Abstract
In the central nervous system, L-type voltage-gated calcium channels (LTCCs) come in two isoforms, namely Cav1.2 and Cav1.3 channels. It has been shown previously that these channels differ in biophysical properties, in subcellular localization, and in the coupling to the gene transcription machinery. In previous work on rat hippocampal neurons we have identified an excitatory cation conductance and an inhibitory potassium conductance as important LTCC coupling partners. Notably, a stimulus-dependent interplay of LTCC-mediated Ca2+ influx and activation of these Ca2+-dependent conductances was found to give rise to characteristic voltage responses. However, the contribution of Cav1.2 and Cav1.3 to these voltage responses remained unknown. Hence, the relative contribution of the LTCC isoforms therein was the focus of the current study on hippocampal neurons derived from genetically modified mice, which either lack a LTCC isoform (Cav1.3 knockout mice) or express a dihydropyridine-insensitive LTCC isoform (Cav1.2DHP−-knockin mice). We identified common and alternate ion channel couplings of Cav1.2 and Cav1.3 channels. Whereas hyperpolarizing Ca2+-dependent conductances were coupled to both Cav1.2 and Cav1.3 channels, an afterdepolarizing potential was only induced by the activity of Cav1.2 channels. Unexpectedly, the activity of Cav1.2 channels was found at relatively hyperpolarized membrane voltages. Our data add important information about the differences between Cav1.2 and Cav1.3 channels that furthers our understanding of the physiological and pathophysiological neuronal roles of these calcium channels. Moreover, our findings suggest that Cav1.3 knockout mice together with Cav1.2DHP−-knockin mice provide valuable models for future investigation of hippocampal LTCC-dependent afterdepolarizations.
Keywords: knockout, dihydropyridine, voltage-gated calcium channel, gene deletion
l-type voltage-gated calcium channel (LTCC)-targeting drugs have a long history in the treatment of cardiovascular diseases. However, it emerged from studies published within about the last decade that LTCCs also play important roles in higher brain functions such as learning and memory, fear conditioning, as well as mood and addictive behavior (9, 11, 15, 38, 50, 51, 54). In addition, evidence accumulated that malfunction of LTCCs may be crucially involved in various neurological diseases such as bipolar disorder, autism, age-dependent memory deficits, Morbus Alzheimer, Morbus Parkinson, and epilepsy (1, 2, 3, 7, 30, 36, 59, 61). What is more, there is now evidence that the implication of LTCCs in these pathogenic conditions may be specifically due to one of the two brain LTCC isoforms, e.g., Cav1.3 channels in Parkinson's disease and Cav1.2 in Alzheimer's disease, bipolar-disorder, and autism, respectively (2, 36, 61). Therefore, isoform specific blockers of LTCCs are currently being developed for therapeutic applications (29). To shed light on the pathological mechanisms of LTCC malfunction, it is necessary to obtain a deeper understanding of the functional differences between Cav1.2 and Cav1.3 channels.
On the cellular level, the neuronal role of LTCCs has been demonstrated to lie in gene regulation (e.g., excitation-transcription coupling), synaptic plasticity (e.g., long-term potentiation), differentiation, and control of electrical excitability (10, 38, 40, 60). Differences between Cav1.2 and Cav1.3 have been identified with respect to biophysical properties and subcellular localization (32), but both LTCC isoforms appear to be involved in the above-mentioned functions, although isoform-specific properties were identified with respect to the coupling to the gene transcription machinery (65, 74).
In our previous work we focused on LTCCs in hippocampal neurons to address physiological details of these channels in excitability (18). We identified an excitatory cation conductance and an inhibitory potassium conductance as important LTCC coupling partners. Notably, a stimulus-dependent interplay of LTCC-mediated Ca2+ influx and activation of these Ca2+-dependent conductances gives rise to characteristic voltage responses. In addition to current-induced depolarizations in tetrodotoxin-silenced neurons, we could also provide evidence that both couplings play a role in normal (18) and abnormal discharge activities (49). However, the contribution of the two central nervous system LTCC isoforms Cav1.2 and Cav1.3 to the coupling-dependent voltage responses has not been addressed so far. Hence, this was the focus of the current study on hippocampal neurons derived from genetically modified mice, which either lack an LTCC isoform [Cav1.3-knockout mice (46)] or express a dihydropyridine-insensitive LTCC isoform variant [Cav1.2DHP−-knockin mice (24); also known as Cav1.2DHP−/− mice (54)]. We provide evidence that the two LTCC isoforms have alternate coupling to Ca2+-dependent conductances in hippocampal neurons, whereas the voltage range of operation seems less differential than commonly thought. Our data add to the current knowledge of the respective roles of Cav1.2 and Cav1.3 channels in neuronal functions, which is of considerable importance in the light of ongoing assiduous efforts to employ isoform-specific LTCC modulators in neurological therapy (2, 28, 29, 71).
MATERIALS AND METHODS
Origin of wild-type and genetically modified mice strains.
C57Bl/6N mice were purchased from Charles River Laboratories (Sulzfeld, Germany). Cav1.3−/− mice (46) and Cav1.2DHP−/− mice (54) were obtained from Joerg Striessnig (Department of Pharmacology and Toxicology, University of Innsbruck, Austria). Before the start of the investigations, mice were sanitized by embryo transfer at the Division for Laboratory Animal Science and Genetics of the Medical University of Vienna (Himberg, Austria). Mice breeding pairs were kept in the in-house laboratory animal facility and were checked daily for litter. Newborn mice were used immediately after birth, because long-term culture of primary hippocampal neurons requires isolation of the cells at this early stage. This ruled out the use of conditional knockout models (e.g., Refs. 38, 66), which have been developed to circumvent the lethality of CNCA1C gene deletion.
Since our approach is based on the use of dihydropyridine-type LTCC modulators (18), Cav1.2DHP−/− mice represented a suitable alternative experimental model. In the modified channel, a threonine to tyrosine mutation in segment IIIS5 of the channel protein eliminates high dihydropyridine sensitivity through a steric mechanism, see Ref. 24 and Drugs.
Cell culture of primary hippocampal neurons.
Hippocampi were dissected after the mice were euthanized by decapitation. Genotypes were confirmed by conventional PCR testing of DNA extracted from the mouse tails. Primary cultures of hippocampal neurons were prepared in the same manner as described previously (18). Hence, all experiments were performed ex vivo. The keeping and killing of the animals was done in full accordance with all rules of the Austrian animal protection law and the Austrian animal experiment law.
Electrophysiology.
Perforated patch measurements were performed using 500 μg/ml amphotericin B (from Streptomyces sp., compound purchased from Sigma-Aldrich, Vienna, Austria) added to the pipette solution which contained the following (in mM): 120 potassium gluconate, 1.5 sodium gluconate, 3.5 NaCl, 1.5 CaCl2, 0.25 MgCl2, 10 HEPES, 10 glucose, and 5 EGTA. pH was adjusted to 7.3 by KOH. Experiments were started only after the series resistance had dropped to the lowest achievable level (e.g., to between 20 and 30 MΩ), which usually occurred within 15–30 min. Membrane voltage was recorded using a Multiclamp 700B amplifier (Axon Instruments) in the current clamp mode exactly as described by us previously with the same electrodes as well as pipette and external solutions (49). To assure that only viable cells were used, the following inclusion criteria had to be met: a membrane voltage of at least −50 mV and the capability of generating overshooting action potentials, which was always tested before the recordings. Experiments were performed at room temperature, and cells were superfused continuously with external solution containing the following (in mM): 140 NaCl, 3 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, and 20 glucose (pH was adjusted to 7.4 by NaOH). LTCC activity was modulated by application of the dihydropyridines isradipine (LTCC antagonist) and Bay K8644 (BayK; LTCC agonist), both at 3 μM in all experiments. Activation of LTCCs was provoked by incremental current injections (typically 5 injections of equally increasing amplitude, e.g., injection 1 of 75 pA to injection 5 of 375 pA in a typical experiment, separated by 30-s intervals) to depolarize the neurons experimentally beyond the LTCC activation threshold. Duration of current injection was 8 s in most of the experiments, but pulse duration was varied in some experiments, as indicated. Unless stated otherwise, the recordings were made in the presence of 500 nM tetrodotoxin (TTX) in the external solution. As in earlier work of our group (18, 49), bona fide LTCC effects were identified as being inhibited by isradipine and augmented by BayK. In neurons of all strains, the dihydropyridine-type modulators did not affect the resting membrane potential [e.g., Vm was −68.8 ± 5.5 mV (means ± SD) in dimethyl sulfoxide (DMSO; control, see Drugs], −68.5 ± 6.0 mV in BayK, and −69.7 ± 7.4 mV in isradipine, as determined from 28 wild-type neurons used in this study). Membrane resistance close to the resting potential also remained unaffected, which enabled onset analysis of LTCC-mediated active voltage responses.
Drugs.
BayK, DMSO, isradipine, and bulk chemicals were purchased from Sigma-Aldrich. Since some of these drugs were dissolved in DMSO, the concentration of this solvent was kept constant at 0.3% in all solutions. Control solution contained 0.3% DMSO only, whereas DMSO-soluble compounds were diluted from concentrated stock solutions so as to obtain the same final concentration of DMSO.
In the majority of the experiments, BayK and isradipine were used at lower micromolar concentrations (e.g., 3 μM). It has been demonstrated (54) that in this concentration range, Cav1.2DHP−/− channels are not potentiated by BayK but can be partially inhibited by isradipine (this difference in residual sensitivity may be due to the more complex structural requirements that are required for effective stimulation by dihydropyridine agonists than for the action of antagonists, see Ref. 64). In our experiments, 3 μM isradipine typically sufficed to eliminate distinct active voltage responses; only responses that are presumably caused by pronounced LTCC-mediated Ca2+-elevations, e.g., sag responses (18), were noted in some instances to persist in the presence of isradipine in Cav1.2DHP−/− neurons (data not shown).
Data analysis and statistics.
Afterpotentials were quantified by measuring the area (mV·ms) between the baseline (=membrane voltage before the current injection) and the recorded trace from the end of the current injection to the repolarization back to the baseline level. GraphPad Prism version 5.03 was used for preparation of the graphs (all data are represented as means ± SE, unless otherwise stated) and for statistical analysis, which was performed using Kruskal-Wallis one-way ANOVA with Dunn's multiple comparison post hoc test, and with Mann Whitney test for the data shown in Fig. 10C.
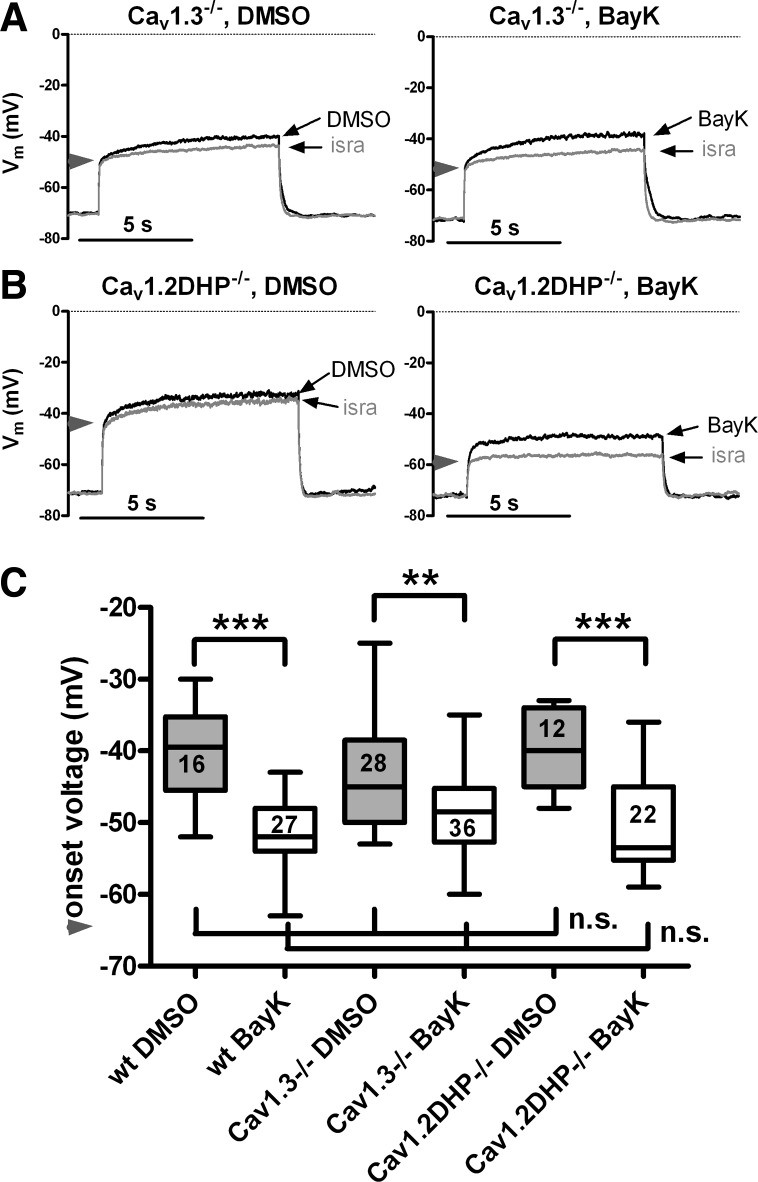
Fig. 10.
Cav1.2 and Cav1.3 channels operate in an overlapping voltage range. Overlay of traces recorded in the presence of isradipine with those recorded when either solvent (DMSO; left) or BayK (right) was present from Cav1.3−/− neurons (A) and Cav1.2DHP−/− neurons (B). Responses were evoked with moderate depolarizations that led to bump responses, as shown for wild-type neurons in Fig. 1, A and B. The horizontal arrowheads on the y-axes indicate the divergence of the 2 overlaid traces, which was taken as an indication of the onset of the LTCC-mediated response. C: box plot of onsets determined for neurons derived from the 3 mouse strains under conditions of unaltered (DMSO) and potentiated (BayK) LTCC channels. Boxed figures indicate the number of experiments. Statistical analysis revealed that there is a highly significant difference between onsets recorded in the presence of DMSO and BayK in neurons of all mice strains (**P < 0.01 and ***P < 0.001). However, no statistical difference was observed when onsets were compared between mice strains, and this was true for both control conditions (DMSO) and in the presence of BayK.
RESULTS
LTCC activity leads to both depolarizing and hyperpolarizing voltage responses.
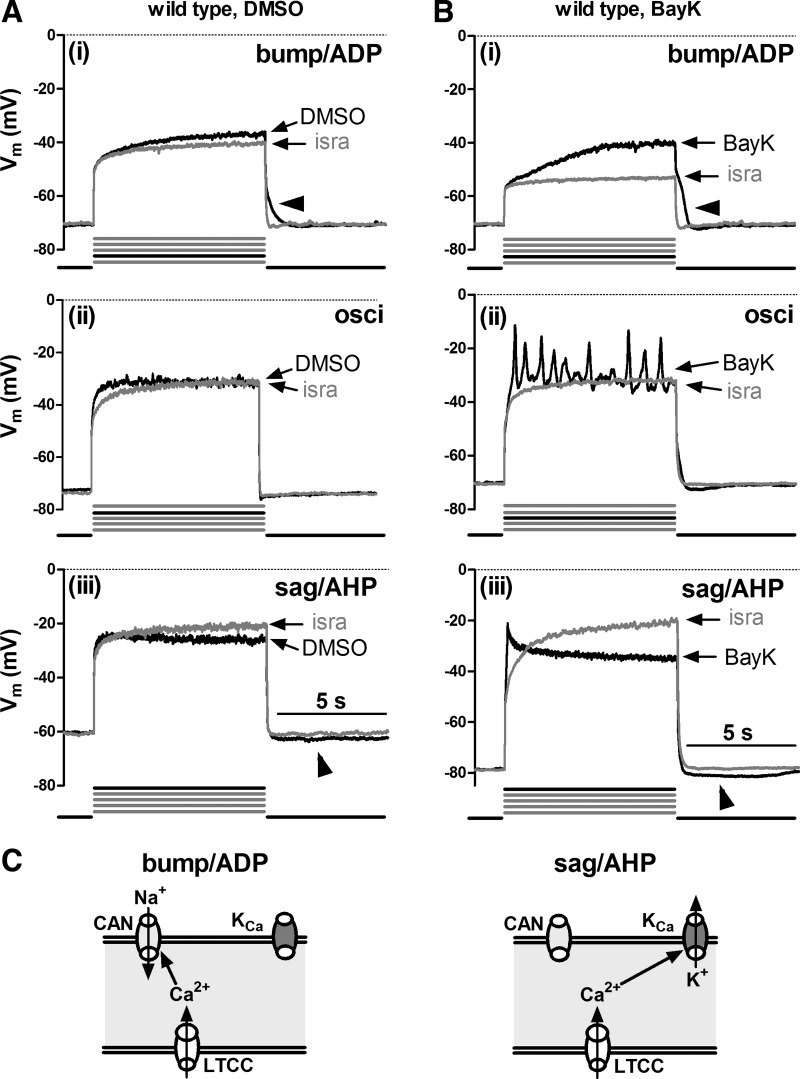
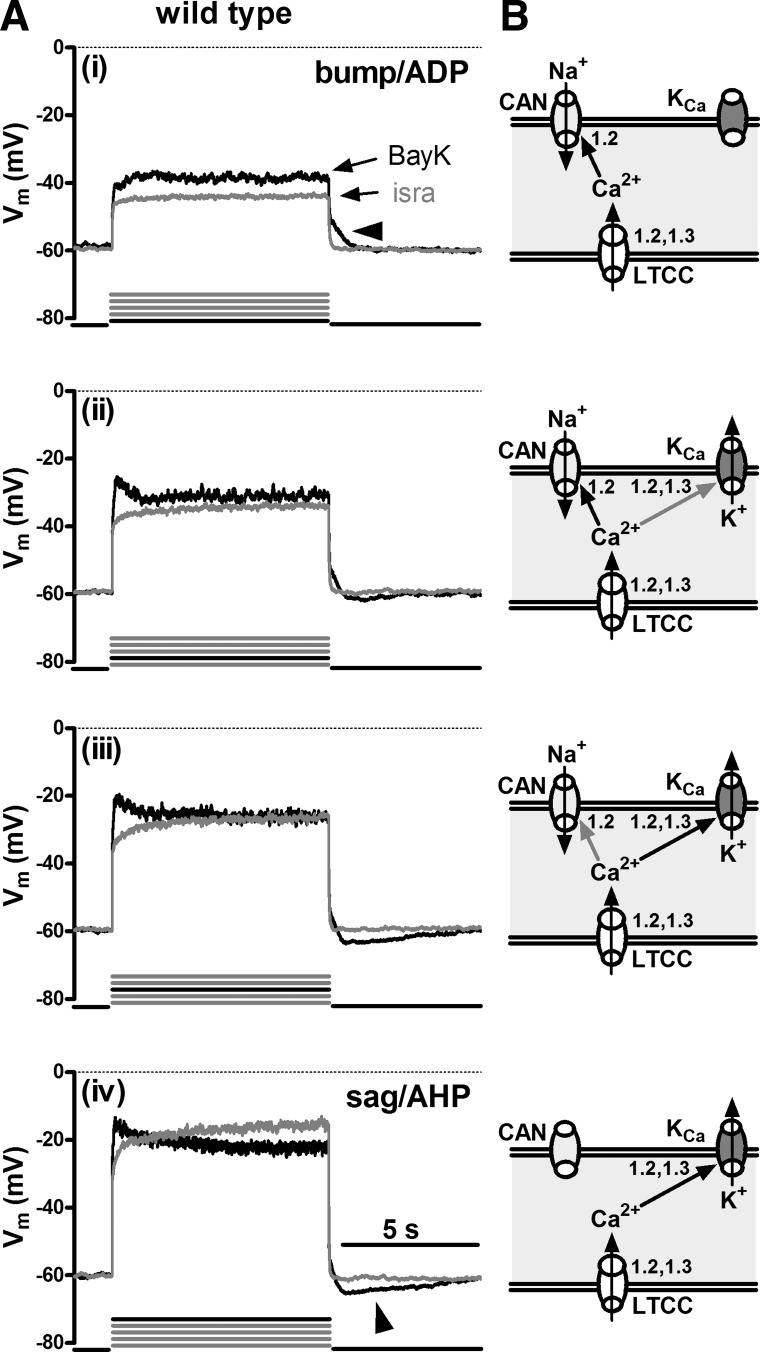
To investigate the effect of LTCC activation on membrane voltage, we depolarized mouse hippocampal neurons in the presence of 500 nM TTX by 8-s long current injections. Depolarization was induced by five successive current injections of increasing amplitude (injections 1 to 5 as described in materials and methods), so as to test for active responses at various levels of depolarization, between the resting membrane potential and about −20 mV. The recordings (n was at least 36 for each mouse strain) were made in the presence of DMSO and after modulation of the activity of LTCCs by addition of BayK (LTCC agonist) and isradipine (antagonist), respectively. Afterwards, overlays of responses obtained in the absence of LTCC activity (i.e., in the presence of isradipine, “isra”-labeled traces) with responses recorded when only solvent (DMSO traces) or when BayK were present (BayK traces) were generated to test for LTCC-mediated changes in the voltage responses. In all but 1 out of 36 neurons derived from wild-type neurons, LTCC-mediated responses were detected. Despite quantitative differences, qualitatively two major types of responses could be identified and were termed according to their appearance as either “bumps” or as “hyperpolarizing sags”: the term bump was used to describe responses where depolarizations rose above the response elicited in the presence of isradipine, irrespective of the magnitude of the difference between the two traces; the term hyperpolarizing sag was used to describe responses that, after an initial rise above the trace recorded in isradipine, hyperpolarize already during the pulse towards or even below the response elicited in the presence of the LTCC inhibitor so that the voltage response declines to or even traverses the isradipine trace in the overlays. In some neurons, the LTCC-mediated response was accompanied by oscillatory activity of variable frequency and amplitude. Figure 1 depicts prototypical examples recorded from different neurons. The exemplary traces also illustrate that bump responses were typically followed by afterdepolarizations (ADPs), whereas sag responses were typically followed by a distinct afterhyperpolarization (AHP) (these afterpotentials are marked with arrowheads in Fig. 1). The response modes could be identified before potentiation of LTCCs by BayK (see Fig. 1A, overlays of DMSO and isradipine traces) but application of BayK pronounced their appearance (Fig. 1B, overlays of BayK and isradipine traces). The bump response was seen in 44% of the neurons (16 out of 36) where it typically occurred at the lowest levels of depolarization (e.g., current injections 1 or 2, as indicated by the bold current pulse below the traces in Fig. 1A). Hyperpolarizing sags appeared when the neurons were depolarized to −35 mV or above (e.g., in current injections 4 and 5) in 75% of the neurons (27 out of 36). Oscillatory activity occurred at intermediate levels of depolarization, where afterpotentials were typically missing or small (see Fig. 1, Aii and Bii).
Fig. 1.
L-type voltage-gated calcium channel (LTCC)-mediated voltage responses in mouse hippocampal neurons. Voltage traces evoked by injection of current in the presence of isradipine (isra; grey traces) are overlaid with those recorded in the same wild-type neuron when only solvent (DMSO; A, black traces) or when Bay K8644 (BayK; B, black traces) was present in the superfusate (B). Overlays in A and B are from different neurons and illustrate the main types of active responses that could be readily classified according to their coarse appearance, namely bumps (i) and hyperpolarizing sags (sag; iii). Bumps and hyperpolarizing sags were followed after termination of the current injection by afterdepolarizations (ADP) and afterhyperpolarizations (AHPs), respectively. ii: voltage responses with associated oscillatory activity (osci). Horizontal arrowheads mark ADPs and oblique arrowheads mark AHPs. Below each pairs of traces, the number of the pulse (black line) out of 5 incremental current injections (grey lines) that typically evoked the depicted response mode is indicated. C: schematic illustrations of the conductances that may underlie the 2 response modes: bump/ADPs are suggested to be due to Ca2+ influx via LTCCs and activation of Ca2+-dependent nonselective cation channels (CAN). Sag/AHPs are suggested to be due to Ca2+ influx via LTCCs and activation of Ca2+-dependent potassium channels (KCa). Vm, membrane potential.
These LTCC-mediated response modes were identical to the ones described by us previously in rat hippocampal neurons (18). In this earlier study, we characterized the underlying conductances and provided evidence that the excitatory “bump” response involves not only LTCC-mediated Ca2+ influx but also a coupling to Ca2+-dependent sodium-permeable channels (e.g., CAN channels). On the other hand, the hyperpolarizing sag response was shown to involve activation of Ca2+-dependent potassium channels (e.g., apamin-sensitive KCa channels). Figure 1C schematically illustrates the respective ion fluxes and channel coupling that may underlie the two response modes.
Both LTCC-dependent response modes persist in Cav1.3−/− neurons.
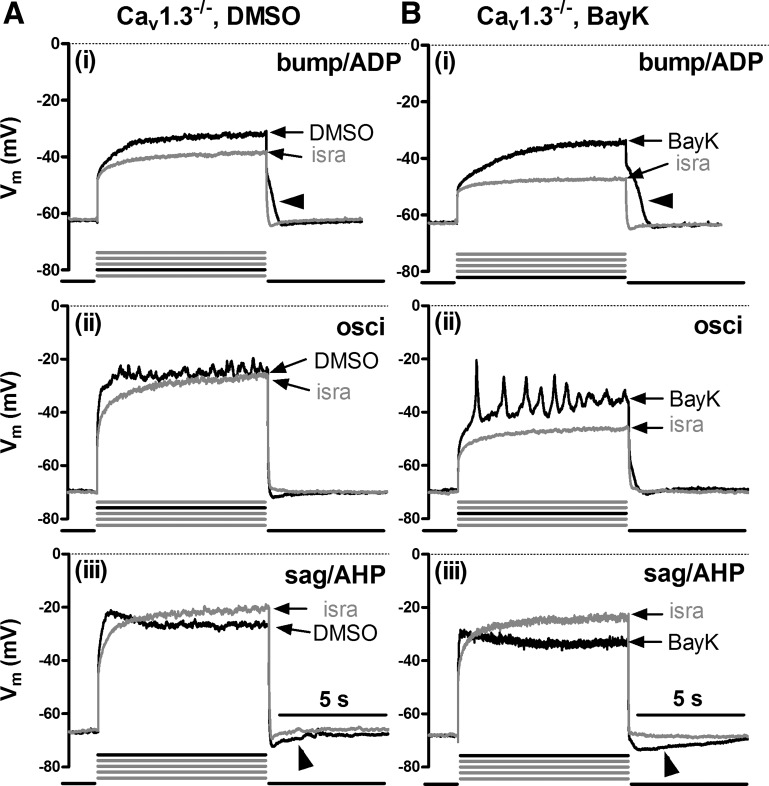
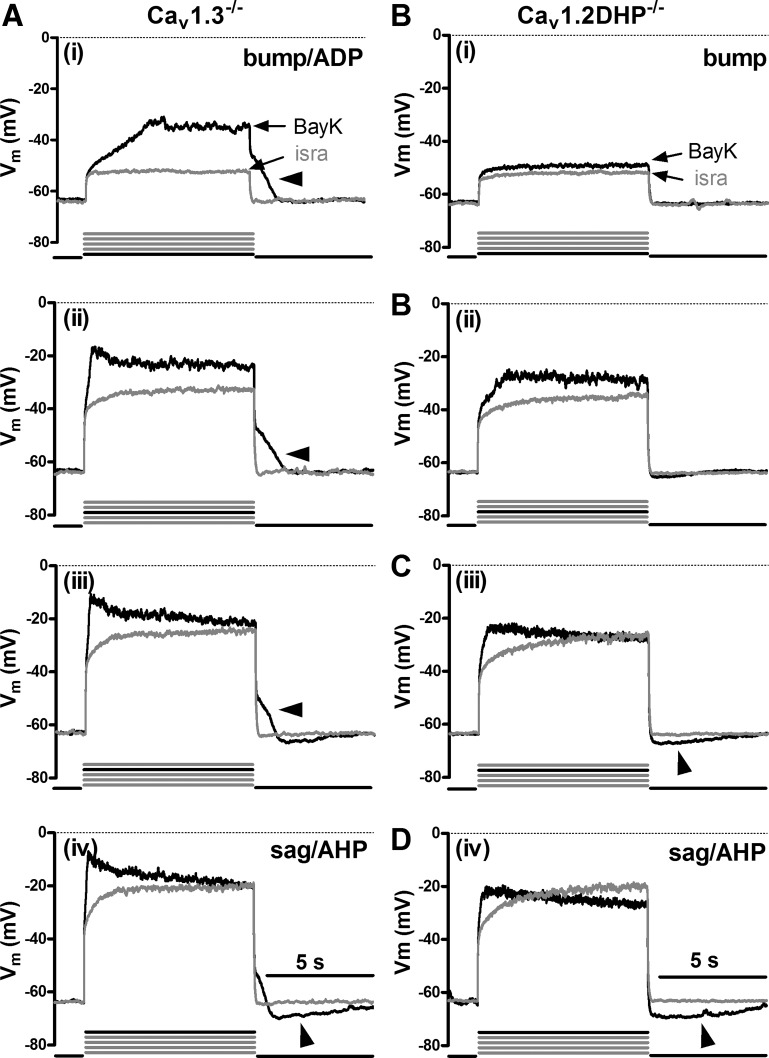
To gain insight into the involvement of specific LTCC isoforms, we performed identical experiments as above on hippocampal neurons derived from Cav1.3−/− mice. Again, all types of responses could be identified, both under control conditions (Fig. 2A) and in the presence of BayK (Fig. 2B). As in wild-type neurons, bumps were followed by ADPs, whereas hyperpolarizing sags were accompanied by AHPs (marked by arrowheads in Fig. 2). Again, the bump response typically occurred at the lowest levels of depolarization (e.g., current injections 1 or 2, as indicated by the bold current pulse below the traces) and was seen in 70% of Cav1.3−/− neurons (32 out of 46). Responses were occasionally associated with oscillatory activity as the level of depolarization was increased. Hyperpolarizing sags appeared when the neurons were depolarized to −35 mV or above (e.g., in current injections 4 and 5) and where seen in 43% of Cav1.3−/− neurons (20 out of 46).
Fig. 2.
LTCC-mediated voltage responses in Cav1.3−/− neurons. Overlays of voltage traces as in Fig. 1 (see there for a description of the labeling) but from different Cav1.3−/− neurons recorded in the presence of isra (grey traces) and either DMSO (A, black traces) or BayK (B, black traces). Arrowheads indicate afterpotentials.
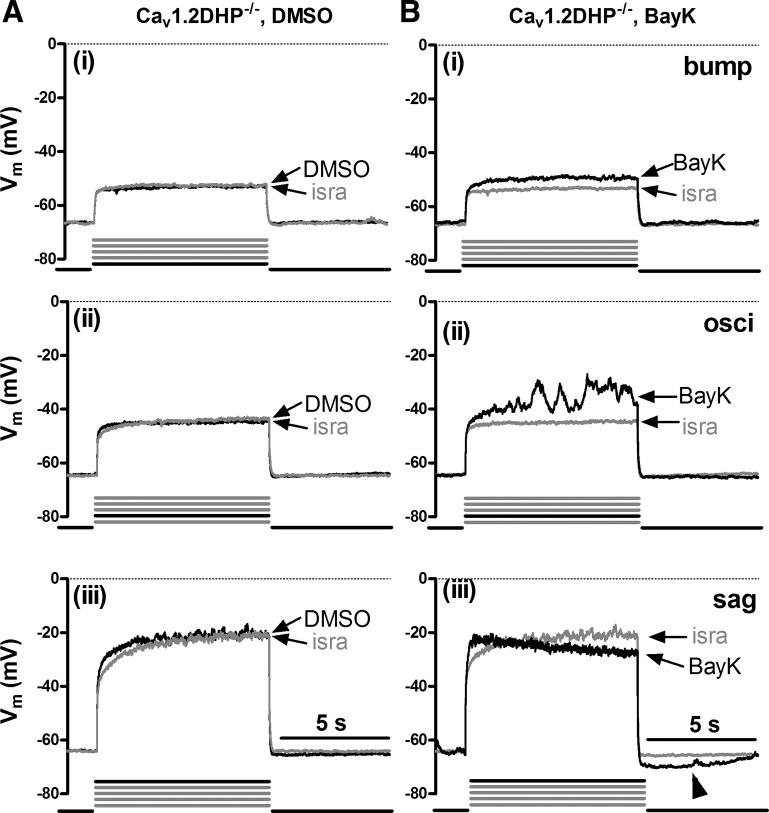
Both LTCC-dependent response modes can be activated by BayK in Cav1.2DHP−/− neurons.
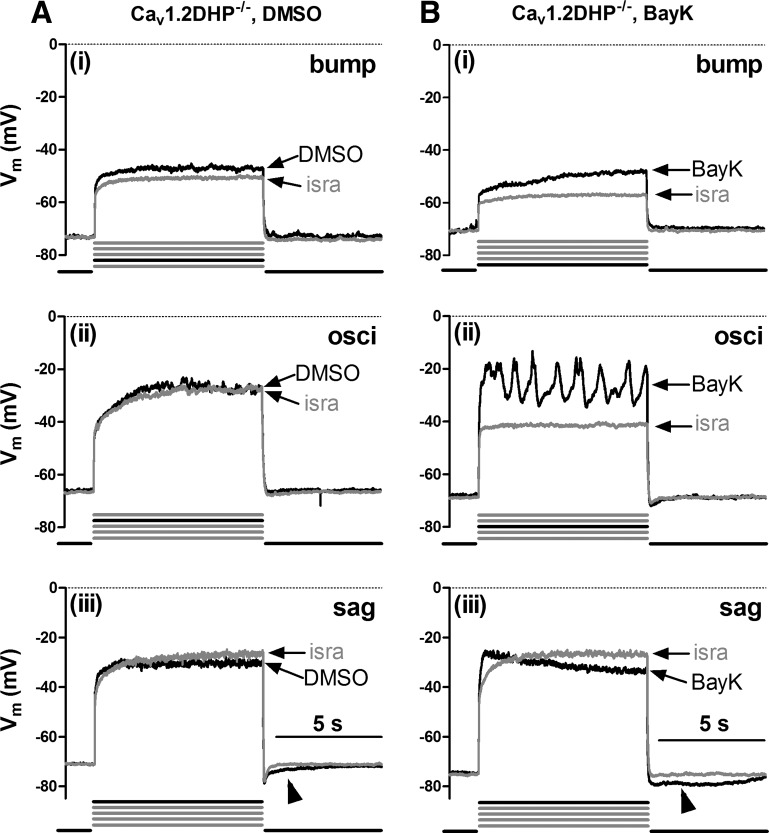
The presence of bumps and hyperpolarizing sags as well as ADPs and AHPs in Cav1.3−/− neurons suggests that these LTCC-induced voltage effects were all mediated by Cav1.2 channels. To explore this possibility, we used neurons derived from knockin mice, which express a Cav1.2 channel mutant that lacks high dihydropyridine sensitivity (Cav1.2DHP−/− neurons, see materials and methods) and took advantage of the pronounciation of the LTCC responses upon application of the channel agonist BayK. Because Cav1.2DHP−/− channels cannot be potentiated by BayK even in the low micromolar range (see Drugs), BayK-induced effects on voltage can only be due to Cav1.3 channel activity. On the other hand, 3 μM isradipine is a concentration high enough to inhibit a considerable fraction of Cav1.2DHP−/− channels (54) and was thus again used for reference recordings. As expected, both bumps and hyperpolarizing sags could be identified under control conditions (Fig. 3A). However, in the presence of BayK LTCC-mediated voltage responses appeared more pronounced even in Cav1.2DHP−/− neurons, with bumps occurring in ∼45% of the neurons (17 out of 38) and hyperpolarizing sags in 71% of the neurons (27 out of 38) (Fig. 3B). This suggests that Cav1.3 channels can also induce both bumps and sag responses. To corroborate this notion, we made direct comparisons of voltage responses in Cav1.2DHP−/− neurons evoked before addition of BayK (when only DMSO was present) and after addition of the dihydropyridine agonist. Figure 4 shows examples from three neurons (representative of 10 similar observations) where under control conditions (DMSO) LTCC-mediated voltage responses were absent or very small (note the largely overlapping traces in the three examples in Fig. 4A), so that it can be clearly seen that only BayK induced the characteristic LTCC-mediated voltage responses in this subset of neurons (Fig. 4B). As in wild-type and Cav1.3−/− neurons, responses were occasionally associated with oscillatory activity as the level of depolarization was increased.
Fig. 3.
LTCC-mediated voltage responses in dihydropyridine-insensitive LTCC isoform variant (Cav1.2DHP−/−) neurons. Overlays of voltage traces as in Fig. 1 (see there for a description of the labeling) but from different Cav1.2DHP−/− neurons recorded in the presence of isra (grey traces) and either DMSO (A, black traces) or BayK (B, black traces). Arrowheads indicate afterpotentials.
Fig. 4.
Cav1.3-mediated voltage responses. The induction of active voltage response by BayK in Cav1.2DHP−/− neurons is demonstrated by a comparison of traces recorded in 3 neurons in the presence of solvent (DMSO) only (A) with traces recorded from the same neurons after addition of BayK (B). All responses are shown in overlays with the corresponding trace obtained when isradipine was present. Ai and Bi, Aii and Bii, Aiii and Biii are from the same neuron. Pronounced differences from the isra traces [bump (i), osci (ii), and sag (iii) responses] were only induced when BayK was present, implicating that they were mediated by Cav1.3 channels.
Differences and similarities in the coupling of Cav1.2 and Cav1.3 channels to afterpotential-mediating conductances.
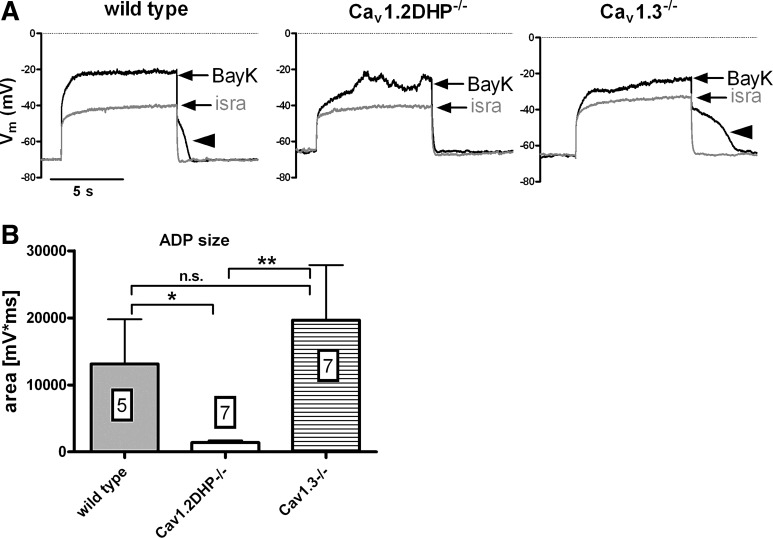
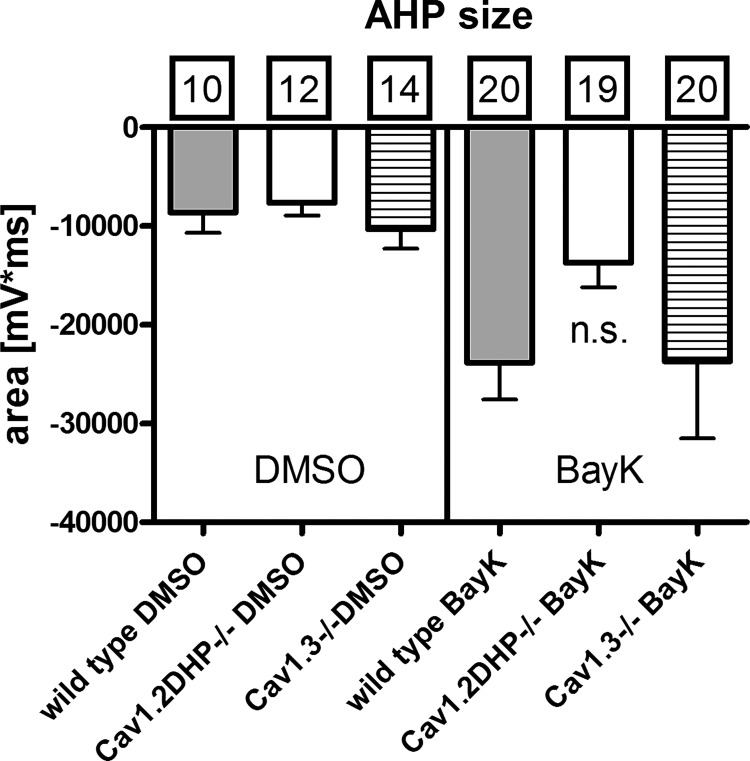
Taking together the results described so far, it can be concluded that bump and hyperpolarizing sag responses can be induced by Ca2+ influx irrespective of whether it occurs through Cav1.2 or Cav1.3 channels. According to the data obtained in rat neurons on the mechanisms underlying the two opposing voltage responses (see scheme in Fig. 1C), this would also suggest that both LTCC isoforms couple to depolarizing CAN channels and inhibitory KCa channels. However, closer inspection of the voltage responses recorded in neurons derived from the two genetically modified mouse strains indicated that there are differences in the respective coupling modes of Cav1.2 and Cav1.3 channels: we noted that in the presence of BayK, bump responses are always associated with readily observable ADPs in Cav1.3−/− neurons but not in Cav1.2DHP−/− neurons. Hence, Cav1.2-mediated Ca2+ influx appears to be required for formation of depolarizing afterpotentials. To evaluate this observation we determined the afterpotential area in recordings from neurons of all mouse strains (see materials and methods for details of the determination). To warrant that only neurons with a discernable LTCC conductance were included, this was performed for recordings with a clear bump response only (Fig. 5A). The result of this analysis is shown in the graph in Fig. 5B. Large depolarizing afterpotential areas were found in both wild-type and Cav1.3−/− neurons, reflecting the occurrence of ADPs as illustrated also in Figs. 1Ai, and 2Bi. Depolarizing afterpotential areas were significantly smaller in Cav1.2DHP−/− neurons, which corresponds to the lack of distinct ADPs in these neurons (see Fig. 3Bi and Fig. 4Bi). In contrast, a similar evaluation of AHPs that accompanied hyperpolarizing sag responses (see Fig. 1, Aiii and Biii, Fig. 2 Aiii and Biii, and Fig. 3, Aiii and Biii) revealed no significant difference among neurons from wild-type and genetically modified mice strains, both under control conditions (n = 10–14) and in the presence of BayK (n ≥ 19; Fig. 6).
Fig. 5.
Size of ADPs in neurons of wild-type and LTCC-gene modified mice. A: overlays of traces recorded in the presence of BayK and isradipine exemplify pronounced bump responses in neurons of wild-type (left), Cav1.2DHP−/− (middle), and Cav1.3−/− mice (right) that were selected for evaluation of the ADP size. Arrowheads indicate distinct depolarizing afterpotentials. B: average area of ADPs (mV·ms) recorded in the presence of BayK in response to 8 s-long current injections is displayed for neurons from the 3 mouse strains as indicated. Boxed figures indicate the number of experiments. Statistical analysis revealed a significant difference between wild-type and Cav1.2DHP−/− data (*P < 0.05), between Cav1.2DHP−/− and Cav1.3−/− data (**P < 0.01), but not between wild-type and Cav1.3−/− data (n.s., not significant).
Fig. 6.
Size of AHPs in neurons of wild-type and LTCC-gene modified mice. The average area of AHPs (mV·ms) recorded in the presence of DMSO (left) and BayK (right) is displayed for neurons from the 3 mouse strains as indicated. Boxed figures indicate the number of experiments. Statistical analysis did not reveal any significant difference between AHP areas of the 3 mice strains in both DMSO and BayK (n.s., not significant).
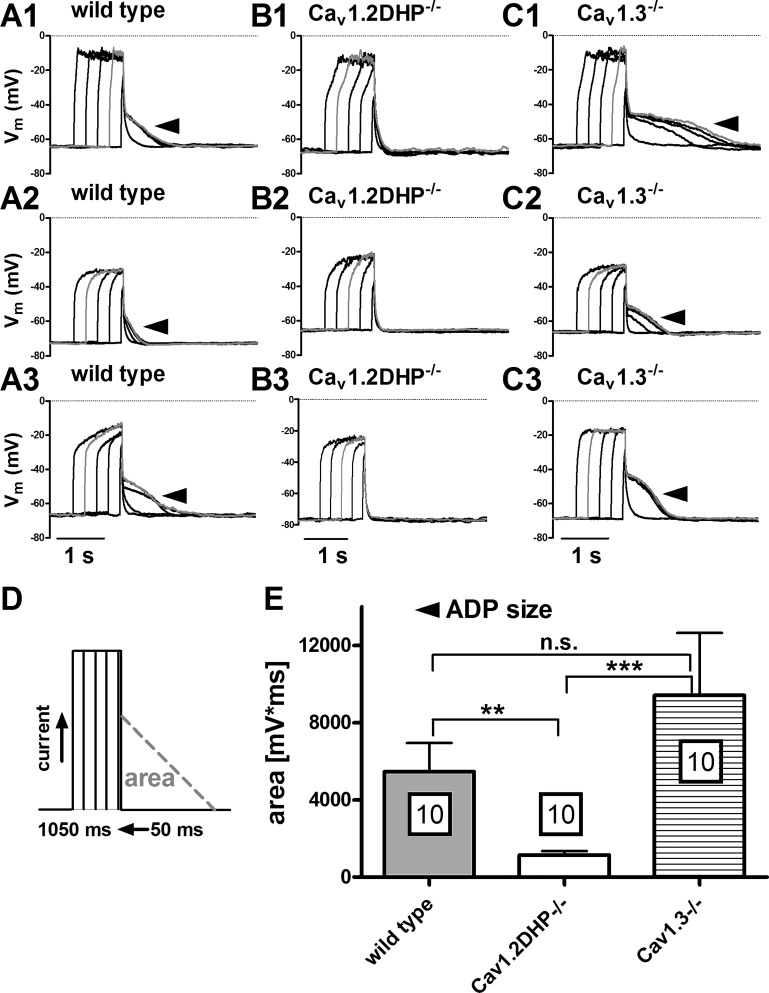
Previous work in our laboratory has shown a bimodal activation pattern for ADPs, which appear already with moderate depolarizations but decrease or give way to AHP potentials as the depolarizing stimulus is increased [both in terms of amplitude and duration (18)]. Hence, we used a second, considerably shorter stimulation paradigm (50 to 1,050 ms, as indicated in Fig. 7D, with current injections large enough to depolarize the neurons to at least −35 mV) to test for ADPs in neurons of the three mice strains. As illustrated in Fig. 7, ADPs were readily evoked in wild-type and Cav1.3−/− neurons (Fig. 7, A1–A3 and C1–C3). Again, distinct ADPs were missing in voltage responses recorded from Cav1.2DHP−/− neurons (Fig. 7, B1–B3). The analysis of afterpotential areas in these recordings is summarized in the graph in Fig. 7E (n = 10 for each strain). Besides the significantly smaller afterpotential areas determined in recordings from Cav1.2DHP−/− neurons, it emerges that afterpotential areas in Cav1.3−/− neurons are at least as large as those of ADPs from the wild-type neurons. Hence, our experiments demonstrate that the ADPs are selectively induced by Ca2+ influx via Cav1.2 channels and not by Ca2+ influx via Cav1.3 channels.
Fig. 7.
ADPs induced by brief depolarizing current injections require Cav1.2 channels. The traces in A–C depict 3 examples each of voltage responses evoked by a series of brief current injections (duration 50 to 1,050 ms, as indicated in D) that are followed by ADPs in wild-type neurons (A1–A3) and neurons derived from Cav1.3−/− neurons (C1–C3) but are missing in neurons form Cav1.2DHP−/− mice (B1–B3). The grey trace in each overlay depicts the response with the largest ADP in the series. In E the average area of the largest ADPs (mV·ms) is displayed for neurons from the 3 mouse strains as indicated. Boxed figures indicate the number of experiments. Statistical analysis revealed a significant difference between wild-type and Cav1.2DHP−/− data (**P < 0.01), between Cav1.2DHP−/− and Cav1.3−/− data (***P < 0.001), but not between wild-type and Cav1.3−/− data (n.s., not significant).
Cav1.2 and Cav1.3 channel-mediated response modes show a similar stimulus dependency.
As indicated above we have previously reported on a dynamic interplay of the two LTCC coupling modes giving rise to a stimulus dependency of the response modes in rat hippocampal neurons. We also observed this stimulus dependency in mice neurons: a transition of LTCC-dependent response modes could be identified in control (DMSO) experiments (not shown) but was best seen in the presence of BayK. As for wild type, transition was manifest in 10 neurons (in other neurons it was incomplete in that bumps or sag responses predominated). A representative example is illustrated in Fig. 8, which depicts four responses of the same neuron to consecutive current injections of increasing amplitude (Fig. 8, Ai–Aiv; see the bold current pulse in the pulse protocol below the traces). The change from bump to sag responses was accompanied by a switch from depolarizing to hyperpolarizing afterpotentials. As indicated in Fig. 8, the bump/ADP responses were evoked with the smallest level of depolarization (e.g., current injection 1 or 2, see the bold current pulse below the traces in Ai). In contrast, sag/AHP responses were prominent at the highest levels of depolarization (Fig. 8Aiv). The schematic drawings in B indicate the proposed switch from excitatory to inhibitory coupling that may underlie the transition between response modes together with the Ca2+ entry routes upon which the couplings are based according to previous (18) and above-mentioned data. However, transition from excitatory to inhibitory response mode was also seen in neurons derived from Cav1.3−/− neurons and in neurons derived from Cav1.2DHP−/− mice (Fig. 9). In Cav1.3−/− neurons, the switch from bump to sag response during the current injection was followed by a decaying ADP that gave way to an AHP as the depolarization was increased (representative of 11 neurons), which was similar to the situation in wild-type neurons. In contrast, in Cav1.2DHP−/− bumps, that were not followed by ADPs, diminished as the depolarization was increased and turned into hyperpolarizing sags, which were accompanied by AHP potentials (also representative of 11 neurons).
Fig. 8.
Transition between response modes in wild-type neurons. A: overlays of voltage responses recorded in the presence of BayK or isradipine by incremental current injections in a wild-type neuron (representative of 10 similar observations) illustrates the transition from bump/ADP (i) to sag/AHP (iv) responses as the level of depolarization was increased (i–iv). Four out of 5 incremental current injections that were sequentially applied in the presence of BayK (black traces) and isradipine (grey traces) are shown. The schema in B schematically illustrate the conductances that may underlie the response modes: transition is suggested to be due to a sequential activation of initially Ca2+ influx via LTCCs (Cav1.2 and Cav1.3) and activation of Ca2+-dependent nonselective cation channels (CAN, via Cav1.2-mediated Ca2+ influx only) and subsequently Ca2+ influx via LTCCs (Cav1.2 and Cav1.3) and activation of Ca2+-dependent potassium channels (KCa, via Ca2+ influx through Cav1.2 and Cav1.3 LTCCs). Grey arrows indicate weak activation, and black arrows indicate strong activation. “1.2” and “1.3” indicate the Ca2+ entry route via Cav1.2 and Cav1.3 channels, respectively.
Fig. 9.
Transition between response modes in Cav1.3−/− and in Cav1.2DHP−/− neurons. The overlays illustrate the transition from bump to sag responses in a Cav1.3−/− neuron (A) and in a Cav1.2DHP−/− neuron (B) in the same manner as described in Fig. 8 for wild-type neurons. Both illustrations are representative for 11 similar observations.
Cav1.2 and Cav1.3 channels operate in a similar voltage range.
In our experiments, we gradually raised Vm of the neurons to depolarized voltages. The resting membrane potential of the neurons was not affected by application of BayK or isradipine (see materials and methods). As for the smallest levels of depolarization, voltage traces also did not differ when recorded under control conditions or after potentiation or block of LTCCs with dihydropyridines. However, at a certain level of depolarization, BayK traces but also DMSO traces started to diverge from the isradipine trace. Close inspection of the point of divergence (in the first out of the 5 pulses in which this occurred) was used for determination of the “onset” of LTCC-mediated voltage responses (see Fig. 10, A and B, for an illustration of this procedure, where arrowheads mark the onsets). We determined the onset for neurons of all three mice strains and subjected the collected data to statistical analysis. In neurons of all strains, onset occurred at significantly less depolarized voltages when BayK was present than in its absence (DMSO control). Hence, addition of BayK led to more hyperpolarized onsets. This is in line with the observation that BayK shifts the half-activation potential (V0.5) of LTCCs to more negative voltages (see for example Refs. 19, 70) and supports the idea that the deflection of the respective traces from the isradipine trace does indeed represent the membrane potential at which LTCC-mediated Ca2+ influx starts to affect membrane voltage. However, when onsets were compared between mice strains under both conditions, no significant difference was found, irrespective of whether LTCCs were potentiated (BayK) or not (DMSO). These data are summarized in the box plot in Fig. 10C. Our recordings demonstrate that in the absence of Cav1.3 channels LTCC-dependent voltage responses can be induced in a subset of neurons already with quite moderate depolarizations, e.g., at membrane voltages more negative than −50 to −53 mV (these values represent the 25% percentiles in the box plot of the data for DMSO and BayK, respectively, see Table 1).
Table 1.
Statistical data on LTCC-response onsets
| Mouse Strain/Condition |
||||||
|---|---|---|---|---|---|---|
| Wild-Type DMSO | Wild-Type BayK | Cav1.3−/− DMSO | Cav1.3−/− BayK | Cav1.2DHP−/− DMSO | Cav1.2DHP−/− BayK | |
| Number of determinations | 16 | 27 | 28 | 36 | 12 | 22 |
| Minimum, mV | −52.00 | −63.00 | −53.00 | −60.00 | −48.00 | −59.00 |
| 25% Percentile, mV | −45.50 | −54.00 | −50.00 | −52.75 | −45.00 | −55.25 |
| Median, mV | −39.50 | −52.00 | −45.00 | −48.50 | −40.00 | −53.50 |
LTCC, L-type voltage-gated calcium channel; BayK, Bay K8644; DHP, dihydropyridine.
Taken together, our results demonstrate that both hippocampal Cav1 channel isoforms couple to hyperpolarizing Ca2+ dependent conductances, so that their activation can induce AHPs. In contrast, only Cav1.2 channels couple to an excitatory Ca2+-dependent conductance, so that of the LTCCs solely this isoform is capable of inducing depolarizing afterpotentials. Moreover, we showed that both hippocampal LTCC isoforms can activate at membrane voltages more negative than −50 to −60 mV, which is more hyperpolarized than voltage drops usually thought to be required for activation of high-voltage-activated calcium channels.
DISCUSSION
Differences in the coupling of Cav1.2 and Cav1.3 channels to Ca2+-dependent conductances.
The response modes previously described in rat primary hippocampal neurons could also be identified in identical form in primary neurons isolated from mouse hippocampus. Moreover, all response modes could be evoked or augmented by BayK in both Cav1.3−/− neurons and in Cav1.2DHP−/− neurons. Earlier (18) we provided evidence that these voltage responses are due to differential coupling of LTCC-mediated Ca2+ influx to de (CAN channels)- or hyperpolarizing conductances (e.g., KCa channels). In Cav1.3−/− neurons, LTCC-mediated Ca2+ influx is through Cav1.2 channels, whereas induction by BayK in Cav1.2DHP−/− neurons means that the activating Ca2+ influx is provided by influx via Cav1.3 channels. Hence both LTCC isoforms can activate the ion fluxes that underlie the described voltage responses. However, in Cav1.2DHP−/− neurons BayK did not induce the distinct ADPs that were seen routinely in wild-type and in Cav1.3−/− neurons. In contrast, AHPs were evoked or enhanced by BayK in both Cav1.3−/− neurons and Cav1.2DHP−/− neurons, suggesting that both LTCC isoforms have functional coupling to AHP-mediating channels. We would like to point out that the recordings used to illustrate the LTCC-mediated voltage responses in neurons of the three mouse strains used in this article were chosen in an entirely arbitrary manner; hence, differences beyond the ones described for depolarizing afterpotentials are visible, for example with respect to amplitudes or the rise and/or fall times of the voltage trajectories. However, none of such differences was associated consistently with only one of the strains. In fact, there was considerable variability in the voltage trajectories within the neurons of each strain [compare for example the bumps responses of wild-type neurons shown in Figs. 1Bi, 5A, and 8Ai; a similar variability was seen in hyperpolarizing sag responses (not shown)], which may be due to the fact that we used primary cultures of dissociated neurons and thus sampled the data from all neuronal types and all subregions of the hippocampus. However, our data show that throughout the hippocampus, all major LTCC-mediated response modes can be elicited irrespective of whether Ca2+ influx occurs via Cav1.2 or Cav1.3 channels. Hence, we found no other phenotypic difference than the lack of BayK-induced ADPs in Cav1.2DHP−/− neurons.
Evidence for isoform-specific roles in the induction of afterpotentials is rare, although there have been reports that AHPs in hippocampal neurons are evoked by Ca2+ influx via Cav1.3 channels rather than Cav1.2 channels (17, 37). This is in contrast to our observation that both channel isoforms can couple to AHP-mediating channels. Probably in certain experimental settings activation of depolarizing Ca2+-dependent channels by Cav1.2 channel-mediated Ca2+ influx is capable of masking simultaneously occurring coupling to AHP-mediating channels. Alternatively, only loss of Cav1.3 channels may enable effective coupling of Cav1.2 channels to these channels. At present we cannot distinguish between these two possibilities. Other studies are also inconclusive regarding compensatory changes of Cav1.2 channels. For example, Clark et al. (8) reported on a lack of effect of Cav1.3 gene deletion on Cav1.2 protein levels in whole brain preparations, whereas Jurkovičová-Tarabová et al. (26) found increased expression levels of Cav1.2 in the lateral superior olive of Cav1.3−/− mice.
In hippocampal neurons depolarizing effects were also noted for Cav1.2 channel activity by Lacinova et al. 2008 (33) but to date not for Cav1.3 channels. Hence, our study for the first time demonstrates Cav1.2-specific activation of ADP potentials and that AHPs can be evoked by Ca2+ influx through both Cav1.2 and Cav1.3 channels. We also provide evidence that Cav1.3 channels can cause neuronal depolarization in a direct manner that is independent of any coupling to an afterpotential-mediating Ca2+-dependent conductance (see Relation between Cav1.2- and Cav1.3-mediated voltage responses and afterpotentials).
Nature of the ADP-mediating conductance.
With respect to rat hippocampal neurons, we have previously obtained the following data regarding the nature of the conductance underlying the above described ADP (18): 1) it is activated upon Ca2+- influx via LTCCs, 2) it is due to Na+ but not Ca2+ ion flux, and 3) it is sensitive to flufenamic acid (≤100 μM). Other studies provided evidence pointing to a relation between such afterpotentials and transient receptor potential (TRP) channels: 1) ADPs in central neurons are due to an ADP-generating conductance (ICAN), which is carried by Ca2+-activated monovalent cation current channels (21, 22, 63). 2) TRPM4b and TRPM5 share many of the hallmarks of ICAN channels, e.g., single-channel conductances between 20 and 35 pS, Ca2+ activation, selectivity for monovalent cations, and low permeability to Ca2+. Other than TRPM4b and TRPM5, there are no other channels described with these features (23). 3) TRPM4 and 5 are the only TRP channels shown to be directly gated by increases in intracellular Ca2+ concentration. 4) In contrast to all other TRPs, both TRPM4 and 5 form ion channels permeable for monovalent cations but impermeable to Ca2+ [see the review by Pedersen et al. (42), for 3 and 4]. Hofmann et al. (23), therefore, noted in 2003 that these two TRP channels are currently the only molecular candidates that could account for ICAN; to our best knowledge, nothing has changed this view in recent years. Hence, TRPM channels can be considered as the most likely candidates for the ion channels that mediate Cav1.2-dependent ADPs in hippocampal neurons, although the lack of “specific” inhibitors obviously precludes final proof of this notion.
Relation between Cav1.2- and Cav1.3-mediated voltage responses and afterpotentials.
Previous results, for example, correlation analysis, indicated that bumps involve the same conductance that mediates depolarizing afterpotentials, with ADPs presumably being caused by a protracted deactivation of the channel contributing to the enhanced depolarization during the current injection (18). Here we show that bumps were induced by BayK also in Cav1.2DHP−/− neurons (which express only BayK-insensitive Cav1.2 channels), where a concomitant ADP did not occur. Hence we suggest that Cav1.3-mediated bumps are caused directly by LTCC-mediated Ca2+ influx. Since Cav1.3 channels are known to carry significant window current (60, 70), their activity can indeed be envisaged to support long-lasting depolarizations. Previously, we found that replacing sodium in the external solution with an impermeant cation led to elimination of ADPs and to profound reductions in bumps of rat hippocampal neurons (18). Taken together with our current data we propose that Cav1.2-mediated bumps may be due to both Ca2+ influx and activation of CAN channels, whereas Cav1.3-mediated bumps may solely be due to Ca2+ influx. In contrast, hyperpolarizing sags were always associated with AHPs, both in rat neurons (18) and neurons derived from wild-type and genetically modified mice (this study), suggesting that both effects on membrane voltage are due to the same hyperpolarizing LTCC-coupled ion conductance. Note that bumps were seen more frequently in Cav1.3−/− neurons than in wild-type and Cav1.2DHP−/− neurons. This may be due to the loss of the Cav1.3-dependent part of a hyperpolarizing drive provided by LTCC coupling, e.g., to KCa channels. In line with this notion, sag responses were less often identified in Cav1.3−/− neurons. Since Jurkovičová-Tarabová et al. (26) reported on an upregulation of Cav1.2 channels in neurons of Cav1.3−/− mice, augmentation of the Cav1.2/CAN-mediated depolarizing drive may also play a role in the relative increase of bump responses.
Role of LTCC-mediated ADPs.
In neuronal cells, the ionic mechanisms that underlie ADPs have been implicated in physiological and pathophysiological electrical activities, e.g., bursting discharge patterns and plateau potentials on the one hand (13, 16, 35, 39, 41) and paroxysmal depolarization shifts (PDS) on the other hand (e.g., 18, 55, 56). By enhancing neuronal firing, ADPs are thought to indirectly contribute to the provision of pronounced Ca2+ elevations for effective release of hormones (48, 69). By promoting plateau potentials, ADP-mediating conductances may be involved in neuronal information processing, for example, providing short-term memory or, by shunting of those excitatory postsynaptic potential that follow the plateau-inducing ones, in establishing low pass filter characteristics (5, 12, 44, 76). Similarly, ADPs were suggested to act in synaptic integration, for example when back-propagating ADP potentials affect coincidentally arriving dendritic synaptic inputs (27, 72). In contrast, the proposed role of an ADP-generating conductance in PDS suggests an involvement in neuropathological processes, because these abnormal electrical events have been suggested to play a role in epileptogenesis (57, 58).
The LTCC-ICAN coupling is one out of several mechanisms how an ADP can be generated, and this kind of ADPs has been linked to bistability in motoneurons of neonatal rats (4), burst firing in dopaminergic neurons (35, 45), and information processing in hippocampal neurons (68). As ADPs in hippocampal neurons were found in this study to depend on the coupling of Cav1.2 channels to an ADP-mediating conductance, this may offer one explanation for the role of Cav1.2 channels in learning and memory (25, 34, 38, 66). Indeed, in hippocampal CA1 networks Cav1.2 but not Cav1.3 channels have been found to enable a N-methyl-d-aspartate receptor-independent form of long-term potentiation (8, 38). In contrast, although proposed earlier on in original articles and textbooks (e.g., Refs. 55, 56), the contribution to PDS has not received experimental proof so far. Hence, the two mouse models identified in this study to have either a prominent coupling of neuronal LTCCs to an ADP-generating conductance (ICAN; Ref. 18), i.e., Cav1.3−/− mice, or to lack LTCC-agonist stimulated ADPs, i.e., Cav1.2DHP−/− mice, can be envisaged to greatly aid in future analysis of the physiological and pathophysiological implications of LTCC-induced ADPs.
However, long-lasting depolarizations may also arise from activation of Cav1.3 channels, because these LTCC subtype was shown to have a lower tendency to inactivate than Cav1.2 channels and may carry significant window current (60, 70). Indeed, LTCC-mediated plateau potentials that were solely attributed to Cav1.3 channels have been described previously (43, 52). Here we observed Cav1.3-mediated long lasting depolarizations in rat hippocampal neurons, i.e., BayK-induced bump responses in Cav1.2DHP−/− neurons. Hence, similar functions (for example generation of plateau potentials) may be carried out by both Cav1.2 and Cav1.3 channels, yet with differing underlying mechanisms. It is possible that such mechanistical differences endow plateau potentials with different dependences on cytoplasmic calcium or membrane voltage or with different “off” kinetics. Note that Cav1.3-mediated long-lasting depolarizations observed in this study, i.e., BayK-induced bump responses in Cav1.2DHP−/− neurons, were not accompanied by ADP potentials, which indicates that Ca2+ influx was rapidly terminated after switching off the current injection. In contrast, the Cav1.2-CAN coupling only terminates with a significant delay (thus giving rise to the ADPs), a feature that is most likely related to the time course of the intracellular Ca2+ elevation.
Cav1.2 and Cav1.3 operate in a similar voltage range.
The onset of LTCC activity in Cav1.3−/− neurons, which must be due to activation of Cav1.2 channels, occurred at potentials negative to −50 mV. This is not significantly different from BayK-induced responses in Cav1.2DHP−/− neurons, which are mediated by Cav1.3 channels. Notably, the onset of LTCC activity in Cav1.3−/− neurons occurred at potentials more negative than the relatively depolarized threshold of activation currently thought to characterize Cav1.2 channels. In heterologous expression systems, several studies indicated considerable differences between neuronal Cav1.2 and Cav1.3 channels in their voltage dependence of activation [see, for example, Ref. 67 (human neuronal alpha1D) and Ref. 62 (rat brain alpha 1C), discussed by Carlin et al. (6)]. Such observations were supported by direct comparison in the laboratories of J. Striessnig (31) and D. Lipscombe (70), with Cav1.3 channels [from human pancreas (J. Striessnig) and rat peripheral neurons (D. Lipscombe), respectively] being activated at considerably less depolarized potentials than rabbit cardiac Cav1.2 channels (both studies). This appears at odds with our finding that central Cav1.2 and Cav1.3 channels operate in the same negative voltage range in hippocampal neurons. It should be noted that the biophysical properties of LTCCs are subject to modulation by transcriptional (e.g., splice variation) and posttranslational (e.g., phosphorylation) mechanisms and may also depend on the exact composition of the calcium channel complex, e.g., association with auxiliary subunits and regulatory proteins (14, 20, 53, 73). Hence, it is possible that while activation by moderate depolarizations is an intrinsic property of Cav1.3 channels, Cav1.2 channels may be tuned to operate in a comparable voltage range by regulatory mechanisms.
Our observation of Cav1.2 channel activity at unexpectedly negative voltages is in line with a recent report by Radzicki et al. (47), who showed that Cav1.2 channels were active at hyperpolarized potentials in hippocampal slices (e.g., at potentials negative to −50 mV), although in their study activation required elevation of the ambient temperature. Moreover, although not specifically noted by the authors, unrelated work on Cav1.2DHP−/− in lateral superior olive neurons indicates the existence of Cav1.2 channels operating at voltages more negative than −40 mV (26). In gene deletion studies, the question arises whether a loss of Cav1.3−/− channels may trigger transcriptional or posttranslational modifications of at least a subpopulation of Cav1.2 channels, thereby changing their voltage-dependence so as to compensate for the elimination of negatively activating Cav1.3 channels. The difference between onset medians determined in the presence of DMSO and in the presence of BayK was notably smaller in Cav1.3−/− neurons (3.5 mV) than in both wild-type neurons and Cav1.2DHP−/− neurons (≥12.5 mV; see Table 1). Thus the ability of LTCC potentiation to shift the average onset to more hyperpolarized voltages was less expressed in neurons lacking Cav1.3 channels, which may be indicative of an increase in a subpopulation of rather low voltage-gated Cav1.2 channels. However, ADPs (here identified to require Cav1.2 activity) were evoked by small depolarizations not only in Cav1.3−/− neurons but also in wild-type neurons (see for example Figs. 1B and Fig. 8A, top traces), suggesting that activation of Cav1.2 channels at relatively negative voltages also occurs in neurons that do express the Cav1.3 LTCC isoform. Taken together, recent data challenge the view that only Cav1.3 but not Cav1.2 channels can operate already at potentials that are usually thought to be to low for activation of cannonical high-voltage-activated calcium channels.
Conclusion.
In the current study we identified common and alternate ion channel couplings of the central LTCC isoforms Cav1.2 and Cav1.3. Additionally, we provide evidence that both LTCC isoforms have the capability to operate at relatively hyperpolarized membrane voltages. Hence, our study adds important pieces of information to our current knowledge of the differences between neuronal Cav1.2 and Cav1.3 channels. Furthermore, our data suggest that neurons of Cav1.3−/− mice may ease the investigation of Cav1.2-mediated hippocampal ADPs, because both depolarizing effects resulting directly from Cav1.3-channel mediated Ca2+ influx and membrane potential changes related to Cav1.3 channel coupling to hyperpolarizing conductances are eliminated. On the other hand, neurons of Cav1.2DHP−/− may aid in further studies of the role of ADPs under conditions of enhanced LTCC activity (49). Hence, these genetically modified mouse strains may provide valuable models for further investigation of hippocampal LTCC-dependent ADPs but potentially also in testing of other LTCC-ADP-related electrophysiological phenomena such as plateau potentials and depolarization shifts in normal and abnormal LTCC activities (49, 75).
GRANTS
This study was supported by a grant from the Austrian Science Fund (FWF, Project P-19710 to H. Kubista).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.H. and L.G. performed experiments; J.H., L.G., and H.K. analyzed data; J.H., L.G., S.B., and H.K. edited and revised manuscript; J.H., L.G., S.B., and H.K. approved final version of manuscript; S.B. and H.K. interpreted results of experiments; S.B. and H.K. drafted manuscript; H.K. conception and design of research; H.K. prepared figures.
ACKNOWLEDGMENTS
We thank Gabriele Gaupmann for excellent technical assistance and Joerg Striessnig (University of Innsbruck, Austria) for advice during the preparation of this article. It is acknowledged that Petra Geier performed a few electrophysiological recordings in the early stages of this study.
REFERENCES
- 1.Amano H, Amano T, Matsubayashi H, Ishihara K, Serikawa T, Sasa M. Enhanced calcium influx in hippocampal CA3 neurons of spontaneously epileptic rats. Epilepsia 42: 345–350, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Anekonda TS, Quinn JF, Harris C, Frahler K, Wadsworth TL, Woltjer RL. L-type voltage-gated calcium channel blockade with isradipine as a therapeutic strategy for Alzheimer's disease. Neurobiol Dis 41: 62–70, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barad M. Later developments: molecular keys to age-related memory impairment. Alzheimer Dis Assoc Disord 17: 168–176, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bouhadfane M, Tazerart S, Moqrich A, Vinay L, Brocard F. Sodium-mediated plateau potentials in lumbar motoneurons of neonatal rats. J Neurosci 33: 15626–15641, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai X, Liang CW, Muralidharan S, Kao JP, Tang CM, Thompson SM. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron 44: 351–364, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Carlin KP, Jones KE, Jiang Z, Jordan LM, Brownstone RM. Dendritic L-type calcium currents in mouse spinal motoneurons: implications for bistability. Eur J Neurosci 12: 1635–1646, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ. ‘Rejuvenation’ protects neurons in mouse models of Parkinson's disease. Nature 447: 1081–1086, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Clark NC, Nagano N, Kuenzi FM, Jarolimek W, Huber I, Walter D, Wietzorrek G, Boyce S, Kullmann DM, Striessnig J, Seabrook GR. Neurological phenotype and synaptic function in mice lacking the Cav1.3 alpha subunit of neuronal L-type voltage-dependent Ca2+ channels. Neuroscience 120: 435–442, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Dao DT, Mahon PB, Cai X, Kovacsics CE, Blackwell RA, Arad M, Shi J, Zandi PP, O'Donnell P, Bipolar Genome Study (BiGS) Consortium. Knowles JA, Weissman MM, Coryell W, Scheftner WA, Lawson WB, Levinson DF, Thompson SM, Potash JB, Gould TD. Mood disorder susceptibility gene CACNA1C modifies mood-related behaviors in mice and interacts with sex to influence behavior in mice and diagnosis in humans. Biol Psychiatry 68: 801–810, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Ascenzo M, Piacentini R, Casalbore P, Budoni M, Pallini R, Azzena GB, Grassi C. Role of L-type Ca2+ channels in neural stem/progenitor cell differentiation. Eur J Neurosci 23: 935–944, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Davis SE, Bauer EP. L-type voltage-gated calcium channels in the basolateral amygdala are necessary for fear extinction. J Neurosci 32: 13582–13586, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dovzhenok A, Kuznetsov AS. Exploring neuronal bistability at the depolarization block. PLoS One 7: e42811, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunmyre JR, Del Negro CA, Rubin JE. Interactions of persistent sodium and calcium-activated nonspecific cationic currents yield dynamically distinct bursting regimes in a model of respiratory neurons. J Comput Neurosci 31: 305–328, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felix R, Gurnett CA, De Waard M, Campbell KP. Dissection of functional domains of the voltage-dependent Ca2+ channel alpha2delta subunit. J Neurosci 17: 6884–6891, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford KA, Wolf ME, Hu XT. Plasticity of L-type Ca2+ channels after cocaine withdrawal. Synapse 63: 690–697, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fossat P, Sibon I, Le Masson G, Landry M, Nagy F. L-type calcium channels and NMDA receptors: a determinant duo for short-term nociceptive plasticity. Eur J Neurosci 25: 127–135, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Gamelli AE, McKinney BC, White JA, Murphy GG. Deletion of the L-type calcium channel Cav1.3 but not Cav12 results in a diminished sAHP in mouse CA1 pyramidal neurons. Hippocampus 21: 133–141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geier P, Lagler M, Boehm S, Kubista H. Dynamic interplay of excitatory and inhibitory coupling modes of neuronal L-type calcium channels. Am J Physiol Cell Physiol 300: C937–C949, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez JP, Fares N, Potreau D. Effects of Bay K 8644 on L-type calcium current from newborn rat cardiomyocytes in primary culture. J Mol Cell Cardiol 28: 2217–2229, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Haase H, Alvarez J, Petzhold D, Doller A, Behlke J, Erdmann J, Hetzer R, Regitz-Zagrosek V, Vassort G, Morano I. Ahnak is critical for cardiac Cav1.2 calcium channel function and its beta-adrenergic regulation. FASEB J 19: 1969–1977, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Haj-Dahmane S, Andrade R. Calcium-activated cation nonselective current contributes to the fast afterdepolarization in rat prefrontal cortex neurons. J Neurophysiol 78: 1983–1989, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Hasuo H, Phelan KD, Twery MJ, Gallagher JP. A calcium-dependent slow afterdepolarization recorded in rat dorsolateral septal nucleus neurons in vitro. J Neurophysiol 64: 1838–1846, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Hofmann T, Chubanov V, Gudermann T, Montell C. TRPM5 is a voltage-modulated and Ca2+-activated monovalent selective cation channel. Curr Biol 13: 1153–1158, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Huber IG, Wappl-Kornherr E, Sinnegger-Brauns MJ, Hoda JC, Walter-Bastl D, Striessnig J. Opposite effects of a single IIIS5 mutation on phenylalkylamine and dihydropyridine interaction with L-type Ca2+ channels. J Biol Chem 279: 55211–55217, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, Rabah D, Kinet JP, Shin HS. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci 13: 482–488, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jurkovičová-Tarabová B, Griesemer D, Pirone A, Sinnegger-Brauns MJ, Striessnig J, Friauf E. Repertoire of high voltage-activated Ca2+ channels in the lateral superior olive: functional analysis in wild-type, Cav1.3−/−, and Cav12DHP−/− mice. J Neurophysiol 108: 365–379, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Kalmbach BE, Chitwood RA, Dembrow NC, Johnston D. Dendritic generation of mGLUR-mediated slow afterdepolarization in layer 5 neurons of prefrontal cortex. J Neurosci 33: 13518–13532, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang S, Cooper G, Dunne SF, Dusel B, Luan CH, Surmeier DJ, Silverman RB. Cav1.3-selective L-type calcium channel antagonists as potential new therapeutics for Parkinson's disease. Nat Commun 3: 1146, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Kang S, Cooper G, Dunne SF, Luan CH, James Surmeier D, Silverman RB. Antagonism of L-type Ca2+ channels Cav1.3 and Cav12 by 1,4-dihydropyrimidines and 4H-pyrans as dihydropyridine mimics. Bioorg Med Chem 21: 4365–4373, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Köhling R, Straub H, Speckmann EJ. Differential involvement of L-type calcium channels in epileptogenesis of rat hippocampal slices during ontogenesis. Neurobiol Dis 7: 471–482, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J. Alpha 1D (Cav1.3) subunits can form L-type Ca2+ channels activating at negative voltages. J Biol Chem 276: 22100–22106, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Lacinová L. Voltage-dependent calcium channels. Gen Physiol Biophys 24, Suppl 1: 1–78, 2005 [PubMed] [Google Scholar]
- 33.Lacinova L, Moosmang S, Langwieser N, Hofmann F, Kleppisch T. Cav1.2 calcium channels modulate the spiking pattern of hippocampal pyramidal cells. Life Sci 82: 41–49, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Langwieser N, Christel CJ, Kleppisch T, Hofmann F, Wotjak CT, Moosmang S. Homeostatic switch in hebbian plasticity and fear learning after sustained loss of Cav1.2 calcium channels. J Neurosci 30: 8367–8375, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee CR, Tepper JM. A calcium-activated nonselective cation conductance underlies the plateau potential in rat substantia nigra GABAergic neurons. J Neurosci 27: 6531–6541, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao P, Soong TW. Cav1.2 channelopathies: from arrhythmias to autism, bipolar disorder, and immunodeficiency. Pflügers Arch 460: 353–359, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Liebmann L, Karst H, Sidiropoulou K, van Gemert N, Meijer OC, Poirazi P, Joëls M. Differential effects of corticosterone on the slow afterhyperpolarization in the basolateral amygdala and CA1 region: possible role of calcium channel subunits. J Neurophysiol 99: 958–968, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Moosmang S, Haider N, Klugbauer N, Adelsberger H, Langwieser N, Müller J, Stiess M, Marais E, Schulla V, Lacinova L, Goebbels S, Nave KA, Storm DR, Hofmann F, Kleppisch T. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci 25: 9883–9982, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morisset V, Nagy F. Ionic basis for plateau potentials in deep dorsal horn neurons of the rat spinal cord. J Neurosci 19: 7309–7316, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moyer JR, Jr, Thompson LT, Black JP, Disterhoft JF. Nimodipine increases excitability of rabbit CA1 pyramidal neurons in an age- and concentration-dependent manner. J Neurophysiol 68: 2100–2109, 1992 [DOI] [PubMed] [Google Scholar]
- 41.Mrejeru A, Wei A, Ramirez JM. Calcium-activated nonselective cation currents are involved in generation of tonic and bursting activity in dopamine neurons of the substantia nigra pars compacta. J Physiol 589: 2497–2514, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium 38: 233–252, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Perrier JF, Mejia-Gervacio S, Hounsgaard J. Facilitation of plateau potentials in turtle motoneurones by a pathway dependent on calcium and calmodulin. J Physiol 528: 107–113, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petersson ME, Fransén E. Long-lasting small-amplitude TRP-mediated dendritic depolarizations in CA1 pyramidal neurons are intrinsically stable and originate from distal tuft regions. Eur J Neurosci 36: 2917–2925, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Ping HX, Shepard PD. Blockade of SK-type Ca2+-activated K+ channels uncovers a Ca2+-dependent slow afterdepolarization in nigral dopamine neurons. J Neurophysiol 81: 977–984, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell 102: 89–97, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Radzicki D, Yau HJ, Pollema-Mays SL, Mlsna L, Cho K, Koh S, Martina M. Temperature-sensitive Cav1.2 calcium channels support intrinsic firing of pyramidal neurons and provide a target for the treatment of febrile seizures. J Neurosci 33: 9920–9931, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts CB, O'Boyle MP, Suter KJ. Dendrites determine the contribution of after depolarization potentials (ADPs) to generation of repetitive action potentials in hypothalamic gonadotropin releasing-hormone (GnRH) neurons. J Comput Neurosci 26: 39–53, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Rubi L, Schandl U, Lagler M, Geier P, Spies D, Gupta KD, Boehm S, Kubista H. Raised activity of L-type calcium channels renders neurons prone to form paroxysmal depolarization shifts. Neuromolecular Med 15: 476–492, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schierberl K, Hao J, Tropea TF, Ra S, Giordano TP, Xu Q, Garraway SM, Hofmann F, Moosmang S, Striessnig J, Inturrisi CE, Rajadhyaksha AM. Cav1.2 L-type Ca2+ channels mediate cocaine-induced GluA1 trafficking in the nucleus accumbens, a long-term adaptation dependent on ventral tegmental area Cav1.3 channels. J Neurosci 31: 13562–13575, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seoane A, Massey PV, Keen H, Bashir ZI, Brown MW. L-type voltage-dependent calcium channel antagonists impair perirhinal long-term recognition memory and plasticity processes. J Neurosci 29: 9534–9544, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simon M, Perrier JF, Hounsgaard J. Subcellular distribution of L-type Ca2+ channels responsible for plateau potentials in motoneurons from the lumbar spinal cord of the turtle. Eur J Neurosci 18: 258–266, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, Dascal N. The roles of the subunits in the function of the calcium channel. Science 253: 1553–1557, 1991 [DOI] [PubMed] [Google Scholar]
- 54.Sinnegger-Brauns MJ, Hetzenauer A, Huber IG, Renström E, Wietzorrek G, Berjukov S, Cavalli M, Walter D, Koschak A, Waldschütz R, Hering S, Bova S, Rorsman P, Pongs O, Singewald N, Striessnig J. Isoform-specific regulation of mood behavior and pancreatic beta cell and cardiovascular function by L-type Ca2+ channels. J Clin Invest 113: 1430–1439, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Speckmann EJ, Walden J. Anti-epileptic effects of organic calcium channel, blockers in animal experiments. In: Epilepsy: Models, Mechanism and Concepts, edited by Schwartzkroin PA. Cambridge, UK: Cambridge Univ. Press, 1993, p. 462–486 [Google Scholar]
- 56.Speckmann EJ, Walden J, Bingmann D. Functional implication of calcium ions in epileptic seizures. Antiepileptic effects of organic calcium antagonists. Arzneimittelforschung 39: 149–156, 1989 [PubMed] [Google Scholar]
- 57.Staley K, Hellier JL, Dudek FE. Do interictal spikes drive epileptogenesis? Neuroscientist 11: 272–276, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Staley KJ, White A, Dudek FE. Interictal spikes: harbingers or causes of epilepsy? Neurosci Lett 497: 247–250, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Straub H, Köhling R, Frieler A, Grigat M, Speckmann EJ. Contribution of L-type calcium channels to epileptiform activity in hippocampal and neocortical slices of guinea-pigs. Neuroscience 95: 63–72, 2000 [DOI] [PubMed] [Google Scholar]
- 60.Striessnig J, Koschak A, Sinnegger-Brauns MJ, Hetzenauer A, Nguyen NK, Busquet P, Pelster G, Singewald N. Role of voltage-gated L-type Ca2+ channel isoforms for brain function. Biochem Soc Trans 34: 903–909, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Surmeier DJ, Schumacker PT. Calcium, bioenergetics, and neuronal vulnerability in Parkinson's disease. J Biol Chem 288: 10736–10741, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tomlinson WJ, Stea A, Bourinet E, Charnet P, Nargeot J, Snutch TP. Functional properties of a neuronal class C L-type calcium channel. Neuropharmacology 32: 1117–1126, 1993 [DOI] [PubMed] [Google Scholar]
- 63.Vogalis F, Harvey JR, Lohman RJ, Furness JB. Action potential afterdepolarization mediated by a Ca2+-activated cation conductance in myenteric AH neurons. Neuroscience 115: 375–393, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Wappl E, Mitterdorfer J, Glossmann H, Striessnig J. Mechanism of dihydropyridine interaction with critical binding residues of L-type Ca2+ channel alpha 1 subunits. J Biol Chem 276: 12730–12735, 2001 [DOI] [PubMed] [Google Scholar]
- 65.Wheeler DG, Groth RD, Ma H, Barrett CF, Owen SF, Safa P, Tsien RW. Cav1 and Cav2 channels engage distinct modes of Ca2+ signaling to control CREB-dependent gene expression. Cell 149: 1112–1124, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.White JA, McKinney BC, John MC, Powers PA, Kamp TJ, Murphy GG. Conditional forebrain deletion of the L-type calcium channel Cav1.2 disrupts remote spatial memories in mice. Learn Mem 15: 1–5, 2008 [DOI] [PubMed] [Google Scholar]
- 67.Williams ME, Feldman DH, McCue AF, Brenner R, Velicelebi G, Ellis SB, Harpold MM. Structure and functional expression of alpha 1, alpha 2, and beta subunits of a novel human neuronal calcium channel subtype. Neuron 8: 71–84, 1992 [DOI] [PubMed] [Google Scholar]
- 68.Wu WW, Chan CS, Disterhoft JF. Slow afterhyperpolarization governs the development of NMDA receptor-dependent afterdepolarization in CA1 pyramidal neurons during synaptic stimulation. J Neurophysiol 92: 2346–2356, 2004 [DOI] [PubMed] [Google Scholar]
- 69.Wu SN, Li HF, Jan CR. Regulation of Ca2+-activated nonselective cationic currents in rat pituitary GH3 cells: involvement in L-type Ca2+ current. Brain Res 812: 133–141, 1998 [DOI] [PubMed] [Google Scholar]
- 70.Xu W, Lipscombe D. Neuronal Cav1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci 21: 5944–5951, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yagami T, Kohma H, Yamamoto Y. L-type voltage-dependent calcium channels as therapeutic targets for neurodegenerative diseases. Curr Med Chem 19: 4816–4827, 2012 [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto R, Ueta Y, Kato N. Dopamine induces a slow afterdepolarization in lateral amygdala neurons. J Neurophysiol 98: 984–992, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Yasuda T, Chen L, Barr W, McRory JE, Lewis RJ, Adams DJ, Zamponi GW. Auxiliary subunit regulation of high-voltage activated calcium channels expressed in mammalian cells. Eur J Neurosci 20: 1–13, 2004 [DOI] [PubMed] [Google Scholar]
- 74.Zhang H, Fu Y, Altier C, Platzer J, Surmeier DJ, Bezprozvanny I. Cav1.2 and Cav1.3 neuronal L-type calcium channels: differential targeting and signaling to pCREB. Eur J Neurosci 23: 2297–2310, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Z, Reboreda A, Alonso A, Barker PA, Séguéla P. TRPC channels underlie cholinergic plateau potentials and persistent activity in entorhinal cortex. Hippocampus 21: 386–397, 2011 [DOI] [PubMed] [Google Scholar]
- 76.Zhou Z, Xiong W, Masurkar AV, Chen WR, Shepherd GM. Dendritic calcium plateau potentials modulate input-output properties of juxtaglomerular cells in the rat olfactory bulb. J Neurophysiol 96: 2354–2363, 2006 [DOI] [PubMed] [Google Scholar]