Abstract
Mitochondria are dynamic organelles, capable of altering their morphology and function. However, the mechanisms governing these changes have not been fully elucidated, particularly in muscle cells. We demonstrated that oxidative stress with H2O2 resulted in a 41% increase in fragmentation of the mitochondrial reticulum in myoblasts within 3 h of exposure, an effect that was preceded by a reduction in membrane potential. Using live cell imaging, we monitored mitochondrial motility and found that oxidative stress resulted in a 30% reduction in the average velocity of mitochondria. This was accompanied by parallel reductions in both organelle fission and fusion. The attenuation in mitochondrial movement was abolished by the addition of N-acetylcysteine. To investigate whether H2O2-induced fragmentation was mediated by dynamin-related protein 1, we incubated cells with mDivi1, an inhibitor of dynamin-related protein 1 translocation to mitochondria. mDivi1 attenuated oxidative stress-induced mitochondrial fragmentation by 27%. Moreover, we demonstrated that exposure to H2O2 upregulated endoplasmic reticulum-unfolded protein response markers before the initiation of mitophagy signaling and the mitochondrial-unfolded protein response. These findings indicate that oxidative stress is a vital signaling mechanism in the regulation of mitochondrial morphology and motility.
Keywords: mitochondria, mitochondrial movement, oxidative stress, mitochondrial morphology
mitochondria are essential organelles for the life and death of eukaryotic cells. They play key roles in aerobic energy production, apoptosis, mitophagy, and cellular signaling. These versatile organelles were once thought to be static and rigid structures. More recently, mitochondria have been appreciated for their dynamic nature. They can change their distribution by moving along cytoskeletal tracks or change their overall morphology. The maintenance and appropriate networking of mitochondria within the cell is mediated by fusion and opposing fission processes. Fusion involves the mixing of mitochondrial material, whereas fission divides the organelle into smaller components. Disruptions in either of these opposing events can lead to developmental defects and disease (29), suggesting that the proper maintenance of mitochondrial morphology is critical for normal cell function.
Mitochondrial fission is orchestrated, in part, by dynamin-related protein 1 (Drp1) (4, 25), a protein that is a GTPase of the dynamin family. All dynamin members are structurally similar but functionally diverse GTP-binding proteins. Drp1 assists in mitochondrial fission by polymerizing into a ring-like structure around the organelle. The cross-bridging of the GTPase domains of adjacent Drp1 proteins results in GTP hydrolysis, constriction, and the ultimate severing of mitochondria (26). It is the phosphorylation status of Drp1 that determines its localization and consequently its effect on mitochondrial structure. Ser637 phosphorylation results in inhibition of Drp1 GTPase activity, preventing the protein from translocating from the cytosol to the sites of mitochondrial division, thereby inhibiting organelle fission (9, 11).
Mitochondria are the primary source of ROS generation. Within these organelles, low levels of ROS are constantly produced during normal respiration when the O2 consumed undergoes a one-electron reduction. In skeletal muscle subject to conditions of chronic muscle disuse (1) and aging (8), increased production of ROS from mitochondria occurs, and this coincides with an increased incidence of mitochondrial fragmentation (19). More direct connections between oxidative stress and mitochondrial morphology have previously been shown in endothelial cells and neurons and, recently, in C2C12 cells, whereby exposure to high levels of H2O2, a major contributor to oxidative stress, induces mitochondrial fragmentation (3, 5, 21).
To promote proper functioning of mitochondria in the face of ROS-induced damage, the cell has quality control mechanisms in place. The endoplasmic reticulum (ER) and mitochondria are exposed to newly synthesized protein and are responsible for their proper folding and assembly. Homeostasis is achieved by balancing the nascent protein load with the folding capacity of the organelle. When the ability to do so is compromised, ER and mitochondrial stress ensue. The adaptive responses to this imbalance, termed the ER-unfolded protein response (ER-UPR) and mitochondrial UPR (mt-UPR), coincide with the upregulation of molecular chaperones allowing for the increased folding capacity of the cell. If the cellular stress is too extensive and cannot be alleviated, the UPR activates cell destructive responses, including apoptosis and the mitophagy pathway (24).
Mitochondria are dynamic organelles, continuously moving and undergoing cycles of fission and fusion, resulting in alterations in their displacement, morphology, and function; however, the mechanisms governing these changes have yet to be fully elucidated. Hence, we sought to determine the effects of ROS on mitochondrial dynamics in myoblasts and to establish the interplay between mitochondrial morphology and stress responses during conditions of oxidative stress.
MATERIALS AND METHODS
Cell culture and treatments.
C2C12 murine skeletal muscle cells were plated into six-well dishes and allowed to proliferate in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin [growth media (GM); Wisent, St-Bruno, QC, Canada]. Upon reaching a confluence of ∼60%, the mito-DsRED2 plasmid (Clontech, Mountain View, CA) was transfected into myoblasts using Lipofectamine 2000 (Life Technologies, Burlington, ON, Canada), thus allowing for the visualization of mitochondria.
Mitochondrial movement was observed in C2C12 cells using an expression vector that encoded a fusion of red fluorescence protein and the mitochondrial targeting sequence from cytochrome c oxidase subunit VIII (mito-dsRED2). Forty-eight hours after transfection with mito-DsRed2, cells were treated with either vehicle (H2O or DMSO) or 20mM N-acetylcysteine (NAC; Sigma-Aldrich, Oakville, ON, Canada) for 60 min while incubated with H2O2. mDivi1 (25 μM, Tocris, Burlington, ON, Canada) was used as a selective inhibitor of Drp1, due to its suppression of Drp1 GTPase activity, thus blocking the self-assembly of the protein (7). Imaging of Drp1 occurred by transfection of the pEYFP-C1-Drp1 plasmid (Addgene, Cambridge, MA) into myoblasts.
Mitochondrial movement analysis.
Mitochondria were visualized using an inverted Nikon Eclipse TE2000-U fluorescent microscope (Nikon, Mississauga, ON, Canada) equipped with a ×100/1.5 oil objective lens while a custom-designed chamber maintained a constant temperature of 37°C with 5% CO2. Mitochondrial dynamics were captured at 2-s intervals for a total time of 5 min using real-time imaging. Analyses were performed using NIS Element AR 3.1 software and were limited to regions of interest in the periphery of cells, where individual mitochondria were readily resolved and could be used for analysis while avoiding the confounding influence of high-density mitochondrial networks.
The percentage of time that mitochondria spent in motion with and without the addition of H2O2 was determined by monitoring the position of the organelle throughout the 5-min capturing time. Mitochondria were considered in motion if movement was >0.01 μm in the 2-s capturing frames and taken as a percentage over the 5-min time course for each movie.
Numbers of fission and fusion events were determined in a 7 × 7-μm area over the time course of 5 min. The average from 3–5 squares, placed in a nonoverlapping fashion, were used to determine the number of fission and fusion events from 6–10 separate cells. A fusion event occurred when a mitochondrion extended its projection and merged with a neighbouring mitochondrion and black pixels no longer separated the organelles. A fission event consisted of a break of the mitochondrial filament, whereby black pixels separate the once continuous mitochondrial network.
Mitochondria-enriched fractions.
Mitochondria-enriched fractions were obtained using an adapted protocol (6, 17). Briefly, the medium was removed from myoblasts and washed with ice-cold PBS (Wisent). Cells were gently scraped in mitochondrial isolation buffer containing 10% 0.1 M Tris-MOPS, 1% EGTA-Tris, and 20% 1 M sucrose (pH 7.4; Sigma-Aldrich). Subsequently, cells were subjected to differential centrifugation, and mitochondria were resuspended in mitochondrial isolation buffer. Mitochondria-enriched fractions were used for immunoblot analysis of mitophagy marker proteins.
Immunoblot analysis.
Protein extracts from myoblasts and isolated mitochondria-enriched fractions were separated with 6–12% SDS-PAGE and subsequently transferred onto nitrocellulose membranes. Membranes were blocked for 1 h with 5% skim milk in 1× Tris-buffered saline-Tween 20 [TBST; 25 mM Tris·HCl (pH 7.5), 1 mM NaCl, and 0.1% Tween 20, Sigma-Aldrich] solution at room temperature followed by an incubation in blocking solution with antibodies directed toward phosphorylated (p-)Drp1 (1:250, Cell Signaling, Whitby, ON, Canada), Drp1 (1:500, BD Transduction Laboratories, Mississauga, ON, Canada), light chain 3 (1:500, Cell Signaling), p62 (1:5,000, Sigma-Aldrich), porin (1:3,000, Abcam, Toronto, ON, Canada), mitochondrial heat shock protein 60 (mtHSP60; 1:500, Enzo Life Sciences, Brockville, ON, Canada), mitochondrial heat shock protein 70 (mtHSP70; 1:1,000, Enzo Life Sciences), chaperonin 10 (Cpn10; 1:500, Enzo Life Sciences), C/EBP homology protein (CHOP; 1:1,000, Cell Signaling), p-eukaryotic translation initiation factor 2α (p-eIF2α; 21:500, Life Technologies), eIF2α (1:500, Cell Signaling), or α-tubulin (1:5,000, Calbiochem, Ottawa, ON, Canada) overnight at 4°C. Subsequently, membranes were washed three times for 5 min with TBST followed by an incubation at room temperature (1 h) with the appropriate secondary antibody conjugated to horseradish peroxidase and washed again three times for 5 min each with TBST. Membranes were developed using Western Blot Luminol Reagent (Santa Cruz Biotechnology), and films were subsequently scanned and quantification via densitometric analysis for the intensity of signals with SigmaScanPro software (version 5, Jandel Scientific, San Rafael, CA).
Membrane potential.
Tetramethylrhodamine ethyl ester (TMRE; Life Technologies) is a cationic dye that is rapidly accumulated by mitochondria due to their change in membrane potential. Depolarization of mitochondria results in a decrease in membrane potential and failure to sequester TMRE. To determine membrane potential, cells were washed two times with PBS. Subsequently, cells were incubated with TMRE (50 nM) for 45 min. After cells were washed twice with PBS, media were replaced with GM without phenol red and imaged. TMRE fluorescence was measured in the dark using a fluorescence microscope at ×60 magnification, and light exposure was kept to the minimum to insure accurate measurements. The change in fluorescence observed after the addition of H2O2 (300 μM, Sigma-Aldrich) was determined and compared relative to untreated cells. The mean from 13–18 cells in 6 separate experiments was used for analysis.
O2 consumption.
O2 consumption was determined based on fluorescence quenching by O2 using OxoPlates (Innovative Instruments, Indian Trail, NC). Cells were added to each well at a density of 8,000 cells/well. The following day, H2O2 (300 μM) was added to the wells containing C2C12 cells, and O2 consumption was assessed at 0, 1, 3, and 5 h. Optical O2 sensors at the bottom of microplates contained an indicator and a reference dye. Wells containing air-saturated (100%) and O2-free (0%) solutions were used for calibration. Fluorescence was assessed for 3.5 h in 3-min intervals in dual kinetic mode (Synergy HT microplate reader, Burlington, ON, Canada), using filter 1 (530/645 nm) and filter 2 (530/590) settings to detect the fluorescence of the indicator and reference dyes, respectively. From these values, ratios were calculated and compared with standard calibrations, thus allowing for the quantification of O2 consumption.
Statistical analyses.
Data were analyzed with GraphPad 4.0 software and expressed as means ± SE. t-Tests and one-way ANOVAs were performed where appropriate, and differences were considered statistically significant if P < 0.05.
RESULTS
Oxidative stress halts mitochondrial movement in C2C12 myoblasts.
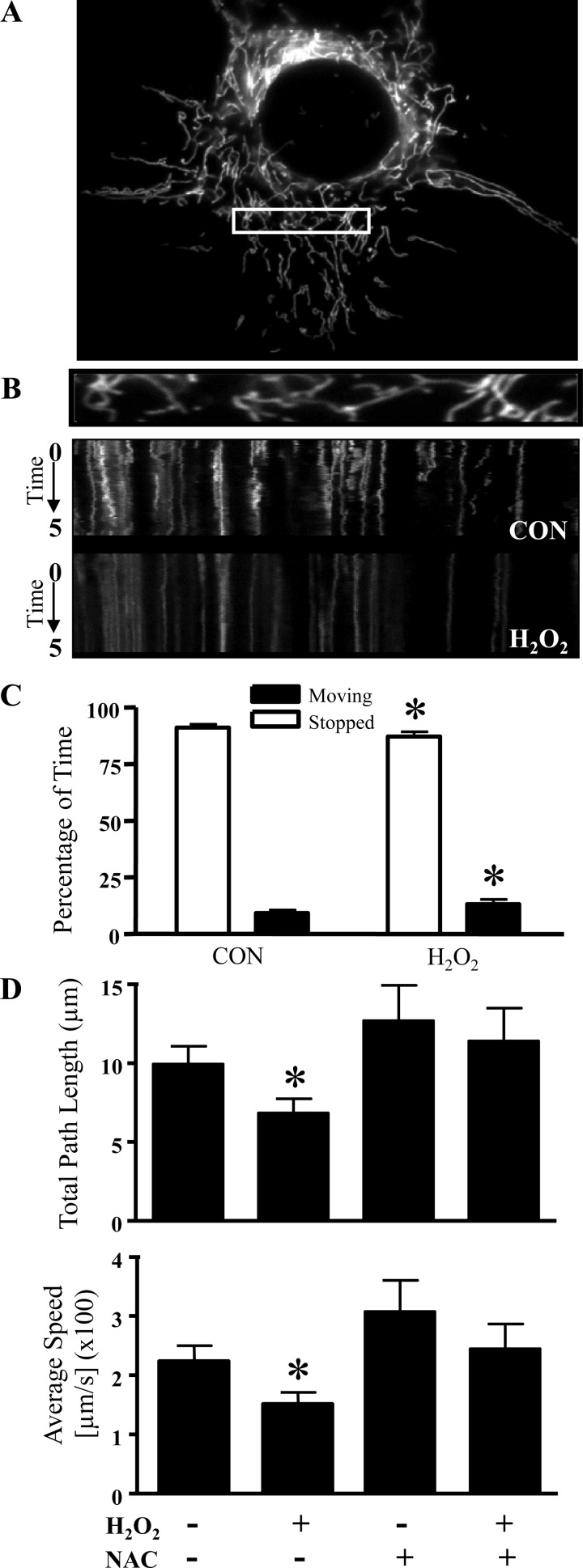
We examined the influence of oxidative stress on mitochondrial dynamics by live cell imaging of cultured myoblasts. By transfecting mito-dsRED2 to visualize mitochondria (Fig. 1A), we determined the percentage of time that mitochondria spent in motion, their total path length, and the average speed traveled by the organelles. To analyze the movement characteristics of individual mitochondria, kymographs were generated from the live cell imaging videos. The side-to-side horizontal oscillations visible over the 5-min data collection revealed the dynamic properties of the organelles (Fig. 1B). We observed that mitochondria remained in constant motion and frequently changed their direction. The addition of H2O2 inhibited mitochondrial movements. Quantification of organelle motility revealed a 42% increase in the percentage of time that mitochondria remained stationary (Fig. 1C). Moreover, H2O2 exposure was able to reduce the total path length and average speed traveled by mitochondria by 31% and 33%, respectively (Fig. 1D, top and bottom). The reduction in mitochondrial movement was attributed to oxidative stress, since the addition of NAC, a free radical scavenger, was able to negate the effects observed with H2O2 alone (Fig. 1D).
Fig. 1.
Oxidative stress halts mitochondrial movement in C2C12 myoblasts. A: representative myoblast co-transfected with mito-DsRED2. B: the first frame (top) in a live cell imaging series with representative kymographs generated from the videos of untreated [control (CON)] and 300 μM H2O2-treated myoblasts (bottom). Time (0–5 min) progresses from top to bottom in the kymograph, whereas the x-axis represents mitochondrial position. Vertical white lines correspond to stationary mitochondria, and horizontal deviations (vibrations from the vertical) depict moving mitochondria. C: the percentage of time that mitochondria spent in motion with and without the addition of 300 μM H2O2 was determined by monitoring the position of the organelle throughout the 5-min capturing time in 10 separate experiments. D: quantification of the total path length (top) and average speed (bottom) traveled by mitochondria assessed in control and 300 μM H2O2-treated myoblasts with or without the presence of N-acetylcysteine (NAC; 60-min pretreatment). The movement of mitochondria was determined and averaged from n = 18–80 mitochondria from 6–10 separate cells. All values are expressed as averages ± SE. *P < 0.05 vs. CON.
Oxidative stress induces mitochondrial fragmentation as well as reductions in membrane potential and O2 consumption.
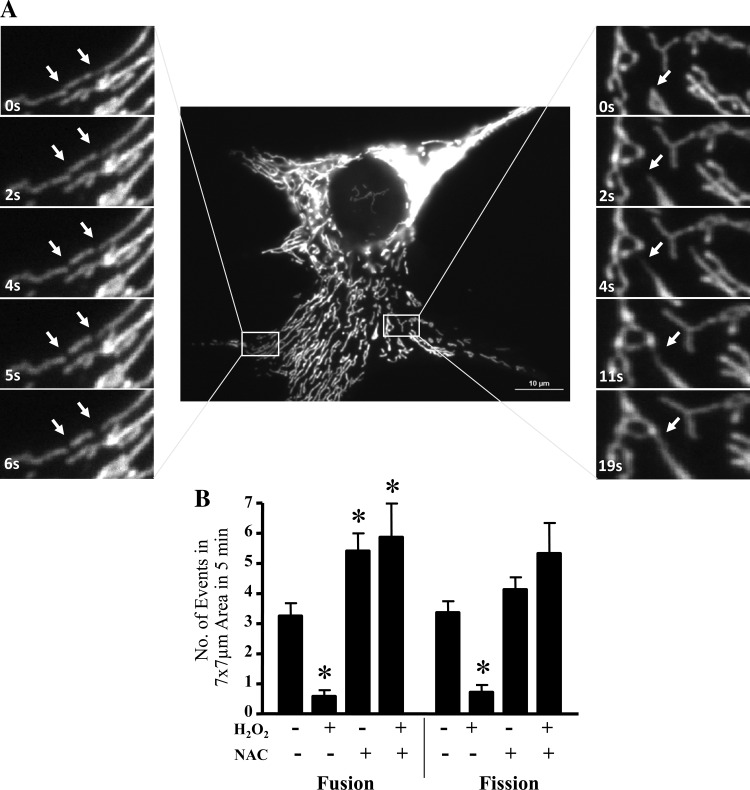
Mitochondrial movement is speculated to be associated with alterations in the morphology of the organelle as a result of fission and fusion events (Fig. 2A). Thus, we investigated whether oxidative stress would change the frequency of fission and fusion. Exposure to H2O2 for 5 min significantly reduced the number of fusion and fission events occurring within C2C12 myoblasts by 5.6- and 4.7-fold, respectively (Fig. 2B). Interestingly, the removal of ROS by NAC restored the rate of fission but enhanced the number of fusion events in both the presence and absence of H2O2.
Fig. 2.
Oxidative stress results in changes to mitochondrial dynamics. A, middle: the first frame from a live cell imaging series of a representative myoblast with time-lapse images of myoblasts expressing mito-DsRed2. Left, representative images of a 6-s time-lapse series of a myoblast undergoing mitochondrial fission. The arrows indicate sites of mitochondrial division. Right, sequential events of mitochondrial fusion monitored over 19 s. The arrow denotes the extension of a mitochondrial filament, resulting in a fusion event. B: numbers of mitochondrial fission and fusion events were determined in a 7 × 7-μm area over the time course of 5 min from 6–10 separate cells. Numbers of fission and fusion events were determined in control and H2O2 (300 μM)-treated myoblasts with or without NAC. Values are expressed as averages ± SE. *P < 0.05 vs. CON (without H2O2 and NAC).
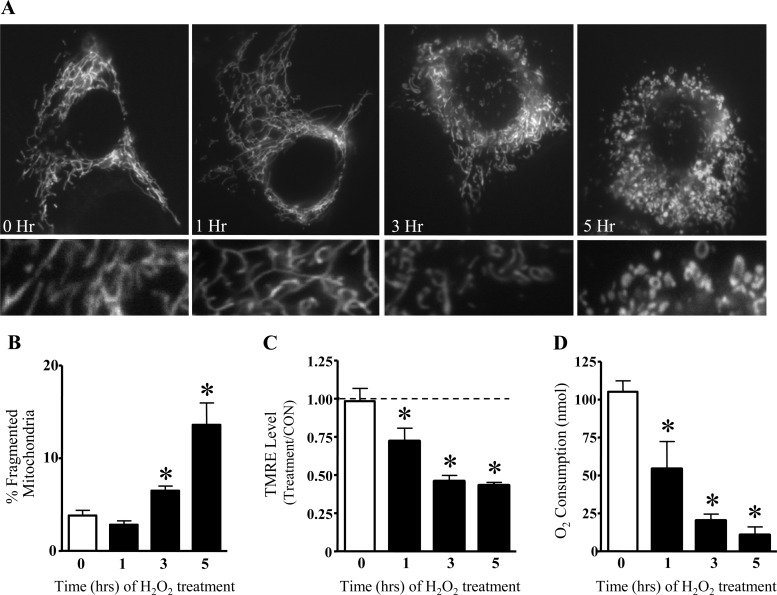
At early time points after the addition of H2O2 (1 h), the morphology of mitochondria was not altered (Fig. 3, A and B). After 3 h of exposure, H2O2 resulted in an increased percentage of fragmented mitochondria relative to the total area of the cell (Fig. 3B). In cells exposed to H2O2 for 5 h, the extent of fragmentation was enhanced by 3.6-fold (Fig. 3B). These changes in mitochondrial morphology were not due to an increase in apoptosis, as cell viability was unaltered up to 5 h after the addition of 300 μM H2O2 (unpublished observations).
Fig. 3.
Oxidative stress induces mitochondrial fragmentation as well as reductions in membrane potential and O2 consumption. Myoblasts were treated with 300 μM H2O2 for 5 h, transfected with mito-DsRed2 for visualization of mitochondria, and imaged immediately or after 1, 3, or 5 h of incubation. Mitochondrial morphology was assessed as the percentage of the cell occupied by either fragmented or network mitochondria. A: representative images for each time point (top) with magnified images of the mitochondrial network condition (bottom). H2O2 altered the morphology of mitochondria, such that more fragmented organelles were observed beginning at 3 h posttreatment. B: percentages (means ± SE) of total cell area displaying fragmented mitochondria from 6 separate experiments. C: membrane potential was analyzed by assessing the fluorescence of Tetramethylrhodamine ethyl ester (TMRE). The mean fluorescence intensity of each condition was expressed as a percentage of the untreated CON. Mean percentages ± SE of 13–18 cells are shown. D: O2 consumption was determined in C2C12 cells treated with 300 μM H2O2 for a total time of 5 h. n = 4. *Significantly different from 0 h (P < 0.05).
Next, we sought to determine the functional implications resulting from acute exposure to oxidative stress. H2O2 (300 μM) induced membrane depolarization, as indicated by reductions in the fluorescence of TMRE, beginning at 1 h after treatment (Fig. 3C). TMRE fluorescence continued to decline to 57% of control values at 5 h. Since alterations in membrane potential often result in changes in O2 consumption, we evaluated the effect of H2O2 on O2 consumption of C2C12 cells at 0, 1, 3, and 5 h of treatment (Fig. 3D). One hour after exposure to H2O2 (300 μM), O2 consumption was reduced by 48% and continued to decline up to 5 h, at which time point respiration was reduced by 90% compared with initial values.
Oxidative stress-induced mitochondrial fragmentation is mediated by Drp1.
To examine the importance of Drp1 during oxidative stress-induced mitochondrial fragmentation, we reduced the activity of Drp1 using mDivi1. When myoblasts were exposed to 100 μM H2O2 for 3 h, mDivi1 attenuated the amount of fragmented mitochondria by 27% (Fig. 4A). These findings indicate that Drp1 activation is responsible, in part, for oxidative stress-induced mitochondrial fragmentation. Next, we examined the expression and translocation profile of Drp1 after H2O2 exposure. In control untreated cells, and at 1 h after H2O2 exposure, Drp1 was visualized throughout the cytosol, as observed by the diffuse yellow fluorescence in the cell (Fig. 4B). After 3–5 h of H2O2 exposure, Drp1 was recruited from the cytosol, concentrated to areas nearby mitochondria, and revealed as an accumulation of Drp1-positive puncta in mitochondrial regions (Fig. 4B). Concomitantly, H2O2 treatment resulted in the dephosphorylation (and activation) of Drp1, which was inhibited by mDivi1 (Fig. 4C). This occurred in the absence of a change in total Drp1 expression.
Fig. 4.
Oxidative stress-induced mitochondrial fragmentation is mediated by dynamin-related protein 1 (Drp1). Mitochondrial morphology for myoblasts expressing mito-DsRed2 after 100 μM H2O2 exposure is shown. A: percentages of fragmented mitochondria were expressed relative to the total area of the cells with or without the presence of mDivi1 (25 μM, 60 min). B: C2C12 myoblasts were treated with and without 300 μM H2O2 for 5 h. Cells were cotransfected with mito-DsRed2 (red) and pEYFP-C1-Drp1 (green) and imaged at ×150 magnification. Visualization of CON myoblasts displayed long, reticular mitochondria with diffuse Drp1. Cells treated with H2O showed small, fragmented mitochondria and an accumulation of Drp1-positive puncta concentrated by mitochondria, in a time-dependent manner. The hatched boxes are magnified on the insert (bottom). Scale bar = 10 μm. C: Drp1 dephosphorylation compared with total Drp1 levels with 100 μM H2O2 exposure. Expression of phosphorylated (p-)Drp1 (Ser637) and total Drp1 was assessed on the same gel with and without 25μM mDivi1 (60-min preincubation). The line on the blot indicates that this image was taken from two separate blots to best illustrate the difference observed in the presence and absence of mDivi1, representative of 6 independent experiments. n = 6 for each condition. *Significantly different from 0 h (P < 0.05); †significantly different from untreated conditions (P < 0.05).
Acute oxidative stress specifically induces the ER-UPR without affecting the mt-UPR or mitophagy proteins.
Alterations in mitochondrial morphology have been found to correspond to an increased incidence of mitophagy (28). Thus, we sought to determine whether the oxidative stress-induced fragmentation also affected mitophagy. During 5 h of exposure to 300 μM H2O2, levels of the mitophagy markers light chain 3-II and p62 were unaltered in mitochondria-enriched fractions from C2C12 myoblasts. However, longer treatments with H2O2 (24 h) were sufficient to evoke an increase in the mitochondrial localization of these mitophagy markers (Fig. 5A).
Fig. 5.
Acute oxidative stress specifically induces the endoplasmic reticulum (ER)-unfolded protein response (UPR) without affecting the mitochondrial (mt)-UPR or mitophagy proteins. A: effect of oxidative stress on mitophagy. Representative Western blots of the mitophagy protein markers light chain 3 (LC3) and p62 were assessed with acute (5 h) and long exposure (24 h) with 300 μM H2O2 in mitochondrial-enriched fractions from C2C12 myoblasts. Porin was used as the loading marker. B: protein expression of markers for the mt-UPR. Levels of mitochondrial heat shock protein 60 (mtHSP60), mitochondrial heat shock protein 70 (mtHSP70), and chaperonin 10 (Cpn10) protein were determined in whole cell lysates after 300 μM H2O2 exposure. Tubulin was used as the loading control. C and D: representative Western blots of the ER-UPR markers C/EBP homology protein (CHOP), p-eukaryotic translation initiation factor 2α (p-eIF2α), and eIF2α (top) as well as graphical quantification (bottom). Tubulin was unchanged between the conditions and was used as a loading control for CHOP. Values are expressed as averages ± SE; n = 6. *P < 0.05 vs. CON.
To determine whether the mt-UPR was altered by acute exposure to oxidative stress, we examined the expression of mitochondrial chaperones involved in protein folding (Fig. 5B). We did not observe any significant changes in levels of mtHSP60, mtHSP70, or Cpn10 in either whole cell (Fig. 5B) or mitochondrial fractions (unpublished observations) by increasing the duration of H2O2 treatment from 1 to 5 h. We next examined the effects of H2O2 on the expression of ER-UPR proteins. At 3 h of H2O2 exposure, a large 12-fold increase in the expression of CHOP was observed, which remained elevated at 5 h by 8.5-fold relative to control values (Fig. 5C). Similarly, phosphorylation of eIF2α was significantly upregulated by 32% in the presence of oxidative stress (Fig. 5D).
DISCUSSION
Mitochondria are dynamic organelles, continuously undergoing cycles of fission and fusion, resulting in alterations in their morphology and function. However, the mechanisms governing these changes have yet to be fully elucidated. In the present study, we examined the effects of oxidative stress on mitochondrial motility in myoblasts. To do so, we used live cell imaging techniques to monitor the dynamics of mitochondria in cells with H2O2 exposure.
H2O2, a major contributor to oxidative damage, was shown to induce mitochondrial arrest and time-dependent fragmentation. It was observed initially 3 h after the addition of H2O2 and was further elevated by 5 h. The delay observed between the addition of H2O2 and the resulting fragmentation suggests the temporal activation of a signaling response, leading to posttranslational modifications of Drp1. Three hours of H2O2 exposure was associated with a decrease in the phosphorylation status of Drp1, thus allowing for the translocation of Drp1 to mitochondria. Since Drp1 protein expression was unaltered in the presence of H2O2, these results indicate that Drp1 is activated by cellular signaling, independent of a change in new protein synthesis.
To validate the importance of Drp1 in oxidative stress-induced mitochondrial fragmentation, we inhibited Drp1 dephosphorylation using the selective inhibitor mDivi1. mDivi1 administration maintained the levels of p-Drp1, concomitantly with an attenuation in H2O2-induced mitochondrial fragmentation. We observed comparable decreases in the number of fission and fusion events 5 min after the addition of H2O2. This is consistent with results seen in endothelial cells, whereby similar reductions in both fission and fusion were observed with H2O2 treatment in a dose- and time-dependent manner (21). Equivalent fission/fusion events have also been measured in senescent human umbilical vein endothelial cells (22), and these parallel changes suggest that mitochondrial dynamics (i.e., fission and fusion) are coordinately regulated.
Mitochondria were less mobile in cells exposed to H2O2. This attenuation in mitochondrial movement was abolished by the addition of NAC, a ROS scavenger. Little is known about the factors that contribute to ROS-mediated mitochondrial immobilization. We speculated that reduced mitochondrial motility could be a consequence of changes in cellular energetics. Thus, we evaluated whether oxidative stress-induced mitochondrial fragmentation and reductions in movement coincided with changes in the membrane potential and O2 consumption of myoblasts. In corroboration with previous reports (13, 27), our results demonstrate that acute exposure to oxidative stress resulted in fragmentation of the mitochondrial network within 3 h of exposure, an effect that was preceded by a reduction in membrane potential and O2 consumption, beginning after 1 h of treatment. Twig et al. (28) originally demonstrated that the decreased membrane potential in fragmented mitochondria was a prerequisite for mitophagy induction. In addition, Chen et al. (10) reported that impaired mitochondrial fusion as a result of mitofusin 1/2 or optic atrophy 1 deficiency resulted in lower rates of respiration (10), resembling the effects found in our H2O2-treated cells. Fragmented mitochondrial structures have been observed by others using similar stressful indicators (14, 16, 19, 20, 23). The resulting fragmented organelles allow for the damaged portions of the mitochondrial network to be isolated from its unaffected parts. Recent findings in HeLa cells have found that mitochondrial fission is required for mitophagy induction under oxidative stress (15). We observed similar results in myoblasts, whereby mitochondrial fragmentation preceded an elevation in mitophagy markers.
Mitochondrial stress has been speculated to result in the activation of at least three cellular responses, including mitophagy, and mt- and ER-UPRs. The UPRs are thought to occur before the initiation of mitophagy. Here, we show that acute exposure to oxidative stress plays a causal role in initiating ER-UPR signaling without an initial effect on the mt-UPR, indicating the specificity of the UPR. CHOP has been shown to be upregulated by ER stress. CHOP is a transcription factor that binds to and activates the promoter of stress response genes, such as c-JNK (18). As such, it is capable of propagating the UPR signal. Additionally, it is also known that CHOP activates the transcription of mt-UPR chaperone genes, such as mtHSP60 (24). We observed an increase in the expression of CHOP with oxidative stress, despite no changes in the levels of mtHSP60. Our acute treatment with H2O2 may not have been potent enough to elicit the mt-UPR but was sufficient to evoke the ER-UPR, in a time-dependent manner. The ER is responsible for the proper folding of vastly more proteins than that of the mitochondrion and, as a result, may be under more stringent control of the protein folding environment.
Our findings indicate that H2O2 exposure leads to a reduction in membrane potential, an event that preceded mitochondrial fragmentation. Subsequently, the oxidative stress-induced decreases in membrane potential lead to reductions in respiration as well as enhanced mitochondrial fragmentation. It is conceivable that reductions in respiration result in enhanced fission (Fig. 6). Previous work has demonstrated that inhibition of electron transport and ATP synthase induces mitochondrial fission and the formation of small, round mitochondria (12). We demonstrated that the mitochondrial fragmentation induced by oxidative stress is mediated, in part, by Drp1. Moreover, these changes in mitochondrial morphology lead to increases in the ER-UPR, which, when persistent, can lead to mitophagy induction and the activation of the mt-UPR.
Fig. 6.
Summary and working hypothesis on the effects of excess ROS on cellular responses based on the temporal changes described by our results. Exposure of the cell to increased levels of ROS resulted in reductions in mitochondrial membrane potential (ΔΨ; left). Subsequently, the ROS-induced decreases in membrane potential lead to reductions in respiration as well as enhanced mitochondrial fragmentation mediated by Drp1. Previous work has shown that inhibition of respiratory components results in enhanced organelle fission. These changes in mitochondrial morphology lead to increases in the ER-UPR, which then activates mitophagy along with an upregulation of the mt-UPR.
In summary, our data indicate that oxidative stress is a vital signaling mechanism in the regulation of the mitochondrial morphology response and motility in myoblasts. Oxidative stress is relevant to the pathophysiology of numerous diseases, including the aging process, since it can damage DNA, proteins, and lipids (2), and plays a key role in age-related tissue dysfunction. During aging, an increased incidence of mitochondrial fragmentation has been observed, with a concomitant increase in the expression of mitochondrial fission proteins (19). Our data suggest that enhanced oxidative stress may underlie the mitochondrial dysfunction and fragmentation observed during the aging process.
GRANTS
This work was supported by a Natural Science and Engineering Research Council of Canada grant (to D. A. Hood). D. A. Hood holds a Canada Research Chair in Cell Physiology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.I. and D.A.H. conception and design of research; S.I. performed experiments; S.I. analyzed data; S.I. and D.A.H. interpreted results of experiments; S.I. prepared figures; S.I. drafted manuscript; S.I. and D.A.H. edited and revised manuscript; S.I. and D.A.H. approved final version of manuscript.
REFERENCES
- 1.Adhihetty PJ, O'Leary MF, Chabi B, Wicks KL, Hood DA. Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J Appl Physiol 102: 1143–1151, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell 120: 483–495, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Graber S, Kovacs I, Lee WD, Waggoner J, Cui J, White AD, Bossy B, Martinou JC, Youle RJ, Lipton SA, Ellisman MH, Perkins GA, Bossy-Wetzel E. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J 25: 3900–3911, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleazard W, McCaffery JM, King EJ, Bale S, Mozdy A, Tieu Q, Nunnari J, Shaw JM. The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nat Cell Biol 1: 298–304, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks C, Cho SG, Wang CY, Yang T, Dong Z. Fragmented mitochondria are sensitized to Bax insertion and activation during apoptosis. Am J Physiol Cell Physiol 300: C447–C455, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter HN, Hood DA. Contractile activity-induced mitochondrial biogenesis and mTORC1. Am J Physiol Cell Physiol 303: C540–C547, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell 14: 193–204, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell 7: 2–12, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem 282: 21583–21587, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem 280: 26185–26192, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep 8: 939–944, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Vos KJ, Allan VJ, Grierson AJ, Sheetz MP. Mitochondrial function and actin regulate dynamin-related protein 1-dependent mitochondrial fission. Curr Biol 15: 678–683, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Fan X, Hussien R, Brooks GA. H2O2-induced mitochondrial fragmentation in C2C12 myocytes. Free Radic Biol Med 49: 1646–1654, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fannjiang Y, Cheng WC, Lee SJ, Qi B, Pevsner J, McCaffery JM, Hill RB, Basanez G, Hardwick JM. Mitochondrial fission proteins regulate programmed cell death in yeast. Genes Dev 18: 2785–2797, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank M, Duvezin-Caubet S, Koob S, Occhipinti A, Jagasia R, Petcherski A, Ruonala MO, Priault M, Salin B, Reichert AS. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochim Biophys Acta 1823: 2297–2310, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1: 515–525, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc 2: 287–295, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Horibe T, Hoogenraad NJ. The chop gene contains an element for the positive regulation of the mitochondrial unfolded protein response. PLOS ONE 2: e835, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iqbal S, Ostojic O, Singh K, Joseph AM, Hood DA. Expression of mitochondrial fission and fusion regulatory proteins in skeletal muscle during chronic use and disuse. Muscle Nerve 48: 963–970, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Jagasia R, Grote P, Westermann B, Conradt B. DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans. Nature 433: 754–760, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Jendrach M, Mai S, Pohl S, Voth M, Bereiter-Hahn J. Short- and long-term alterations of mitochondrial morphology, dynamics and mtDNA after transient oxidative stress. Mitochondrion 8: 293–304, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Jendrach M, Pohl S, Voth M, Kowald A, Hammerstein P, Bereiter-Hahn J. Morpho-dynamic changes of mitochondria during ageing of human endothelial cells. Mech Ageing Dev 126: 813–821, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell 15: 5001–5011, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pellegrino MW, Nargund AM, Haynes CM. Signaling the mitochondrial unfolded protein response. Biochim Biophys Acta 1833: 410–416, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smirnova E, Shurland DL, Ryazantsev SN, van der Bliek AM. A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol 143: 351–358, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strack S, Cribbs JT. Allosteric modulation of Drp1 mechanoenzyme assembly and mitochondrial fission by the variable domain. J Biol Chem 287: 10990–11001, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeyama N, Miki S, Hirakawa A, Tanaka T. Role of the mitochondrial permeability transition and cytochrome C release in hydrogen peroxide-induced apoptosis. Exp Cell Res 274: 16–24, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27: 433–446, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, Zappia M, Nelis E, Patitucci A, Senderek J, Parman Y, Evgrafov O, Jonghe PD, Takahashi Y, Tsuji S, Pericak-Vance MA, Quattrone A, Battaloglu E, Polyakov AV, Timmerman V, Schroder JM, Vance JM. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet 36: 449–451, 2004 [DOI] [PubMed] [Google Scholar]