase in the stMSCvect cells (Fig. 2B).
The autocrine activation of Hh signaling within the MSC cell lines and the differential expression of the Hh pathway components suggested that the Hh pathway may mediate the transition of MSCs from a benign to malignant cell type. One way in which this could occur is through the induction of cell proliferation. Proteins involved the regulation of the cell cycle are among the targets of the transcription factor Gli1 (34). We observed a significant increase in cell cycle genes cyclin B1 and cyclin D1 in both the wtMSCShh and stMSCvect cells compared with wtMSC and stMSCShhKO cells (Fig. 2C). In addition, the percentage of the cell population in G0/G1, S, and G2/M was determined by flow cytometry in each cell line. The profile for the wtMSCShh and stMSCvect cell lines was very similar, showing an increase in the percentage of cells in S phase compared with wtMSCs, while knockdown of Shh gene expression in the stMSCShhKO cell line was able to revert levels of proliferation in the population back to wtMSC levels (Fig. 2, D and E). These data suggest that the Shh signaling pathway may be a key regulator of MSC phenotype through the induction of autocrine signaling and resultant increases in cell proliferation.
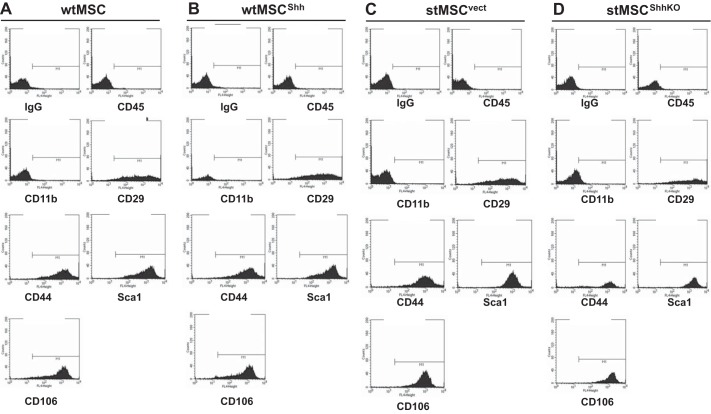
To verify that the knockdown of Shh in stMSCs did not alter the differentiation of the cells, an expression pattern for the classical CD markers (CD29, CD106, CD105, CD44, and CD73) and Sca-1 using flow cytometry was performed using cultured wtMSC (Fig. 3A), wtMSCShh (Fig. 3B), stMSCvect (Fig. 3C), and stMSCShhKO (Fig. 3D) cells. Additionally, all cell lines were negative for the hematopoietic markers CD45 and CD11b (Fig. 3). Collectively, these data suggest that knockdown of the Shh protein in stMSCs did not change the differentiation of these cells.
Fig. 3.
Characterization of the transduced wtMSC and stMSC cell lines. An expression pattern for the classical cluster-of-differentiation (CD) markers (CD29, CD106, CD105, CD44, CD73 CD45, and CD11b) and Sca-1 was analyzed using flow cytometry and wtMSC (A), wtMSCShh (B), stMSCvect (C), and stMSCShhKO cells (D).
MSC-induced tumor growth was mediated by Hh signaling in GKO mice.
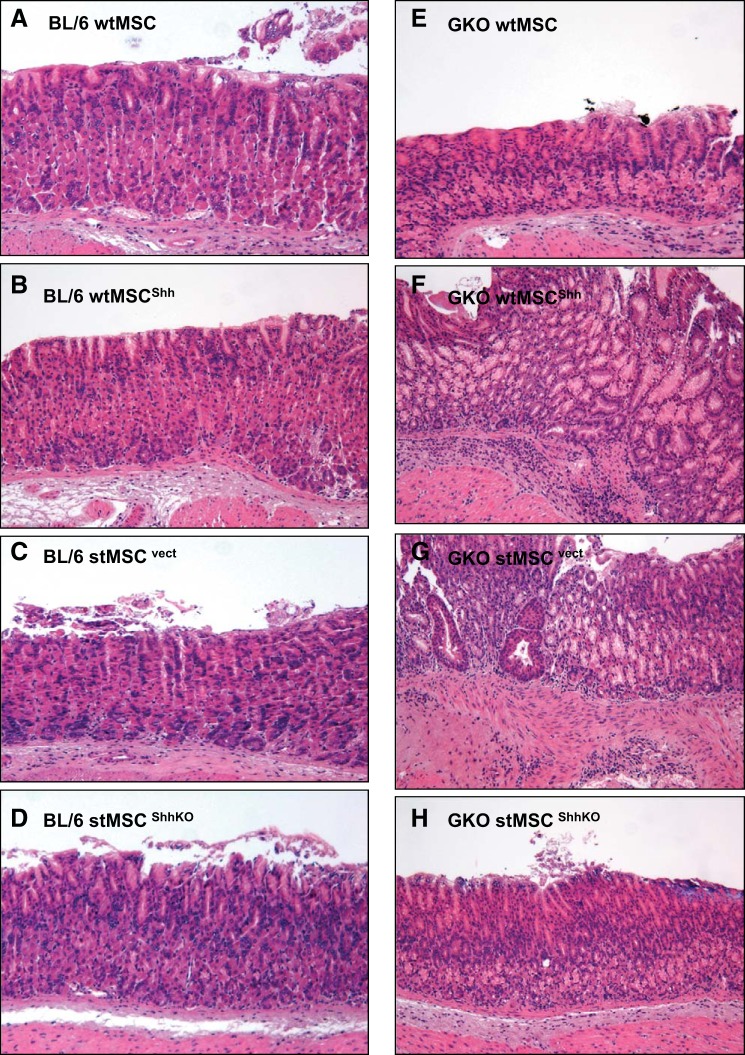
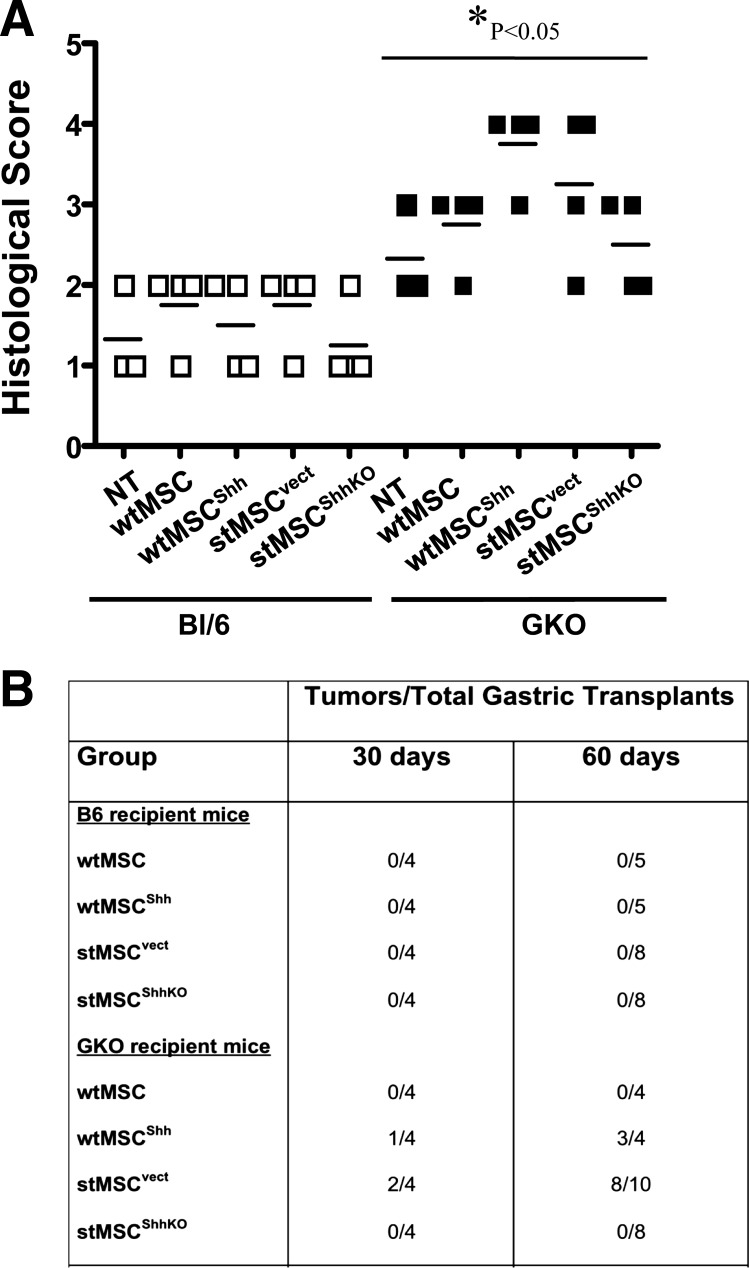
The GKO mouse model was ideal to study the action of MSC-derived Shh on the epithelium within a chronically inflamed stomach. wtMSC, wtMSCShh, stMSCvect, or stMSCShhKO cells were transplanted into the stomach wall of either BL/6 or GKO mice at 3 mo of age. After only 30 days posttransplantation with either wtMSCShh or stMSCvect cells, histological evaluation revealed that the GKO mice developed gastric tumors with highly dysplastic gastric glands and gastric lesions that were invasive into the submucosa (Fig. 4, F and G), compared with the normal stomachs of BL/6 mice transplanted with wtMSCShh (Fig. 4B) or stMSCvect cells (Fig. 4C). Although GKO mice transplanted with either wtMSC (Fig. 4E) or stMSCShhKO (Fig. 4H) cells exhibited atrophic gastritis typically observed in this animal model (35, 38), no dysplasia or neoplastic transformation was observed. Normal histology was observed in the stomachs of wtMSC (Fig. 4A)- and stMSCShhKO (Fig. 4D)-transplanted BL/6 animals. Histological scores documented the development of atrophic gastritis in the GKO mice and were significantly greater in the transplanted GKO mice compared with transplanted BL/6 mice (Fig. 5A). In addition, on average 75–80% of GKO mice transplanted with wtMSCShh or stMSCvect cells developed tumors 60 days postsurgery, while those mice transplanted with either wtMSCs or stMSCShhKO cells did not develop tumors (Fig. 5B).
Fig. 4.
Histological changes within the stomachs of BL/6 and GKO gastric transplanted mice. H&E stains of stomach sections collected from noninflamed BL/6 mice gastric transplanted with wtMSC (A), wtMSCShh (B), stMSCvect (C), and stMSCShhKO (D). H&E stains of stomach sections collected from inflamed GKO mice gastric transplanted with wtMSC (E), wtMSCShh (F), stMSCvect (G), and stMSCShhKO (H).
Fig. 5.
Histological evaluation of stomachs collected from BL/6 and GKO mice transplanted with wtMSCs and stMSCs. A: histological scores of stomach sections collected from BL/6 and GKO mice 30 days posttransplantation with wtMSC, wtMSCShh, stMSCvect, and stMSCShhKO. B: tumor incidence in BL/6 and GKO recipient mice transplanted with wtMSC, wtMSCShh, stMSCvect, and stMSCShhKO cells 30 and 60 days postsurgery.
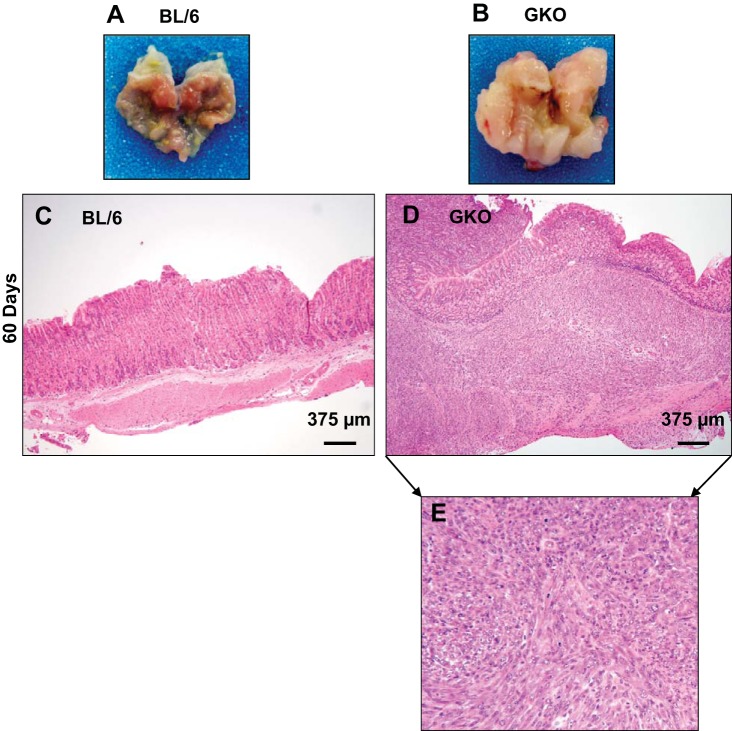
By 60 days posttransplantation, the GKO stMSCvect-transplanted group exhibited aggressive tumor development while the GKO stMSCShhKO-transplanted group still maintained gastric morphology consistent with the chronic inflammation that develops in this animal model (Fig. 6, A and B). The neoplasms that developed were characterized by a whirling pattern of pleomorphic spindle shaped cells with large nuclei and high mitotic activity infiltrating into the muscular wall and into the mucosa consistent with a sarcoma phenotype.
Fig. 6.
Morphological changes in stomachs collected from BL/6 and GKO mice 60 days after gastric transplants. Representative gross morphology of stomachs collected from BL/6 (A) and GKO (B) mice gastric transplanted with stMSCvect. H&E stains of stomach sections collected from noninflamed BL/6 (C) and GKO (D) mice gastric transplanted with stMSCvect cells. Higher magnification of stomach section shown in D is shown in E.
MSC-derived Shh promotes the proliferation of epithelial and mesenchymal CD44-positive cell populations.
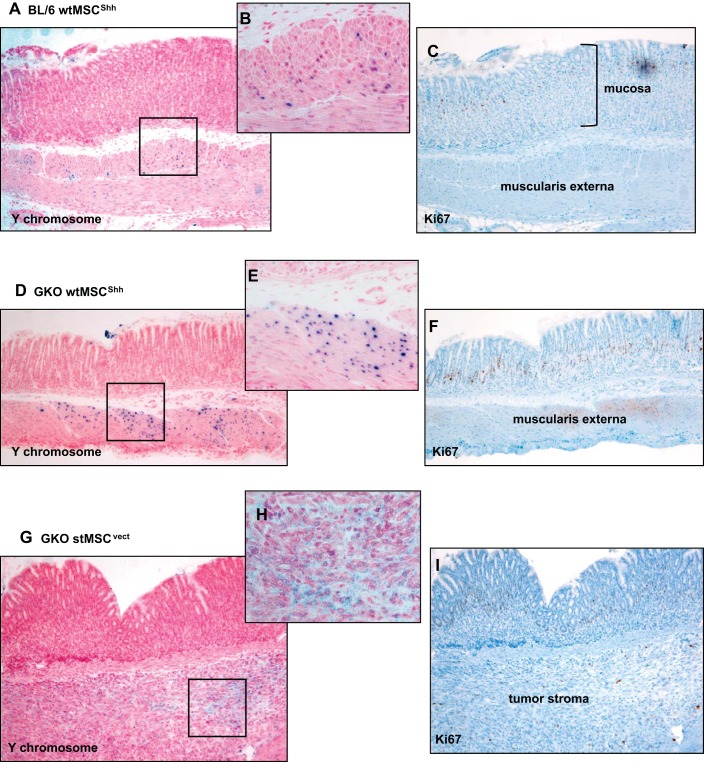
Figure 2 demonstrates the autocrine pro-proliferative effect of Hh signaling on MSCs. Therefore, to identify whether the tumors were derived from the engraftment of transplanted MSCs, female recipient BL/6 or GKO mice were gastric transplanted with MSCs derived from male mice and in situ hybridization for the Y chromosome was performed (Fig. 7, A–H). In all groups of BL/6 mice, Y chromosome-positive MSCs engrafted within the muscularis mucosa (shown in the representative micrographs in Fig. 7, A and B). In GKO mice transplanted with either wtMSCShh or stMSCvect, Y chromosome-positive cells were localized within the muscularis mucosa 30 days posttransplantation (shown in the representative micrographs in Fig. 7, D and E) and within the tumor stroma 60 days posttransplantation (shown in the representative micrographs in Fig. 7, G and H). It was obvious in both the BL/6 (Fig. 7C) and GKO (Fig. 7, F and I) mice that proliferating Ki67-positive cells were present within the epithelium and were negative for the Y chromosome. These data demonstrated that once engrafted within the mucosa, MSCs may have lost their proliferative capacity that was observed in vitro. Therefore, tumor development observed was like to not be induced by the engraftment of proliferating MSCs.
Fig. 7.
In situ hybridization for Y chromosome and Ki67 immunostaining of stomachs collected from BL/6 and GKO recipient mice. A: representative images of in situ hybridization for Y chromosome using stomach sections collected from BL/6 recipient mice transplanted with male wtMSCShh cells. Highlighted area in A is shown as a higher magnification in B. C: Ki67 immunostaining of BL/6 recipient mice transplanted with wtMSCShh cells. D: representative images of in situ hybridization for Y chromosome using stomach sections collected from GKO recipient mice transplanted with male wtMSCShh cells. Highlighted area in D is shown as a higher magnification in E. F: Ki67 immunostaining of BL/6 recipient mice transplanted with wtMSCShh cells. G: representative images of in situ hybridization for Y chromosome using stomach sections collected from GKO recipient mice transplanted with male stMSCvect cells. Highlighted area in D is shown as a higher magnification in H. I: Ki67 immunostaining of BL/6 recipient mice transplanted with stMSCvect cells.
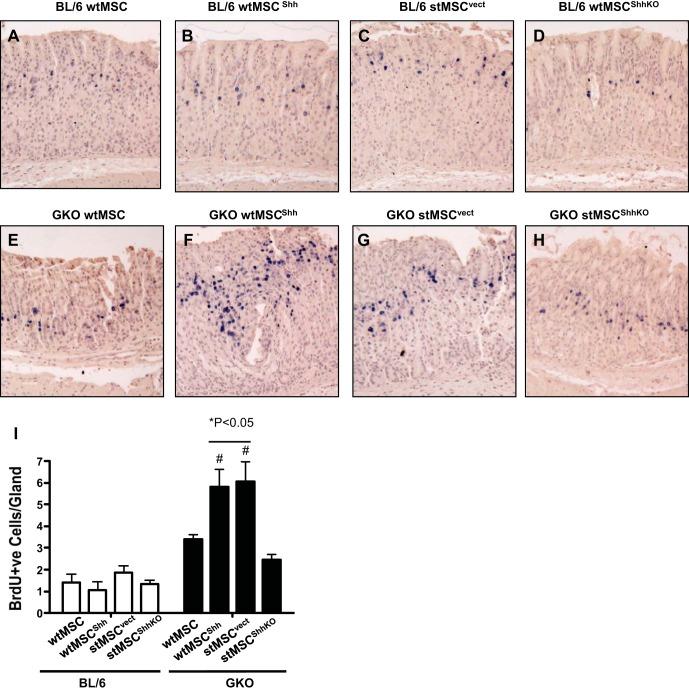
In gastric biopsies obtained from GKO mice transplanted with stMSCvect cells, a consistent increase in epithelial cell proliferation overlapping with the isthmus region of gastric glands was observed at 30 days compared with other experimental groups (Fig. 8, A–I). In the 30-day time course, an expansion in this same region was observed in GKO stomachs with transplantation of wtMSCShh cells and was lost with transplantation of wtMSC and stMSCShhKO cells. These findings suggest the MSC-derived Shh promotes proliferation, and this response is indicates that Shh from MSCs promotes epithelial proliferation (Fig. 8, A–I).
Fig. 8.
Epithelial cell proliferation in mice gastric transplanted with wtMSC, wtMSCShh, stMSCvect, or stMSCShhKO cells. Stomachs were isolated from mice 60 days after cell transplants. Sections were stained with an anti-bromodeoxyuridine (anti-BrdU, blue) antibody. Stomachs collected from BL/6 mice transplanted with wtMSC (A), wtMSCShh (B), stMSCvect (C), and stMSCShhKO (D). Stomach sections collected from inflamed GKO mice gastric transplanted with wtMSC (E), wtMSCShh (F), stMSCvect (G), and stMSCShhKO (H). I: quantification of stomach sections immunostained using an anti-BrdU antibody in gastric transplanted BL/6 and GKO mice. *P < 0.05, compared with BL/6 group; #P < 0.05, compared with GKO mice transplanted with stMSC cells; n = 4–6 mice per group.
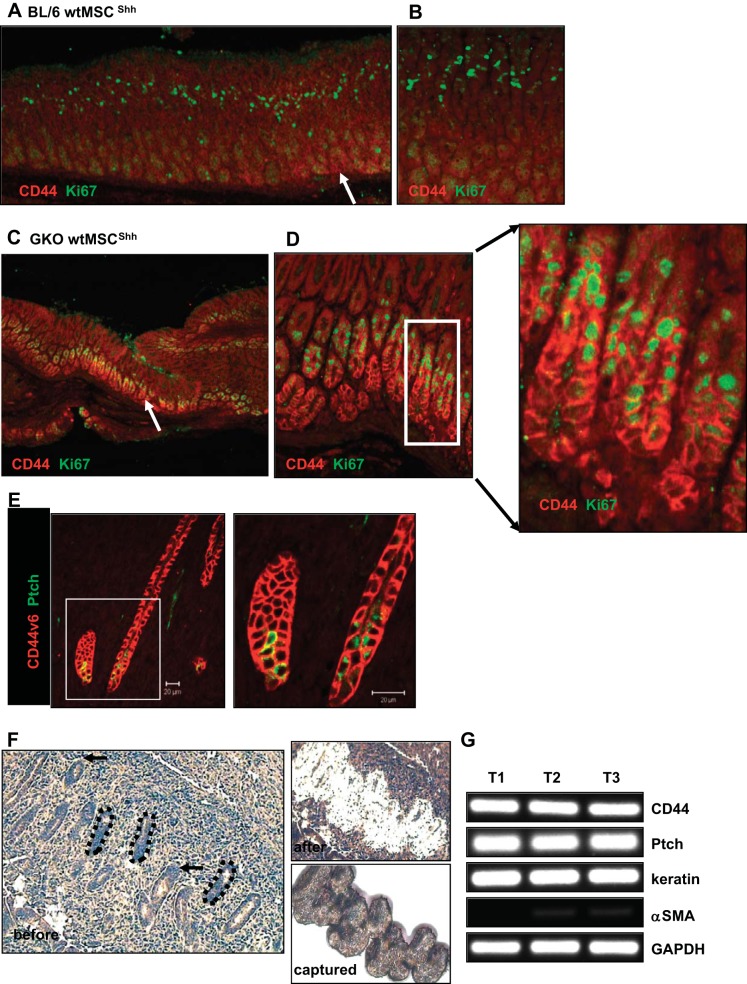
CD44 has been identified as a marker of gastric cancer stem cells, and increased expression of CD44 in response to H. pylori infection is associated with gastric carcinoma (11, 15, 27). Recently an expansion of CD44-positive undifferentiated cells has been reported in response to tamoxifen-induced parietal cell atrophy and Helicobacter infection (16). Based on this, gastric sections from BL/6 and GKO mice were stained using antibodies against CD44 and Ki67 (Fig. 9, A–D). There was an expansion of CD44-positive proliferating glands within the stomachs of GKO mice transplanted with either wtMSCShh cells (Fig. 9, C and D) compared with BL mice transplanted with wtMSCShh cells (Fig. 9, A and B). The expansion of proliferating CD44-positive cells within the GKO mouse stomachs was not observed in those animals transplanted with either wtMSC or stMSCShhKO cells (data not shown).
Fig. 9.
Expression of CD44 and Ki67 in stomachs collected from BL/6 and GKO recipient mice. Representative images of immunostaining using antibodies specific for CD44 (red) and Ki67 (green) using stomach sections collected from BL/6 recipient mice (A) transplanted with wtMSCShh cells (higher magnification in B) and GKO recipient mice (C) transplanted with wtMSCShh cells (higher magnification in D). Arrows show CD44-positive cells. E: immunostaining of CD44v6 (red) and Ptch (green) in GKO recipient mice transplanted with stMSCvect cells. F: paraffin-embedded sections collected from gland-like epithelium within the tumor stroma were stained and the ultraviolet cutting laser was used to first ablate the cells surrounding the region (before and after). An infrared laser was then used to capture the cells from surface pit, neck, and base epithelium (captured). G: gene expression of CD44, Ptch, cytokeratin 20 (keratin), and α-smooth muscle actin (α-SMA) was analyzed by qRT-PCR using RNA collected by Laser Capture Microdissection from 3 representative tumors (T1, T2, and T3) in GKO recipient mice transplanted with stMSCvect cells.
A number of variant isoforms of CD44 are expressed and can serve as a prognostic factor and unique biomarker of gastric cancer development (6). CD44 and the expression of the variant isoforoms, in particular CD44v6, are closely related to Helicobacter infection and gastric cancer progression (6, 11, 15). Therefore, stomach sections collected from GKO mice transplanted with either wtMSCShh or stMSCvect cells were immunostained using an antibody against CD44v6 and Hh receptor Ptch 60 days posttransplantation. CD44v6 was in fact detected only in tumor bearing GKO wtMSCShh or stMSCvect stomachs. CD44v6 staining cells clustered in gland-like structures within the epithelium and tumor stroma and were positive for Ptch (Fig. 9E). Expression of Ptch was confirmed by extracting RNA from these morphologically distinct gland-like structures captured from within the tumor stroma using LCM (Fig. 9F). The captured cells from three representative tumors expressed both CD44 and Ptch (Fig. 9G). While these cells expressed epithelial cell marker keratin, expression of the mesenchymal marker α-smooth muscle actin was expressed at low levels, suggesting that the CD44 ± Ptch ± cells were captured from epithelial glands. Collectively, these data suggest that Hh signaling derived from MSCs may induce the proliferation of CD44-positive cells within the gastric epithelium and tumor stroma.
DISCUSSION
Although it is well documented that MSCs migrate to the site of gastric inflammation (9, 10, 14, 25), the role of these cells in cancer development is largely unknown as is the effect they have on the gastric epithelium. What is known is that MSCs may contribute to an environment that is compatible with the development of gastric cancer by providing an immunosuppressive response to H. pylori infection (19). Alternatively, MSCs are reported to serve as the source of cancer-associated fibroblasts (25). Here we report two major findings: 1) Shh provides a proliferative stimulus for MSCs, and 2) MSC-derived Shh may provide a proliferative stimulus within the gastric epithelium associated with tumor development.
Our studies demonstrate that Shh provides a proliferative stimulus for MSCs. The role of autocrine Hh signaling within MSCs was demonstrated by elevated Ptch, Hhip, Gli1, and Gli2 expression with the overexpression of Shh ligand observed in both wtMSCShh and stMSCvect cells. The autocrine activation of Hh signaling within these MSC cell lines suggested that the Hh pathway is likely to mediate the transition of MSCs from a benign to malignant cell type. There is strong evidence that gastric cancer originates from transformed BM-MSCs in human (4, 32) and mouse (14), yet the mechanism of such an in vivo transformation is largely unclear. We proposed that one way in which this could occur is through the induction of cell proliferation. Proteins involved in cell cycle, such as cyclin D1, are among the targets of the transcription factor Gli1 (39). Indeed, cell cycle analysis showed that wtMSCShh and stMSCvect cell lines exhibited an increase in the percentage of cells in S phase compared with wtMSCs and stMSCShhKO cells. In vitro studies show that TGF-β, often secreted during chronic gastritis, may contribute to BM-MSC transformation (25). In support of these findings we have observed that Shh pathway activity within BM-MSCs is downstream of the TGF-β signaling (9). Therefore, Shh signaling may be a key regulator of MSC phenotype in response to chronic inflammation through the induction of autocrine signaling and resultant increases in cell proliferation (Fig. 10).
Fig. 10.
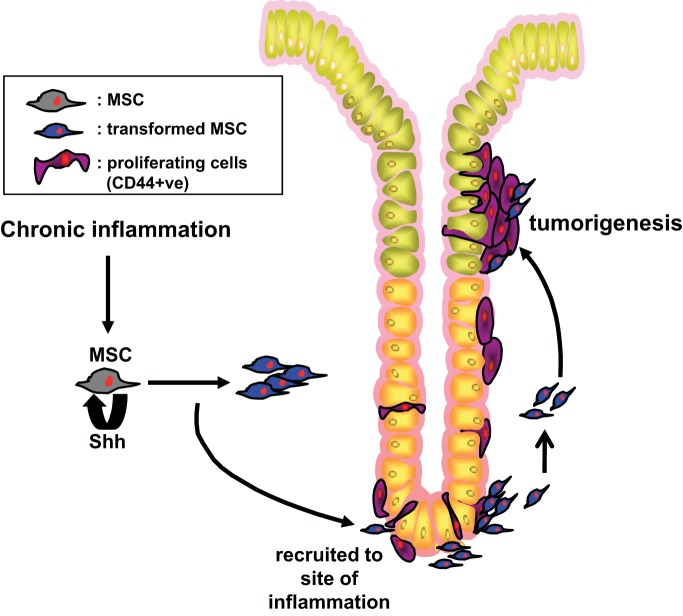
Proposed model for tumor development in the stomach in response to MSC-derived Shh. Aberrant proliferation of MSCs is mediated by autocrine Shh signaling. MSCs may then promote the proliferation of CD44-positive cells, a potential gastric epithelial stem cell population via a mechanism that is dependent on Shh and inflammation.
MSC-derived Shh also provides a proliferative stimulus within the gastric epithelium that may be associated with tumor development, a response that is sustained by chronic inflammation. The active proliferation observed in MSCs overexpressing Shh was not maintained after their transplantation and engraftment within the stomach. Rather, Shh-expressing stMSC and wtMSCShh cells promoted epithelial cell proliferation within the stomach in response to chronic inflammation. A significant expansion in CD44-positive proliferating cells was observed in response to chronic inflammation within the GKO stomach in the presence of Shh-expressing wtMSCShh and stMSCvect cells. Further analysis of these CD44-positive cells revealed the strong expression of Hh receptor Ptch, thus providing evidence that these cells are Hh responsive. CD44 is a cell surface adhesion molecule expressed on a variety of cells including gastric epithelial cells. Recently, CD44 has been identified as a gastric cancer stem cell marker (15, 27) and our data may suggest a possible interaction between recruited MSCs and CD44-positive cells. In support of this notion, CD44-positive cells isolated from human gastric cancer cell lines promote malignancy and form differentiated gastric cancer in SCID mice (27). Moreover, atrophy of the acid-secreting parietal cell also induces an expansion in a population of CD44-positive undifferentiated cells in the gastric isthmus where stem cells normally reside (16). Indeed, the striking observation in the GKO mice transplanted with wtMSCShh and stMSCvect cells emphasizes the role of the MSCs as a pro-proliferative and tumor-promoting cell. Here we demonstrate that MSCs may promote the proliferation of CD44-positive cells, a potential gastric epithelial stem cell population, via a mechanism that is dependent on Shh and inflammation (Fig. 10).
To identify whether the tumors were derived from the engraftment of transplanted MSCs, female recipient BL/6 or GKO mice were gastric transplanted with MSCs derived from male mice and in situ hybridization for the Y chromosome was performed. It was obvious from our data that the proliferating cells were confined to the epithelium, while the Y chromosome positive cells were observed either within the muscularis mucosa or within the tumor stroma. Therefore, once engrafted within the mucosa, MSCs do not proliferate suggesting that tumor development was not induced by the engraftment and expansion of proliferating MSCs.
The current study uses gastric serosal transplantation of stMSCs and wtMSCs to study the role of MSC-derived Shh on the gastric epithelial cell proliferation. The use of an animal model to examine the role of MSC-derived Shh allows for contextual examination of the role of Hh in vivo. Site delivery of the cells allows for a controlled setting to understand the direct role of the cells on gastric epithelial cell proliferation. In addition, transplantation of MSCs within the inflamed gastric mucosa accelerated cancer progression in a murine model that in vivo would occur over the course of 1 yr with chronic inflammation (35). While our data may be the first indication of an interaction between MSCs expressing Shh and CD44-positive cells, an important limitation is that the role of endogenous MSCs in the development of gastric cancer was not addressed in this model. Bolus dosing of cells does not recapitulate the neoplastic process. An alternative approach may be to study this in vitro using a coculture system between MSCs and newly developed gastric organoids cultures (2, 20).
In summary, to establish that MSC-derived Shh acts as a pro-proliferative stimulus on the gastric epithelium, we generated several BM-MSC cell lines expressing variable levels of Shh ligand. Both the wtBM-MSCs and the stMSCs with low levels of Shh expression failed to induce epithelial proliferation and tumor development. In contrast, the MSC cell lines expressing high Shh ligand expression induced proliferation of CD44-positive epithelial cells and neoplastic changes within the epithelium. Therefore, we conclude that MSC-derived Shh within the environment of the inflamed gastric mucosa may be sufficient to promote tumor development by inducing the proliferation of CD44-positive cells. CD44 has been identified as a putative gastric epithelial stem cell marker (16). In addition, CD44 is identified as a gastric cancer stem cell marker that labels an epithelial cell population responsive to H. pylori (15, 27).
GRANTS
This work was supported by the American Cancer Society Research Scholar Award 119072-RSG-10-167-01-MPC (to Y. Zavros), Albert J. Ryan Foundation Fellowship (to J. Donnelly), and in part by the Digestive Health Center Cincinnati Children's Medical Health Center (DHC: Bench to Bedside Research in Pediatric Digestive Disease) CHTF/SUB National Institutes of Health (NIH) Grant DK-078392 and Research Flow Cytometry Core in the Division of Rheumatology at Cincinnati Children's Hospital Medical Center (NIH Grant AR-47363). All flow cytometric data were acquired using equipment maintained by the Research Flow Cytometry Core in the Division of Rheumatology at Cincinnati Children's Hospital Medical Center, supported in part by NIH Grant AR-47363.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.M.D., A.C.E., R.F., and Y.Z. conception and design of research; J.M.D., A.C.E., R.F., J.L., and Y.Z. performed experiments; J.M.D., A.C.E., R.F., G.P.B., J.L., and Y.Z. analyzed data; J.M.D., R.F., C.X., G.P.B., J.L., J.H., and Y.Z. interpreted results of experiments; J.M.D., R.F., J.L., and Y.Z. prepared figures; J.M.D. and Y.Z. drafted manuscript; J.M.D., R.F., C.X., G.P.B., J.L., J.H., and Y.Z. edited and revised manuscript; J.M.D., A.C.E., R.F., C.X., G.P.B., J.L., J.H., and Y.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Kumar Shanmukhappa, Betsy DiPasquale, and Diane Hauck for assistance with the protocols for the in situ hybridization for the Y chromosome and Ki67 immunostaining (Cincinnati Children's Hospital Medical Center, Pathology Research Core).
REFERENCES
- 1.Aruffo A, IS, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell 61: 1303–1313, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Barker N., Huch M., Kujala P., van de Wetering M., Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6: 25–36, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, Beachy PA. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature 425: 846–851, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Cao H, Xu W, Qian H, Zhu W, Yan Y, Zhou H, Zhang X, Xu X, Li J, Chen Z, Xu X. Mesenchymal stem cell-like cells derived from human gastric cancer tissues. Cancer Lett 274: 61–71, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet 2: 58–60, 1975 [DOI] [PubMed] [Google Scholar]
- 6.da Cunha CB, Oliveira C, Wen X, Gomes B, Sousa S, Suriano G, Grellier M, Huntsman DG, Carneiro F, Granja PL, Seruca R. De novo expression of CD44 variants in sporadic and hereditary gastric cancer. Lab Invest 90: 1604–1614, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Dai P, Akimaru H, Tanaka Y, Maekawa T, Nakafuku M, Ishii S. Sonic Hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J Biol Chem 274: 8143–8152, 1999 [DOI] [PubMed] [Google Scholar]
- 8.De Gasperi R, Rocher AB, Sosa MA, Wearne SL, Perez GM, Friedrich VL, Hof PR, Elder GA. The IRG mouse: a two-color fluorescent reporter for assessing Cre-mediated recombination and imaging complex cellular relationships in situ. Genesis 46: 308–317, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnelly JM, Chawla A, Houghton J, Zavros Y. Sonic hedgehog mediates the proliferation and recruitment of transformed mesenchymal stem cells to the stomach. PLoS One 8: e75225, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnelly JM, Engevik AC, Engevik M, Schumacher MA, Xiao C, LY, Worrell RT, Zavros Y. Gastritis promotes an activated bone marrow-derived mesenchymal stem cell with a phenotype reminiscent of a cancer-promoting cell. Dig Dis Sci 59: 569–582, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan X, Long A, Goggins M, Fan X, Keeling P, Kelleher D. Expression of CD44 and its variants on gastric epithelial cells of patients with Helicobacter pylori colonisation. Gut 38: 507–512, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friis-Hansen L, Rehfeld JF. Impaired feedback of gastric functions in carboxypeptidase E-deficient mice. Biochem Biophys Res Commun 267: 638–642., 2000 [DOI] [PubMed] [Google Scholar]
- 13.Houghton J, Li H, Fan X, Liu Y, Liu JH, Rao VP, Poutahidis T, Taylor CL, Jackson EA, Hewes C, Lyle S, Cerny A, Bowen G, Cerny J, Moore N, Kurt-Jones EA, Erdman SE. Mutations in bone marrow-derived stromal stem cells unmask latent malignancy. Stem Cells Dev 19: 115–1166, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houghton JS, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science 306: 1568–1571, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 19: 387–400, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Khurana SS, Riehl TE, Moore BD, Fassan M, Rugge M, Romero-Gallo J, Noto J, Peek RMJ, Stenson WF, Mills JC. The hyaluronic acid receptor CD44 coordinates normal and metaplastic gastric epithelial progenitor cell proliferation. J Biol Chem 288: 16085–16097, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li HF, Kovi RC, Jo Y, Moquin B, Konz R, Stoicov C, Kurt-Jones E, Grossman SR, Lyle S, Rogers AB, Montrose M, Houghton J. Spontaneous expression of embryonic factors and p53 point mutations in aged mesenchymal stem cells: a model of age-related tumorigenesis in mice. Cancer Res 67: 10889–10898, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Li HC, Stoicov C, Rogers AB, Houghton J. Stem cells and cancer: evidence for bone marrow stem cells in epithelial cancers. World J Gastroenterol 12: 363–371, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin R, Ma H, Ding Z, Shi W, Qian W, Song J, Hou X. Bone marrow-derived mesenchymal stem cells favor the immunosuppressive T cells skewing in a helicobacter pylori model of gastric cancer. Stem Cells Dev 22: 2836–2848, 2013 [DOI] [PubMed] [Google Scholar]
- 19a.Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Mahe MM, Aihara E, Schumacher MA, Zavros Y, Montrose MH, Helmrath MA, Sato T, Shroyer NF. Establishment of gastrointestinal epithelial organoids. Curr Protoc Mouse Biol 3: 217–240, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature 287: 795–801, 1980 [DOI] [PubMed] [Google Scholar]
- 22.Okumura T, Wang SS, Takaishi S, Tu SP, Ng V, Ericksen RE, Rustgi AK, Wang TC. Identification of a bone marrow-derived mesenchymal progenitor cell subset that can contribute to the gastric epithelium. Lab Invest 89: 1410–1422, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan Y, Bai CB, Joyner AL, Wang B. Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol 26: 3365–3377, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian H, Le Blanc K, Sigvardsson M. Primary mesenchymal stem and progenitor cells from bone marrow lack expression of CD44 protein. J Biol Chem 287: 25795–25807, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, Friedman R, Varro A, Tycko B, Wang TC. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 19: 257–272, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spaeth EL, Labaff AM, Toole BP, Klopp A, Andreeff M, Marini FC. Mesenchymal CD44 expression contributes to the acquisition of an activated fibroblast phenotype via TWIST activation in the tumor microenvironment. Cancer Res 73: 5347–5359, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 27: 1006–1020, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Brink GR. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev 87: 1343–1375, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell 100: 423–434, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Wang F, Scoville D, He XC, Mahe MM, Box A, Perry JM, Smith NR, Lei NY, Davies PS, Fuller MK, Haug JS, McClain M, Gracz AD, Ding S, Stelzner M, Dunn JC, Magness ST, Wong MH, Martin MG, Helmrath M, Li L. Isolation and characterization of intestinal stem cells based on surface marker combinations and colony-formation assay. Gastroenterology 145: 383–395, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao C, Ogle SA, Schumacher MA, Orr-Asman MA, Miller ML, Lertkowit N, Varro A, Hollande F, Zavros Y. Loss of parietal cell expression of sonic hedgehog induces hypergastrinemia and hyperproliferation of surface mucous cells. Gastroenterology 138: 550–561, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu X, Zhang X, Wang S, Qian H, Zhu W, Cao H, Wang M, Chen Y, Xu W. Isolation and comparison of mesenchymal stem-like cells from human gastric cancer and adjacent non-cancerous tissues. J Cancer Res Clin Oncol 137: 495–504, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, Nannini-Pepe M, Kotkow K, Marsters JC, Rubin LL, de Sauvage FJ. A paracrine requirement for hedgehog signalling in cancer. Nature 455: 406–410, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Yoon J, Kita Y, Frank D, Majewski R, Konicek B, Nobrega M, Jacob H, Walterhouse D, Iannaccone P. Gene expression profiling leads to identification of GLI1-binding elements in target genes and a role for multiple downstream pathways in GLI1-induced cell transformation. J Biol Chem 277: 5548–5555, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Zavros Y, Eaton KA, Kang W, Rathinavelu S, Katukuri V, Kao JY, Samuelson LC, Merchant JL. Chronic gastritis in the hypochlorhydric gastrin-deficient mouse progresses to adenocarcinoma. Oncogene 24: 2354–2366, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Zavros Y, Waghray M, Tessier A, Bai L, Todisco A, L, Gumucio D, Samuelson LC, Dlugosz A, Merchant JL. Merchant reduced pepsin a processing of sonic hedgehog in parietal cells precedes gastric atrophy and transformation. J Biol Chem 282: 33265–33274, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Zavros Y, Orr MA, Xiao C, Malinowska DH. Sonic hedgehog is associated with H+,K+-ATPase-containing membranes in gastric parietal cells and secreted with histamine stimulation. Am J Physiol Gastrointest Liver Physiol 295: G99–G111, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zavros Y, Rieder G, Ferguson A, Samuelson LC, Merchant JL. Genetic or chemical hypochlorhydria is associated with inflammation that modulates parietal and G-cell populations in mice. Gastroenterology 122: 119–133., 2002 [DOI] [PubMed] [Google Scholar]
- 39.Zhong J, Chen S, Xue M, Du Q, Cai J, Jin H, Si J, Wang L. ZIC1 modulates cell-cycle distributions and cell migration through regulation of sonic hedgehog, PI(3)K and MAPK signaling pathways in gastric cancer. BMC Cancer 16: 290, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]