Abstract
Incomplete β-oxidation of fatty acids in mitochondria is a feature of insulin resistance and type 2 diabetes mellitus (T2DM). Previous studies revealed that plasma concentrations of medium- and long-chain acylcarnitines (by-products of incomplete β-oxidation) are elevated in T2DM and insulin resistance. In a previous study, we reported that mixed d,l isomers of C12- or C14-carnitine induced an NF-κB-luciferase reporter gene in RAW 264.7 cells, suggesting potential activation of proinflammatory pathways. Here, we determined whether the physiologically relevant l-acylcarnitines activate classical proinflammatory signaling pathways and if these outcomes involve pattern recognition receptor (PRR)-associated pathways. Acylcarnitines induced the expression of cyclooxygenase-2 in a chain length-dependent manner in RAW 264.7 cells. l-C14 carnitine (5–25 μM), used as a representative acylcarnitine, stimulated the expression and secretion of proinflammatory cytokines in a dose-dependent manner. Furthermore, l-C14 carnitine induced phosphorylation of JNK and ERK, common downstream components of many proinflammatory signaling pathways including PRRs. Knockdown of MyD88, a key cofactor in PRR signaling and inflammation, blunted the proinflammatory effects of acylcarnitine. While these results point to potential involvement of PRRs, l-C14 carnitine promoted IL-8 secretion from human epithelial cells (HCT-116) lacking Toll-like receptors (TLR)2 and -4, and did not activate reporter constructs in TLR overexpression cell models. Thus, acylcarnitines have the potential to activate inflammation, but the specific molecular and tissue target(s) involved remain to be identified.
Keywords: acylcarnitine, inflammation, pattern recognition receptors, TLR, β-oxidation
low-grade chronic inflammation, ectopic accumulation of lipids in tissues other than adipose, and inefficient or incomplete mitochondrial long-chain fatty acid (LCFA) β-oxidation are key features of insulin resistance and type 2 diabetes mellitus (T2DM) (16, 23). Excess accumulation of fatty acids or their derivatives in extra-adipose tissues can cause lipotoxicity, although the molecular mechanisms remain to be elucidated (17, 19, 20). In addition, other metabolic intermediates such as diacylglycerols (DAGs) and ceramides derived from saturated fatty acids are also implicated in lipotoxicity (21). It remains possible that additional metabolites participate in the inflammation or lipotoxicity associated with these conditions.
Previous studies have demonstrated a correlation between the obese, insulin-resistant state and incomplete β-oxidation of LCFA in rodent models (7), and our recent study comparing weight-matched obese T2DM and nondiabetic individuals showed an almost threefold increased plasma concentration of medium-chain acylcarnitines (MCFA-carnitines; e.g., C8-, C10-, C12, and C14-carnitine), by-products of incomplete fatty acid metabolism (1, 12). In rodent obesity models, muscle medium-chain fatty acid (MCFA) C14-carnitine moieties reach estimated concentrations of 5–10 μM, with some LCFA-carnitine concentrations around 5–10 times higher (as calculated from data in Refs. 3 and 7). These data suggest that a mismatch in fuel usage and accumulation of acylcarnitines in T2DM may underlie some of the consequences of the disease state.
Evidence gained recently suggests that members of the pattern recognition receptor (PRR) family [Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-containing proteins (NODs)] mediate infection-induced inflammation as well as sterile inflammation (inflammation with noninfectious origin) by recognizing pathogen-associated molecular patterns (PAMPs) and endogenous molecules derived from tissue injury, respectively, activating downstream signaling and stimulating the release of proinflammatory cytokines and cyclooxygenase-2 (COX-2) expression. Results from genetic, clinical, and biochemical studies revealed that TLR-mediated inflammation is an important determinant in altering the risk of the development of many chronic diseases including insulin resistance and diabetes. Lee and colleagues (8–10) have shown that saturated MCFA (C10:0, C12:0, C14:0, components of the Lipid A moiety of LPS) and LCFA induce the proinflammatory state in cultured immune cells largely via increased NF-κB-regulated transcriptional events through activation of cell surface TLRs and intracellular NOD proteins.
Considering the important role for TLRs as targets of saturated FA-induced “sterile inflammation” via increased NF-κB-regulated transcriptional events, we have hypothesized that TLR activation may underlie proinflammatory effects of acylcarnitine. Our preliminary proof-of-principle studies, using d,l-MCFA-carnitines in murine RAW 264.7 cells (monocyte/macrophage cell line containing necessary components for PRR signaling and a good model for elucidating PRR signaling pathways), suggested that MCFA-carnitines can trigger proinflammatory pathways implicated in insulin resistance (1). To confirm and extend those observations and to better understand potential mechanisms, we determined whether the naturally occurring form of C14 acylcarnitine (myristoyl-l-carnitine) stimulates proinflammatory signaling pathways in murine macrophages (RAW 264.7) and a human colon epithelial cell line (HCT-116). To evaluate the possibility that PRRs or their downstream signaling pathways are involved, PRR reporter gene assays and chemical inhibitor studies were also performed. Additionally, we determined whether knockdown of MyD88 (a key PRR signaling adapter) or the PRR TLR2 attenuates acylcarnitine-induced expression of proinflammatory markers. Finally, since docosahexaenoic acid (DHA) has been shown to attenuate saturated fat-induced inflammation (22), DHA was tested to determine whether it diminishes C14 acylcarnitine-induced stimulation of proinflammatory signaling pathways in macrophages.
MATERIALS AND METHODS
Reagents.
LPS (cat. no. 421), TNFα (cat. no. 315-01A), and MDP (cat. no. G-1055) were purchased from List Biological Laboratories (Campbell, CA), PeproTech (Rocky Hill, NJ), and BACHEM Bioscience (King of Prussia, PA), respectively. Pam3CSK4 (cat. no. tlrl-pms), Poly(I:C) (cat. no. tlrl-picw), and C12-IEDAP (cat. no. tlrl-c12dap) were purchased from Invivogen (San Diego, CA). Polymyxin B and antibody for β-actin were purchased from Sigma (St. Louis, MO). Antibodies for JNK (cat. no. 9252), ERK (cat. no. 9102), and phospho-ERK (cat. no. 4370) were purchased from Cell Signaling Technology (Danvers, MA). Phospho-JNK (cat. no. 07-175) antibody was purchased from Millipore. Rabbit polyclonal anti-COX-2 immune serum was prepared using a synthetic COX-2 polypeptide as immunogen as previously described (10). Premium select fetal bovine serums (FBS) and horse serum were purchased from Atlanta Biologicals (Lawrenceville, GA) and HyClone (Logan, UT), respectively. Bovine serum albumin (cat. no. 30-AB79, lot no. A11020301) was purchased from Fitzgerald Industries International (Acton, MA). l-Acylcarnitines of various chain lengths and l-carnitine were purchased from Crystal Chem (Downers Grove, IL). Palmitic acid and myristic acid (cat. no. P5585, lot no. 020M15811 and cat. no. M8005-10g lot no. 047K1434) were purchased from Sigma (St. Louis, MO). The cytotoxicity assay Toxilight and PyroGene Recombinant Factor C Endotoxin Detection Assay kits were purchased from Lonza (Basel, Switzerland), and the nonesterified fatty acid (NEFA) assay kits were purchased from Wako (Richmond, VA) and run according to the manufacturers' instructions.
Cell culture.
RAW 264.7 cells (a murine monocytic cell line containing necessary components for TLR/PRR signaling and a good model for elucidating inflammatory signaling pathways) and HCT-116 cells (a human colonic epithelial cell line that responds to most PRR agonists, and is typically used as a model of limited TLR2 and TLR4 signaling) were purchased from ATCC (Manassas, VA). These cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% (vol/vol) FBS (premium select FBS, cat. no. S11595, lot no. K0109; Atlanta Biologicals, Lawrenceville, GA), 100 U/ml penicillin, and 100 μg/ml streptomycin. RAW 264.7 and HCT116 cells were maintained at 37°C in a 5% CO2 atmosphere.
Generation of stable control (nontargeting) and MyD88 and TLR2 knockdown RAW 264.7 cells.
Gene silencing was achieved using a lentivirally mediated short hairpin RNA (shRNA) strategy to knock down MyD88 or TLR2 expression. A set of commercially available lentiviral particles designed to knock down murine MyD88 (Sigma-Aldrich, no. SHCLNV-NM_010851) or TLR2 (SHCLNV-NM_011905), available as part of the MISSION validated lentiviral-based shRNA libraries of The RNAi Consortium (TRC), were used. RAW 264.7 cells were plated in 24-well plates at 60,000 cells/well. Following the manufacturer's instructions, lentiviral particles for five different shRNA constructs as well as a nontargeting control (NT, no. SHC002V) were incubated individually with ExpressMag beads (Sigma) for 15 min to conjugate the virus to the magnetic beads. The conjugate was then added to the cells, and the cell culture plate was placed on the magnetic plate to concentrate the bead-virus complex on the cell layer. After 24 h of incubation, the medium was replaced with RAW 264.7 growth medium containing 4 μg/ml puromycin to select for transduced cells. After a minimum of 5 days of selection with puromycin, cells were analyzed by for knockdown of specific target. Gene expression for both MyD88 and TLR2 and Western blotting for MyD88 were performed to assess degree of knockdown in cell line pools. Functional knockdown was assessed by using known agonists (LPS and Pam3CSK4) followed by measurement of COX-2 protein induction and/or agonist-mediated cytokine secretion. Two cell pools with the greatest degree of knockdown as well as the NT cell pool were used in the acylcarnitine treatment experiments.
Acylcarnitine treatment.
RAW 264.7 cells were seeded at 1.5 × 106 per well in six-well plates. HCT-116 cells were seeded at 1 × 105 per well in 24-well plates. The day following seeding, cells were serum starved in 0.25% FBS-DMEM medium for 4–6 h and then treated with l-acylcarnitine (various acyl chain lengths) for the indicated times and doses detailed in the figure legends. After treatments, the medium supernatants were collected and frozen at −20°C or −80°C until assayed for cytokine levels by ELISA or Milliplex. Conditioned medium was also used in the Toxilight cytotoxicity assay that measures the activity of released adenylate kinase (AK), from damaged and dying cells. For immunoblotting, cells were rinsed with ice-cold PBS twice and then lysed by sonication in cold cell lysis buffer (Cell Signaling Technology) containing 20 mM Tris·HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and 100 μM PMSF. The lysate supernatants were collected by centrifugation at 12,000 g for 10 min at 4°C.
Immunoblotting.
Cell lysates were subjected to 10% SDS-PAGE (Tris-glycine or Bis-Tris gels) followed by transfer of the proteins to polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). The membrane was blocked in 20 mM Tris·HCl (pH 7.4), 150 mM NaCl, and 0.05% (vol/vol) Tween 20 containing 5% nonfat milk (COX-2, β-actin), or 5% BSA (phospho-antibodies JNK and ERK). The membrane was probed with primary antibody for 1 h at room temperature or overnight at 4°C followed by incubation with horseradish peroxidase-conjugated secondary antibody (Amersham Biosciences, Piscataway, NJ) for 1 h at room temperature. The proteins were detected by the ECL Western blot detection reagents (Amersham Biosciences) followed by exposure to X-ray film (Eastman Kodak) or by imaging using a Bio-Rad ChemiDoc XRS (CCD camera) system.
Cytokine analysis.
Media supernatants from l-acylcarnitine-treated RAW 264.7 cells (collected from immunoblotting experiments) were assayed for cytokine secretion using a Milliplex MAP Mouse Cytokine/Chemokine kit (MPXMCYTO-70K; Millipore, Billerica, MA) on a Bio-Plex system with xMAP Luminex technology. Alternatively, ELISA assays were carried out for TNFα (eBioscience, San Diego, CA) or IL-8 (BD Biosciences) from the cell culture medium supernatants using an ELISA kit and a Synergy 2 plate reader (BioTek, Winooski, VT) following the manufacturers' instructions.
Total RNA isolation and gene expression analyses.
Total RNA was extracted using a RiboPure Kit (Invitrogen) according to the manufacturer's instructions. Total RNA (900 ng) per reaction was used for cDNA synthesis with the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen). Gene expression measured by quantitative real-time PCR utilized gene-specific Taqman primers and FAM-MGB-labeled probes run in triplicate for each sample. Amplification cycle number (CT) of ribosomal 18S RNA was used to correct for template loading. For each gene, individual sample ΔCT values were subtracted from computed average control group ΔCT to derive a sample's ΔΔCT and transformed to a linear distribution and expressed as percent vehicle control or fold of control.
Reactive oxygen species analysis.
The levels of reactive oxygen species (ROS) were examined by confocal microscopy. Briefly, RAW 264.7 cells were seeded at 1 × 105 cells per well onto a coverslip placed in a well of 24-well plates in DMEM containing 10% FBS. The cells were serum starved for 6 h and then treated with 10 μM CM-H2DCFDA in PBS for 30 min at 37°C. After a washing with warm PBS three times, cells were treated as described in figure legends for 45 min in 0.25% FBS-DMEM. The cells were washed with ice-cold PBS three times, fixed in 10% formalin for 30 min at 4°C, and washed again with cold PBS three times. Coverslips were mounted on glass slides, and confocal microscopy was performed with a Zeiss LSM 510 microscope with 40 × 1.3 oil objective lens using laser excitation at 488 and 543 nm. For mitochondrial ROS analysis, RAW 264.7 cells were serum starved for 6 h then treated with rotenone (500 nM), l-carnitine (25 μM), or l-C14 carnitine (10 or 25 μM) for 16 h. Thirty minutes before the end point, cells were loaded with 2.5 μM MitoSOX dye in DMEM. RAW 264.7 cells were then washed with cold PBS and scraped into PBS with 1 mM EDTA. Cells were spun at 180 g for 3 min at 4°C and resuspended in FACS buffer before being analyzed by flow cytometry (FSC: E-1, 8.5, lin SSC: 400, 1.0, lin FL-2: 475,0, log).
Alternatively, acute effects were determined by first loading cells with 2.5 μM MitoSOX dye in DMEM for 30 min. Cells were rinsed with warm PBS and then treated with rotenone (500 nM), l-carnitine (25 μM), or l-C14 carnitine (10 or 25 μM) for 30, 60, 120, or 180 min. RAW cells were washed with PBS, scraped into the medium, spun, and resuspended in FACS buffer and analyzed by flow as described above.
TLR screening.
The responses of TLRs to l-C14 carnitine were tested utilizing human embryonic kidney 293 (HEK-293-Blue) cell lines functionally expressing a specific human TLR protein with a stable reporter gene driven by a NF-κB-inducible promoter (NF-κB-SEAP). A recombinant HEK-293 cell line for the reporter gene only (NF-κB control) was used as negative control. Positive control ligands were: HKLM (heat-killed Listeria monocytogenes) at 108 cells/ml for HEK-293-hTLR2, LPS K12 (100 ng/ml) for HEK-293-hTLR4, and flagellin (1 μg/ml) for HEK-293-hTLR5, CL097 (1 μg/ml) for HEK-293-TLR7 and HEK-293-TLR8, CpG ODN 2006 (1 μg/ml) for HEK-293-TLR9, and TNFα (1 μg/ml) for NF-κB control reporter line as well as l-C14 carnitine at 25 μM. Assay was conducted in medium containing 10% or 0.25% FBS. TLR ligand screenings were performed by InvivoGen (San Diego, CA).
Expression vectors.
pDisplay, pDisplay-TLR1, pDisplay-TLR2, and pDisplay-TLR6 were obtained from Adeline Hajjar (University of Washington, Seattle, WA). (2X)-NF-κB-luciferase reporter construct was provided by Frank Mercurio (Signal Pharmaceuticals, San Diego, CA). pRSV-β-galactosidase plasmid was from Jongdae Lee (University of California, San Diego, CA). The plasmid DNA from these expression vectors was prepared in large scale for transfection using the EndoFree Plasmid Maxi kit (Qiagen, Valencia, CA).
TLR transfections and luciferase assays.
Transient transfections were carried out using SuperFect transfection reagent (Qiagen) according to the manufacturer's recommendations. HEK-293T cells were seeded at 2 × 105 per well on 24-well plates and cotransfected the following day with 10 ng each of pDisplay-TLR2 and pDisplay-TLR1, or pDisplay-TLR2 and pDisplay-TLR6, in addition to 50 ng (2X)-NF-κB-luciferase reporter and 10 ng pRSV-β-galactosidase expression vectors. Twenty nanograms of pDisplay empty vector was used in addition to the above amounts of (2X)-NF-κB-luciferase and pRSV-β-galactosidase expression vectors for transfection as controls. Twenty-four hours after transfection, the cells were serum starved in 0.25% FBS-DMEM medium for 6 h followed by 12–16 h of treatment with positive control agonists or l-C14 carnitine in the same low-serum medium. The cells were lysed. Luciferase and β-galactosidase enzyme activities were determined from the lysate supernatants using the luciferase and β-galactosidase enzyme assay systems (Promega, Madison, WI) according to the manufacturer's instructions. Luciferase activity was normalized by β-galactosidase activity to correct differences in transfection efficiency among samples. Each of the experiments was repeated at least three times.
Data analysis.
Each of the experiments was repeated at least twice. Data from the ELISA, cytokine/chemokine analyses, ROS, and reporter gene assays are presented as means ± SE and were analyzed by one-way ANOVA with Dunnett's post-test comparing against control value and multicomparison t-tests with Holm-Sidak correction for effect of KD or DHA or two-tailed t-test using Prism 6 (GraphPad Software) as indicated in the figure legends.
RESULTS
Effect of acylcarnitines on indexes of inflammation.
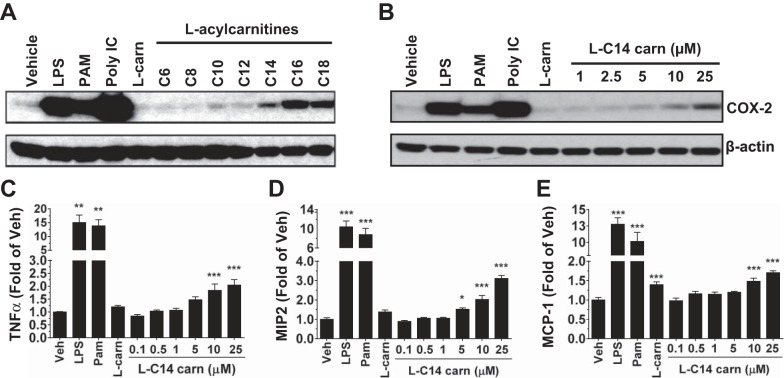
To determine if l-acylcarnitines can induce proinflammatory mediators in RAW 264.7 cells, we examined the expression of COX-2 (one of the important TLR target gene products) after treatment with a single dose (25 μM) of l-acylcarnitines of various chain lengths. l-Acylcarnitines induced COX-2 differentially depending on acyl chain length (Fig. 1A). Although l-C16 and l-C18 acylcarnitine induced the highest expressions of COX-2, there appeared to be an increase in cytotoxicity (as measured by toxicity assay from conditioned medium; data not shown) at the 25 μM concentration of l-C16 and l-C18 used but not with l-C14 or any of the PRR agonists tested. Therefore, all further studies were conducted with l-C14 carnitine as a representative acylcarnitine by-product of incomplete β-oxidation. Known PRR agonists including LPS (TLR4), PAM3CSK4 (TLR2), and poly(I:C) (TLR3) were also used as positive controls in these experiments and showed robust induction of all inflammatory related signaling pathways. To determine the threshold concentration for induction of the expression of other proinflammatory markers, media cytokines, and gene transcripts were analyzed following 24-h treatment with l-C14 carnitine (0–25 μM) in RAW 264.7 cells. l-C14 carnitine increased COX-2 (Fig. 1B) and media concentrations of TNFα, MIP2 (murine IL-8 analog), and MCP1 in a dose-dependent manner, with significant increases seen at concentrations as low as 5 μM (Fig. 1, C–E). l-C14 carnitine also induced the expression of multiple proinflammatory gene transcripts in a dose-dependent manner, including IL-1β, Ptgs2 (COX-2), IL-6, TNFα, Ccl2 (MCP1), Cxcl2 (MIP2), and Ccl5 (RANTES) (Table 1).
Fig. 1.
Induction of the proinflammatory gene product COX-2 by l-acylcarnitines in a chain length manner and l-C14 carnitine induction of COX-2 and secretion of TNFα, MIP2, and MCP1 in a dose-dependent manner in murine monocyte/macrophages. RAW 264.7 cells were serum-starved for 6 h (0.25% FBS/DMEM) then treated with LPS (TLR4 ligand, 0.2 ng/mL), PAM3CSK4 (TLR2 ligand, 2 ng/mL), Poly (I:C) (TLR 3 ligand, 0.25 μg/mL), 25 μM l-carnitine (l-carn) or (A) 25 μM of various chain lengths of l-acylcarnitine or (B) various doses of l-C14 carnitine (l-C14 carn, 0–25 μM) for 18 h. Whole cell lysates were analyzed by immunoblotting using COX-2 or β-actin antibodies. (Representative blot from 3 separate experiments). Media concentrations of proinflammatory cytokines TNFα, MIP2, and MCP1 (C, D, & E respectively) were measured by multiplex assay (Milliplex MAP). Controls: LPS (0.2 ng/mL), PAM (2 ng/mL), 25 μM l-carnitine. n = 6 over 3 experiments. One way ANOVAs with Dunnett's posttest for positive controls and for l-C14 carnitine dose response: *P < 0.05 **P < 0.01, ***P < 0.001 vs. vehicle-treated control (Veh); mean ± SE. Data are expressed as fold of vehicle control.
Table 1.
Induction of proinflammatory gene products by l-C14 carnitine in RAW 264.7 cells
| IL-1β | COX-2 | IL-6 | TNFα | MCP1 | MIP2 | RANTES | |
|---|---|---|---|---|---|---|---|
| Vehicle | 100 (2.6) | 100 (7.2) | 100 (4.5) | 100 (2.3) | 100 (5.3) | 100 (3.4) | 100 (4.2) |
| LPS | 5,482‡ (1,182.3) | 5,250‡ (1,040.8) | 5,989‡ (1,584.4) | 293‡ (24) | 9,542‡ (1,007.5) | 1,032‡ (127.7) | 997‡ (108.5) |
| Pam | 2,546† (355.3) | 2,149† (253.9) | 3,471† (1,067.5) | 216‡ (13.7) | 5,509‡ (577.5) | 567‡ (44.3) | 593‡ (65.8) |
| l-Carn | 107 (6.6) | 119 (12.6) | 118 (11.2) | 117 (6.9) | 120 (7.1) | 112 (10) | 114 (5.6) |
| l-C14 carn, 1 μM | 91 (3.2) | 95 (8.6) | 91 (4) | 99 (7) | 98 (5.4) | 107 (7.8) | 106 (7) |
| l-C14 carn, 5 μM | 140 (8.1) | 172 (21.2) | 164* (13.8) | 126* (9.7) | 137† (11.3) | 150† (11.9) | 155‡ (7) |
| l-C14 carn, 10 μM | 190† (17.6) | 217† (32.2) | 182† (15.7) | 137† (9.5) | 149‡ (9.4) | 173‡ (17) | 166‡ (18) |
| l-C14 carn, 25 μM | 323‡ (46.2) | 336‡ (61.1) | 265‡ (38.8) | 156‡ (13.4) | 205‡ (4.6) | 201‡ (20.5) | 207‡ (17.5) |
Data are expressed as mean mRNA abundance [%Vehicle, normalized to 18S (SE)].RAW 264.7 cells were serum-starved and treated with l-C14 carnitine (l-C14 carn, 0-25 μM), LPS, PAM, or free carnitine (l-Carn) for 18 h. Total RNA was extracted, and proinflammatory gene transcripts IL-1β (Il1b), COX-2 (Ptgs2), IL-6 (Il6), TNFα (Tnf), MCP1 (Ccl2), MIP2 (Cxcl2), and RANTES (Ccl5) were measured by qPCR using Taqman primer/probes. LPS (0.2 ng/ml), PAM3CSK4 (2 ng/ml), 25 μM l-carnitine (n = 6/treatment over 3 experiments). One way ANOVA with Dunnett's posttest
P < 0.05,
P < 0.01,
P < 0.001 vs. vehicle-treated control.
To rule out the possibility that the induction of the inflammatory markers was due to contamination of endotoxin in the cell culture reagents, RAW 264.7 cells were pretreated with the endotoxin inhibitor polymixin B (7.5 μg/ml) for 1 h prior to treatment with various concentrations of l-C14 carnitine. Polymixin B completely abolished LPS-induced TNFα secretion, but l-C14 carnitine-induced TNFα secretion was not affected (data not shown). To further rule out endotoxin contamination, all l-acylcarnitine preparations and accessory reagents were tested for endotoxins by using the PyroGene Recombinant Factor C Endotoxin Detection Assay and were found to have undetectable levels of endotoxin. These results indicate that l-C14 carnitine effects on COX-2 protein induction, cytokine production, and induction of proinflammatory gene transcripts were not due to endotoxin contamination in the reagents used in the assays.
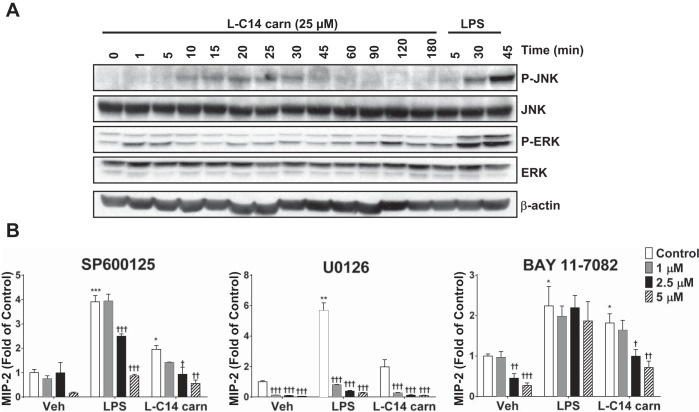
Our previous studies demonstrated that d,l-C12 or C14-carnitines activated NF-κB signaling in RAW 264.7 cells, which could involve PRRs or downstream pathways. Activation of key signaling molecules in PRR pathways was determined by measuring phosphorylation of ERK and JNK. As shown in Fig. 2A, l-C14 carnitine (25 μM) induced phosphorylation of ERK and JNK in a time-dependent manner. To further verify the role of PRR-related signaling pathways, JNK, ERK1/2, and NF-κB inhibitors (SP600125, U0126, and Bay 11-7082, respectively) were utilized to determine effects on cytokine release (MIP2). The maximal induction of media cytokine MIP2 (murine IL-8 analog) by l-C14 carnitine was blunted by each of the inhibitors tested. However, it should be noted that all inhibitors, at least at the higher concentrations, also reduced the basal MIP2 response in the vehicle-treated controls (Fig. 2B).
Fig. 2.
l-C14 carnitine increases phosphorylation of ERK and JNK in a time-dependent manner: MAP kinases and NF-κB contribution to l-C14 carnitine induced cytokine production. RAW 264.7 cells were serum-starved for 6 h (0.25% FBS/DMEM) then treated with LPS (100 ng/mL) or 25 μM l-C14 carn for indicated times (A). Whole cell lysates were prepared and analyzed for P-JNK, total JNK, P-ERK, total ERK. (Representative blot from 3 separate experiments). (B) RAW 264.7 cells were serum-starved for 3 h (0.25% FBS/DMEM) and then pretreated with JNK (Panel 1), ERK (Panel 2) or NF-κB (Panel 3) inhibitors for 1 h prior to treatment with LPS (100 ng/mL), or 25 μM l-C14 acylcarnitine (l-C14 carn) for 24 h. Media concentrations of proinflammatory cytokine MIP2 were measured by multiplex assay (Milliplex MAP); n = 4–6/treatment over 2–3 experiments. t-Tests for effect of treatment: *P < 0.05 **P < 0.01, ***P < 0.001. One way ANOVA with Dunnett's posttest for effect of inhibitor within treatment group: †P < 0.05 ††P < 0.01, †††P < 0.001 vs. vehicle-treated control (Veh); mean ± SE. Data are expressed as fold of vehicle control.
Effects of l-C14 carnitine on ROS production.
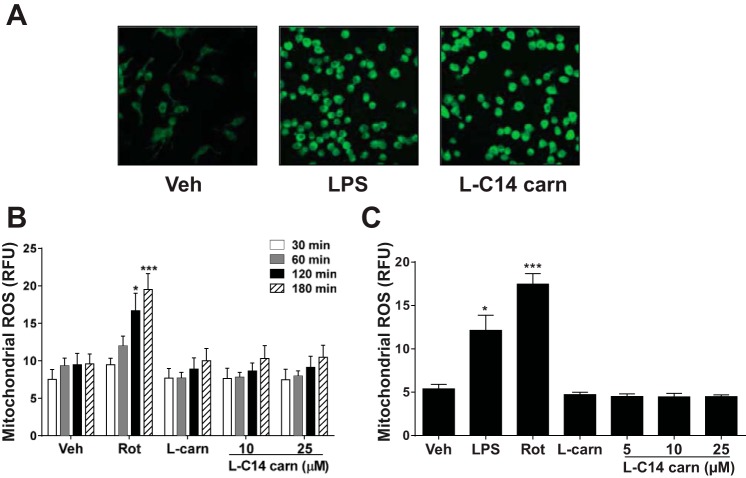
One potential mechanism by which acylcarnitines induce inflammation may be mediated through ROS generation. Generation of NADPH oxidase-dependent ROS generation is one of the well-documented downstream signaling pathways of TLR activation (24). Confocal microscopic analysis of total cellular ROS by visualization using CM-H2DCFDA showed that there was an l-C14 carnitine-mediated increase in ROS production (Fig. 3A). However, l-C14 carnitine did not induce mitochondria-derived ROS production acutely (30–180 min; Fig. 3B) or chronically (24 h; Fig. 3C), suggesting that l-C14 carnitine-induced ROS may be mediated through alternate ROS producing pathways.
Fig. 3.
l-C14 carnitine induced reactive oxygen species (ROS) but not mitochondrial ROS in murine monocyte/macrophages. RAW 264.7 cells were serum-starved for 6 h (0.25% FBS/DMEM) then treated with LPS (100 ng/mL), 25 μM l-C14 acylcarnitine (l-C14 carn), or 25 μM l-carnitine for 45 min. (A) ROS production was determined from living cells by confocal microscopy as described in the Methods section. (B) RAW 264.7 cells were loaded with MitoSOX dye and then treated with rotenone (500 nM), l-carnitine (25 μM) or various doses of l-C14 carnitine (10 or 25 μM) for 30, 60, 120, or 180 min and then analyzed by flow cytometry. (C) RAW 264.7 cells were treated with LPS (1 μg/ml), rotenone (100 nM), l-carnitine (25 μM), or various doses of l-C14 carnitine (5, 10, or 25 μM) for 16 h. Cells were loaded with MitoSOX dye and analyzed by flow cytometry. One-way ANOVA with Dunnett's posttest *P < 0.05 **P < 0.01, ***P < 0.001 vs. vehicle-treated control (Veh). Data are expressed as RFU ± SE and n = 3.
Effects of l-C14 carnitine on PRR-mediated signaling pathways.
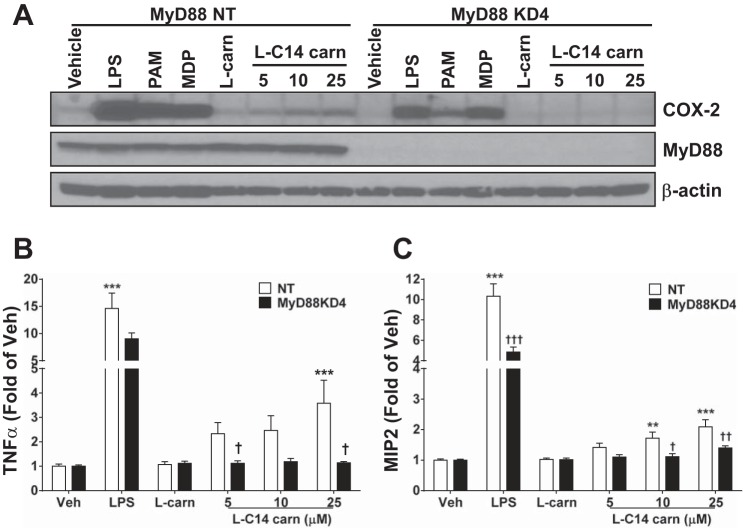
Since saturated LCFA and MCFA activate select PRRs, including some TLRs (8, 17), we assessed whether acylcarnitines trigger inflammatory responses through a similar mechanism. Since all TLRs except TLR3 activate MyD88-dependent signaling pathways, which in turn activate NF-κB and MAPK pathways, we determined whether l-C14 carnitine activates MyD88-dependent signaling pathways. l-C14 carnitine failed to induce COX-2 expression in the immortalized MyD88−/− macrophage cell line; however, l-C14 carnitine also failed to induce COX-2 expression in the immortalized wild-type control cell line (data not shown). Thus, we prepared control (NT) and MyD88 knockdown (KD) RAW 264.7 cell lines using lentiviral-mediated shRNA constructs. MyD88 was successfully silenced: MyD88 KD4 mRNA expression level was 5% of NT control (data not shown), and MyD88 protein expression was dramatically reduced (Fig. 4A). The results showed that l-C14 carnitine-induced COX-2 and the relative induction of cytokine release were diminished in MyD88 KD cells compared with the control NT cell line (Fig. 4, A–C). These results were verified using a second shRNA MyD88 construct (MyD88 KD6; data not shown).
Fig. 4.
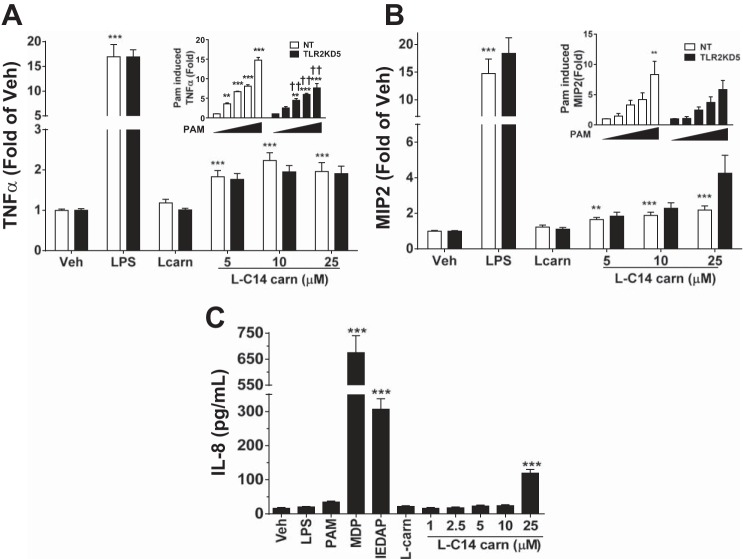
Induction of COX-2 and proinflammatory cytokines are reduced in MyD88 knock-down RAW 264.7 cells. Stable MyD88 KD cells were generated by lentiviral mediated shRNA in RAW 264.7 cells. Cells were then serum-starved for 4–6 h (0.25% FBS/DMEM) then treated with LPS (0.2 ng/mL), PAM3CSK4 (2 ng/mL), MDP (25 μg/mL), 25 μM l-carnitine (l-carn) or l-C14 carnitine (l-C14 carn, 5–25 μM) for 18 h. Whole cell lysates were analyzed by immunoblotting using COX-2 or β-actin antibodies (A) (Representative blot from 3 separate experiments). Media concentrations of proinflammatory cytokines TNFα or MIP2 (B & C respectively) were measured by multiplex assay (Milliplex MAP). n = 6 over 3 experiments. One way ANOVAs with Dunnett's posttest for positive controls and for l-C14 carnitine dose response: *P < 0.05 **P < 0.01, ***P < 0.001 vs. vehicle-treated control (Veh); mean ± SE. Multi-comparison t-tests with Holm-Sidak correction for effect of KD †P < 0.05 ††P < 0.01, †††P < 0.001 vs. non-targeting (NT); mean ± SE. Data are expressed as fold of KD vehicle control.
TLR2 is a MyD88-dependent PRR and shown to be activated by saturated MCFA. Thus, we investigated whether l-C14 carnitine stimulates proinflammatory pathways through TLR2 activation. Stable TLR2 knockdown (TLR2 KD) RAW 264.7 cell lines were prepared. While 5 TLR2 KD cell lines were generated, only one line, TLR2KD5, had sufficient enough TLR2 silencing (reduced to 28% of NT control, data not shown) to blunt the Pam3CSK4 (control agonist) cytokine response. As expected, LPS-induced cytokine release was not affected by TLR2 KD, but activation by PAM3CSK3 was attenuated (insets, Fig. 5, A and B) compared with the NT control line. The relative l-C14 carnitine-induced inflammatory response in the TLR2KD5 cell line was not diminished (Fig. 5, A and B). Furthermore, we evaluated the acylcarnitine response in the HCT-116 cell line as a tool to assess the relevance of TLR2 and TLR4; this cell line responds to most PRR agonists but is functionally TLR2 and -4 deficient. HCT-116 cells failed to secrete IL-8 in response to agonism by either TLR2 ligand Pam3CSK4 or TLR4 ligand LPS yet maintained their responsiveness to NOD1/2 agonists (C12-iE-DAP/MDP) and l-C14 carnitine (Fig. 5C), suggesting that neither TLR2 nor TLR4 is exclusively required in this cell type for acylcarnitine- mediated proinflammatory responses.
Fig. 5.
Induction of proinflammatory cytokines is unaltered in TLR2 knock-down RAW cells and human colonic epithelial cells lacking TLR2 and TLR4. Stable non-targeting (NT) and TLR2 knockdown cells were generated by lentiviral mediated shRNA in RAW 264.7 cells. Cells were then serum-starved for 4–6 h (0.25% FBS/DMEM) then treated with LPS (0.2 ng/mL), 25 μM l-carnitine (l-carn) or l-C14 carnitine (l-C14 carn, 5–25 μM) for 18 h. Media concentrations of proinflammatory cytokines TNFα and MIP2 (A & B respectively) were measured by multiplex assay (Milliplex MAP). n = 6 over 3 experiments. Additionally, NT and TLR2KD cells were treated with PAM3CSK4 of various doses (0, 0.25, 0.5, 1 & 2 ng/mL) and media concentrations of TNFα and MIP2 (respective insets of A and B) were measured. One way ANOVAs with Dunnett's posttest for positive controls and for l-C14 carnitine dose response, or PAM3CSK4 dose response (inset): *P < 0.05 **P < 0.01, ***P < 0.001 vs. vehicle-treated control (Veh); mean ± SE. Multi-comparison t-tests with Holm-Sidak correction for effect of KD: †P < 0.05 ††P < 0.01, †††P < 0.001 vs. non-targeting (NT); mean ± SE. Data are expressed as fold of KD vehicle control. HCT116 cells were serum-starved for 6 h (0.25% FBS/DMEM) then treated with various doses of l-C14 carnitine (l-C14 carn, 0–25 μM) for 18 h (C). Media concentrations of proinflammatory cytokine IL-8 were measured by ELISA assay (BD Bioscience). Controls: LPS (1 μg/mL), PAM3CSK4 (1 μg/mL), MDP (25 μg/mL), C12-IEDAP (20 μg/mL), 25 μM l-carnitine. Data are expressed as pg/mL; mean ± SEM. One way ANOVAs with Dunnett's posttest for positive controls and for l-C14 carnitine dose response: *P < 0.05 **P < 0.01, ***P < 0.001 vs. vehicle (Veh); mean ± SE (n = 6 over 3 experiments).
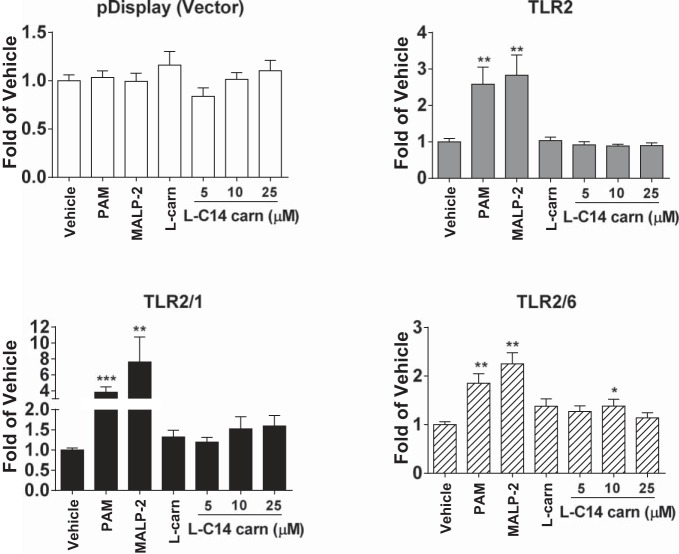
To further support the hypothesis that TLR2 is not a necessary and sufficient mediator of the l-C14 carnitine proinflammatory effects, l-C14 carnitine also did not transactivate a NF-κB reporter when HEK-293T cells were transfected with TLR2/1 or TLR2/6 plasmids (Fig. 6). In fact, single TLR reconstitution/promoter reporter gene assays in HEK-293-Blue cells for TLR2, TLR3, TLR4/MD2, TLR5, TLR7, and TLR9 showed no transactivation of a NF-κB/AP-1 reporter gene, suggesting that the effects of l-C14 carnitine are not mediated through these TLRs when expressed individually in this ligand screening assay system (Table 2).
Fig. 6.
l-C14 carnitine does not induce NFκB in TLR 2/1 or 2/6 in transfected HEK-293T cells. HEK-293T cells were cultured in 10% FBS/DMEM medium and cotransfected with TLR2 and TLR1, TLR2 and TLR6 in addition to NF-κB-luciferase reporter and β-galactosidase expression vectors. After 24 h, the cells were serum-starved in 0.25% FBS/DMEM for 6 h and then treated with l-C14 carnitine for 12 h. The cell lysates were assayed for luciferase and β-galactosidase activities. Values are expressed as RLA (relative luciferase activity). Controls: Pam (PamCSK4, TLR2/1 agonist, 10 ng/mL), MALP-2 (TLR2/6 agonist, 10 ng/mL), LPS (TLR4 agonist, 50 ng/mL). One way ANOVAs with Dunnett's posttest for positive controls and for l-C14 carnitine dose response: *P < 0.05 **P < 0.01, ***P < 0.001 vs. vehicle-treated control (Veh); mean ± SE. Data are expressed as fold of vehicle control.
Table 2.
Screening for TLR activation by l-C14 carnitine
| 293/TLR/NLR Cell Line | l-C14 carn | Positive Control |
|---|---|---|
| hTLR2 | 0.83 (0.066) | 16.63 (0.466) |
| hTLR3 | 0.96 (0.004) | 12.55 (0.722) |
| hTLR4(MD2-CD14) | 0.85 (0.003) | 17.17 (0.993) |
| hTLR5 | 0.99 (0.031) | 10.80 (1.812) |
| hTLR7 | 0.87 (0.022) | 19.73 (0.379) |
| hTLR8 | 0.91 (0.006) | 20.81 (0.412) |
| hTLR9 | 0.90 (0.063) | 19.77 (1.919) |
| NF-κB control cells | 1.04 (0.027) | 32.83 (0.560) |
Data are expressed as fold of vehicle-treated control cells; values are means (SD); n = 2/treatment. HEK-293-Blue TLR cell lines were serum starved for 6 h and then treated with 25 μM l-C14 carnitine or appropriate positive controls: HKLM (heat-killed listeria monocytogenes) at 108 cells/ml for HEK-293-hTLR2, LPS K12 (100 ng/ml) for HEK-293-hTLR4, and flagellin (1 μg/ml) for HEK-293-hTLR5, CL097 (1 μg/ml) for HEK-293-TLR7 and HEK-293-TLR8, CpG ODN 2006 (1 μg/ml) for HEK-293-TLR9, and TNFα (1 μg/ml).
Inhibition of l-C14 carnitine-induced expression of proinflammatory marker gene products by DHA in RAW 264.7 cells.
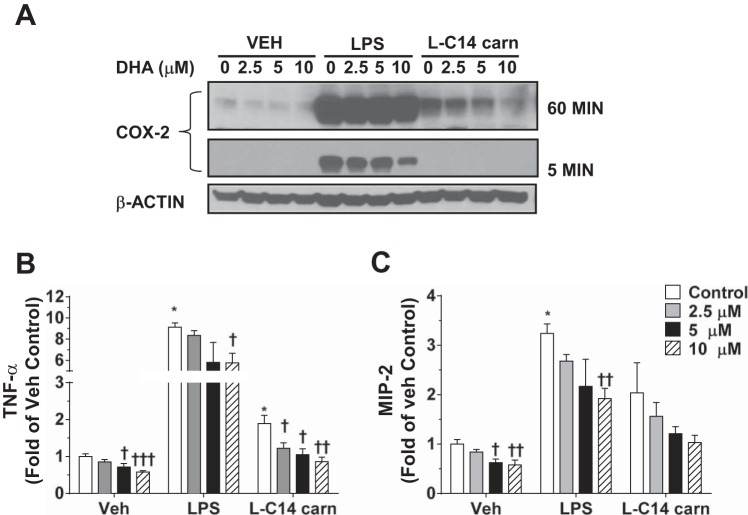
Since DHA has been shown to inhibit receptor-mediated signaling pathways derived from cytokine receptor activation, TLRs, or T cell receptors (9), we tested the effect of DHA pretreatment on l-C14 carnitine-induced expression of COX-2 and cytokines. Although DHA was capable of inhibiting the l-C14 carnitine-mediated COX-2 induction, as well as TNFα and MIP2 responses in a dose-dependent manner (Fig. 7, A–C), it also dampened the overall basal cytokine production in control cells.
Fig. 7.
DHA inhibits l-C14 carnitine-induced COX-2 and secretion of TNFα and MIP2. RAW 264.7 cells were pretreated with various doses of DHA for 1 h. Cells were co-treated with DHA and LPS (0.2 ng/mL) or 25 μM l-C14 carnitine for 24 h in a low serum media (0.25% FBS). Cells were serum-starved (0.25% FBS) for 6 h prior to l-C14 carnitine treatment. Whole cell lysates were analyzed by immunoblotting using COX-2 or β-actin antibodies (A). Representative blot at either 5 min or 60 min exposure from 3 independent experiments. Media concentrations of proinflammatory cytokines TNFα (B) and MIP2 (C) were measured by multiplex assay (Milliplex MAP). n = 6 over 3 experiments and values are mean ± SE (n = 4–8). T-tests for effect of treatment: *P < 0.05 **P < 0.01, ***P < 0.001. Multi-comparison t-tests with Holm-Sidak correction for effect of DHA within treatment group: †P < 0.05 ††P < 0.01, †††P < 0.001; mean ± SE. Data are expressed as fold of vehicle control.
DISCUSSION
The factors underlying lipotoxicity in T2DM tissues remain to be fully understood, but there is a growing consensus that by-products of inefficient LCFA oxidation and abnormal lipid storage are involved. The T2DM condition is associated with chronic systemic and tissue level inflammation (2). Our previous studies demonstrated that MCFA-carnitines, by-products of incomplete LCFA combustion, are elevated in plasma from humans with T2DM, and a racemic mixture of C12 or C14 acylcarnitine-transactivated NF-κB in RAW 264.7 cells (1). In the current study, we confirmed and extended those findings to illustrate that biologically relevant l-MCFA-acylcarnitines can activate a suite of markers associated with proinflammatory pathways. Herein, it was demonstrated that acylcarnitines induce the expression of the proinflammatory gene product COX-2 and various cytokines in RAW 264.7 cells in acyl chain length and concentration-dependent manner. In addition, l-C14 carnitine also induced JNK and ERK pathways. These novel results support the idea that MCFA and LCFA carnitines may play a role in inflammatory processes in T2DM and other conditions characterized by inordinate accumulation of acylcarnitines.
Although unlikely, it is theoretically possible that fatty acid hydrolysis products from the acylcarnitine preparations occurred and could confound interpretations, since MCFA and LCFA activate some PRRs. However, analyses of acylcarnitine and free fatty acid levels in the preparations used herein indicated that the media contained the targeted acylcarnitine concentrations and that free fatty acid concentrations were nil (data not shown). Further support that the proinflammatory effects observed are indeed mediated by l-C14 carnitine itself and not hydrolysis products is the observation that the concentrations of l-C14 carnitine needed to induce COX-2 expression and other inflammation-associated factors (as low as 5 μM) are ∼10 times lower than those required by BSA-solubilized C14:0 or the sodium salt of C14:0 (myristic acid) to elicit a comparable effect (data not shown). The preparations were also not found to contain endotoxin contamination, and acylcarnitine effects were not abolished by the LPS-binding inhibitor polymixin B. Collectively, these data indicate that acylcarnitines are distinct bioactive molecules capable of eliciting a proinflammatory response in cultured cells.
Since many proinflammatory responses are mediated by transcriptional activation of cytokine genes via the AP-1 and NF-κB response elements in their promoters (11, 18), we examined the upstream signaling of these key transcription factor complexes, phosphorylation of ERK and JNK (MAPKs). Time-dependent phosphorylation of each of these molecules occurred after treatment with l-C14 carnitine, suggesting that common pathways in classical inflammatory signaling are also activated in acylcarnitine-mediated inflammation. Consistent with this view, by using pathway inhibitors it could be demonstrated that the MAPK pathways contribute to the full acylcarnitine-mediated cytokine response in murine RAW 264.7 cells.
Previous studies have demonstrated that saturated fatty acids including lauric (C12) and palmitic (C16), both as sodium salts and BSA-conjugated forms, induce ROS in RAW 264.7 cells (5, 8). Furthermore, apocynin, an inhibitor of NADPH oxidase, suppressed not only C12- or C16-induced phosphorylation of JNK and ERK but also COX-2 and TNFα expression, leading us to question whether the same is true for l-C14 carnitine. Interestingly, l-C14 carnitine induced an increase in cellular ROS; however, mitochondrial ROS did not show a similar increase. This indicates that an alternative pathway (e.g., NADPH oxidase-, peroxisome-, or endoplasmic reticulum-mediated production) may be responsible for the increase in cellular ROS observed. Consistent with the current results, Aguer et al. recently demonstrated ROS activation by acylcarnitines in myotubes in culture (C. Aguer, C.S. McCoin, T.A. Knotts, R. McPherson, R. Dent, D. Hwang, S.H. Adams, and M.E. Harper, personal communication). Future studies utilizing inhibitors and siRNA knockdown of molecules involved in ROS production pathways are warranted to determine the exact pathways involved and to understand whether induction of ROS is important to proinflammatory or other bioactivities of acylcarnitines.
To test whether PRR-associated signaling molecules were involved in the acylcarnitine-mediated proinflammatory response, the role of a critical multi-PRR adapter protein MyD88 was evaluated. Data from RAW 264.7 MyD88 knockdown (MyD88 KD) cells suggests a role for MyD88-associated pathways in the proinflammatory effects of acylcarnitines, since COX-2 expression and cytokine secretion were blunted in response to l-C14 carnitine with loss of MyD88. However, the possibility that diminished responsiveness of l-C14 carnitine is a secondary effect, due to globally-dampened inflammation in MyD88 KD cells, cannot be ruled out. Secondly, using TLR2 KD cells, overall inflammation was reduced but l-C14 carnitine was still able to induce a response over basal levels, suggesting that l-C14 carnitine-mediated proinflammatory signaling is not solely mediated through activation of TLR2. Immortalized macrophages from wild-type and KO mice were used to test the role of important inflammatory factors. When we treated C57BL/6 control and KO immortalized macrophages with l-C14 carnitine we failed to see an induction or cytokine secretion. While commonly used, the C57BL/6 wild-type macrophage line lacked sensitivity and required much higher doses of many known agonists such as LPS or PAM3CSK4 (when compared with other cell lines) limiting the experimental capabilities in this context.
To gain additional information about potential target signaling, we took several other approaches. First, we leveraged the use of the HCT-116 cell line that does not express TLR2 or TLR4 nor respond to their agonists but has been shown to express MyD88 (6). Secretion of IL-8 was significantly increased from HCT-116 cells treated with l-C14 carnitine, indicating that l-C14 carnitine-induced HCT-116 cell IL-8 expression is mediated through signaling pathways other than TLR2 or TLR4. Second, TLR reconstitution-reporter studies in HEK293 cells using TLR2, TLR2/1 or TLR2/6 also failed to show activation by l-C14 carnitine. Third, since RAW 264.7 cells are TLR5- and IL-receptor-deficient (13) (confirmed as a lack of cytokine response to the TLR5 activator flagellin and the IL-R activator IL-1β, as well as negligible levels of TLR5 and IL-R by qPCR; data not shown), it is unlikely that the acylcarnitine-associated inflammation in these cells is TLR5- or IL-R dependent. Fourth, TLR reconstitution/reporter assays in HEK293 cells suggested that none of the individual TLRs tested (TLR2, TLR3, TLR4/MD2, TLR5, TLR7, or TLR9) were sufficient to support an acylcarnitine response. However, we cannot rule out that the 293 cells lack an important component required for acylcarnitine-mediated proinflammatory signaling that is not required by the positive control agonists. Overall, these data suggest that TLR2, TLR3, TLR4/MD2, TLR5, TLR7, TLR9, or IL-R are not necessary and sufficient for the acylcarnitine- mediated proinflammatory responses in the cell types tested.
Another potential mechanism for acylcarnitine- mediated induction of inflammatory pathways is its activity on the plasma membrane through detergent-like effects, as has been reported for fatty acids like palmitate or other amphiphilic compounds. Indeed, D,L C12- and C14-acylcarnitines have been characterized to form micelles at 1 and 0.2 mM, respectively, in water (850 or 75 µM in 0.2M KCl) (25), which may interact with cellular membranes. A more recent paper using 13C-NMR suggested that C14-carnitine can incorporate into model membranes at high rates but does not cause disorganization of bilayer structure. Therefore, the authors hypothesized that acylcarnitine effects are not merely the result of membrane disruption but by interference with membrane functions or signaling (4). However, while the responses that we observed may be in part due to a “detergent effect,” this is unlikely to explain the specific activation of signaling pathways including the necessity of the downstream signaling component MyD88. Furthermore, the concentrations tested here are similar to those found in muscle tissues isolated from a rodent obesity model (C14-carnitine 5–10 μM (3, 7)) further emphasizing the potential physiological importance in disease states.
Since DHA blocks saturated fatty acid mediated proinflammatory effects (5), we tested the ability of DHA to inhibit acylcarnitine- mediated COX-2 induction and cytokine secretion. DHA has been shown to activate GPR120, leading to inhibition of TAK1, a common downstream signaling component of TLR and TNFα receptor (14). DHA has also been shown to interfere with translocation of TLR4 into lipid rafts induced by LPS (24). Thus, our results demonstrating inhibition of l-C14 carnitine-induced expression of COX-2 or cytokines suggest a possibility that acylcarnitines may engage some of the same pathways shared by proinflammatory factors such as TNFα, TLR ligands, and/or T-cell receptors. However, it does not elucidate the mechanism of the inhibition by DHA, and it was noted that DHA attenuated basal cytokine release suggesting a general dampening of inflammation.
The finding that acylcarnitines activate proinflammatory pathways raises the possibility that under some contexts, accumulation of these metabolites within immune cells could contribute to pathophysiological outcomes. Yet, the in situ free concentrations of many metabolites, including acylcarnitines, are difficult to determine in tissues, and it is important to note that in our cell models acylcarnitines were applied in the culture media. With a rodent obesity models, muscle medium-chain fatty acid (MCFA) C14-carnitine moieties reach estimated concentrations of 5–10 μM, with some LCFA-carnitine concentrations around 5–10 times higher (calculated from (3, 7)). Furthermore, the total tissue concentrations of several acylcarnitines of longer chain length in mice were shown to be much greater than that of l-C14 carnitine used in our study (15). Herein we demonstrated that l-C14 carnitine, representing a class of MCFA-carnitines found to be elevated in blood of humans with T2DM and in blood and tissues of obese rodents with insulin resistance, induces COX-2, proinflammatory cytokines and ERK/JNK/NF-κB pathways in RAW 264.7 cells at extracellular concentrations as low as 5 μM (and most was gone from the media within 18–24 h). In summary, our results suggest an intriguing possibility that acylcarnitines, the by-products of incomplete β-oxidation of long-chain fatty acids, mediate at least in part undesirable consequences of dysregulated fatty acid oxidation in mitochondria by activating proinflammatory signaling pathways.
GRANTS
This work was supported by USDA-Agricultural Research Service Intramural Projects 5306-51530-019-00 and 5306-51530-017-00D, NIH-NIDDK R01DK078328 (SHA) and R01DK078328-02S1 (SHA, DHH), DK064007 (DHH), an International Life Sciences Institute Future Leader Award (SHA) and American Diabetes Association Grant 1-12-BS-02 (SHA, DHH). USDA is an equal opportunity provider and employer.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.M.R., T.A.K., K.D.O.-M., C.S.M., S.H., S.H.A., and D.H.H. conception and design of research; J.M.R., T.A.K., K.D.O.-M., C.S.M., S.H., D.S., and S.S. performed experiments; J.M.R., T.A.K., K.D.O.-M., C.S.M., S.H., D.S., and S.S. analyzed data; J.M.R., T.A.K., K.D.O.-M., C.S.M., S.H., D.S., S.H.A., and D.H.H. interpreted results of experiments; J.M.R., T.A.K., K.D.O.-M., and C.S.M. prepared figures; J.M.R., T.A.K., K.D.O.-M., S.H.A., and D.H.H. drafted manuscript; J.M.R., T.A.K., K.D.O.-M., C.S.M., S.H.A., and D.H.H. edited and revised manuscript; J.M.R., T.A.K., K.D.O.-M., C.S.M., S.H., D.S., S.S., S.H.A., and D.H.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Pieter Oort for completing both the endotoxin and NEFA assays. In addition we would like to thank Dr. Charles L. Hoppel for media acylcarnitine analyses. We acknowledge Ryan Snodgrass for assistance with flow cytometry method development.
REFERENCES
- 1.Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, Hwang DH, Newman JW, Garvey WT. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr 139: 1073–1081, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 17: 4–12, 2006 [PubMed] [Google Scholar]
- 3.De Vogel-van den Bosch J, Hoeks J, Timmers S, Houten SM, van Dijk PJ, Boon W, Van Beurden D, Schaart G, Kersten S, Voshol PJ, Wanders RJ, Hesselink MK, Schrauwen P. The effects of long- or medium-chain fat diets on glucose tolerance and myocellular content of lipid intermediates in rats. Obesity (Silver Spring) 19: 792–799, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Ho JK, Duclos RI, Jr, Hamilton JA. Interactions of acyl carnitines with model membranes: a (13)C-NMR study. J Lipid Res 43: 1429–1439, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Huang S, Rutkowsky JM, Snodgrass RG, Ono-Moore KD, Schneider DA, Newman JW, Adams SH, Hwang DH. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J Lipid Res 53: 2002–2013, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kfoury A, Le Corf K, El Sabeh R, Journeaux A, Badran B, Hussein N, Lebecque S, Manie S, Renno T, Coste I. MyD88 in DNA repair and cancer cell resistance to genotoxic drugs. J Natl Cancer Inst 105: 937–946, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45–56, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem 276: 16683–16689, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Lee JY, Zhao L, Youn HS, Weatherill AR, Tapping R, Feng L, Lee WH, Fitzgerald KA, Hwang DH. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem 279: 16971–16979, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Lee SH, Soyoola E, Chanmugam P, Hart S, Sun W, Zhong H, Liou S, Simmons D, Hwang D. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem 267: 25934–25938, 1992 [PubMed] [Google Scholar]
- 11.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol 2: 725–734, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledo FG, DeLany JP. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring) 18: 1695–1700, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizel SB, Honko AN, Moors MA, Smith PS, West AP. Induction of macrophage nitric oxide production by Gram-negative flagellin involves signaling via heteromeric Toll-like receptor 5/Toll-like receptor 4 complexes. J Immunol 170: 6217–6223, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142: 687–698, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341: 1241214, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schrauwen P, Hesselink MK. Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes 53: 1412–1417, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Senn JJ. Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem 281: 26865–26875, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol 4: E131–136, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116: 3015–3025, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinha S, Perdomo G, Brown NF, O'Doherty RM. Fatty acid-induced insulin resistance in L6 myotubes is prevented by inhibition of activation and nuclear localization of nuclear factor kappa B. J Biol Chem 279: 41294–41301, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res 45: 42–72, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Thies F, Miles EA, Nebe-von-Caron G, Powell JR, Hurst TL, Newsholme EA, Calder PC. Influence of dietary supplementation with long-chain n-3 or n-6 polyunsaturated fatty acids on blood inflammatory cell populations and functions and on plasma soluble adhesion molecules in healthy adults. Lipids 36: 1183–1193, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med 14: 222–231, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem 284: 27384–27392, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yalkowsky SH, Zografi G. Some micellar properties of long-chain acylcarnitines. J Colloid Interface Sci 34: 525–533, 1970 [DOI] [PubMed] [Google Scholar]