Abstract
Adipose triglyceride lipase (ATGL), the rate-limiting enzyme for triacylglycerol (TG) hydrolysis, has long been known to be a phosphoprotein. However, the potential phosphorylation events that are involved in the regulation of ATGL function remain incompletely defined. Here, using a combinatorial proteomics approach, we obtained evidence that at least eight different sites of ATGL can be phosphorylated in adipocytes. Among them, Thr372 resides within the hydrophobic region known to mediate lipid droplet (LD) targeting. Although it had no impact on the TG hydrolase activity, substitution of phosphorylation-mimic Asp for Thr372 eliminated LD localization and LD-degrading capacity of ATGL expressed in HeLa cells. In contrast, mutation of Thr372 to Ala gave a protein that bound LDs and functioned the same as the wild-type protein. In nonstimulated adipocytes, the Asp mutation led to decreased LD association and basal lipolytic activity of ATGL, whereas the Ala mutation produced opposite effects. Moreover, the LD translocation of ATGL upon β-adrenergic stimulation was also compromised by the Asp mutation. In accord with these findings, the Ala mutation promoted and the Asp mutation attenuated the capacity of ATGL to mediate lipolysis in adipocytes under both basal and stimulated conditions. Collectively, these studies identified Thr372 as a novel phosphorylation site that may play a critical role in determining subcellular distribution as well as lipolytic action of ATGL.
Keywords: lipolysis, triglyceride, lipid metabolism, adipose triglyceride lipase, phosphorylation, lipid droplet
lipid droplets (LDs) are intracellular depots for neutral lipids such as triacylglycerols (TGs) and cholesteryl esters and play essential roles in providing energy substrate during times of nutrient deprivation or enhanced energy demand (13). A key process in energy catabolism is the lipolytic breakdown of TGs in adipose tissue and the release of free fatty acids (FFAs) and glycerol. To date, three enzymes, namely adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL), and monoglyceride lipase (MGL), have been found to be critical for lipolysis at different steps in adipocytes (12, 16, 27). ATGL initiates TG breakdown, resulting in the release of one fatty acid (FA) and diacylglycerol (DG). HSL hydrolyzes DG to FA and monoacylglycerol (MG), the latter of which then is converted to glycerol and FA by MGL.
In adipocytes, catecholamines acting on β-adrenergic receptors and insulin functioning through insulin receptor represent the most potent stimulatory and inhibitory hormones, respectively, in lipolysis (12, 16, 27). In the basal state, a majority of ATGL and HSL proteins reside in the cytoplasm of adipocytes. β-Adrenergic stimulation leads to phosphorylation of perilipin 1 by protein kinase A (PKA) and subsequent translocation of ATGL and HSL to the LD surface (3–5, 7, 24, 26), thereby stimulating lipolysis. Reportedly, the NH2-terminal region of ATGL, which contains the proposed catalytic dyad Ser47 and Asp166, is critical for catalytic activity, whereas the COOH-terminal region of ATGL controls LD localization (6, 22). Although ATGL activation at the LD surface by comparative gene identification-58 (CGI-58) was well studied (9, 10), the exact mechanisms underlying the exclusion of ATGL from LDs in the basal state have remained unclear.
ATGL is known to be a phosphoprotein (29). Previous studies have identified two phosphorylation sites, Ser406 and Ser430, in its COOH-terminal region (2). Earlier work has shown that phosphorylation of Ser406 by AMP-activated protein kinase (AMPK) activates the enzymatic action of ATGL (1). However, more recent studies have provided evidence that Ser406 is a direct target for PKA, and its phosphorylation correlates with lipolytic activation in response to β-adrenergic stimulation (18, 21). In the present study, we used an adenovirus-mediated expression system combined with a mass spectrometry-based proteomic approach to systemically analyze the phosphorylation of ATGL in adipocytes. We identified Thr372 as a novel site that, when phosphorylated, prevents the LD localization of ATGL.
MATERIALS AND METHODS
Antibodies and reagents.
The rabbit monoclonal antibody against ATGL (catalog no. 2439) was purchased from Cell Signaling Technology. Mouse monoclonal c-Myc antibody and c-Myc antibody-conjugated agarose were obtained from Santa Cruz Biotechnology (catalog nos. sc-40 and sc-40 AC). Horseradish peroxidase-linked secondary antibodies were from Pierce Chemical. The protease inhibitor mini tablets (catalog no. 11 836 170 001) were obtained from Roche Diagnostics. Lipolysis assay kit (catalog no. LIP-1-NC-L1) was purchased from Zenbio. 3H-labeled triolein was from Perkin-Elmer. Reagents for tissue culture were obtained from Invitrogen. Isoproterenol, insulin, dexamethasone, 3-isobutyl-1-methylxanthine, and triiodothyronine were purchased from Sigma-Aldrich.
RNA extraction, PCR cloning of cDNA, and site-directed mutagenesis.
Total RNA was prepared from mouse 3T3-L1 adipocytes using the RNeasy Mini kit (Qiagen) according to the manufacturer's instructions. cDNA was prepared from mRNA using SuperScript Reverse Transcriptase protocol (Invitrogen). The sequence containing the complete open-reading frame of mouse ATGL was amplified by PCR using Pfusion High Fidelity DNA polymerase (New England Biolabs). The PCR products containing ATGL cDNA were cleaved by BglII/XhoI. The digested products were purified and ligated in frame to the BamHI (5′) and XhoI (3′) sites of the eukaryotic expression vector of pKMyc to generate constructs with a Myc epitope tag fused to the 5′ end of the cDNA (pKM-ATGL). This pKM-ATGL was used as a template for site-directed mutagenesis to generate T372D (forward: 5′-GTGGATGAAAGAG CAGGACGGTAGCATCTGCCAG-3′; reverse: 5′-CTGGCAGATGCTACCGTCCTGCTCTTT CATCCAC-3′), T372A (forward: 5′-GGATGAA AGAGCAGGCGGGTAGCATCTGCC-3′; reverse: 5′-GGCAGATGCTACCCGCCTG CTCTTTCATCC-3′), and S47A (forward: 5′-CATCTACGGAGCCGCGGCAGGGGCGCTC-3′; reverse: 5′-GAGCGCCCCTGCCGCGGCTCC GTAGATG-3′).
Cell culture.
HeLa cells (ATCC) were cultured in DMEM supplemented with 10% fetal bovine serum (FBS). Mouse 3T3-L1 preadipocytes were maintained in high-glucose DMEM supplemented with 10% newborn calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Differentiation to adipocytes was induced by treatment of postconfluent cells with 10% FBS, 1 μg/ml insulin, 1 μM dexamethasone, and 0.5 mM isobutyl-1-methylaznthine. The differentiation medium was withdrawn 3 days later and replaced with medium supplemented with 10% FBS and 1 μg/ml insulin. After 2 days in insulin-containing medium, the cells were then cultured in DMEM containing 10% FBS. T37i brown preadipocytes were cultured and differentiated as described previously (26). Briefly, T37i preadipocytes were cultured in DMEM-Ham's F-12 medium supplemented with 10% FBS, 2 mM glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 20 mM HEPES. Differentiation into brown adipocytes was achieved under standard conditions by incubating subconfluent undifferentiated preadipocytes with 2 nM triiodothyronine and 20 nM insulin for 5 days.
Transient transfection of HeLa cells.
Transient transfection was performed using Lipofectamine 2000 reagent according to the manufacturer's instructions. For immunofluorescence studies, 0.25 μg of each DNA construct was transfected into cells cultured on coverslips at low density in six-well dishes. Transfected cells were incubated with 400 μM oleic acid overnight and followed by 4-h nutrient starvation. Cells were fixed immediately thereafter for immunofluorescence staining. For TG hydrolase activity assays, 10 μg of each DNA construct was used in transfection of subconfluent cells cultured in 100-mm dishes. Cells were lysed 16–18 h posttransfection.
Adenoviral infection of adipocytes.
Recombinant adenovirus containing mouse ATGL and its variants T372D and T372A under the control of a cytomegalovirus(CMV) promoter (Ad-Myc-ATGL, Ad-Myc-ATGL T372D, and Ad-Myc-ATGL T372A) were custom generated by Vector Biolabs. A CMV-null virus (Ad-Null) was also obtained for use in control experiments. Infection of differentiated adipocytes was performed by using a previously published method with minor modifications (11, 26). The adenoviral stocks were diluted in serum-free DMEM containing 5.0 μg/ml polybrene to 2 × 107 plaque-forming units/ml for immunofluorescence and lipolysis assays and 6 × 107 plaque-forming units/ml for the immunoprecipitation experiment. Adipocytes plated in six-well dishes were washed once with PBS, and 1.5 ml of preincubated virus mix was added to each well. Cells were centrifuged at 800 g for 1 h at room temperature, followed by incubation at 37°C. Fresh DMEM containing 10% FBS (1.5 ml/well) was added after 4 h, and cells were incubated for 24 h before use.
Assay for TG hydrolase activity.
HeLa cells in 10-cm dishes were washed twice in ice-cold PBS and then lysed on ice by sonication in 0.5 ml of cell extraction buffer [0.25 M sucrose, 1 mM EDTA, 1 mM DTT, and protease inhibitors (1 minitablet per 7 ml of volume)]. The cell extracts were clarified by centrifugation at 1,000 g for 15 min. The TG hydrolase activity against 3H-labeled triolein was measured as described previously by mixing 0.1 ml of extracts with 0.1 ml of substrate solution (15, 29).
Immunofluorescence staining and confocal microscopy.
HeLa cells and adipocytes were maintained at proper densities on glass coverslips placed in six-well dishes. Following the fixation with 3% paraformaldehyde in PBS for 20 min, cells were permeabilized by 0.5% triton X-100 for 5 min, quenched with 100 mM glycine in PBS for 20 min, and then blocked with 1% BSA in PBS for 1 h. The cells were then exposed to primary antibody (anti-Myc; Santa Cruz Biotechnology) for 2 h at room temperature. Following three washes with PBS, the cells were treated for 1 h with Alexa Fluor secondary antibodies diluted to 2 μg/ml in blocking solution. To visualize lipid droplets, Bodipy 493/503 dye was added at final concentrations of 1 and 0.3 μg/ml for HeLa and adipocyte cells, respectively, during the incubation with secondary antibodies. Samples were mounted on glass slides with Vectashield mounting medium and examined under a Zeiss LSM 510 inverted confocal microscope. All images presented were of representative Z sections.
For quantitative analysis of lipid content and Myc-ATGL localization, integrated density per area (fluorescence intensity) was measured by using Image J software. Relative lipid content was quantified by fluorescence intensity of Bodipy 493/503 (green). Briefly, integrated density per cell area in transfected and neighboring untransfected cells, was measured and their ratio was used to represent the relative lipid content. At least three different images were used for each condition. Localization of Myc-ATG was quantified based on the intensity of anti-Myc immunofluorescence. The ratio of fluorescence intensity on LD surface and cytosol was used to represent the localization. At least four different cells were measured for each condition.
Cell lysis, immunoblotting, and immunoprecipitation.
HeLa, 3T3-L1 and T37i cells were washed twice with ice-cold PBS and lysed at 4°C in a buffer containing 50 mM Tris·HCl (pH 8.0), 1% Triton X-100, 20 mM sodium pyrophosphate, 135 mM NaCl, 10 mM NaF, 10 mM Na3VO4, 1 mM EDTA, and protease inhibitors (1 tablet per 7 ml of buffer). The lysates were clarified by centrifugation at 10,000 g for 10 min. For immunoblotting, the clarified lysates were mixed with an equal volume of 2× SDS sample buffer. The solubilized proteins were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Individual proteins were blotted with primary antibodies at appropriate dilutions. Peroxide-conjugated secondary antibodies were incubated with the membrane at a dilution of 1:5,000. The signals were then visualized by enhanced chemiluminescence (ECL Reagents; GE Healthcare). For immunoprecipitation, the clarified lysate was mixed with the appropriate amount of c-Myc conjugated to agarose beads (sc-40 AC; Santa Cruz Biotechnology) for 3 h at 4°C. Following extensive washes with the same lysis buffer, the agarose beads were mixed with 1× SDS sample buffer, and the protein was resolved by SDS-PAGE.
Assay for lipolysis.
Lipolysis was measured as the rate of glycerol and FFA release. Ten days after differentiation, 3T3-L1 adipocytes in six-well plates were infected with different adenoviruses, and the next morning cells were washed twice with serum- and phenol red-free DMEM containing 2% FA-free BSA and then incubated in 2.5 ml of the same medium in the presence or absence of 10 μM isoproterenol. Aliquots of the culture medium were collected after 3 h. The amounts of glycerol and FFAs released were determined by using a lipolysis assay kit (Zenbio) according to the manufacturer's instructions. Lysates were then prepared from the remaining cells. Protein concentrations in the lysates were determined and used to normalize the lipolytic signals. ATGL and its variant expressions were checked by Western blot to insure comparable expression.
In-gel digestion and mass spectrometry.
The immunoprecipitated samples were boiled in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and resolved by 4–15% 1D-SDS-PAGE. The proteins were then visualized by Coomassie blue staining (Sigma, St. Louis, MO). In-gel digestion and mass spectrometry were performed as described previously (25). Briefly, the gel portions containing Myc-ATGL were excised, destained, dehydrated, dried, and subjected to trypsin digestion overnight. The resultant peptides were desalted and analyzed by on-line HPLC on a linear trap quadrupole-Fourier transform ion cyclotron resonance (LTQ-FTICR). Phosphorylation sites were located using Scaffold PTM (version 1.0.3; Proteome Software, Portland, OR), a program based on the Ascore algorithm. Sites with Ascore ≥13 (P ≤ 0.05) were considered confidently localized.
Statistical analysis.
All data are presented as means ± SE. Statistical analysis was performed using one-way ANOVA. Bonferroni post hoc analysis was performed where appropriate. Statistical significance was set a priori at P < 0.05.
RESULTS
Myc-tag does not affect ATGL function.
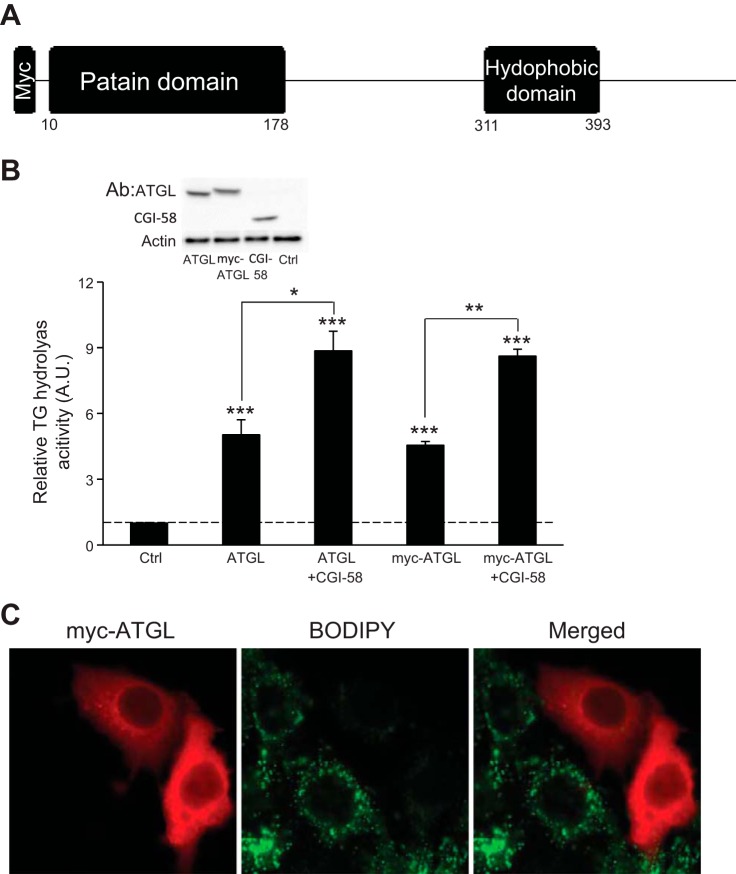
To obtain a sufficient amount of protein for phosphorylation mapping, we employed a strategy in which epitope-tagged fusion protein is expressed in cells and then immunoisolated from cell extracts with a tag-specific antibody. To ensure that such a tag would not lead to functional interference, we constructed plasmids that contained mouse ATGL cDNA without any epitope tag and fused at the NH2 terminus with a Myc epitope (Fig. 1A). We transfected HeLa cells with an empty vector, a nontagged ATGL, or the Myc-ATGL plasmid in the presence or absence of a CGI-58 plasmid. As shown in Fig. 1B, transfection with nontagged ATGL and Myc-ATGL resulted in expression of ectopic ATGL protein at similar levels as well as a nearly 4.5- and 4.2-fold increase, respectively, in the intracellular TG hydrolase activity over that with an empty vector. Moreover, coexpression of CGI-58 was able to similarly activate the activity of nontagged and Myc-tagged ATGL (Fig. 1B). To evaluate the effects on LD turnover, we examined the morphological changes in the intracellular LDs by staining cells with Bodipy 493/503, a nonpolar probe selective for neutral lipids such as TGs. HeLa cells accumulated multiple small LDs upon treatment with oleic acid (Fig. 1C). Expression of Myc-ATGL, as revealed by immunofluorescence staining, resulted in a drastic reduction in both the size and the number of LDs in the transfected cells compared with those in the neighboring untransfected cells (Fig. 1C). Taken together, these results demonstrate that Myc-tagged ATGL retains intact TG hydrolase activity as well as ability to mediate LD turnover in living cells.
Fig. 1.
Myc-adipose triglyceride lipase (ATGL) has intact triacylglycerol (TG) hydrolase activity. A: schematic structure of Myc-tagged mouse ATGL with functional domains. B: extracts of HeLa cells transfected with control (Ctrl) vector, ATGL, Myc-ATGL, or comparative gene identification-58 (CGI-58) were mixed in various combinations. TG hydrolase activity was measured using 3H-labeled triolein as substrate and normalized with the total protein levels of the cell extracts. Data are shown as means ± SD and represent 3 independent experiments.*P < 0.05; **P < 0.01; ***P < 0.001. Expression of ATGL, Myc-ATGL, and CGI-58 were analyzed by anti-ATGL/CGI-58 immunoblotting, using β-actin as a loading control. C: HeLa cells transfected with Myc-ATGL were incubated under growth conditions with 400 μM oleic acid complexed to albumin for 16 h. Immunofluorescence staining with anti-Myc antibody (red) was performed to reveal Myc-ATGL. Lipid droplets (LDs) were costained with Bodipy 493/503 fluorescence dye (green).
Identification of phosphorylation sites in ATGL in adipocytes.
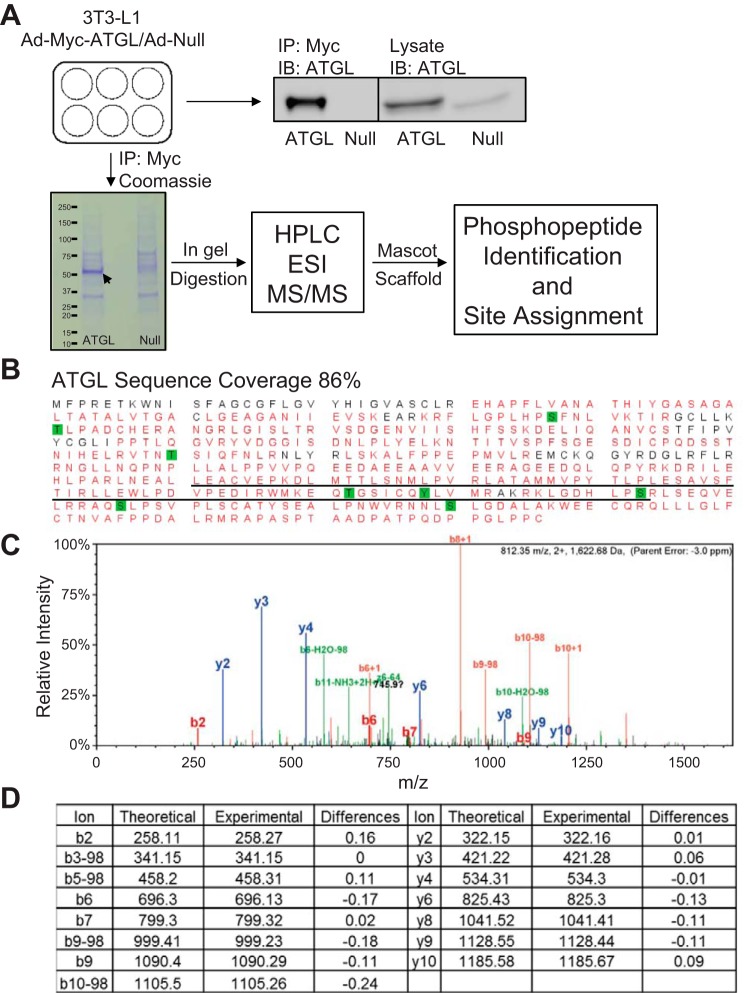
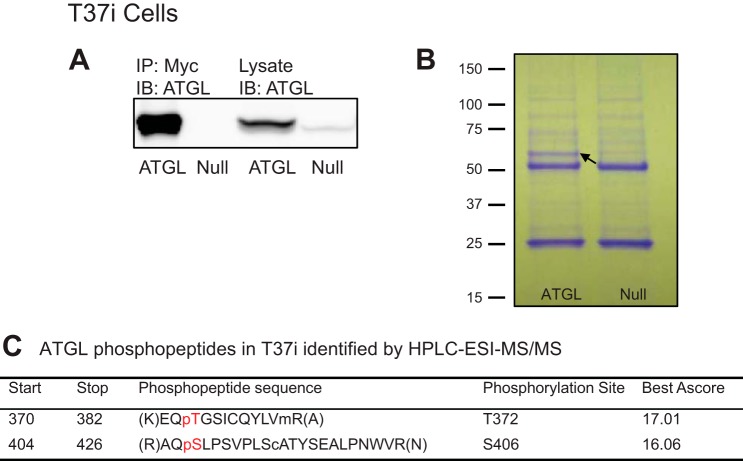
Since lipolysis is mediated by ATGL in white and brown adipocytes, we next attempted to comprehensively map the ATGL phosphorylation sites in both cell types according to an experimental design depicted in Fig. 2A. To this end, we overexpressed Myc-ATGL in fully differentiated 3T3-L1 and T37i adipocytes. 3T3-L1 adipocytes have been used extensively as a model system for investigation of ATGL-mediated lipolysis. T37i cells, on the other hand, have served as a valid model for brown adipocytes (26). To achieve high-efficiency expression, we infected adipocytes with a recombinant adenovirus encoding Myc-tagged ATGL (Ad-Myc-ATGL) that was under the control of a CMV promoter. A null virus (Ad-null) was used as a control. Following adenoviral infection, cells were extracted, and Myc-ATGL protein was isolated by immunoprecipitation. Immunoblotting analysis showed that Ad-Myc-ATGL-infected cells expressed Myc-ATGL at a level that was significantly greater than that of endogenous protein in Ad-null-infected cells (Figs. 2A and 3A). In addition, only Myc-ATGL was immunoprecipitated specifically by anti-Myc immunoprecipitation. Coomassie blue stain clearly revealed the presence of a protein band matching the molecular weight of Myc-ATGL in samples isolated from Ad-Myc-ATGL-infected cells but not from Ad-null-infected cells (Figs. 2A and 3B). The gel areas corresponding to this band were excised and then subjected to trypsin digestion followed by HPLC-electrospray ionization (ESI)-mass spectrometry (MS)/MS analysis. For the protein band isolated from 3T3-L1 adipocytes, the resultant spectrum confirmed the presence of ATGL with 86% sequence coverage (Fig. 2B). Table 1 lists the ATGL phosphopeptides detected by HPLC-ESI-MS/MS and their respective predominant phosphorylation sites. A total of eight phosphorylation sites were detected. Aside from Ser406 and Ser430, which were reported previously (1, 2, 21), Ser87, Thr101, Thr210, Thr372, Tyr378, and Ser393 appeared to be novel phosphorylation sites in the mouse ATGL. Among them, five are conserved in human protein, except for Thr101. The MS/MS spectra for the peptide containing phosphorylated Thr372 as well as the theoretical and experimental m/z values for detected fragment ions are shown in Fig. 2, C and D, respectively. For the Myc-ATGL protein band isolated from T37i brown adipocytes, HPLC-ESI-MS/MS analysis identified phosphopeptides containing Thr372 and Ser406 (Fig. 3B). Therefore, ATGL is phosphorylated on Thr372 and Ser406 in both 3T3-L1 and T37i adipocytes.
Fig. 2.
Comprehensive view of ATGL phosphorylation in 3T3-L1 adipocytes by HPLC-ESI-mass spectrometry/mass spectrometry (MS/MS). A: workflow for identification of ATGL phosphopeptide using adenoviral infection, followed by either immunoprecipitation (IP)/SDS-PAGE/immuoblotting (IB) or IP/SDS-PAGE/Coomassie stain in-gel digestion and mass spectrometric analysis. The Coomassie band for Myc-ATGL protein is indicated by the arrow. B: combined coverage map of ATGL, including unmodified and phosphorylated peptides, was obtained. Detected peptides are in red, serine/threonine/tyrosine phosphorylation sites are highlighted in green, and the LD-targeting hydrophobic domain is underlined. Results are combined experiments using 3T3-L1 adipocytes (n = 8). C: tandem mass spectrum of a novel phosphorylation site, p-Thr372, in ATGL. D: the theoretical and experimental m/z values for detected fragment ions of ATGL peptide containing p-Thr372. Loss of H3PO4 (−98 units) from the indicated fragment.
Fig. 3.
Identification of ATGL phosphorylation sites in T37i brown adipocytes. T37i adipocytes were infected with either Ad-Myc-ATGL or Ad-null virus. A: Myc-ATGL was detected in cell extracts and anti-Myc immunoprecipitates by immunoblotting. B: immunoprecipitated proteins were resolved by SDS-PAGE and stained with Coomassie blue dye. The band for Myc-ATGL protein is indicated by the arrow. C: results from HPLC-ESI-MS/MS analysis. The preceding p indicates phosphorylated amino acid. Sites with Ascore of >13 have a P value of <0.05 and >95% certainty for correct phosphorylation site localization. Sites with Ascore of >19 have a P value of <0.01 and >99% certainty for correct phosphorylation site localization (3, 11, 28).
Table 1.
ATGL phosphopeptides identified by HPLC-ESI-MS/MS
| Phosphopeptide Sequence | Start | Stop | Phosphorylation Site | Best Ascore |
|---|---|---|---|---|
| (R)FLGPLHPpSFNLVK(T) | 72 | 92 | S87* | 1000 |
| (K)pTLPADCHER(A) | 101 | 109 | T101* | 1000 |
| (R)VTNpTSIQFNLR(N) | 207 | 217 | T210* | 17.01 |
| (R)KLGDHLPpSRLSEQVELR(R) | 386 | 402 | S393* | 15.01 |
| (K)EQpTGSICQYLVmR(A) | 370 | 382 | T372* | 20.41 |
| (K)EQTGSICQpYLVmR(A) | 370 | 382 | Y378* | 53.26 |
| (R)AQpSLPSVPLSCATYSEALPNWVR(N) | 404 | 426 | S406 | 20.81 |
| (R)NNLpSLGDALAK(W) | 427 | 437 | S430 | 1000 |
ATGL, adipose triglyceride lipase; ESI; electrospray ionization; MS, mass spectrometry. The preceding p in each phosphopeptide sequence indicates phosphorylated amino acid.
The role of Thr372 in the LD localization of ATGL and ATGL-mediated degradation of LDs.
The identification of Thr372 as a novel ATGL phosphorylation site prompted us to investigate the functional relevance of this phosphorylation. To mimic its phosphorylated state, we generated a Thr372 to the Asp (T372D) point mutant of ATGL. In parallel, we also constructed a Thr to Ala (T372A) mutant to represent the nonphosphorylatable form. We expressed Myc-tagged wild-type ATGL, ATGL/T372D, or ATGL/T372A in HeLa cells and first determined their respective TG hydrolase activity. As shown in Fig. 4A, the total TG hydrolase activity was similar in the extracts of cells transfected with different variants of ATGL. Additionally, activation of ATGL/T372A mutant by CGI-58 was comparable with that of wild-type protein, whereas there was a modest reduction in the activation of ATGL/T372D by CGI-58 (Fig. 4A). The results indicate that phosphorylation of Thr372 does not considerably influence the TG hydrolase activity of ATGL regardless of the presence of CGI-58.
Fig. 4.
Localization and activity of Thr372 variants of ATGL in HeLa cells. A: extracts of HeLa cells transfected with empty vector or different variants of Myc-ATGL were mixed with extracts containing or not containing CGI-58. TG hydrolase activity against 3H-labeled triolein was measured and normalized with the total protein levels of the cell extracts. The activities resulting from ATGL overexpression were calculated based on the differential between the activity detected in cells transfected with empty vector and the activity in ATGL-transfected cells. Data are shown as means ± SD and represent 3 independent experiments. B: HeLa cells transfected with different Myc-ATGL variants were incubated under growth conditions with 400 μM of oleic acid for 16 h. Immunofluorescence staining with anti-Myc antibody (red) was performed to reveal Myc-ATGL. LDs were costained with Bodipy 493/503 dye (green). C: relative green fluorescent signal (Bodipy) per cell in cells transfected with different Myc-ATGL variants. Data are shown as means ± SD and represent 3 independent images. *P < 0.05. WT, wild type; AU, arbitrary units.
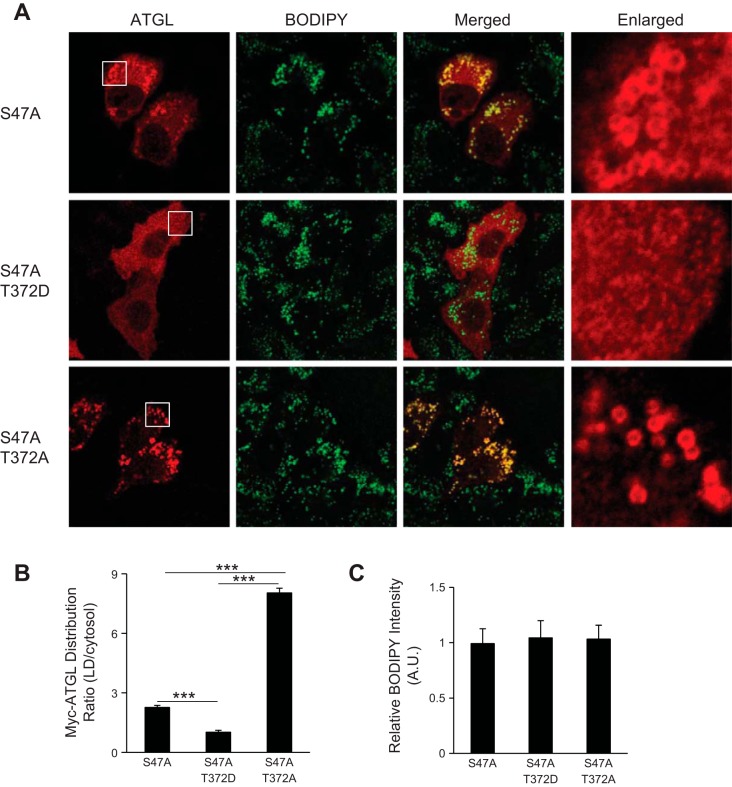
We also examined by immunofluorescence microscopy whether the mutations of Thr372 would affect the ability of ATGL to degrade LDs. Upon oleic acid loading, HeLa cells transfected with either wild-type ATGL or ATGL/T372A showed a marked reduction in both the size and the number of LDs compared with the adjacent untransfected cells (Fig. 4, B and C). Interestingly, expression of ATGL/T372D mutant was unable to cause similar degradation of LDs. To further study the involvement of Thr372 in the localization of ATGL to the surface of LDs, we mutated the active Ser47 in the GXSXG catalytic motif to eliminate the TG hydrolase activity from all three variants of ATGL. As expected, ATGL/S47A single mutant was localized mainly at the surface of LDs along with a small portion distributed diffusively throughout the cytoplasm (Fig. 5, A and B). By contrast, ATGL/S47A/T372D resided diffusively throughout the cytoplasm with no noticeable presence at the LD surface. ATGL/S47A/T372A double mutant, on the other hand, was localized predominantly to LDs, and no obvious cytoplasm localization was observed (Fig. 5, A and B). These results suggest that the LD localization of ATGL may indeed depend on the phosphorylation status of Thr372. There were no significant changes in the total intensity of Bodipy staining in cells expressing these different ATGL mutants (Fig. 5C), indicating similar capacity of these cells to accumulate TGs.
Fig. 5.
ATGL/T372D loses LD localization in HeLa cells. A: HeLa cells transfected with ATGL/S47A, ATGL/S47A/T372D, or ATGL/S47A/T372A were incubated under growth conditions with 400 μM oleic acid for 16 h. Immunofluorescence staining with anti-Myc (red) antibody was performed. LDs were costained with Bodipy 493/503 dye (green). B: relative intensity of red immunofluorescence (anti-Myc) on LD surface and cytosol in cells transfected with different Myc-ATGL variants. Data are shown as means ± SD and represent 4 independent cell images. ***P < 0.001. C: relative fluorescent intensity of Bodipy per cell in cells transfected with different Myc-ATGL variants. Data are shown as means ± SD and represent 3 independent images.
The impact of Thr372 phosphorylation on ATGL subcellular localization in adipocytes.
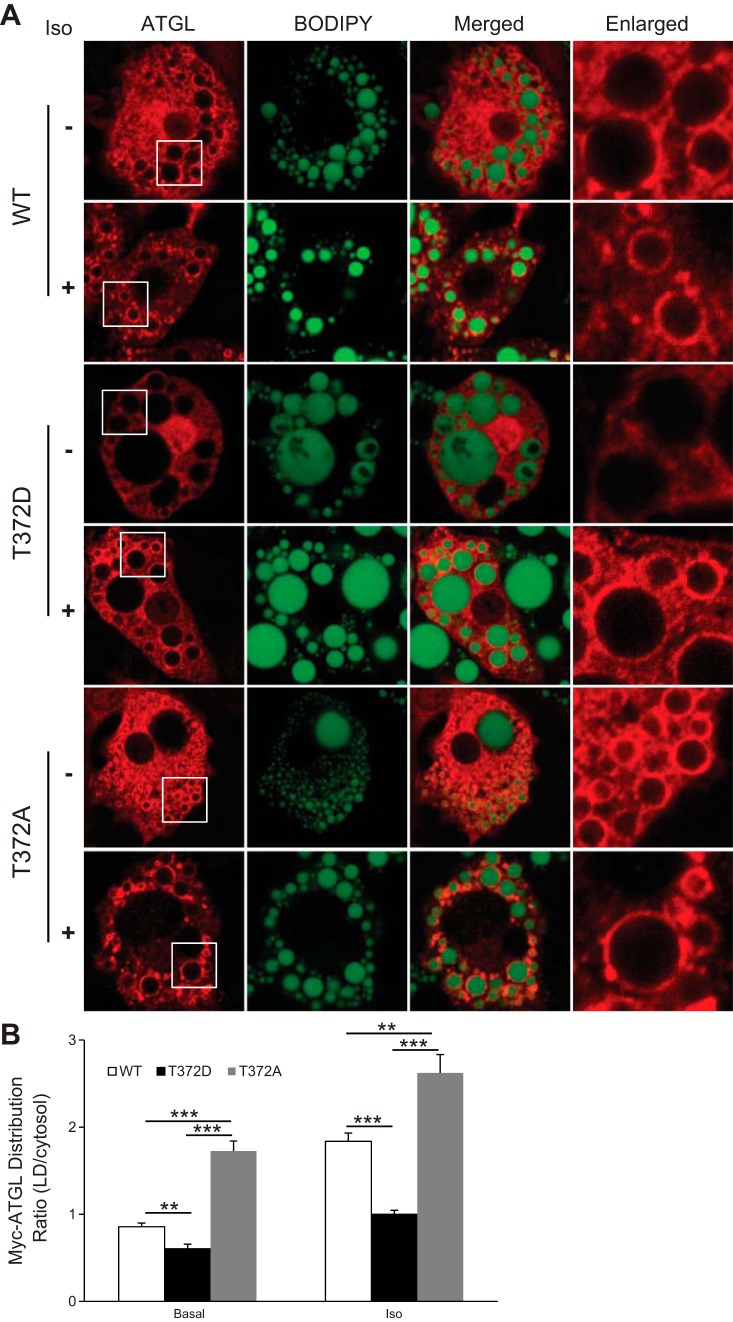
To examine the role of Thr372 phosphorylation in LD targeting of ATGL in adipocytes, we generated recombinant adenoviruses encoding Myc-tagged ATGL/T372D and ATGL/T372A. After a 24-h infection, 3T3-L1 adipocytes were treated with or without the β-adrenergic agonist isoproterenol and then analyzed by immunofluorescence microscopy (Fig. 6, A and B). In the basal state, wild-type ATGL displayed a diffusive cytosolic distribution pattern along with a weak rim-like staining surrounding LDs. In comparison, ATGL/T372D exhibited a diffusive distribution pattern with no basal presence around the LDs (Fig. 6, A and B). Isoproterenol treatment expectedly caused a marked relocalization of wild-type ATGL to LDs. As a result, considerably fewer amounts of wild-type ATGL remained in the cytoplasm after the treatment. In contrast, a large portion of ATGL/T372D was still localized cytoplasmically after the stimulation, although there was an observable LD translocation (Fig. 6, A and B). In comparison, T372A mutation resulted in a constitutive localization of ATGL to the LDs independently of lipolytic stimulation. Therefore, phosphorylation of Thr372 may play a critical role in deciding both basal and hormone-responsive localization of ATGL to LDs in adipocytes.
Fig. 6.
Effect of Thr372 mutations on LD localization of ATGL in 3T3-L1 adipocytes. A: 3T3-L1 adipocytes were infected with adenovirus encoding Myc-tagged WT ATGL, ATGL/T372D, or ATGL/T372A; 24 h later, cells were treated with or without 1 μM of isoproterenol for 15 min. Immunofluorescence staining with anti-Myc (red) antibody was performed to reveal localization of ATGL. LDs were costained with Bodipy 493/503 fluorescence dye (green). B: relative intensity of red immunofluorescence (anti-myc) on LD surface and cytosol in cells transfected with different Myc-ATGL variants. Data are shown as means ± SD and represent 4 independent cell images. **P < 0.01; ***P < 0.001.
Effect of Thr372 mutations on ATGL-mediated adipocyte lipolysis.
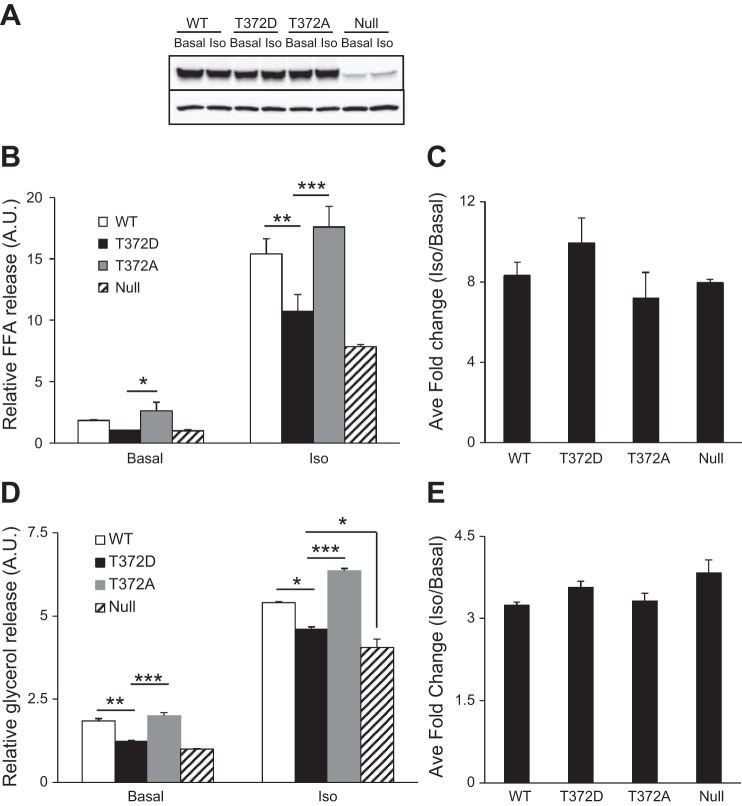
To investigate the potential impact of Thr372 phosphorylation on lipolysis, we infected 3T3-L1 adipocytes singly with Ad-Myc-ATGL or its phosphorylated variants. Cells infected with Ad-Null virus were used as a control. As shown in Fig. 7A, infection with Ad-Myc-ATGL led to a profound increase in the expression of ATGL when compared with the levels of endogenous protein in Ad-null-infected cells. Comparable expression was observed in cells infected with virus encoding either ATGL/T372D or ATGL/T372A (Fig. 7A). Basal FFA release from cells expressing wild-type ATGL and ATGL/T372A was increased by 85 and 163%, respectively, compared with that from Ad-Null infected cells (Fig. 7B). However, there was no significant increase in basal FFA release from cells expressing the T372D variant. In response to isoproterenol stimulation, FFA releases were increased by 96, 124, and 37% in cells expressing wild-type ATGL, ATGL/T372A, and ATGL/T372D, respectively (Fig. 7B). The effects of ATGL and its variants on glycerol release followed a similar trend (Fig. 7D). Thus, under both basal and stimulated conditions, T372A mutation promotes whereas T372D mutation attenuates the lipolytic action of ATGL in adipocytes. Furthermore, no significant changes were observed in the fold stimulation of different ATGL variants by isoproterenol (Fig. 7, C and E), suggesting that additional mechanisms are involved in regulating the responsiveness of ATGL action to β-adrenergic stimulation.
Fig. 7.
Effect of Thr372 mutations on basal and stimulated lipolysis in 3T3-L1 adipocytes. 3T3-L1 adipocytes were infected with adenovirus encoding Myc-tagged WT ATGL, ATGL/T372D, or ATGL/T372A; 24 h later, cells were treated with or without 1 μM of isoproterenol for 3 h. A: expression of WT ATGL and the mutants were analyzed by anti-ATGL immunoblotting, using β-actin as a loading control. B and D: free fatty acids (FFA; B) and glycerol release (D) were measured and normalized with that from Ad-Null-infected cells. Data are shown as means ± SD and represent 3 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001. C and E: average fold change of FFA (C) and glycerol (E) release 3 h after isoproterenol stimulation.
DISCUSSION
The present study provides a global phosphorylation profile of ATGL in adipocytes with a proteomic approach. A total of eight phosphorylation sites, including Ser406 and Ser430, that were previously reported to be phosphorylated (1, 2, 21) were detected in ATGL expressed in 3T3-L1 and T37i adipocytes. Site-directed mutagenesis analysis found that phosphorylation of Thr372, residing in the COOH-terminal hydrophobic region, is important for ATGL subcellular distribution in LDs vs. cytoplasm. Importantly, our data demonstrate that Thr372 phosphorylation functions to prevent the LD localization of ATGL as well as ATGL-mediated TG hydrolysis in nonadipocyte cells as well as in adipocytes. To our knowledge, this represents the first evidence showing that ATGL can be negatively regulated by phosphorylation in mammalian cells.
Among the newly identified phosphorylation sites, Ser87, Thr101, and Thr210 reside in the NH2-terminal patatin-like domain of mouse ATGL. Ser87 and Thr210 are conserved in the human isoform, and the functional impact of their phosphorylation has yet to be elucidated. The remaining five phosphorylation sites are present in the COOH-terminal regulatory region. Ser406 and Thr372 are the only two sites whose phosphorylation was shared by ATGL expressed in 3T3-L1 white adipocytes and T37i brown adipocytes. Although earlier evidence indicates that phosphorylation of Ser406 positively regulates the activity of ATGL (1, 21), the kinase that mediates this phosphorylation remains a topic of debate. Previous work by Ahmadian et al. (1) has demonstrated that Ser406 is phosphorylated by AMPK, and the phosphorylation is required for lipolysis-linked FA oxidation and thermogenesis in BAT. However, recent studies by Pagnon et al. (21) have shown that Ser406 is a direct target of PKA instead of AMPK, and its phosphorylation in adipocytes is increased by β-adrenergic stimulation. Although our analysis qualitatively confirmed the phosphorylation of Ser406, we were unable to quantitatively analyze this phosphorylation in response to activation of either PKA or AMPK due to the limited recovery of the proteolytic peptides containing Ser406 (data not shown).
Thr372 is located in the COOH-terminal hydrophobic domain of ATGL. The sequence motif containing Thr372 does not resemble a canonical target for any known kinase. The hydrophobic region is a putative lipid-binding region that has been well known for its role in targeting ATGL to LDs (6, 17, 23). ATGL mutations have been identified in humans with neutral lipid storage disease characterized by excessive TG deposition in nonadipose tissues and myopathy (8, 22). Most such mutations result in premature stop codons, leaving the catalytic site of the enzyme intact while generating truncated proteins lacking the COOH-terminal region. Whole cell TG hydrolase activity is often retained, whereas the LD-associated ability is lost (8). Likewise, the internal deletion mutant lacking the hydrophobic domain (amino acids 315–360) was disabled in the binding to LDs (17), whereas fusing the COOH-terminal region of ATGL conferred LD localization to green fluorescent protein (19). By using phosphorylated mimicking and nonphosphorylatable mutants, we showed here that phosphorylation of Thr372 in the hydrophobic region was involved in the regulation of ATGL localization to LDs. As observed previously with an ATGL variant lacking the hydrophobic mutant (17), point mutations of Thr372 elicited no effect on the TG hydrolase activity of ATGL against triolein substrate in vitro. Instead, the phosphorylation-mimicking mutant T372D, when expressed in HeLa cells, was disabled in both binding to LDs and mediating LD degradation. By contrast, the nonphosphorylatable mutant T372A exhibited a full LD-degrading capability like the wild-type protein. The finding that the lipase-dead double mutants (i.e., S47A/T372D and S47A/T372A) exhibited completely opposite LD localization implicates that Thr372 phosphorylation can regulate ATGL's ability to degrade LDs through influencing its recruitment to LDs.
Two lines of evidence are in support of a similar regulatory role of Thr372 phosphorylation in adipocytes. First, similar to what was observed in HeLa cells, overexpressed T372D was retained in the cytoplasm of 3T3-L1 adipocytes in the basal state, whereas T372A mutant tended to be enriched at the surface of LDs under the same condition. In accordance, T372A mutant possessed a greater ability than the wild-type protein to mediate basal lipolysis. By contrast, such ability was compromised when Thr372 was mutated to the phosphorylation-mimicking Asp. Second, the Asp mutation in ATGL attenuated its lipolytic action in response to β-adrenergic stimulation. We speculate that the decreased capacity of T372D to mediate hormone-stimulated lipolysis may be due to its inefficient LD translocation, considering that this mutation did not affect coactivation of ATGL by CGI-58. On the other hand, it is noteworthy that the LD translocation of ATGL in response to stimulation was not completely abolished by T372D mutation. The fact that expression of neither of Thr372 variant impacted the fold stimulation of lipolysis by isoproterenol also suggests that additional mechanisms are involved in mediating the action of ATGL in response to β-adrenergic signals. In this regard, our recent study has shown that ATGL translocates to LDs via a perilipin 1-dependent mechanism (3). It is possible that such mechanism may help at least partially overcome the hindrance caused by Thr372 phosphorylation.
In conclusion, with mass spectrometry and mutagenesis approaches, we have identified Thr372 as a novel phosphorylation site that plays a critical role in the control of LD localization of ATGL. Although the phosphorylation of ATGL ortholog in Caenorhabditis elegans and yeast has been known to inhibit lipolysis (14, 20), this is the first evidence that phosphorylation of ATGL can negatively regulate its lipolytic capacity in mammalian cells. It would be tempting to hypothesize that Thr372 phosphorylation is elevated in the basal or fed state and downregulated in response to conditions that stimulate lipolysis. In this regard, further studies are necessary to determine the nutritional and hormonal cues that regulate phosphorylation/dephosphorylation of Thr372 as well as the responsible kinase(s). The success of such studies may depend on the development of a phosphorylation-specific antibody, since the high hydrophobicity of the Thr372 peptide has prevented in our hands its consistent HPLC recovery and mass spectrometric quantification. Furthermore, although the presence of Thr372 within the LD-targeting domain of ATGL strongly suggests a direct impact of its phosphorylation on LD localization, the possibility of such an effect resulting from phosphorylation at other positions cannot be fully excluded. The functional roles of other identified phosphorylation sites also are interesting subjects for future investigation.
GRANTS
This work was supported by research grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-089178) and the American Diabetes Association (1-10-JF-30) to J. Liu.
DISCLOSURES
All authors declare that they have no conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
X.X., P.L., X.Z., B.L.H., L.J.M., and J.L. conception and design of research; X.X., X.Z., B.L.H., and A.M.S. performed experiments; X.X., P.L., L.J.M., and J.L. analyzed data; X.X., P.L., X.Z., B.L.H., and J.L. interpreted results of experiments; X.X. prepared figures; X.X. and J.L. drafted manuscript; X.X. and J.L. edited and revised manuscript; X.X., P.L., X.Z., B.L.H., A.M.S., L.J.M., and J.L. approved final version of manuscript.
REFERENCES
- 1.Ahmadian M, Abbott MJ, Tang T, Hudak CS, Kim Y, Bruss M, Hellerstein MK, Lee HY, Samuel VT, Shulman GI, Wang Y, Duncan RE, Kang C, Sul HS. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab 13: 739–748, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartz R, Zehmer JK, Zhu M, Chen Y, Serrero G, Zhao Y, Liu P. Dynamic activity of lipid droplets: protein phosphorylation and GTP-mediated protein translocation. J Proteome Res 6: 3256–3265, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Beausoleil SA, Villen J, Gerber SA, Rush J, Gygi SP. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat Biotechnol 24: 1285–1292, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Bezaire V, Mairal A, Ribet C, Lefort C, Girousse A, Jocken J, Laurencikiene J, Anesia R, Rodriguez AM, Ryden M, Stenson BM, Dani C, Ailhaud G, Arner P, Langin D. Contribution of adipose triglyceride lipase and hormone-sensitive lipase to lipolysis in hMADS adipocytes. J Biol Chem 284: 18282–18291, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brasaemle DL, Levin DM, Adler-Wailes DC, Londos C. The lipolytic stimulation of 3T3-L1 adipocytes promotes the translocation of hormone-sensitive lipase to the surfaces of lipid storage droplets. Biochim Biophys Acta 1483: 251–262, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Cornaciu I, Boeszoermenyi A, Lindermuth H, Nagy HM, Cerk IK, Ebner C, Salzburger B, Gruber A, Schweiger M, Zechner R, Lass A, Zimmermann R, Oberer M. The minimal domain of adipose triglyceride lipase (ATGL) ranges until leucine 254 and can be activated and inhibited by CGI-58 and G0S2, respectively. PLoS One 6: e26349, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egan JJ, Greenberg AS, Chang MK, Wek SA, Moos MC, Jr, Londos C. Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet. Proc Natl Acad Sci USA 89: 8537–8541, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer J, Lefevre C, Morava E, Mussini JM, Laforet P, Negre-Salvayre A, Lathrop M, Salvayre R. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat Genet 39: 28–30, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Granneman JG, Moore HP, Granneman RL, Greenberg AS, Obin MS, Zhu Z. Analysis of lipolytic protein trafficking and interactions in adipocytes. J Biol Chem 282: 5726–5735, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Granneman JG, Moore HP, Krishnamoorthy R, Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl). J Biol Chem 284: 34538–34544, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huttlin EL, Jedrychowski MP, Elias JE, Goswami T, Rad R, Beausoleil SA, Villen J, Haas W, Sowa ME, Gygi SP. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 143: 1174–1189, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolditz CI, Langin D. Adipose tissue lipolysis. Curr Opin Clin Nutr Metab Care 13: 377–381, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Krahmer N, Farese RV, Jr, Walther TC. Balancing the fat: lipid droplets and human disease. EMBO Mol Med 5: 905–915, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurat CF, Wolinski H, Petschnigg J, Kaluarachchi S, Andrews B, Natter K, Kohlwein SD. Cdk1/Cdc28-dependent activation of the major triacylglycerol lipase Tgl4 in yeast links lipolysis to cell-cycle progression. Mol Cell 33: 53–63, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab 3: 309–319, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res 50: 14–27, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu X, Yang X, Liu J. Differential control of ATGL-mediated lipid droplet degradation by CGI-58 and G0S2. Cell Cycle 9: 2719–2725, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mason RR, Meex RC, Lee-Young R, Canny BJ, Watt MJ. Phosphorylation of adipose triglyceride lipase Ser404 is not related to 5′-AMPK activation during moderate-intensity exercise in humans. Am J Physiol Endocrinol Metab 303: E534–E541, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Murugesan S, Goldberg EB, Dou E, Brown WJ. Identification of diverse lipid droplet targeting motifs in the PNPLA family of triglyceride lipases. PLoS One 8: e64950, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narbonne P, Roy R. Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature 457: 210–214, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Pagnon J, Matzaris M, Stark R, Meex RC, Macaulay SL, Brown W, O'Brien PE, Tiganis T, Watt MJ. Identification and functional characterization of protein kinase A phosphorylation sites in the major lipolytic protein, adipose triglyceride lipase. Endocrinology 153: 4278–4289, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Schweiger M, Lass A, Zimmermann R, Eichmann TO, Zechner R. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am J Physiol Endocrinol Metab 297: E289–E296, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Schweiger M, Schoiswohl G, Lass A, Radner FP, Haemmerle G, Malli R, Graier W, Cornaciu I, Oberer M, Salvayre R, Fischer J, Zechner R, Zimmermann R. The C-terminal region of human adipose triglyceride lipase affects enzyme activity and lipid droplet binding. J Biol Chem 283: 17211–17220, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Hu L, Dalen K, Dorward H, Marcinkiewicz A, Russell D, Gong D, Londos C, Yamaguchi T, Holm C, Rizzo MA, Brasaemle D, Sztalryd C. Activation of hormone-sensitive lipase requires two steps, protein phosphorylation and binding to the PAT-1 domain of lipid droplet coat proteins. J Biol Chem 284: 32116–32125, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie X, Yi Z, Bowen B, Wolf C, Flynn CR, Sinha S, Mandarino LJ, Meyer C. Characterization of the Human Adipocyte Proteome and Reproducibility of Protein Abundance by One-Dimensional Gel Electrophoresis and HPLC-ESI-MS/MS. J Proteome Res 9: 4521–4534, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang X, Lu X, Lombes M, Rha GB, Chi YI, Guerin TM, Smart EJ, Liu J. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab 11: 194–205, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, Madeo F. FAT SIGNALS—lipases and lipolysis in lipid metabolism and signaling. Cell Metab 15: 279–291, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhai B, Villen J, Beausoleil SA, Mintseris J, Gygi SP. Phosphoproteome analysis of Drosophila melanogaster embryos. J Proteome Res 7: 1675–1682, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306: 1383–1386, 2004 [DOI] [PubMed] [Google Scholar]