Abstract
The present project was designed to investigate phosphorylation of p70S6K1 in an animal model of skeletal muscle overload. Within 24 h of male Sprague-Dawley rats undergoing unilateral tenotomy to induce functional overloading of the plantaris muscle, phosphorylation of the Thr389 and Thr421/Ser424 sites on p70S6K1 was significantly elevated. Since the Thr421/Ser424 sites are purportedly mammalian target of rapamycin complex 1 (mTORC1) independent, we sought to identify the kinase(s) responsible for their phosphorylation. Initially, we used IGF-I treatment of serum-deprived HEK-293E cells as an in vitro model system, because IGF-I promotes phosphorylation of p70S6K1 on both the Thr389 and Thr421/Ser424 sites in skeletal muscle and in cells in culture. We found that, whereas the mTOR inhibitor TORIN2 prevented the IGF-I-induced phosphorylation of the Thr421/Ser424 sites, it surprisingly enhanced phosphorylation of these sites during serum deprivation. JNK inhibition with SP600125 attenuated phosphorylation of the Thr421/Ser424 sites, and in combination with TORIN2 both the effect of IGF-I and the enhanced Thr421/Ser424 phosphorylation during serum deprivation were ablated. In contrast, both JNK activation with anisomycin and knockdown of the mTORC2 subunit rictor specifically stimulated phosphorylation of the Thr421/Ser424 sites, suggesting that mTORC2 represses JNK-mediated phosphorylation of these sites. The role of JNK in mediating p70S6K1 phosphorylation was confirmed in the animal model noted above, where rats treated with SP600125 exhibited attenuated Thr421/Ser424 phosphorylation. Overall, the results provide evidence that the mTORC1 and JNK signaling pathways coordinate the site-specific phosphorylation of p70S6K1. They also identify a novel role for mTORC1 and mTORC2 in the inhibition of JNK.
Keywords: MAPK signaling, muscle hypertrophy, tenotomy
resistance exercise is a growth-promoting stimulus that leads to enhanced muscle fiber size and hypertrophy of mammalian skeletal muscle (6, 7, 17). The hypertrophic response results from stimulation of various pro-growth signaling pathways coupled with enhanced rates of protein synthesis (6, 7, 17, 18). One of the major signaling pathways essential in the hypertrophic growth response, is the serine/threonine kinase mechanistic target of rapamycin (mTOR) (15). This kinase functions in two distinct multi-protein complexes: mTOR in complex 1 (mTORC1), which contains the regulatory associated protein of mTOR (raptor) and proline-rich Akt substrate of 40 kDa (PRAS40); and mTOR in complex 2 (mTORC2), which contains rapamycin-insensitive companion of mTOR (rictor) and stress-activated MAP kinase-interacting protein-1 (Sin1) (15). Activation of mTORC1 enables the kinase to phosphorylate its well-established and characterized downstream effector the 70-kDa ribosomal protein S6 kinase (p70S6K1), which plays a vital role in the regulation of mRNA translation, cellular proliferation/growth, and muscle hypertrophy (11, 13, 16, 29, 33).
The kinase p70S6K1 regulates cell and organismal growth (28, 33) through phosphorylation of substrates that promote protein synthesis. While ribosomal protein S6 (rpS6) represents the most extensively studied substrate of p70S6K1, the kinase also phosphorylates eukaryotic initiation factor 4B, programed cell death protein-4, eukaryotic elongation factor 2 kinase, insulin receptor substrate 1, and rictor (8, 11, 14, 23, 26). Mice lacking p70S6K1 are smaller at birth and exhibit impaired growth, such that adults are significantly smaller than their wild-type littermates (36). Furthermore, Drosophila deficient in the S6 kinase gene present with delays in development and a reduction in body size due to reduced cell size compared with their wild-type counterparts (29). Thus, the coordinate activation of p70S6K1 likely plays a critical role in the growth-promoting processes associated with muscle hypertrophy.
Regulation of p70S6K1 involves complex multisite phosphorylation of at least seven residues (19). Full activation of the kinase requires phosphorylation of three critical sites: phosphorylation of the linker domain at the turn motif (Ser371) and hydrophobic motif (Thr389), as well as the catalytic domain at the activation loop (Thr229) (28). It is well established that mTORC1 is the protein kinase responsible for phosphorylating p70S6K1 at Thr389, however, the hierarchy of phosphorylation events that precede phosphorylation of Thr389 remain somewhat controversial. Like other AGC kinases, p70S6K1 exhibits a bilobal fold structure with coordinating NH2- and COOH-terminal lobes (31). Activation of p70S6K1 involves priming phosphorylation of multiple proline-directed sites (Ser411, Ser418, Thr421, and Ser424) located in a basic pseudo-substrate domain of the COOH-terminal lobe, which bears significant homology to the phosphorylated region of rpS6. In the inactive confirmation, this domain serves an autoinhibitory function by folding over the NH2-terminal lobe (4). Mitogen-induced phosphorylation of the pseudo-substrate domain relieves autoinhibition to induce a conformational change in p70S6K1 that facilitates phosphorylation at Thr389 by mTORC1, phosphorylation of Thr229 by phosphoinositide-dependent kinase-1 (PDK1), and ultimately full activation (32).
Thr421 and Ser424 represent the best-studied phosphorylation sites within the COOH-terminal autoinhibitory domain of p70S6K1, and currently available phosphospecific antibodies do not distinguish between the two. Phosphorylation of the Thr421/Ser424 combined sites is both rapamycin insensitive and independent of the TOR signaling (TOS) motif that is required for mTORC1-mediated phosphorylation of other substrates, including p70S6K1 Thr389 (34). Thus, it is presumed that mTORC1 is not responsible for phosphorylation of these sites. Both Thr421 and Ser424 are followed by a proline in the primary sequence of p70S6K1. A number of kinases phosphorylate serine or threonine residues when they are followed by a proline. Of the proline-directed kinases, the mitogen-activated protein kinases (MAPK) are of particular interest because they are present in skeletal muscle in multiple forms, including extracellular signal-regulated kinases (ERK)1 and -2, p38 MAPK, ERK5, big MAPK, and c-Jun NH2-terminal kinase (JNK) (24). Moreover, resistance exercise activates MAPKs and induces inflammatory-mediated responses that contribute to physiological processes such as muscle hypertrophy, lipid metabolism, insulin sensitivity, and glucose homeostasis (22, 24, 35).
The goal of the present study was to assess p70S6K1 phosphorylation in a model of skeletal muscle hypertrophy and to identify the kinase(s) responsible for the observed changes. We found that phosphorylation of the Thr389 and Thr421/Ser424 sites was enhanced within 24 h following functional overloading of the plantaris muscle (37). Of significance, we present evidence that the mTOR and JNK signaling pathways coordinate in the site-specific phosphorylation of p70S6K during stimulation of cell growth. Specifically, both mTORC1 and JNK contribute to the phosphorylation of the p70S6K1 autoinhibitory domain at Thr421/Ser424, whereas mTORC2 inhibits the phosphorylation of this domain by JNK.
MATERIALS AND METHODS
Animal care and use.
The Institutional Animal Care and Use Committee of The Pennsylvania State University College of Medicine approved the animal facilities and experimental procedures used in the present study. Adult male Sprague-Dawley rats (∼200–260 g) were housed in temperature- and humidity-controlled holding facilities. Prior to the tenotomy procedure, animals were acclimated to a reversed 12:12-h light-dark cycle (lights off at 0700) for 1 wk. Rats were provided standard rodent chow (Harlan-Teklad 8604, Indianapolis, IN) and water ad libitum.
Experimental design of animal studies.
One week prior to the experimental procedure, rats were provided standard rodent chow (Harlan-Teklad 8604) for a period of 3 h at the start of the dark cycle; water was provided ad libitum. On the day of the surgery (following the 3-h feeding period), rats were anesthetized with isoflurane (3%) supplemented with oxygen via nasal inhalation. Unilateral tenotomy was performed on each rat, as previously described (37), by severing the tendons of the soleus and gastrocnemius muscles at the Achilles to induce overload of the plantaris muscle. As an internal control, a sham surgical procedure was performed on the contralateral leg of the animals that included all procedures performed on the tenotomized leg except that the gastrocnemius and soleus tendons were not severed. Stainless steel surgical staples (Mik Ran Precision) and Vet-Bond Tissue Adhesive (3M Animal Care Products, St. Paul, MN) were then applied to each wound following the tenotomy or sham operation. Animals were administered a subcutaneous injection of an analgesic (Buprenex, 0.03 mg/kg body mass) 12 h postsurgery. Twenty-four hours after the tenotomy intervention, animals were again anesthetized, and the plantaris muscle from each leg was excised for subsequent analysis. Where indicated, rats received SP600125 (50 mg/kg body mass) dissolved in N-methyl-2-pyrrolidone subcutaneously (Sigma Aldrich, St. Louis, MO) or a vehicle-only control 6 h prior to the harvesting of muscle tissue.
Cell culture.
HEK-293E cells were seeded in 12-well Cell-Bind plates (Corning Cell Bind Surfaces, Corning, NY) or 100-mm culture dishes in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin for 48 h. Inducible raptor (iRapKO) and rictor (iRicKO) knockdown mouse embryonic fibroblasts (MEF) were kindly provided by Dr. Michael N. Hall (University of Basel). Raptor and rictor knockdown was induced by daily treatment of cells with 1 μM 4-hydroxytamoxifen (4-OHT) for 3 days prior to experimentation. Cells were serum deprived for 2 h, after which the following were added where indicated to the medium for 30 min: 10 nM TORIN2 (TOCRIS Bioscience), 10 μM U0126 (Promega), 50 μM SP600125 (Sigma-Aldrich), 10 μM SB203580, 20 mM anisomycin (Sigma-Aldrich), or 100 nM rapamycin (Sigma-Aldrich). For cell culture experiments, all inhibitors were prepared in the vehicle dimethyl sulfoxide (DMSO). As a control, cells were treated with DMSO in the absence of inhibitors. Cells were then treated with 10 ng/ml IGF-I as indicated for 30 min and harvested in 1× Laemmli sample buffer. Lysates were subjected to Western blot analysis as described in the next section. Where indicated, cell lysates were collected in Lambda phosphatase buffer containing 10 μl/ml Sigma Protease Inhibitor Cocktail, pH 7.4, and centrifuged at 1,000 g for 3 min, as previously described (12). Fifty microliters of the supernatant fraction was combined with 10 μl of Lambda phosphatase (New England BioLabs) for 1 h at 30°C, and the dephosphorylated sample was subjected to Western blot analysis.
Western blot analysis.
The plantaris was homogenized in 7 volumes of homogenization buffer consisting of 50 mM HEPES, pH 7.4, 0.1% Triton X-100, 4 mM EGTA, 10 mM EDTA, 15 mM sodium pyrophosphate, 100 mM β-glycerophosphate, and 25 mM sodium fluoride, as previously described (25). The homogenate was centrifuged at 2,000 g for 3 min at 4°C, and the resulting supernatant was combined with an equal volume of 2× Laemmli sample buffer. Muscle supernatant fractions and cell culture lysates were evaluated by Western blot analysis with primary antibodies that recognize the following proteins when they are phosphorylated on the specified residues: p70S6K1 Thr421/Ser424, p70S6K1 Thr389, c-Jun Ser63, Akt Ser473, p38 Thr180/Tyr182, ERK Thr202/Tyr204, and JNK Thr183/Tyr185, all of which were from Cell Signaling Technology. Antibodies against GAPDH and α-tubulin were from Santa Cruz Biotechnology.
Statistical analysis.
Data are presented as means ± SE. One- or two-way ANOVA was used to compare differences among groups. When statistically significant differences (P < 0.05) were observed by ANOVA, a Student's t-test was performed post hoc.
RESULTS
Tenotomy enhances phosphorylation of both the Thr389 and Thr421/Ser424 sites on p70S6K1.
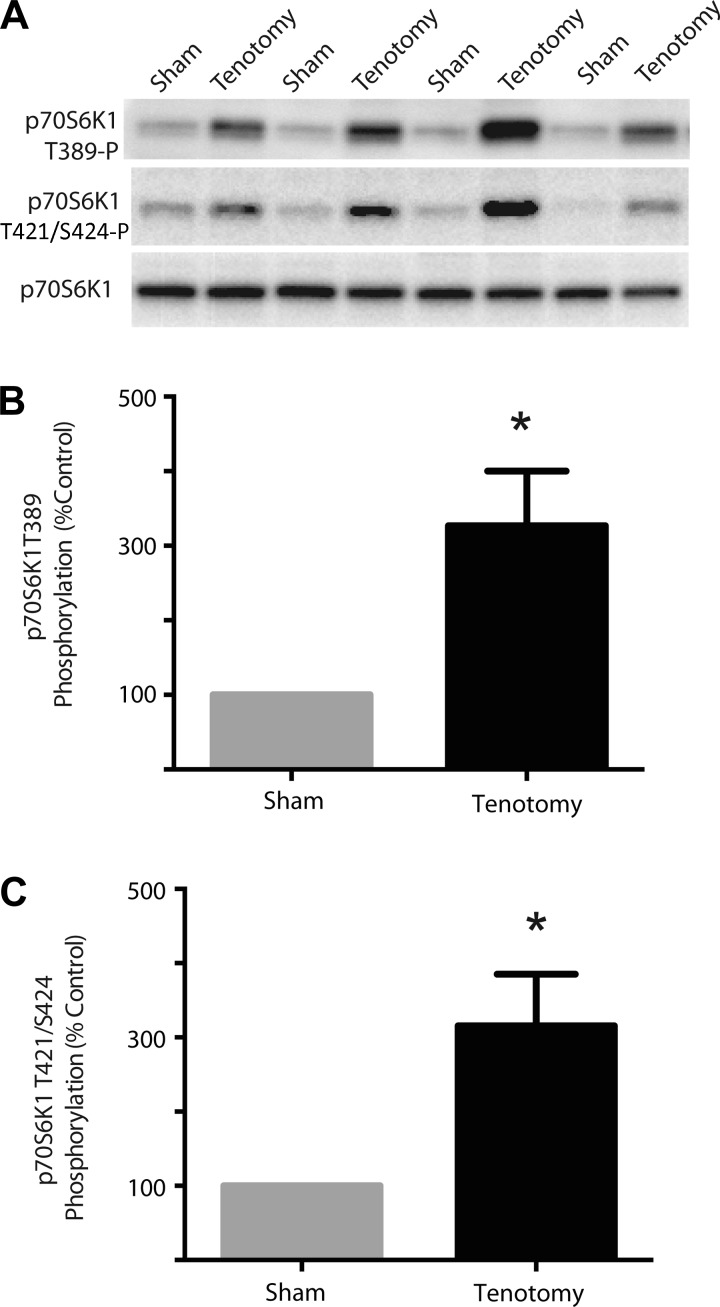
Phosphorylation of p70S6K1 at the Thr389 site in the plantaris muscle of animals that underwent tenotomy was enhanced to 327% of the contralateral limb (Fig. 1, A and B). Similarly, phosphorylation of p70S6K1 at the Thr421/Ser424 sites in the plantaris muscle of animals that underwent tenotomy was enhanced to 316% compared with the plantaris the contralateral limb (Fig. 1, A and C). Since phosphorylation of p70S6K1 at the Thr421/Ser424 sites is rapamycin insensitive and also occurs independently of the TOS motif in p70S6K1 (34), we sought to identify the pathway/kinases responsible for phosphorylation of these residues in response to functional overloading.
Fig. 1.
70-kDa rpS6 kinase-1 (p70S6K1) phosphorylation in plantaris muscle following tenotomy-induced hypertrophy. Plantaris from a tentomized and the contralateral limb were harvested 24 h following the surgical procedure to induce functional overload. Muscle samples were then prepared for analysis of p70S6K1 phosphorylation. A: Western blot analysis of supernatant fractions of muscle homogenates using the phosphospecific anti-Thr389 and -Thr421/Ser424 p70S6K1 antibodies as well as a total p70S6K1 antibody. Quantitation of the results for p70S6K1 phosphorylation at the Thr389 (B) and Thr421/Ser424 sites (C) expressed relative to total p70S6K1. Results represent means ± SE (n = 7). Statistical significance: *P < 0.05 vs. sham.
Inhibition of mTOR enhances p70S6K1 phosphorylation at the Thr421/Ser424 sites.
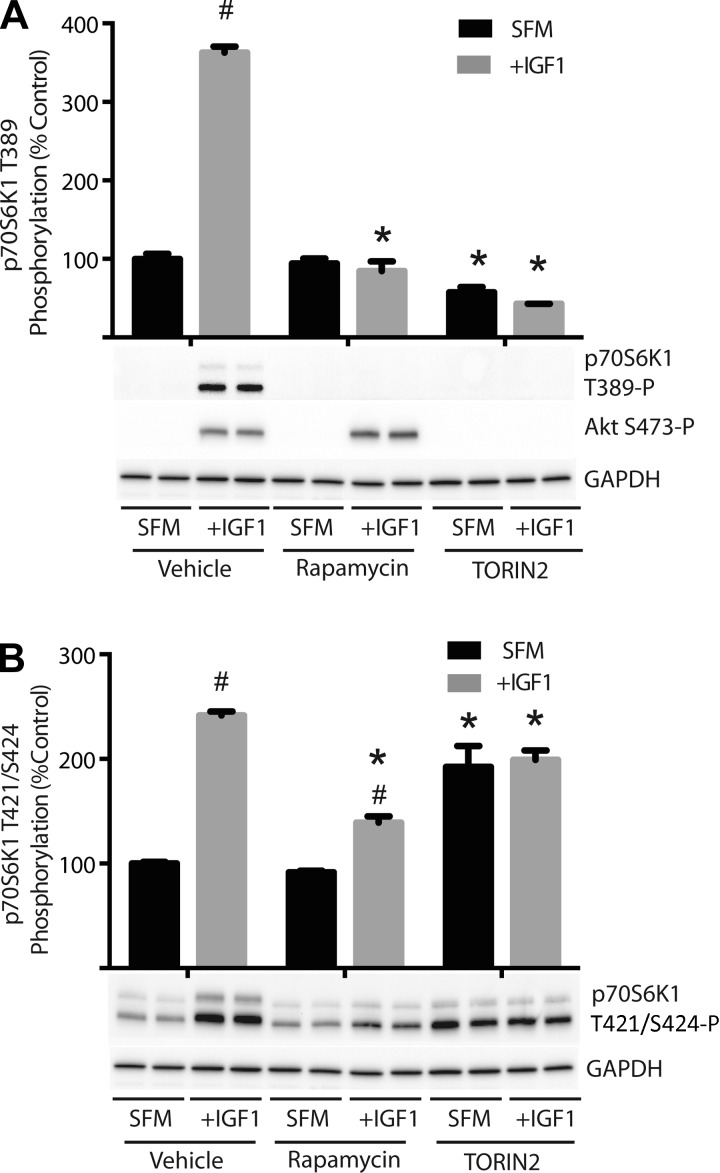
To further investigate phosphorylation of p70S6K1 at the Thr421/Ser424 sites, HEK-293E cells were chosen as a model system in order to better manipulate signaling pathways under pro-growth conditions. Cells were initially treated with rapamycin, which inhibits mTORC1, or TORIN2, which inhibits both mTORC1 and mTORC2. Following serum deprivation, phosphorylation of p70S6K1 on Thr389, an mTORC1-dependent site, and phosphorylation of Akt on Ser473, a site that is directly phosphorylated by mTORC2, were undetectable (Fig. 2A). However, when cells were administered the growth factor IGF-I, phosphorylation of both proteins was enhanced (Fig. 2A). In contrast to Thr389, phosphorylation of p70S6K1 at the Thr421/Ser424 sites was still detected in cells following serum deprivation, but, similarly to Thr389, IGF-I administration intensified phosphorylation of these sites by 242% compared with untreated cells (Fig. 2B). Administration of rapamycin prior to IGF-I administration had no effect on IGF-I-induced phosphorylation of Akt at Ser473; however, phosphorylation of p70S6K1 at the Thr389 site was blocked completely (Fig. 2A). To a large extent, rapamycin also prevented the IGF-I-induced elevation in phosphorylation of the Thr421/Ser424 sites, as the hormone stimulated phosphorylation of these sites by only 52% in the presence of the inhibitor (Fig. 2A). Treatment of the cells with TORIN2 prevented the IGF-I-induced stimulation of both p70S6K1 Thr389 and Akt Ser473 phosphorylation (Fig. 2A). Surprisingly, during serum deprivation, treatment with TORIN2 enhanced phosphorylation of p70S6K1 at the Thr421/Ser424 sites by 192% compared with untreated cells, and IGF-I stimulation did not further enhance phosphorylation of these sites (Fig. 2B). Thus, phosphorylation of p70S6K1 at the Thr421/Ser424 sites can occur through a rapamycin-insensitive mechanism; however, mTORC1 does appear to mediate the IGF-I-mediated enhancement in phosphorylation of these sites.
Fig. 2.
Phosphorylation of p70S6K1 at Thr421/Ser424 is maintained in the presence of rapamycin and TORIN2. HEK-293E cells were incubated in serum-free medium (SFM) for 2 h to repress phosphorylation of p70S6K1. During the last 30 min of serum deprivation, cells were treated with rapamycin (100 nM) or TORIN2 (10 nM) as indicated. Activation of mTOR complex 1 (mTORC1) signaling was achieved by treating cells with IGF-I as indicated. Phosphorylation of p70S6K1 at the Thr389 and Thr421/Ser424 sites and Akt at Ser473 was assessed by Western blot analysis with phosphospecific antibodies 30 min after administration of IGF-I (IGF1). Quantitation of the results for p70S6K1 phosphorylation at the Thr389 (A) and Thr421/Ser424 sites (B) expressed relative to GAPDH. Representative blots are shown. Values are means ± SE (n = 4). Statistical significance: #P < 0.05 vs. SFM; *P < 0.05 vs. vehicle.
Inhibition of JNK attenuates phosphorylation of p70S6K1.
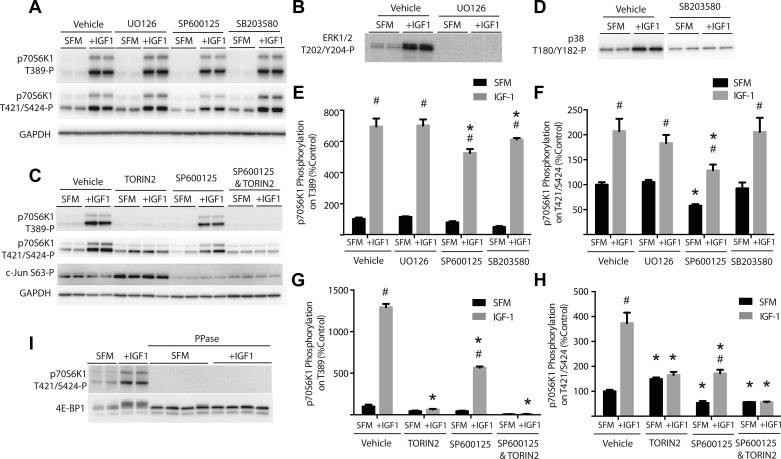
The Thr421 and Ser424 residues are both followed by a proline in the primary sequence of p70S6K1, suggesting the possible involvement of a MAPK in the phosphorylation of one or both sites. Indeed, enhanced phosphorylation of both JNK (178 ± 19%, P < 0.05, n = 7) and ERK1/2 (82 ± 31%, P < 0.05, n = 7) was observed in the plantaris muscle of animals that underwent tenotomy compared with the plantaris of the contralateral limb, and there was a trend toward enhanced phosphorylation of p38 (46 ± 24%, P = 0.07, n = 7). To further assess the possible role of a MAPK in mediating phosphorylation of the Thr421 and Ser424 sites, cells were serum deprived and IGF-I treated in the presence of the MAPK inhibitors U0126 (MEK inhibitor), SP600125 (JNK inhibitor), and SB203580 (p38 inhibitor). As expected, p70S6K1 phosphorylation at the Thr389 and Thr421/Ser424 sites was low in cells incubated in serum-free medium and was enhanced by the addition of IGF-I (Fig. 3A). Compared with vehicle, treatment with U0126, SP600125, or SB203580 repressed phosphorylation of ERK1/2 phosphorylation at Thr202/Tyr204 (Fig. 3B), c-Jun phosphorylation at Ser63 (Fig. 3C), and p38 phosphorylation at Thr180/Tyr182 (Fig. 3D), respectively, in serum-depleted medium and in response to treatment with IGF-I. Whereas treatment with IGF-I enhanced phosphorylation of ERK at Thr202/Tyr204 (Fig. 3B) and p38 at Thr180/Tyr182 (Fig. 3D), it did not enhance c-Jun phosphorylation at Ser63 (Fig. 3C). The IGF-I-mediated enhancement of p70S6K1 phosphorylation at Thr389 was observed in the presence of all three MAPK inhibitors; however, SP600125 caused a slight, but significant, attenuation of hormone-induced Thr389 phosphorylation compared with vehicle (Fig. 3E). Importantly, treatment with SP600125, but not SB203580 or U0126, repressed p70S6K1 phosphorylation at the Thr421/Ser424 sites by 42% during serum deprivation (Fig. 3F). Moreover, neither U0126 nor SB203580 treatment altered p70S6K1 phosphorylation at the Thr421/Ser424 sites in response to IGF-I, whereas in cells treated with SP600125 the magnitude of the IGF-I-induced increase was attenuated by 38% (Fig. 3F). Collectively, the finding that inhibitors of JNK, but not of ERK or p38, repressed p70S6K1 phosphorylation on Thr421/Ser424 and that the IGF-I-induced increase in Thr421/Ser424 phosphorylation was enhanced in cells in which JNK was inhibited implicate JNK in the maintenance of p70S6K1 phosphorylation at the Thr421/Ser424 sites during serum deprivation and also suggests that it contributes to IGF-I-induced phosphorylation of these residues.
Fig. 3.
Inhibition of JNK attenuates phosphorylation of p70S6K1 at the Thr421/Ser424 sites. HEK-293E cells were incubated in SFM for 2 h to repress phosphorylation of p70S6K1. During the last 30 min of serum deprivation, cells were treated with U0126 (10 μM), SP600125 (50 μM), SB203580 (10 μM), TORIN2 (10 nM), or a combination of SP600125 and TORIN2, as indicated. Activation of mTORC1 signaling was achieved by treating cells with IGF-I as indicated. Phosphorylation of p70S6K1 at the Thr389 and Thr421/Ser424 sites (A and C), ERK1/2 at Thr202/Tyr204 (B), c-Jun at Ser63 (F), and p38 at Thr180/Tyr182 (D) was assessed by Western blot analysis with phosphospecific antibodies 30 min after administration of IGF-I. Results of p70S6K1 phosphorylation at the Thr389 (E and G) and Thr421/Ser424 sites (F and H) were quantitated relative to GAPDH. Dephosphorylation of p70S6K1 at Thr421/Ser424 was performed with Lambda phosphatase (PPase) (I). Representative blots are shown. Values are means ± SE (n = 4). Statistical significance: #P < 0.05 vs. SFM; *P < 0.05 vs. vehicle.
Inhibition of mTOR with TORIN2 enhances phosphorylation of c-Jun at Ser63.
To expand upon the observation that inhibition of either mTORC1 or JNK repressed phosphorylation of the Thr421/Ser424 sites on p70S6K1, cells were treated with TORIN2, SP600125, or a combination of SP600125 and TORIN2 under the conditions described above. As previously observed, TORIN2 treatment prevented, whereas the addition of SP600125 diminished, the IGF-I-mediated enhancement in phosphorylation of p70S6K1 at the Thr389 site (Fig. 3G). Remarkably, TORIN2 treatment produced a significant increase in phosphorylation of c-Jun at Ser63, suggesting that inhibition of mTOR serves to activate JNK (Fig. 3C). Treatment with TORIN2 also enhanced phosphorylation of the Thr421/Ser424 sites upon serum deprivation, an effect that was blocked by the combined treatment with SP600125 (Fig. 3H). Furthermore, whereas IGF-I treatment enhanced the phosphorylation of the Thr421/Ser424 sites in the presence SP600125, the combined treatment with TORIN2 prevented this effect (Fig. 3H), as only a weak signal for Thr421/Ser424 phosphorylation was detected by Western blot analysis. We initially thought this weak antigenicity might be due to fidelity of the phosphoantibody (i.e., a weak interaction with p70S6K1 in the absence of Thr421/Ser424 phosphorylation), however, no signal was detected following treatment with Lambda phosphatase (Fig. 3I). Importantly, SP600125 did not completely ablate c-Jun Ser63 phosphorylation; thus, the possibility existed that the inhibitor did not completely block JNK activation (5). Alternatively, another MAPK, such as p38, could also be responsible for the observed phosphorylation of c-Jun at Ser63 in the presence of SP600125, given that p38 and JNK share similar downstream substrates (22).
Activation of JNK enhances p70S6K1 phosphorylation.
To further investigate the role of JNK in promoting phosphorylation of p70S6K1, cells were treated with anisomycin (a potent activator of JNK), TORIN2, SP600125, or a combination thereof following 2 h of serum deprivation and addition of IGF-I for 30 min. IGF-I was sufficient to enhance phosphorylation of p70S6K1 at the Thr389 site in all experimental conditions except when cells were treated with TORIN2, confirming its ability to inhibit mTORC1 without regard for the activation status of JNK (Fig. 4A). In the absence of anisomycin, phosphorylation of c-Jun at Ser63 was not observed; however, when cells were treated with anisomycin, phosphorylation of this site was enhanced (Fig. 4A). Treatment with TORIN2 further enhanced c-Jun Ser63 phosphorylation in the presence of anisomycin, suggesting that inhibition of mTOR promotes JNK kinase activity (Fig. 4A; compare lanes 5–8 with lanes 13–16). Most strikingly, anisomycin treatment stimulated phosphorylation of the Thr421/Ser424 sites on p70S6K1 by 571% under the serum-deprived condition, which was further enhanced in response to IGF-I treatment (Fig. 4B). Furthermore, SP600125 treatment attenuated the phosphorylation of p70S6K1 at the Thr421/Ser424 sites induced by anisomycin administration. Notably, combined treatment with both TORIN2 and SP600125 was additive in suppressing the anisomycin-IGF-I-induced stimulation of p70S6K1 phosphorylation at the Thr421/Ser424 sites (Fig. 4B), further suggesting that both mTOR and JNK target these sites.
Fig. 4.
Activation of JNK enhances phosphorylation of p70S6K1 at Thr421/Ser424. HEK-293E cells were incubated in SFM for 2 h to repress phosphorylation of p70S6K1. During the last 30 min of serum deprivation, cells were treated with anisomycin (20 mM), SP600125 (50 μM), TORIN2 (10 nM), or a combination as indicated. Activation of mTORC1 signaling was achieved by treating cells with IGF-I as indicated. A: phosphorylation of p70S6K1 at the Thr389 and Thr421/Ser424 sites, c-Jun at Ser63, and GAPDH expression assessed by Western blot analysis 15 min after the administration of IGF-I. B: quantitation of p70S6K1 phosphorylation at the Thr421/Ser424 sites relative to GAPDH. Representative blots are shown. Values are means ± SE (n = 4). Statistical significance: #P < 0.05 vs. SFM; *P < 0.05 vs. vehicle.
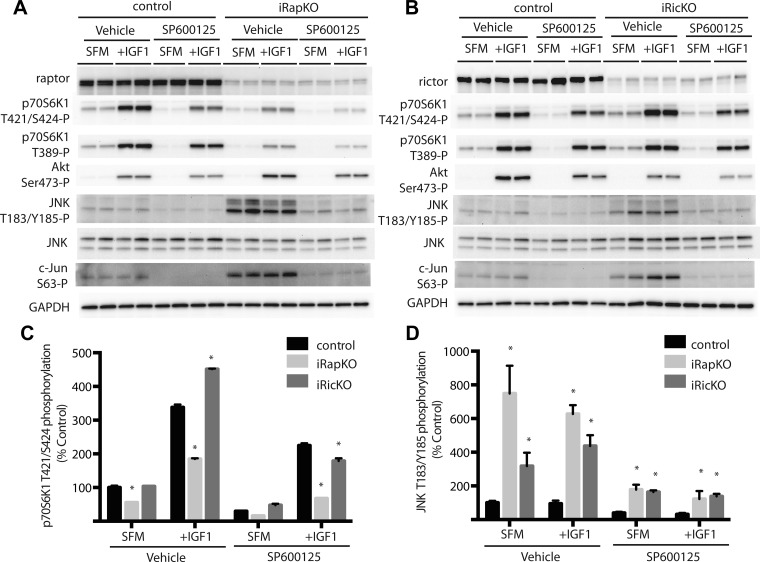
Knockdown of raptor, but not rictor, attenuates phosphorylation of p70S6K1 at the Thr421/Ser424 sites.
Two previously described MEF cell lines (10), iRapKO and iRicKO, were used to evaluate the role of mTORC1 and mTORC2 in phosphorylation of the Thr421/Ser424 sites. Raptor and rictor knockdown was induced in iRapKO and iRicKO MEF, respectively, by treating cells with 4-OHT (Fig. 4, A and B). To further investigate the role of mTORC1 and mTORC2 in phosphorylation of the Thr421/Ser424 sites, iRapKO and iRicKO MEF were serum deprived for 2 h and treated with a combination SP600125 and/or IGF-I for 30 min. Compared with control cells, raptor knockdown repressed the phosphorylation of both the Thr389 and the Thr421/Ser424 sites on p70S6K1 during serum deprivation and in response to IGF-I (Fig. 5, A and C). Remarkably, rictor knockdown enhanced phosphorylation of the Thr421/Ser424 sites on p70S6K1 in response to IGF-I (Fig. 5, B and C). Specifically, IGF-I-induced phosphorylation of these sites was repressed by 46% in iRapKO cells and enhanced by 33% in iRicKO cells compared with control cells (Fig. 5C). Notably, phosphorylation of both JNK at the Thr183/Tyr185 sites and c-Jun at Ser63 were enhanced by both raptor and rictor knockdown, and these effects were repressed by SP600125 (Fig. 5, A, B, and D). Importantly, rictor knockdown failed to enhance phosphorylation of the Thr421/Ser424 sites on p70S6K1 in the presence of SP600125 (Fig. 5, C and D). Overall, these findings are consistent with a model (Fig. 6) where mTORC1 promotes and mTORC2 represses the phosphorylation of the Thr421/Ser424 sites on p70S6K1.
Fig. 5.
mTORC1 and JNK promote phosphorylation of p70S6K1 at the Thr421/Ser424 sites. MEF (mouse embryonic fibroblasts) with inducible raptor (iRapKO) and rictor (iRicKO) knockdown were used to evaluate the role of mTORC1 and mTORC2 in p70S6K1 Thr421/Ser424 phosphorylation. Raptor and rictor knockdown was induced in iRapKO and iRicKO MEF, respectively, by treating cells with 1 μM 4-hydroxytamoxifen (4-OHT) every 24 h for 72 h. iRapKO (A), iRicKO (B), and wild-type control MEF were serum deprived for 2 h and treated with SP600125 and/or IGF-I as indicated. Phosphorylation of p70S6K1 at the Thr421/Ser424 and Thr389 sites, Akt at Ser473, JNK at Thr183/Tyr185, and c-Jun at Ser63, as well as raptor, rictor, JNK, and GAPDH expression were assessed by Western blot analysis. Representative blots are shown. Quantitation of p70S6K1 phosphorylation at the Thr421/Ser424 sites (C) and JNK at the Thr183/Tyr185 sites relative to GAPDH. Values are means ± SE (n = 4). Statistical significance: *P < 0.05 vs. control.
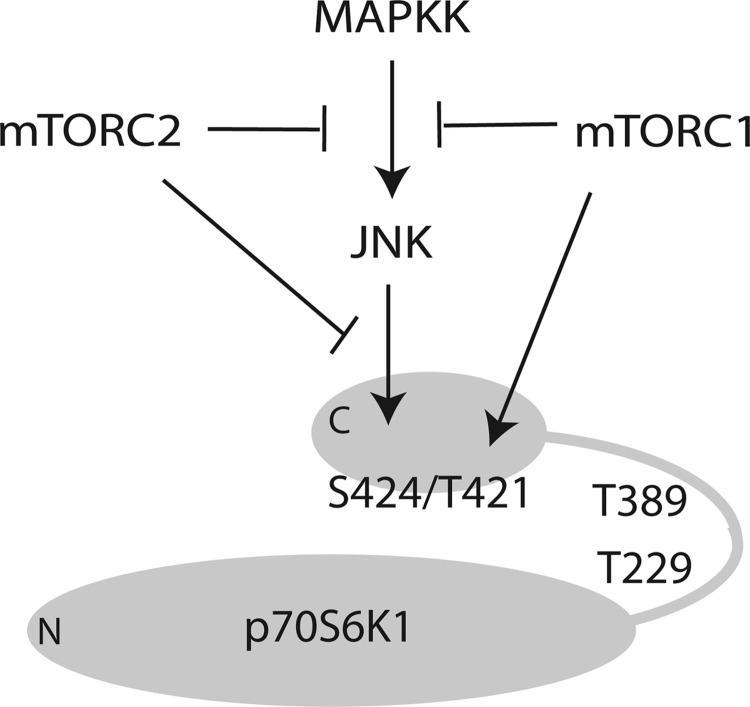
Fig. 6.
Working model for coordinate phosphorylation of p70S6K1 on Thr421/Ser424 by mTORC1 and JNK. Activation of p70S6K1 involves phosphorylation of multiple proline-directed sites including Thr421 and Ser424 located in a pseudo-substrate domain of the COOH-terminal lobe (C), which when dephosphorylated serves an autoinhibitory function by folding over the NH2-terminal lobe (N). JNK and mTORC1 participate in phosphorylation of this pseudo-substrate domain at Thr421 and Ser424 to induce a conformational change in p70S6K1 that facilitates phosphorylation at Thr389 by mTORC1, phosphorylation of Thr229 by phosphoinositide-dependent kinase-1 (PDK1), and ultimately full activation.
Functional overload enhances JNK phosphorylation.
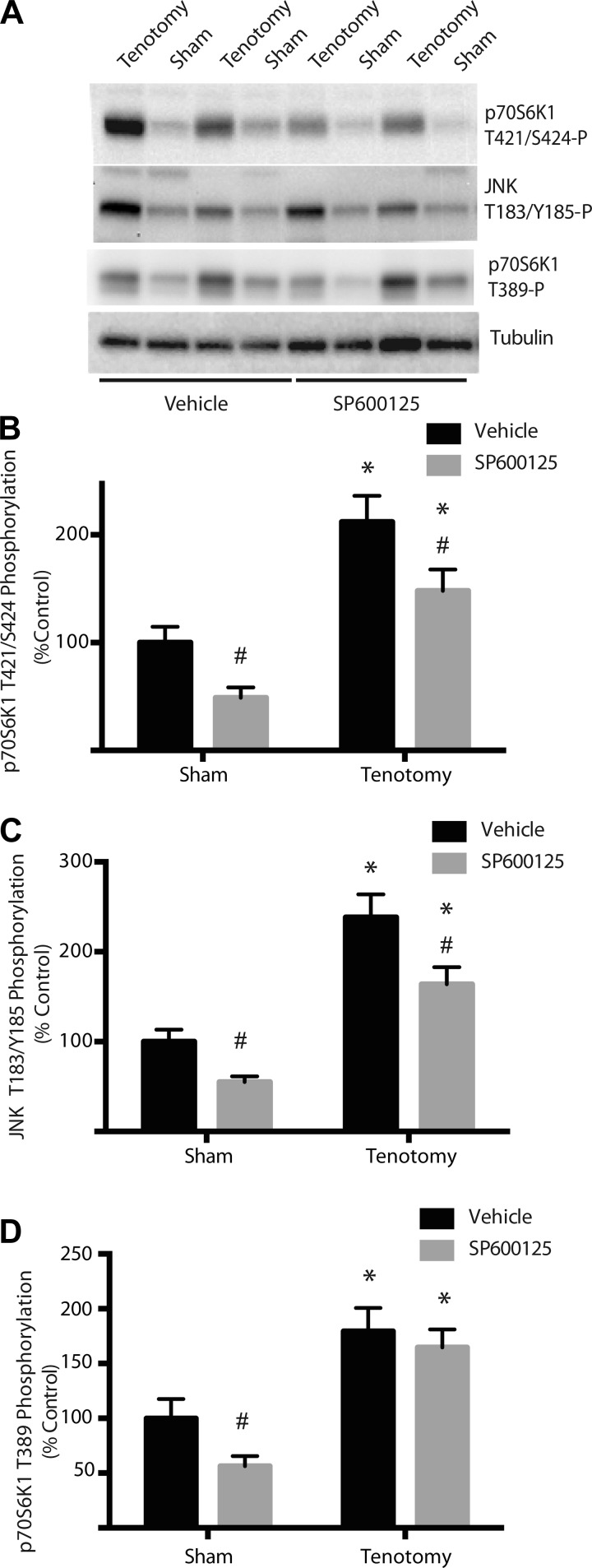
To confirm an in vivo role of JNK in the phosphorylation of the Thr421/Ser424 sites on p70S6K1, rats were administered SP610025 at a time point that was both 24 h after functional overloading (tenotomy) and 6 h prior to tissue harvest. In agreement with the results shown in Fig. 1, phosphorylation of the Thr421/Ser424 sites on p70S6K1 in the plantaris muscle was enhanced by tenotomy to 212% of that observed in a contralateral sham operated limb (Fig. 7, A and B). Notably, tenotomy also induced phosphorylation of JNK at Thr183/Tyr185 (Fig. 7, A and C). In rats treated with SP600125, phosphorylation of the Thr421/Ser424 sites on p70S6K1 was attenuated by 51% in muscle from the leg subjected to the sham operation compared with a 30% attenuation following tenotomy (Fig. 7A). Remarkably, the change in phosphorylation of the Thr421/Ser424 sites was mirrored by a similar attenuation of JNK phosphorylation at Thr183/Tyr185: 45% reduction in muscle from the sham leg and 31% reduction upon tenotomy. Taken together, these data support the conclusion that JNK contributes to the phosphorylation of the Thr421/Ser424 sites on p70S6K1 in response to functional overload. Furthermore, inhibition of JNK with SP600125 significantly attenuated phosphorylation of p70S6K1 on Thr389 (Fig. 7, A and D).
Fig. 7.
Enhanced p70S6K1 phosphorylation at the Thr421/Ser424 sites in plantaris muscle undergoing tenotomy-induced hypertrophy. Plantaris muscle from both the tenotomized and the contralateral limb were harvested 24 h following the surgical procedure. As indicated, rats received SP600125 (50 mg/kg body mass) 6 h prior to harvest of muscle tissue. Muscle samples were then prepared for analysis of p70S6K1 phosphorylation. A: Western blot analysis of supernatant fractions of muscle homogenates, using phosphospecific anti Thr389 and Thr421/Ser424 p70S6K1 antibodies as well as anti-tubulin. Quantitation of the results for p70S6K1 phosphorylation at the Thr421/Ser424 sites (B), JNK phosphorylation at Thr183/Tyr185 (C), and p70S6K1 phosphorylation at Thr389 (D). Results represent means ± SE (n = 9). Statistical significance: #P < 0.05 vs. vehicle; *P < 0.05 vs. sham.
DISCUSSION
In male Sprague-Dawley rats, functional overload of the plantaris muscle resulted in enhanced phosphorylation of both the Thr389 and Thr421/Ser424 sites on p70S6K1. While it is well established that activation of mTORC1 is responsible for phosphorylation of the Thr389 site, the results of the present study show that both mTORC1 and JNK act to coordinate the phosphorylation of the p70S6K1 autoinhibitory domain at the Thr421/Ser424 sites. A limitation of the present study and others is that commercially available antibodies do not distinguish between phosphorylation of the Thr421 and Ser424 sites; thus, it is not possible to be certain whether a given kinase targets one or the other or both sites. Another limitation is the unavailability of a specific chemical inhibitor of JNK. Although SP600125 is the most selective inhibitor of JNK available, it also has off-target effects, including inhibition of p70S6K1 as effectively as it does JNK (3). This limitation precluded attempts to demonstrate a role for JNK in signaling downstream of p70S6K1.
Over 20 years ago, it was established that proline-directed mitogen-regulated MAPKs act to phosphorylate a 37-residue peptide with a sequence corresponding to the autoinhibitory domain of p70S6K1 in vitro (30); however, whether or not MAP kinases phosphorylate the native protein in a physiological setting remained unresolved. During preparation of this paper, Zhang et al. (38) reported that JNK not only phosphorylates recombinant p70S6K1 in vitro, but that TNF-α induced phosphorylation of p70S6K1, as assessed employing the same antiphospho-p70S6K1 Thr421/Ser424 antibody used in the present study, was inhibited by either SP600125 or JNK knockout in cells in culture. Importantly, neither JNK knockout nor SP600125 completely ablated phosphorylation of p70S6K1, suggesting that another kinase also participates in phosphorylation of one or more of these sites. The data presented herein extend the previous study (38) by demonstrating that JNK contributes to p70S6K1 phosphorylation at the Thr421/Ser424 sites in a physiologically relevant model (i.e., muscle hypertrophy) and establishes that mTORC1 also participates in phosphorylation of these sites. Coordinating roles for mTORC1 and JNK in p70S6K1 phosphorylation is particularly appealing, given that both are activated in muscle in response to resistance exercise and that p70S6K1 is a pro-growth kinase (24, 28, 35).
Phosphorylation of the p70S6K1 autoinhibitory domain plays a vital role in altering the kinase's bilobal confirmation and allowing mTORC1 and PDK1 access to the hydrophobic motif and activation loop, respectively (32). In the present study, JNK inhibition not only repressed p70S6K1 phosphorylation at the Thr421/Ser424 sites but also attenuated phosphorylation of the Thr389 site during serum deprivation and in response to IGF-I treatment. Phosphomimetic substitution of the four proline-directed sites (Ser411, Ser418, Thr421, and Ser424) located in this domain by mutation to aspartate and glutamate increases basal kinase activity (15) but is insufficient for maximal activation of p70S6K1, as the resulting D3E mutant exhibits mitogen responsiveness (9) and rapamycin sensitivity (15). Furthermore, alanine substitution of all four sites produces a fivefold reduction in S6K1 activity (15), suggesting a significant contribution of autoinhibitory domain phosphorylation to p70S6K1 activation.
Schalm et al. (34) observed that p70S6K1 phosphorylation at the Thr421/Ser424 sites was not significantly affected by an F5A mutation that disrupts the TOS motif (amino acids 5–9; FDIDL) required for Thr389 phosphorylation. Furthermore, as confirmed in the present study, rapamycin only partially inhibits the mitogen-induced enhancement in the phosphorylation of the Thr421/Ser424 sites (21, 34). Thus, it was presumed that p70S6K1 phosphorylation at the Thr421/Ser424 sites was independent of mTORC1. However, in the present study we observed the surprising finding that the mTOR active site inhibitor TORIN2, which inhibits both mTORC1 and mTORC2, was sufficient to completely prevent the IGF-I-induced phosphorylation of p70S6K1 at the Thr421/Ser424 sites, and in contrast enhanced phosphorylation of these sites during serum deprivation. Thus, mTOR not only contributes to phosphorylation of Thr421 and/or Ser424 in a TOS motif-independent manner, but mTORC1 and/or mTORC2 also likely functions in the repression of another kinase (i.e., JNK) that participates in the phosphorylation of the Thr421/Ser424 sites. In the present study, mTORC1 inhibition via raptor knockdown repressed phosphorylation of the Thr421/Ser424 sites on p70S6K1, and mTORC2 inhibition via rictor knockdown had the opposite effect. Furthermore, the stimulatory effects of rictor knockdown on phosphorylation of the Thr421/Ser424 sites on p70S6K1 were ablated by JNK inhibition. Overall, these findings support a model wherein mTORC1 and JNK contribute to the phosphorylation of p70S6K1 at the Thr421/Ser424 sites, whereas mTORC2 inhibits JNK-mediated phosphorylation of these sites.
Activation of JNK requires phosphorylation of the Thr183/Tyr185 sites, leading to translocation of the kinase to the nucleus, where it phosphorylates transcription factors such as c-Jun (20). Dual mTORC1/mTORC2 inhibition with AZD8055 has been previously reported to occur concomitantly with phosphorylation of JNK at the Thr183/Tyr185 sites (27). The results of the present study corroborate those of the previous report by demonstrating that TORIN2 promoted c-Jun phosphorylation and extend the previous finding by demonstrating that the enhancement is mediated by both mTOR complexes, as either raptor or rictor knockdown was sufficient to promote phosphorylation of both the Thr183/Tyr185 sites on JNK and Ser63 on c-Jun. Although the interplay between mTOR and the MAPK signaling cascades remains poorly understood, it follows from the data presented herein that both mTORC1 and mTORC2 likely repress activation of one or more of the MAPKKs responsible for activation of JNK. However, although knockdown of either raptor or rictor was sufficient to induce JNK and c-Jun phosphorylation, only rictor knockdown was associated with enhanced phosphorylation of p70S6K1 at the Thr421/Ser424 sites. Thus, unlike mTORC1, mTORC2 must also act downstream of JNK to repress phosphorylation of p70S6K1 at Thr421/Ser424. This finding could also be explained if mTORC1 and mTORC2 activate distinct isoforms of JNK and only certain JNK isoforms can phosphorylate p70S6K1, or if JNK needs to be present in a specific scaffolding complex to target p70S6K1 and only mTORC2 signals to that complex.
p70S6K1 is involved in numerous processes within the cell that promote cellular proliferation and hypertrophy in part through increased synthesis of the machinery required for translation (28). Acute resistance exercise training significantly enhances phosphorylation of p70S6K1, and importantly, the phosphorylation status of this kinase following a single bout of resistance exercise directly correlates with the magnitude of muscle hypertrophy obtained after chronic resistance exercise (2). High-intensity exercise protocols and those associated with muscle damage also induce activation of the JNK signaling pathway (1). Based on the findings of the present and previous studies (38), JNK activation would promote phosphorylation of the COOH-terminal tail of p70S6K1. Thus, it is likely that the coordinated JNK/mTORC1-mediated phosphorylation of p70S6K1 at the Thr421/Ser424 sites acts to increase the availability of the final two phosphorylation sites on p70S6K1 (Thr389 and Thr229) to promote full kinase activity and maximal muscle growth. Therefore, JNK activation may play a pivotal role in the magnitude of skeletal muscle hypertrophy following resistance exercise.
GRANTS
This work was supported by National Institutes of Health Grants DK-15658 (to L. S. Jefferson) and EY-023612 (to M. D. Dennis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.D.M., M.D.D., S.R.K., and L.S.J. conception and design of research; T.D.M., M.D.D., and B.S.G. performed experiments; T.D.M. and M.D.D. analyzed data; T.D.M., M.D.D., S.R.K., and L.S.J. interpreted results of experiments; T.D.M. and M.D.D. drafted manuscript; T.D.M., M.D.D., B.S.G., S.R.K., and L.S.J. edited and revised manuscript; T.D.M., M.D.D., B.S.G., S.R.K., and L.S.J. approved final version of manuscript; M.D.D. prepared figures.
ACKNOWLEDGMENTS
We thank Dr. Michael N. Hall (University of Basel) for kindly providing the iRapKO and iRicKO MEF. We also thank Sharon Rannels, Holly Lacko, Lydia Kutzler, and Chen Yeng for assistance in performance of the studies described herein.
REFERENCES
- 1.Aronson D, Boppart MD, Dufresne SD, Fielding RA, Goodyear LJ. Exercise stimulates c-Jun NH2 kinase activity and c-Jun transcriptional activity in human skeletal muscle. Biochem Biophys Res 251: 106–110, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Baar K, Esser K. Phosphorylation of p70S6k correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol 276: C120–C127, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J 408: 297–315, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee P, Ahmad MF, Grove JR, Kozlosky C, Price DJ, Avruch J. Molecular structure of a major insulin/mitogen-activated 70-kDa S6 protein kinase. Proc Natl Acad Sci USA 87: 8550–8554, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA 98: 13681–13686, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Bolster DR, Kubica N, Crozier SJ, Williamson DL, Farrell PA, Kimball SR, Jefferson LS. Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle. J Physiol 553: 213–220, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem 269: 5360–5368, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Cheatham L, Monfar M, Chou MM, Blenis J. Structural and functional analysis of pp70S6k. Proc Natl Acad Sci USA 92: 11696–11700, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cybulski N, Zinzalla V, Hall MN. Inducible raptor and rictor knockout mouse embryonic fibroblasts. Methods Mol Biol 821: 267–278, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Dennis MD, Jefferson LS, Kimball SR. Role of p70S6K1-mediated phosphorylation of eIF4B and PDCD4 proteins in the regulation of protein synthesis. J Biol Chem 287: 42890–42899, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dennis MD, Schrufer TL, Bronson SK, Kimball SR, Jefferson LS. Hyperglycemia-induced O-GlcNAcylation and truncation of 4E-BP1 protein in liver of a mouse model of type 1 diabetes. J Biol Chem 286: 34286–34297, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowling RJ, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E, Wang X, Larsson O, Selvaraj A, Liu Y, Kozma SC, Thomas G, Sonenberg N. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science 328: 1172–1176, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farrell PA, Hernandez JM, Fedele MJ, Vary TC, Kimball SR, Jefferson LS. Eukaryotic initiation factors and protein synthesis after resistance exercise in rats. J Appl Physiol (1985) 88: 1036–1042, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Ferrari S, Pearson RB, Siegmann M, Kozma SC, Thomas G. The immunosuppressant rapamycin induces inactivation of p70s6k through dephosphorylation of a novel set of sites. J Biol Chem 268: 16091–16094, 1993 [PubMed] [Google Scholar]
- 16.Foster KG, Fingar DC. Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem 285: 14071–14077, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem 37: 1974–1984, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Gordon BS, Kelleher AR, Kimball SR. Regulation of muscle protein synthesis and the effects of catabolic states. Int J Biochem 45: 2147–2157, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han JW, Pearson RB, Dennis PB, Thomas G. Rapamycin, wortmannin, and the methylxanthine SQ20006 inactivate p70s6k by inducing dephosphorylation of the same subset of sites. J Biol Chem 270: 21396–21403, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Ichijo H. From receptors to stress-activated MAP kinases. Oncogene 18: 6087–6093, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Iijima Y, Laser M, Shiraishi H, Willey CD, Sundaravadivel B, Xu L, McDermott PJ, Kuppuswamy D. c-Raf/MEK/ERK pathway controls protein kinase C-mediated p70S6K activation in adult cardiac muscle cells. J Biol Chem 277: 23065–23075, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298: 1911–1912, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Julien LA, Carriere A, Moreau J, Roux PP. mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Mol Cell Biol 30: 908–921, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramer HF, Goodyear LJ. Exercise, MAPK, and NF-κB signaling in skeletal muscle. J Appl Physiol (1985) 103: 388–395, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Kubica N, Bolster DR, Farrell PA, Kimball SR, Jefferson LS. Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2Bepsilon mRNA in a mammalian target of rapamycin-dependent manner. J Biol Chem 280: 7570–7580, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Le Bacquer O, Petroulakis E, Paglialunga S, Poulin F, Richard D, Cianflone K, Sonenberg N. Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2. J Clin Invest 117: 387–396, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, Song XM, Ji YY, Jiang H, Xu LG. The dual mTORC1 and mTORC2 inhibitor AZD8055 inhibits head and neck squamous cell carcinoma cell growth in vivo and in vitro. Biochem Biophys Res 440: 701–706, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J 441: 1–21, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Montagne J, Stewart MJ, Stocker H, Hafen E, Kozma SC, Thomas G. Drosophila S6 kinase: a regulator of cell size. Science 285: 2126–2129, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Mukhopadhyay NK, Price DJ, Kyriakis JM, Pelech S, Sanghera J, Avruch J. An array of insulin-activated, proline-directed serine threonine protein-kinases phosphorylate the p70-S6 kinase. J Biol Chem 267: 3325–3335, 1992 [PubMed] [Google Scholar]
- 31.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 11: 9–22, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Price DJ, Mukhopadhyay NK, Avruch J. Insulin-activated protein kinases phosphorylate a pseudosubstrate synthetic peptide inhibitor of the p70 S6 kinase. J Biol Chem 266: 16281–16284, 1991 [PubMed] [Google Scholar]
- 33.Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci 31: 342–348, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Schalm SS, Blenis J. Identification of a conserved motif required for mTOR signaling. Curr Biol 12: 632–639, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Shi H, Scheffler JM, Zeng C, Pleitner JM, Hannon KM, Grant AL, Gerrard DE. Mitogen-activated protein kinase signaling is necessary for the maintenance of skeletal muscle mass. Am J Physiol Cell Physiol 296: C1040–C1048, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Shima H, Pende M, Chen Y, Fumagalli S, Thomas G, Kozma SC. Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J 17: 6649–6659, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomson DM, Gordon SE. Impaired overload-induced muscle growth is associated with diminished translational signalling in aged rat fast-twitch skeletal muscle. Physiol J 574: 291–305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Gao ZG, Ye JP. Phosphorylation and degradation of S6K1 (p70S6K1) in response to persistent JNK1 activation. Bba-Mol Basis Dis 1832: 1980–1988, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]