Abstract
A reversible inhibition of mitochondrial respiration by complex I inhibition at the onset of reperfusion decreases injury in buffer-perfused hearts. Administration of acidic reperfusate for a brief period at reperfusion decreases cardiac injury. We asked if acidification treatment decreased cardiac injury during reperfusion by inhibiting complex I. Exposure of isolated mouse heart mitochondria to acidic buffer decreased the complex I substrate-stimulated respiration, whereas respiration with complex II substrates was unaltered. Evidence of the rapid and reversible inhibition of complex I by an acidic environment was obtained at the level of isolated complex, intact mitochondria and in situ mitochondria in digitonin-permeabilized cardiac myocytes. Moreover, ischemia-damaged complex I was also reversibly inhibited by an acidic environment. In the buffer-perfused mouse heart, reperfusion with pH 6.6 buffer for the initial 5 min decreased infarction. Compared with untreated hearts, acidification treatment markedly decreased the mitochondrial generation of reactive oxygen species and improved mitochondrial calcium retention capacity and inner mitochondrial membrane integrity. The decrease in infarct size achieved by acidic reperfusion approximates the reduction obtained by a reversible, partial blockade of complex I at reperfusion. Extracellular acidification decreases cardiac injury during reperfusion in part via the transient and reversible inhibition of complex I, leading to a reduction of oxyradical generation accompanied by a decreased susceptibility to mitochondrial permeability transition during early reperfusion.
Keywords: mitochondrial permeability transition pore, reactive oxygen species, cytochrome c, ischemia
ischemia-reperfusion leads to myocardial infarction and compromises cardiac function (28). Mitochondrial dysfunction contributes to myocardial injury during ischemia-reperfusion (43, 65). Cardiac injury is decreased by interventions applied at the onset of reperfusion by ischemic postconditioning (67) and pharmacological therapy (3, 26, 49, 57). Ischemic postconditioning is effective by modulating the pathological biochemical behavior of mitochondria that have been damaged by preceding ischemia, including attenuating the onset of mitochondrial permeability transition pore (MPTP) opening during early reperfusion (13, 47). Pharmacologic inhibition of complex I only for a brief period at the onset of reperfusion by the reversible complex I inhibitor amobarbital attenuates cardiac injury in buffer-perfused hearts (15, 57). The modulation of complex I activity in vivo during ischemia-reperfusion by other inhibitors including HMR-1098 (48), volatile anesthetics (4), nitrosyl-modifiers of cysteine (50), and cell-permeable nitric oxide (NO) donors (44, 54) also results in cardioprotection. Genomic modulation of complex I activity (60), but not complex IV activity (24), decreases mitochondrial injury during ischemia and subsequent myocardial injury during reperfusion. Thus attenuation of electron flux through complex I in the pathological setting of ischemia (37), and especially during early reperfusion, decreases cardiac injury and enhances functional recovery.
MPTP opening is a key mechanism of injury during ischemia-reperfusion (43, 65). Opening is favored by ischemia-induced damage to the electron transport chain (ETC) (10, 13, 47) and is blunted during ischemia by the acidic intracellular environment, whereas opening is favored when intracellular pH is normalized during early reperfusion (43, 65). Ischemic postconditioning results in a delayed recovery of intracellular pH during early reperfusion (18, 31), suggesting that a short period of intracellular acidification at the onset of reperfusion should be beneficial and reduce mitochondrial-driven cardiac injury. Indeed, the perfusion of acidic buffer at the onset of reperfusion decreases cardiac injury in the isolated heart (18, 31). Acidification may attenuate MPTP opening during early reperfusion, but direct evidence regarding the effect of acidification treatment on MPTP opening is still lacking.
At the onset of reperfusion, normalization of intracellular pH in the setting of reperfusion induces intracellular calcium overload and a burst of ROS generation that strongly favors MPTP opening (65). A delayed intracellular pH recovery inhibits Na+-H+ exchange and results in less calcium overload during reperfusion (31, 33). The decreased calcium overload during acidification may explain decreased MPTP opening. However, oxidative stress also is a key contributor to the induction of MPTP opening during reperfusion (53). The effect of acidification on the generation of reactive oxygen species (ROS) in cardiac mitochondria has not been studied. In the present study, acidification decreased mitochondrial respiration through inhibition of complex I activity. We demonstrated that a similar partial blockade of complex I during early reperfusion achieved by low-dose amobarbital administration exerts substantial cardioprotection. Thus acidification applied at the onset of reperfusion contributes to the decrease in cardiac injury via the attenuation of electron flux through complex I, in turn decreasing the production of ROS and, finally, decreasing the susceptibility to calcium-induced MPTP opening, accompanied by less cardiac injury during reperfusion.
METHODS
Isolation of mouse heart mitochondria.
The Animal Care and Use Committees of the McGuire Veterans Affairs (VA) Medical Center and Virginia Commonwealth University approved the study. Male C57BL/6 mice [2–3 mo of age (22–28 g)] were anesthetized with pentobarbital sodium (100 mg/kg ip) and anticoagulated with heparin (1,000 IU/kg ip). Mouse hearts were quickly placed in cold buffer A [composition in mM: 100 KCl, 50 3-(N-morpholino)propanesulfonic acid (MOPS), 1 EGTA, 5 MgSO4, and 1 mM ATP]. After removal of blood by washing with buffer A, the heart was blotted dry, weighed, and homogenized using a polytron tissue homogenizer at 10,000 rpm for 2.5 s in the presence of trypsin (5 mg/g tissue). The homogenate was incubated for 15 min at 4°C, and then the same volume of buffer B [buffer A + 0.2% bovine serum albumin (BSA)] was added, and the mixture was centrifuged at 500 g for 10 min. The supernatant was again centrifuged at 3,000 g to pellet mitochondria. The mitochondrial pellet was first washed with buffer B, resuspended in KME (100 mM KCl, 50 mM MOPS, 0.5 mM EGTA), and centrifuged at 3,000 g to yield the final mitochondrial pellet. Mitochondria were resuspended in KME for study. This method yielded approximately 3–4 mg of mitochondrial protein per 100 mg murine heart. Protein content was measured using the Lowry method. The isolation procedure required ∼2 h. Mitochondria were kept on ice and used within 4 h (60).
Mitochondrial oxidative phosphorylation.
Oxygen consumption in mitochondria was measured using a Clark-type oxygen electrode at 30°C as previously described (60). Mitochondria were incubated in 80 mM KCl, 50 mM MOPS, 1 mM EGTA, 5 mM KH2PO4, and 1 mg defatted, dialyzed bovine serum albumin/ml at pH 7.4. Glutamate (20 mM) + malate (10 mM) (complex I substrate), succinate (20 mM) plus 7.5 μM rotenone (complex II substrate), and N,N,N′,N′ tetramethyl p-phenylenediamine (TMPD; 1 mM)-ascorbate (10 mM) (complex IV substrate) + rotenone were used.
Measurement of electron transport chain enzyme activities and aconitase activity.
The following enzyme activities were measured in detergent-solubilized heart mitochondria using previously described methods (30, 36): NADH-cytochrome c reductase (NCR), rotenone sensitive; complex I, complex II, complex III, and citrate synthase (CS). The coupled aconitase-isocitrate dehydrogenase assay was used (30, 51). The reaction components were 50 mM Tris-HCl (pH 8.1), 1 mM sodium citrate, 1 mM MnCl2, 1 mM NADP+, isocitrate dehydrogenase (1.34 U/ml), and mitochondria (50 μg/ml). The production of NADPH was detected by the change in absorbance at 340 nm for 10 min at 37°C. Rabbit subsarcolemmal mitochondria (40) were used for these studies because the amount of mitochondria generated from one mouse heart was not sufficient to complete the incubation experiments.
Detection of H2O2 production in isolated mouse heart mitochondria.
H2O2 production from intact mitochondria was measured using the oxidation of the fluorogenic indicator Amplex red in the presence of horseradish peroxidase (16). Glutamate + malate and succinate (+ rotenone) were used as complex I and complex II substrates, respectively. The concentration of substrates was the same as that used to measure oxidative phosphorylation. Rotenone and antimycin A were added into the incubation medium to detect maximal H2O2 generation from complex I and complex III, respectively.
Isolation of cardiomyocytes and measurement of oxygen uptake.
Cardiac myocytes were isolated from adult C57BL/6J mice (59). Briefly, hearts were excised and retrograde perfused via the aorta at 37°C. First, hearts were perfused for 10 min with oxygenated Ca2+-free Tyrode solution (146 mM NaCl, 4 mM KCl, 0.5 mM MgCl2, 5 mM HEPES, 5.5 mM glucose; pH 7.25) supplemented with 10 mM 2,3-butanedione monoxime (BDM), followed by Tyrode solution containing 0.75 mg/ml collagenase (type 2), 20 μM CaCl2, and 5 mg/ml fatty acid-free BSA for ∼12 min. The ventricles were then isolated and cut into ∼1–2 mm2 pieces in fresh Tyrode/enzyme solution supplemented with 0.025 mg/ml protease (Type XIV) and gently shaken in a water bath at 37°C for 5 min. After filtering through a 210-μm nylon mesh, myocytes were washed and stored in MiR05 mitochondrial respiration medium (OROBOROS Instruments, Innsbruck, Austria) (0.5 mM EGTA, 110 mM sucrose, 3 mM MgCl2, 20 mM taurine, 60 mM K-lactobionate, 10 mM KH2PO4, 20 mM HEPES, and 1 g/l BSA; pH 7.1). The yield of rod-shaped, quiescent myocytes was >70%.
Freshly isolated cardiomyocytes (∼15,000 cells) were suspended in MiR05 mitochondrial respiration medium containing 0.5 mM EGTA, 110 mM sucrose, 3 mM MgCl2, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES, and 1 g/l BSA (pH 7.1). Oxidative phosphorylation was determined using OROBOROS (Oxygraph-2k, Innsbruck, Austria). Pyruvate/malate (5 mM/2 mM) were used as complex I substrates. After permeabilization of cardiomyocytes with digitonin (10 μg/ml), consumption of oxygen was strongly activated by 2 mM ADP. Successive additions of rotenone (0.5 μM), succinate (complex II substrate, 10 mM), 2-thenoyltrifluoroacetone (TTFA; 40 μM), TMPD/ascorbate (0.3 mM/3 mM, respectively), and azide (15 mM) allowed determination of inhibitor-sensitive rates of oxidative phosphorylation for complex I, II, and IV substrates. Oxygen consumption was evaluated by the OROBOROS DataLab4 software (OROBOROS Instruments, Innsbruck, Austria) and expressed as picomoles oxygen per second per milligram. To test if acidification decreased the rate of respiration of in situ mitochondria in cardiac myocytes, the rate of oxidative phosphorylation was measured in digitonin-permeabilized myocytes at buffer pH 7.1, 6.8, 6.6, and 6.4.
Preparation of mouse hearts for perfusion.
Hearts from male C57BL/6 mice were excised and perfused retrograde via the aorta in the Langendorff mode with modified Krebs-Henseleit (K-H) buffer (115 mM NaCl, 4.0 mM KCl, 2.0 mM CaCl2, 26 mM NaHCO3, 1.1 mM MgSO4, 0.9 mM KH2PO4, and 5.5 mM glucose), gassed with 95% O2-5% CO2 to adjust pH to 7.35–7.45 (12). Hearts were paced at 420 beats/min. The cardiac function was monitored with a balloon inserted into the left ventricle, and data were recorded digitally with Powerlab (AD Instruments, Colorado Springs, CO).
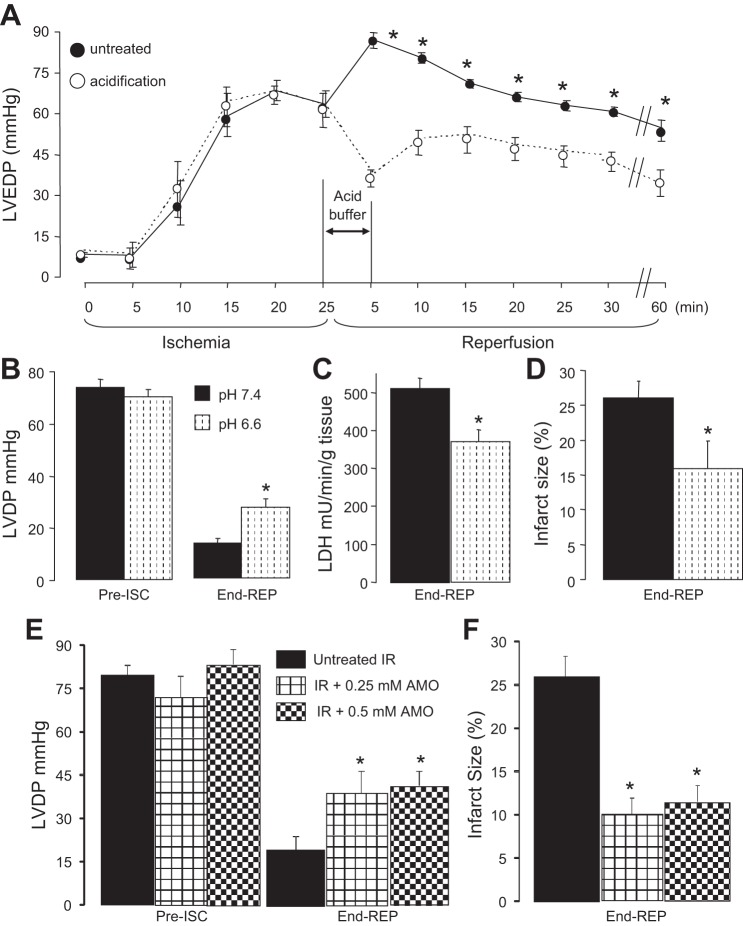
In the untreated ischemia-reperfusion group, hearts were perfused for 15 min equilibration with K-H buffer (pH 7.43 ± 0.01), followed by 25-min global ischemia at 37°C and 60 min reperfusion. In the acidification during early reperfusion group, acidic K-H buffer (pH 6.61 ± 0.01) was perfused for 5 min at the onset of reperfusion followed by normal pH buffer for the remainder of reperfusion (see Fig. 5). The pH of the acidic buffer was adjusted by lowering bicarbonate concentration and increasing sodium chloride to maintain osmolarity constant while maintaining the same gas mixture containing 5% CO2 (31, 33). Time control hearts were perfused without ischemia. Lactate dehydrogenase (LDH) release into coronary effluent during the entire reperfusion period and myocardial infarct size (12) measured at 60 min reperfusion were used to determine the extent of cardiac injury. Cardiac mitochondria were isolated at 5 or 30 min of reperfusion.
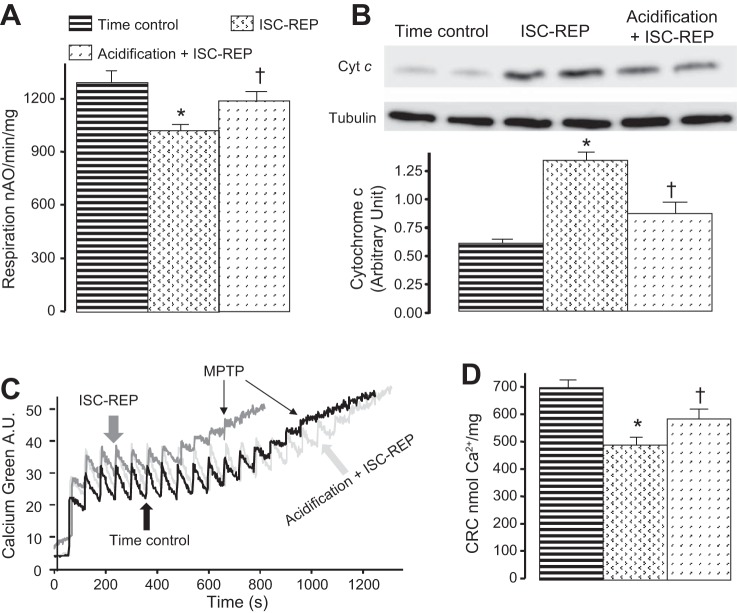
Fig. 5.
Acidic reperfusion improved oxidative phosphorylation and calcium retention capacity (CRC) with decreased loss of cytochrome c from mitochondria assessed during early reperfusion immediately following the resumption of pH 7.4 perfusion. Reperfusion with acidic buffer preserved oxidative phosphorylation using N,N,N′,N′ tetramethyl p-phenylenediamine (TMPD)-ascorbate (A) in part due to decreased release of cytochrome c from mitochondria (B). Acidic reperfusion improved CRC compared with untreated ischemia-reperfusion (ISC-REP) (C, D). MPTP, mitochondrial permeability transition pore. Values are means ± SE. *P <0.05 vs. time control; †P < 0.05 vs. untreated; n = 5 in each group.
Calcium retention capacity (CRC) in isolated mitochondria.
CRC was used to assess calcium-induced mitochondrial permeability transition pore opening in isolated mitochondria (47). CRC was evaluated in mitochondria (125 μg/ml) incubated in medium containing 150 mM sucrose, 50 mM KCl, 2 mM KH2PO4, 5 mM succinic acid in 20 mM Tris/HCl, pH 7.4 by sequential pulses of a known amount of calcium (5 nmol). Extramitochondrial Ca2+ concentration was recorded with 0.5 μM Calcium Green-5N (Life Technologies) and fluorescence monitored with excitation and emission wavelengths set at 500 and 530 nm, respectively.
Relative estimation of mitochondrial inner membrane potential.
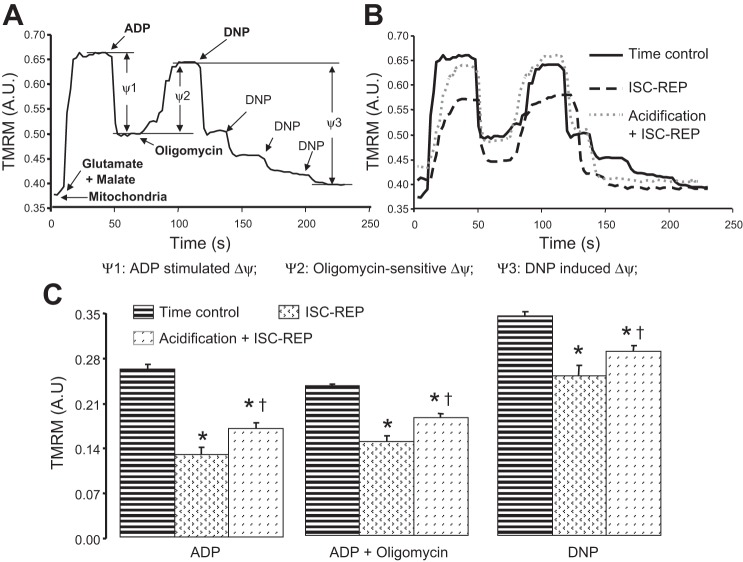
Mitochondrial inner membrane potential (Δψ) was assessed using the fluorogenic indicator tetramethylrhodamine methyl ester (TMRM) (13, 52). Freshly isolated mitochondria (0.2 mg/ml) were incubated in a single cuvette with continuous stirring at 30°C with sequential additions of glutamate (10 mM) + malate (5 mM), ADP (2 mM), oligomycin (1 μM), and DNP (0.3 mM) in buffer (100 mM KCl, 50 mM MOPS, 5 mM KPi, 1 mM EGTA). Relative changes in Δψ were assessed by the change in the 573 nm/546 nm fluorescence ratio of TMRM (13, 52). Fluorescence changes before and after ADP addition (ψ1), oligomycin addition (ψ2), and DNP titration (ψ3) are as shown in Fig. 5A. Relative membrane potential was measured at 30°C to parallel conditions under which oxidative phosphorylation was measured. Comparison experiments performed using control mouse heart mitochondria showed essentially the same results if the conditions of the present study (30°C, 0.2 mg protein/ml, 0.1 μM TMRM) or the conditions of Scaduto and Grotyohann (13, 52) (28°C, 0.2 mg protein/ml, 0.3 μM TMRM) were used (data not shown).
Statistical analysis.
Data are expressed as means ± SE (56). For all analyses, differences between groups were compared by one-way ANOVA. For cardiac functional analysis, differences between groups were compared by two-way ANOVA. When a significant F value was obtained, means were compared using the Student-Newman-Keuls test of multiple comparisons. Statistical significance was defined as a value of P < 0.05.
RESULTS
Acidification decreased the rate of oxidative phosphorylation and electron transport complex enzyme activities in isolated cardiac mitochondria.
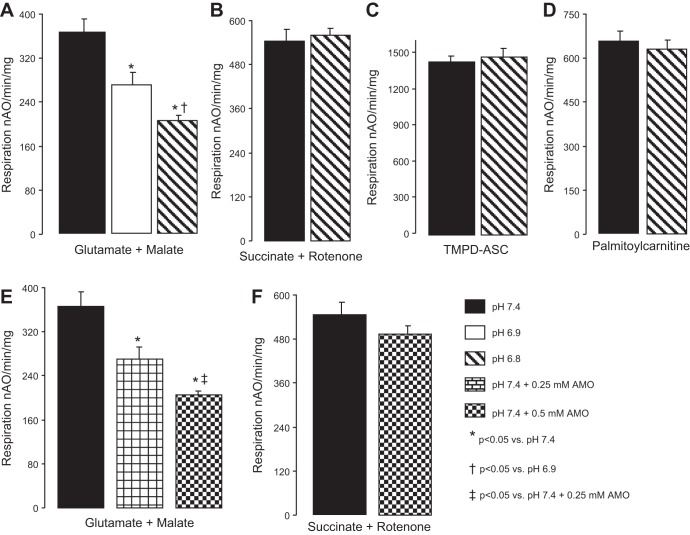
The rate of oxidative phosphorylation was significantly decreased in intact mouse heart mitochondria exposed to acidic extramitochondrial buffer in a pH-dependent manner using glutamate + malate as a complex I substrate (Fig. 1A). Acidification did not alter the rate of oxidative phosphorylation when succinate + rotenone (complex II, Fig. 1B) or TMPD-ascorbate + rotenone (complex IV, Fig. 1C) were used as substrates. The oxidation of palmitoylcarnitine was not altered in mitochondria exposed to acidification (Fig. 1D). These results suggested that acidification decreased the rate of respiration by selectively inhibiting complex I.
Fig. 1.
An extramitochondrial acidic environment decreased oxidative phosphorylation in cardiac mitochondria. Acidification decreased ADP-stimulated oxidative phosphorylation only when glutamate + malate as complex I substrate was used (A), whereas respiration with a complex II substrate (B), a complex IV substrate (C), or a fatty acid substrate (D) were unaffected. Amobarbital (AMO), a cell-permeable, rapid and reversible inhibitor of complex I, was used in a dose response to cause a partial inhibition of complex I-driven respiration (E) that did not affect the electron transport chain distal to complex I (F). Values are means ± SE; n = 5 in each group; nAO, nanoatoms O (atomic oxygen).
Amobarbital (2 mM) completely inhibited complex I activity in isolated mitochondria (6). Since acidification only partially inhibited complex I activity in isolated heart mitochondria, exposure to a lower concentration of amobarbital was used to obtain a similar extent of complex I inhibition. The rate of oxidative phosphorylation using glutamate + malate was decreased in the presence of 0.25 and 0.5 mM amobarbital compared with untreated mitochondria (Fig. 1E). Succinate oxidation was not affected by these concentrations of amobarbital (Fig. 1F). Thus lower concentrations of amobarbital lead to partial inhibition of complex I-stimulated respiration.
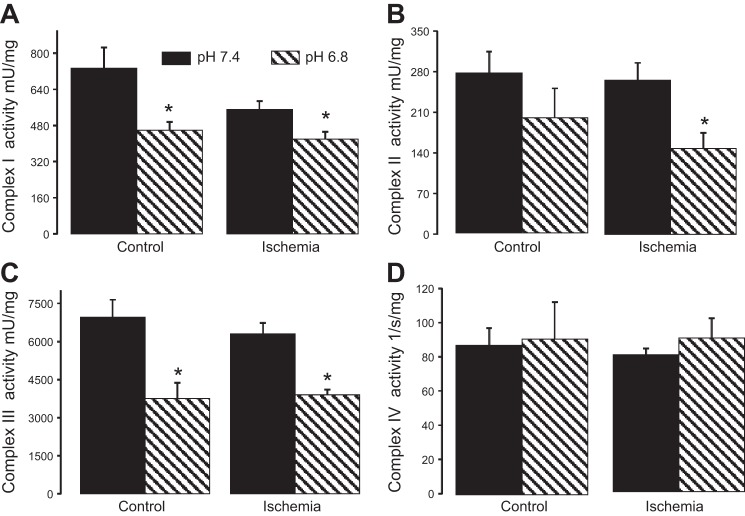
To further localize the site of inhibition of the ETC, the enzyme activities of complexes I–IV were measured in detergent solubilized heart mitochondria in the presence of acidic buffer (pH 6.8) and normal pH buffer (pH 7.4). Exposure of solubilized mitochondria to an acidic environment (pH 6.8) significantly decreased the activity of complex I (Fig. 2A) but not complex II in control mitochondria compared with normal pH buffer (Fig. 2B). Acidification also decreased complex III activity (Fig. 2C). The activity of cytochrome oxidase was not altered by acidification in heart mitochondria (Fig. 2D). These results provide direct evidence that exposure to an acidic environment resulted in a decrease in the activities of complex I and complex III.
Fig. 2.
Acidification selectively inhibits complexes I and III of the electron transport chain. Exposure to an acidic environment (pH 6.8) decreased the enzyme activity of complex I (A) and complex III (C) both at baseline and following ischemia. Complex IV (D) was unaffected by acidic pH. Complex II (B) was not inhibited by acidic pH in the baseline state but was inhibited in mitochondria isolated from hearts following ischemia. Values are means ± SE; n = 5 in each group *P < 0.05 vs. pH 7.4.
Acidification applied at the onset of reperfusion must interact with mitochondria that have sustained damage from the preceding period of ischemia (38, 40). Therefore, the effect of acidification on ETC activities was studied in ischemia-damaged mitochondria. Acidic buffer decreased the activities of complex I, complex II, and complex III in subsarcolemmal mitochondria isolated following 30 min of ischemia (39, 40) compared with pH 7.4 buffer (Fig. 2). However, acidification did not affect NADH dehydrogenase activity of complex I (mU/mg) in either control [means ± SE, 1,663 ± 60 (pH 7.4) vs. 1,670 ± 76 (pH 6.8); P = NS, n = 4 in each group] or ischemia-damaged mitochondria [mean ± SE, 1,546 ± 36 (pH 7.4) vs. 1,530 ± 75 (pH 6.8); P = NS, n = 4]. Thus reperfusion with persistent intracellular acidification is predicted to induce a partial blockade of complex I, localized distal to the NADH dehydrogenase portion of the complex. In addition to deceasing electron flow into complex III via the blockade of complex I, acidification also directly inhibits complex III in ischemia-damaged mitochondria. Thus an acidic extramitochondrial environment has the potential to decrease the production of ROS from complex I and complex III of mitochondria, the major sources of ROS following ischemia (9, 11, 16).
Acidification decreases the net release of H2O2 from cardiac mitochondria.
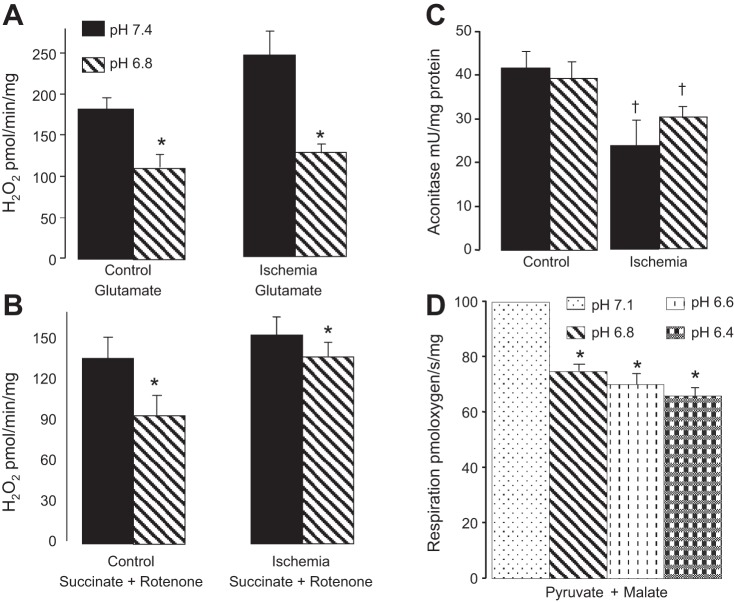
The uncontrolled formation of cytotoxic ROS from the ETC contributes to cardiac injury, especially during early reperfusion (2, 12, 64). In isolated mitochondria, acidification dramatically decreased maximal ROS generation from control and ischemia-damaged mitochondria when glutamate was used as complex I substrate (Fig. 3A). Acidification also decreased the net release of H2O2 when succinate + rotenone was used as substrate but to a lesser extent (Fig. 3B).
Fig. 3.
Acidification decreased the generation of reactive oxygen species from mitochondria. An acidic extramitochondrial environment decreased the net release of H2O2 from control and ischemia-damaged mitochondria in the presence of either complex I (A) or complex II substrates (B). Acidification did not alter aconitase activity in control or ischemia-damaged mitochondria, suggesting that acidification does not increase superoxide generation that is directed into mitochondrial matrix (C). However, aconitase activity was reduced by ischemia. Acidification decreased oxidative phosphorylation in digitonin permeabilized myocytes when pyruvate + malate was used as a complex I substrate, indicating that intracellular pH alters complex I activity in situ. The partial block of respiration observed on acidification from pH 7.1 to 6.8 was not significantly increased by acidification to pH 6.6 or 6.4 (D). Values are means ± SE. *P <0.05 vs. pH 7.4 or pH 7.1 (D); †P < 0.05 vs. nonischemic; n = 5 in A–C; n = 6 in D.
Inhibition of complex I decreased the net release of ROS from complex III in isolated mitochondria, but it can increase superoxide generation into the mitochondrial matrix directly from complex I (16, 55). The activity of aconitase is sensitive to superoxide generation within the mitochondrial matrix (11), and a decrease in aconitase activity was used as a marker for increased superoxide within the mitochondrial matrix (11, 55). Aconitase activity was measured in mitochondria incubated with normal and acidic buffer (11). Incubation in acidic media did not lead to a decrease in aconitase activity compared with normal pH buffer in either control or ischemia-damaged mitochondria (Fig. 3C). Aconitase activity was decreased in mitochondria following ischemia compared with mitochondria from nonischemic hearts (Fig. 3C), suggesting that ischemia-mediated damage of the ETC increased superoxide generation directed toward the mitochondrial matrix. Aconitase activity was not affected by acidification, indicating that acidification did not paradoxically increase ROS release into the mitochondrial matrix. Unfortunately, a decrease in ROS directed toward the matrix may not lead to an improvement in aconitase activity. Thus exposure of mitochondria to acidic pH clearly decreases ROS release from mitochondria, indicating production directed toward the intermembrane space is decreased; it remains possible that production directed into the matrix may not be decreased.
Acidification decreased the rate of oxidative phosphorylation in situ in isolated cardiac myocytes.
Cardiac myocytes were isolated from nonischemic mouse hearts, and the rate of oxidative phosphorylation in different pH buffers was measured after digitonin permeabilization. The concentration of digitonin used (10 μg/ml) permeabilized only the sarcolemmal membrane (59). Intracellular pH in intact cardiac myocytes is ∼7.1 (31). Thus we used pH 7.1 buffer as the control (mean ± SE: pyruvate + malate, 1,083 ± 64 nAO (nanoatom molecular oxygen)· min−1·million cells−1).The rate of oxidative phosphorylation was decreased by 30% in mitochondria in situ upon exposure to an acidic environment (pH 6.8) compared with a normal pH buffer when pyruvate + malate was used as a complex I substrate. Further decreases in pH to 6.6 and 6.4 did not decrease respiration compared with pH 6.8 (Fig. 3D). Acidification also slightly decreased oxidative phosphorylation in myocytes when succinate + rotenone was used as complex II substrate (data not shown). Thus simulated intracellular acidification resulted in a partial blockade of electron transport activity in mitochondria in situ.
A brief period of reperfusion with acidic buffer decreased myocardial injury.
Reperfusion with an acid pH buffer at the onset of and during early reperfusion decreased cardiac injury in isolated rabbit (18) and rat hearts (31). Perfusion of acidic buffer (pH 6.6) for 2 min at the onset of reperfusion maintained intracellular pH at ∼6.4 and decreased cardiac injury in isolated hearts (18, 31). Since there were no differences in the rate of oxidative phosphorylation of in situ cardiac mitochondria in permeabilized cardiac myocytes between pH 6.8, 6.6, and 6.4, we chose pH 6.6 to test if acidification decreased cardiac injury in the isolated mouse heart. There were no differences in left ventricular developed pressure (LVDP, mmHg) between treatment groups at 15 min equilibration, nor in diastolic pressure at the end of ischemia (Fig. 4A). The similar degree of ischemic contracture indicates that hearts in both groups sustained a similar degree of ischemic damage before treatment. Reperfusion with pH 6.6 buffer decreased diastolic pressure during reperfusion compared with untreated hearts (Fig. 4A). Acidification also improved cardiac contractile recovery during reperfusion (Fig. 4B) and markedly decreased both LDH release (Fig. 4C) and infarct size (Fig. 4D) compared with untreated hearts. Thus reperfusion with acidic pH also decreased cardiac injury in the buffer-perfused mouse heart.
Fig. 4.
Reperfusion with an acidic buffer decreased cardiac injury in buffer-perfused mouse hearts. Acidic buffer (pH 6.6) was given during the initial 5 min of reperfusion (A). This treatment decreased diastolic pressure during reperfusion compared with untreated hearts. This treatment improved the recovery of cardiac contractile function during reperfusion (B) and decreased cardiac injury shown by a decreased release of lactate dehydrogenase (LDH) during reperfusion (C) and a reduction in myocardial infarct size (D). Reperfusion with low concentrations of amobarbital that result in a partial blockade of complex I-stimulated respiration also results in a similar improvement in contractile recovery (E) and reduction in infarct size (F) compared with untreated ischemic-reperfused hearts. Values are means ± SE. ISC, ischemia; REP, reperfusion. *P < 0.05 vs. untreated hearts.
To directly test if partial inhibition of complex I decreased cardiac injury in buffer-perfused hearts, amobarbital (0.25 and 0.5 mM) was administered to hearts during the initial 5 min of reperfusion. Compared with untreated hearts, amobarbital treatment improved cardiac function during reperfusion (Fig. 4E) and decreased infarct size (Fig. 4F). Hence, partial inhibition of complex I at the onset of reperfusion decreased cardiac injury. The extent of cardioprotection was similar to that observed with reperfusion with acidic buffer, which also resulted in a similar partial inhibition of complex I.
Extracellular acidification during early reperfusion decreased the release of cytochrome c from mitochondria during reperfusion.
Ischemia-reperfusion leads to a release of cytochrome c from mitochondria (40). Cardiac mitochondria and cytosol fractions were isolated following 5 min reperfusion. The content of cytochrome c in cytosol obtained from untreated reperfused hearts was increased, whereas acidification treatment at the onset of reperfusion reduced the cytochrome c content in cytosol (Fig. 5B). Thus acidification treatment decreased the loss of cytochrome c from mitochondria during early reperfusion, indicating protection of the distal ETC.
Extracellular acidification during early reperfusion leads to a decrease in susceptibility to MPTP opening.
Calcium retention capacity (CRC) was used to assess the susceptibility to MPTP opening in isolated mitochondria (47). Isolated mitochondria from control, untreated, and acidification-treated hearts were studied at pH 7.4. A representative tracing of CRC measurement is shown in Fig. 5C. The CRC of mitochondria from untreated ischemia-reperfusion hearts was significantly decreased compared with nonischemic hearts (Fig. 5D). Acidification at the onset of reperfusion significantly improved CRC compared with untreated hearts (Fig. 5D). The decreased susceptibility to MPTP opening in mitochondria from hearts reperfused with acidic buffer is not a result of the transient extracellular and resulting intracellular acidification because measurements were made at pH 7.4. Thus reperfusion with acidic buffer protected mitochondria, indicated by a decreased susceptibility to opening of the MPTP.
Acidification improved oxidative phosphorylation in mitochondria following ischemia-reperfusion.
Cardiac ischemia damages the distal electron transport in buffer-perfused hearts (40). Mouse heart mitochondria were isolated at 5 or 30 min of reperfusion, and oxidative phosphorylation was measured in a physiological pH environment (59). Five minutes of reperfusion decreased the rate of ADP-stimulated respiration in mitochondria oxidizing TMPD-ascorbate compared with time control mitochondria, and acidification treatment significantly improved the rate of TMPD-ascorbate stimulated respiration compared with untreated hearts (Fig. 5A). This result is in line with the improved retention of cytochrome c in mitochondria isolated from hearts treated with acidic reperfusion. On the other hand, acidification did not significantly alter oxidative phosphorylation using glutamate + malate (complex I) or succinate + rotenone (complex II) substrates compared with that in untreated ischemia-reperfused hearts (data not shown). Thus reperfusion with acidic buffer protected the distal segment of the ETC as reflected in improved mitochondrial oxidative physiology.
For untreated hearts undergoing ischemia-reperfusion, at 30 min reperfusion, ADP-stimulated state 3 respiration was decreased, ADP-limited state 4 respiration was increased, and consequently, the respiratory control ratio (RCR) was decreased compared with nonischemic time controls (Table 1). Reperfusion with acidic buffer significantly improved ADP-limited state 4 respiration and resulted in an improved RCR vs. that of untreated ischemia-reperfusion hearts. However, the rate of state 4 and RCR in mitochondria from acidic buffer-treated hearts remained lower than time control (Table 1). The recovery of state 3 respiratory rates indicates that blockade of respiration by extracellular acidification was only transient and reversed following normalization of extracellular pH. Importantly, improved coupling of respiration and decreased state 4 respiration rates indicates that acidification during early reperfusion protected the integrity of the inner mitochondrial membrane.
Table 1.
The rate of oxidative phosphorylation in mouse heart mitochondria following 25 min ischemia and 30 min reperfusion using glutamate + malate as complex I substrates
| State 3 | State 4 | RCR | ADP/O | |
|---|---|---|---|---|
| Time control (n = 6) | 267 ± 10 | 25 ± 1 | 11.3 ± 0.6 | 3.01 ± 0.07 |
| IR (n = 6) | 144 ± 10* | 37 ± 2* | 3.9 ± 0.3* | 2.80 ± 0.07 |
| Acidification + IR (n = 9) | 156 ± 9* | 30 ± 1*† | 5.4 ± 0.5*† | 2.96 ± 0.07 |
Values are means ± SE. RCR, respiratory control ratio; ADP/O, adenosine diphosphate-to-oxygen phosphorylation ratio.
P < 0.05 vs. time control;
P < 0.05 vs. ischemia-reperfusion (IR).
MPTP opening increases the permeability of both inner and outer mitochondrial membranes. Permeation of the inner mitochondrial membrane leads to the loss of inner membrane potential. A relative estimation of inner membrane potential was used to further assess the functional integrity of the inner membrane. Representative original tracings of the TMRM estimation of mitochondrial inner membrane potential are shown in Fig. 6B. Inner membrane potential was more depolarized in mitochondria studied at the end of reperfusion compared with time controls in the presence of ADP, oligomycin, and DNP, indicating increased permeability of the inner membrane (Fig. 6C). Acidification treatment significantly improved the relative estimate of inner membrane potential compared with untreated ischemia-reperfused hearts (Fig. 6C), supporting an improved functional integrity of the inner mitochondrial membrane. These results provide additional evidence that acidification treatment decreased irreversible MPTP opening during early reperfusion, leading to an improved capacity of mitochondria to support formation of a phosphorylating membrane potential as reperfusion continued.
Fig. 6.
Acidic reperfusion preserved mitochondrial inner membrane integrity assessed by the membrane potential indicator TMRM measured following more prolonged periods of reperfusion. A: a representative trace of TMRM fluorescence as an indicator of mitochondrial inner membrane potential. The addition of ADP stimulated oxidative phosphorylation with a depolarization of inner membrane potential. Membrane potential was restored when oligomycin was used to inhibit oxidative phosphorylation at complex V. Dinitrophenol (DNP), an uncoupler, resulted in a depolarized inner membrane potential. B: representative traces of TMRM fluorescence from mitochondria isolated from time control, untreated ischemia-reperfusion, and acidic reperfusion-treated hearts. The changes of fluorescence intensity of TMRM in the presence of ADP, oligomycin, and DNP were compared in C. Ischemia-reperfusion (ISC-REP) significantly decreased the relative inner membrane potential in the presence of ADP, oligomycin, and DNP compared with the time control group. Acidic reperfusion treatment substantially improved the relative measurement of inner membrane potential as a functional index of mitochondrial integrity compared with mitochondria from untreated hearts. Values are means ± SE; *P < 0.05 vs. time control; †P < 0.05 vs. untreated; n = 5 in each group.
DISCUSSION
In the present study, we found that exposure of mitochondria to an acidic extramitochondrial environment, simulating acidification of the cytosol, inhibited respiration due to a decrease in the activity of complex I. The decrease in complex I activity was accompanied by less ROS generation from both control and ischemia-damaged mitochondria. Simulated intracellular acidification in permeabilized cardiac myocytes decreased oxidative phosphorylation driven by a complex I substrate, indicating that acidification attenuated complex I activity in situ as well. Moreover, acidic reperfusion decreased cardiac injury in the isolated mouse heart by inhibiting MPTP opening as shown by improved CRC early in reperfusion, decreased cytochrome c release from mitochondria (integrity of outer mitochondrial membrane), and preserved inner membrane potential (integrity of inner mitochondrial membrane) during reperfusion. Thus partial blockade of electron transport at complex I via extracellular acidification at the onset of reperfusion suppressed mitochondrial-driven cardiac injury. The mechanism of protection likely included prevention of MPTP opening, in part via the reduced generation of ROS. The cardiac protection was reproduced by partial blockade of complex I activity achieved by the use of low concentrations of the rapid and reversible complex I inhibitor amobarbital.
An acidic environment inhibits complex I activity.
Acidification inhibited respiration in mouse heart mitochondria when glutamate + malate was used as complex I substrate but not with succinate as complex II substrate. This finding suggested that an acidic environment led to decreased electron flux through complex I, and the decrease was confirmed by direct measurement of complex I activity. In addition, acidification directly decreased complex III activity. The unaltered succinate oxidation in control mitochondria suggests that the partial inhibition of complex III was consistent with the notion that greater than 50% inhibition of complex III is required to decrease respiration (38). If exposure of intact mitochondria to the acidic milieu results in a greater acidification of the intermembrane space than the matrix, there may be a further increase in the proton gradient across the inner membrane that should further attenuate electron flow through complex I leading to additional relative blockade. Acidification decreased oxidative phosphorylation in isolated myocytes using a complex I substrate, indicating that respiration in situ can be modulated in the cardiac myocyte at complex I by manipulating pH.
Acidification decreases complex I activity in both control and ischemia-damaged mitochondria, but it does not affect NADH dehydrogenase activity. These results suggest that the sites affected by acidification are distal to the flavin mononucleotide (FMN) located in the NADH dehydrogenase portion of the complex. Thus iron-sulfur proteins or the distal tightly bound quinones are potential candidate sites for inhibition of activity (11, 45, 63). The iron-sulfur centers are proximal to the site of ischemic damage to complex I in untreated mitochondria proposed to be located at the two tightly bound quinones (11). Thus inhibition of activity at or proximal to the site of ischemic damage is consistent with the observed acidification-induced decrease in oxidant generation from complex I.
The rate of oxidation of a fatty acid substrate, palmitoylcarnitine, is not altered by acidic pH (Fig. 1). Reducing equivalents from palmitate not only enter the electron transport chain via complex I but also via complex II and electron-transfer flavoprotein, both located distal to the acid pH-induced blockade at complex I and consistent with unaltered rates of succinate-stimulated respiration. Thus acidification appears to provide a partial and selective attenuation of electron transport in contrast to a complete blockade of oxidative metabolism. The partial inhibition of complex I by acidification does not appear to attenuate fatty acid oxidation. In the intact heart with available fatty acid substrates, this mechanism may provide a key energy supply during in situ acidification by funneling reducing equivalents into electron transport distal to complex I, whereas key complex I-driven mechanisms of injury are attenuated.
Ischemia results in intracellular acidification to approximately pH 6.4 after 40 min of ischemia in buffer-perfused rat hearts (31). Intracellular pH quickly (within 2 min) returns to preischemic values when normal pH perfusate is used for reperfusion. In contrast, in the isolated perfused rat heart, 3 min of perfusion with acidic buffer (pH 6.4) delays intracellular pH recovery (31). Based upon studies of respiration in permeabilized myocytes, an intracellular pH of 6.4–6.8 will inhibit electron flux through complex I. Intracellular pH will return to preischemic values within 5 min even if acidic buffer perfusion is extended to 15 min (31). Thus we chose a 5-min perfusion period to delay intracellular pH recovery during the critical early reperfusion period. Based upon our studies of isolated and in situ mitochondria, reperfusion with acidic buffer can inhibit complex I activity during this critical period by modulating intracellular pH in situ.
Acidification decreases the generation of ROS during reperfusion.
Complex I and complex III are the major sites of ROS production (16), including from the ETC damaged by ischemia (11). The limitation of electron flow into complex III due to complex I blockade decreases ROS generation in intact mitochondria (16). ROS generated from complex I are released into the matrix, whereas ROS from the complex III Qo center are released into the intermembrane space. The decrease in net H2O2 production from mitochondria in an acidic environment related to partial inhibition of complex I most likely represents attenuation of ROS production directly from complex I (indicated by the lack of loss of matrix aconitase activity) (Fig. 2) in concert with decreased electron transport into complex III (decreased extra mitochondrial release of ROS).
Both the iron-sulfur proteins (23) and quinone sites (45, 63) are potential loci of superoxide generation within complex I (11, 63). Blockade of electron transport at the quinone binding site by rotenone increases superoxide generation directed into the mitochondrial matrix and leads to decreased aconitase activity (11, 55), not observed in the present study either at baseline or following ischemia. This finding suggests that acidification probably does not block complex I at the quinone binding site. Thus acidification most likely decreases complex I activity and ROS production due to inhibition at the iron-sulfur proteins.
Acidification also decreases the ROS generation from intact mitochondria when succinate + rotenone is the substrate, an index of ROS production from complex III. Inhibition of complex III at the Qi center using antimycin A increases ROS generation, whereas inhibition at the Qo center decreases ROS production (16, 27). Acidification decreases ROS from complex III, suggesting that acid exposure decreases complex III activity and ROS production by inhibiting the Qo center.
In the present study, acidification decreased maximal ROS generation in ischemia-damaged mitochondria when glutamate was used as a complex I substrate, suggesting that in addition to attenuating ROS production from complex I, acidic exposure also attenuated ROS production from complex III likely via both decreased electron flow into complex III and potentially via direct inhibition of the Qo site.
The decreased aconitase activity in the ischemia-damaged mitochondria compared with nonischemic mitochondria (Fig. 2) is likely a result of the period of untreated ischemia that occurred before the mitochondrial incubation experiments that simulate reperfusion. Unfortunately, aconitase activity will not clearly improve above initial levels if ROS production decreases. Since ischemic damage to complex I increases superoxide generation directed toward the mitochondrial matrix (11), this finding at least suggests that acidification did not paradoxically increase ROS production directed into the matrix. Acidic exposure may have a limited effect on superoxide released into the matrix. Thus acidification applied at the onset of reperfusion most likely decreases the net release of ROS from mitochondria by blocking flux into complex III via inhibition of complex I and decreasing ROS production from the Qo site of complex III.
Potential mechanisms of intracellular acidification in response to reperfusion with an acidic perfusate.
In the isolated perfused rat heart, reperfusion with acidic buffer (pH 6.4) delays intracellular pH recovery (31). Physiological CO2/HCO3− buffers are used, so CO2 is a potential agent that could equilibrate extracellular and intracellular pH across the plasma membrane. In contrast to other physiological situations, the extracellular acidification achieved by acidic reperfusion only needs to perpetuate the intracellular acidification already present from ischemia. Thus, while systems including NH3/NH4+ (46, 62) or CO2/HCO3− (22) may acidify intracellular pH from normal values in response to extracellular pH, including by membrane diffusion of the gaseous component, it appears that regulation of intracellular pH occurs via cardiomyocyte plasma membrane anion exchangers that affect chloride/hydroxide and chloride/bicarbonate exchange (21). Cardiac myocytes contain transmembrane anion exchangers of the SLC26 superfamily (42). The major anion exchanger in cardiac myocytes is Slc26a6 (1, 20). This protein forms the predominant cardiac chloride-hydroxyl and chloride-bicarbonate exchange mechanism (1) and interacts with and is stimulated by cardiac CFTR (7, 35). In addition, Na-H exchange (NHE) is present in heart and contributes to pH regulation (3). Although the exact role in preserving intracellular acidification in the presence of extracellular acidosis awaits experiments using Slc26a6 knockout mice (34), previous work already supports that Slc26a6 is a key contributor to the recovery of intracellular pH following ischemia (1).
Acidification at the onset of reperfusion blocks complex I and prevents MPTP opening to decrease cardiac injury.
Reversible total blockade of electron transport at complex I by amobarbital treatment for a brief period at the onset of reperfusion (15, 57) decreases injury in isolated hearts. In the present study, we found that complex I activity can be modulated by adjusting extracellular pH at the onset of reperfusion. The cardiac protection of a brief period of extracellular acidification at reperfusion was reproduced by a brief period of partial inhibition of complex I achieved by perfusion with low-dose amobarbital. Thus it is highly likely that the decreased complex I activity due to acidification treatment contributed to the decrease in myocardial injury during reperfusion. MPTP opening contributes to cell death during reperfusion. Increased ROS generation and calcium overload are key factors that induce MPTP opening (43, 65). In the present study, acidification treatment markedly improved CRC and inner membrane integrity following reperfusion, supporting the idea that acidification treatment decreased MPTP opening. Delaying intracellular pH normalization using an NHE inhibitor decreased calcium overload during reperfusion (3). Acidification treatment significantly delays intracellular pH recovery during reperfusion (32), suggesting that acidification decreases MPTP opening during reperfusion in part by reducing calcium overload (18). However, oxidative stress also contributes a key role in the induction of MPTP opening. In the present study, we provided evidence that acidification decreased the generation of ROS in ischemia-damaged mitochondria. Thus the findings of the current study with observations in systems ranging from complex I activity in isolated mitochondria to the behavior of the intact heart provide solid support for a role of acidic reperfusion in cardiac protection by decreasing the MPTP opening via decreased generation of ROS from the ETC, which would complement the decreased calcium overload. The present study is in line with emerging work that increasingly connects the attenuation of electron transport through complex I with resistance to MPTP opening in cellular systems (41, 61) and in the intact heart (13, 17).
The present study provides a likely integrative mechanism for the protection of ischemic postconditioning. Postconditioning modulates the ischemia-damaged ETC to attenuate oxidative damage, MPTP opening, and cardiac injury (10, 13, 47). Postconditioning perpetuates the intracellular acidosis present from the preceding ischemia into reperfusion (18), with likely transient inhibition of complex I and ROS production the probable mechanisms of the previously observed attenuation of oxidative damage and MPTP opening (10, 13, 47).
In addition to MPTP opening, opening of other channels at the inner mitochondrial membrane including calcium-sensitive potassium channel (58) and the mitochondrial ATP-sensitive potassium channel (25) affect cardiac injury during reperfusion. The effect of acidification on the activity of these channels during reperfusion remains to be investigated.
The milieu of the reperfused myocyte is complex, and inhibition of electron transport is one of several potentially protective mechanisms of action of transient intracellular acidification at the onset of reperfusion. Acidic reperfusion activates the cGMP/PKG signal transduction pathway which activates cardioprotection (33). Intracellular acidification prevents relocation of intracellular calpains (32). Attenuation of calpain activation, including in mitochondria (14), leads to decreased damage to the ETC (5), decreases susceptibility to MPTP (19), and decreases caspase-independent cell death (8, 29, 66). The current study expands our understanding of the mechanism whereby extracellular acidification at the onset of reperfusion protects jeopardized myocardium.
Conclusions.
The maintenance of intracellular acidification at the onset of reperfusion decreased myocardial injury by attenuating MPTP opening through decreased ROS generation via a reversible and partial blockade of electron transport at complex I. Consequently, there was enhanced retention of cytochrome c, improved inner mitochondrial membrane integrity, and ultimately decreased cardiac injury in the buffer-perfused heart. The current study reinforces previous mechanisms of cardioprotection achieved by intracellular acidification and also provides a likely mechanism whereby ischemic postconditioning protects myocardium via attenuation of MPTP opening.
The manipulation of complex I activity is a valuable approach to decrease cardiac injury during the early reperfusion period. Perpetuation of acidosis from the preceding ischemia via reperfusion with extracellular perfusate or modulation of cardiac cell membrane chloride/anion exchangers may provide an approach to modulate the ETC and protect reperfused myocardium. This approach provides an alternative to the direct, reversible inhibition of electron transport during early reperfusion to reduce cardiac injury (2, 15, 57).
GRANTS
This work was supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs (E. J. Lesnefsky), a Scientist Development Grant (11SDG5120011) from the American Heart Association (Q. Chen), Program Project 2PO1-AG-15885 from the National Institutes of Health (E. J. Lesnefsky), and the Pauley Heart Center, Virginia Commonwealth University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.S., Q.C., E.J.L. conception and design of research; A.X., K.S., M.W.M., T.R., E.B., Y.H., B.K., C.M., P.N.D., Q.C. performed experiments; A.X., K.S., M.W.M., T.R., E.B., Y.H., B.K., C.M., P.N.D., C.M.B., Q.C., E.J.L. analyzed data; A.X., K.S., M.W.M., T.R., E.B., B.K., P.N.D., C.M.B., Q.C., E.J.L. interpreted results of experiments; K.S., M.W.M., Q.C. prepared figures; A.X., K.S., M.W.M., Q.C., E.J.L. drafted manuscript; C.M.B., Q.C., E.J.L. edited and revised manuscript; A.X., K.S., M.W.M., T.R., E.B., Y.H., B.K., C.M., P.N.D., C.M.B., Q.C., E.J.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciate the excellent technical support of J. Thompson.
REFERENCES
- 1.Alvarez BV, Kieller DM, Quon AL, Markovich D, Casey JR. Slc26a6: a cardiac chloride-hydroxyl exchanger and predominant chloride-bicarbonate exchanger of the mouse heart. J Physiol 561: 721–734, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, Tritto I, Cirillo P, Condorelli M, Chiariello M. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem 268: 18532–18541, 1993 [PubMed] [Google Scholar]
- 3.An J, Varadarajan SG, Camara A, Chen Q, Novalija E, Gross GJ, Stowe DF. Blocking Na+/H+ exchange reduces [Na+]i and [Ca2+]i load after ischemia and improves function in intact hearts. Am J Physiol Heart Circ Physiol 281: H2398–H2409, 2001 [DOI] [PubMed] [Google Scholar]
- 4.An J, Varadarajan SG, Novalija E, Stowe DF. Ischemic and anesthetic preconditioning reduces cytosolic [Ca2+] and improves Ca2+ responses in intact hearts. Am J Physiol Heart Circ Physiol 281: H1508–H1523, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Arrington DD, Van Vleet TR, Schnellmann RG. Calpain 10: a mitochondrial calpain and its role in calcium-induced mitochondrial dysfunction. Am J Physiol Cell Physiol 291: C1159–C1171, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Chance B, Williams GR, Hollunger G. Inhibition of electron and energy transfer in mitochondria. I. Effects of Amytal, thiopental, rotenone, progesterone, and methylene glycol. J Biol Chem 238: 418–431, 1963 [PubMed] [Google Scholar]
- 7.Chen H, Liu LL, Ye LL, McGuckin C, Tamowski S, Scowen P, Tian H, Murray K, Hatton WJ, Duan D. Targeted inactivation of cystic fibrosis transmembrane conductance regulator chloride channel gene prevents ischemic preconditioning in isolated mouse heart. Circulation 110: 700–704, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Chen M, Won DJ, Krajewski S, Gottlieb RA. Calpain and mitochondria in ischemia/reperfusion injury. J Biol Chem 277: 29181–29186, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol 292: C137–C147, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Lesnefsky EJ. Blockade of electron transport during ischemia preserves bcl-2 and inhibits opening of the mitochondrial permeability transition pore. FEBS Lett 585: 921–926, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol 294: C460–C466, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J Pharmacol Exp Ther 319: 1405–1412, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Chen Q, Paillard M, Gomez L, Li H, Hu Y, Lesnefsky EJ. Postconditioning modulates ischemia-damaged mitochondria during reperfusion. J Cardiovasc Pharmacol 59: 101–108, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Chen Q, Paillard M, Gomez L, Ross T, Hu Y, Xu A, Lesnefsky EJ. Activation of mitochondrial mu-calpain increases AIF cleavage in cardiac mitochondria during ischemia-reperfusion. Biochem Biophys Res Commun 415: 533–538, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Q, Ross T, Hu Y, Lesnefsky EJ. Blockade of electron transport at the onset of reperfusion decreases cardiac injury in aged hearts by protecting the inner mitochondrial membrane. J Aging Res 2012: 753949, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: Central role of complex III. J Biol Chem 278: 36027–36031, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Chouchani ET, Methner C, Nadtochiy SM, Logan A, Pell VR, Ding S, James AM, Cocheme HM, Reinhold J, Lilley KS, Partridge L, Fearnley IM, Robinson AJ, Hartley RC, Smith RA, Krieg T, Brookes PS, Murphy MP. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med 19: 753–759, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen MV, Yang XM, Downey JM. The pH hypothesis of postconditioning: staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation 115: 1895–1903, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Ding WX, Shen HM, Ong CN. Calpain activation after mitochondrial permeability transition in microcystin-induced cell death in rat hepatocytes. Biochem Biophys Res Commun 291: 321–331, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Duan D. Phenomics of cardiac chloride channels: the systematic study of chloride channel function in the heart. J Physiol 587: 2163–2177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duan DY, Liu LL, Bozeat N, Huang ZM, Xiang SY, Wang GL, Ye L, Hume JR. Functional role of anion channels in cardiac diseases. Acta Pharmacol Sin 26: 265–278, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Filosa JA, Dean JB, Putnam RW. Role of intracellular and extracellular pH in the chemosensitive response of rat locus coeruleus neurones. J Physiol 541: 493–509, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Genova ML, Ventura B, Giuliano G, Bovina C, Formiggini G, Parenti Castelli G, Lenaz G. The site of production of superoxide radical in mitochondrial Complex I is not a bound ubisemiquinone but presumably iron-sulfur cluster N2. FEBS Lett 505: 364–368., 2001 [DOI] [PubMed] [Google Scholar]
- 24.Gomez L, Paillard M, Price M, Chen Q, Teixeira G, Spiegel S, Lesnefsky EJ. A novel role for mitochondrial sphingosine-1-phosphate produced by sphingosine kinase-2 in PTP-mediated cell survival during cardioprotection. Basic Res Cardiol 106: 1341–1353, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grover GJ, Garlid KD. ATP-sensitive potassium channels: a review of their cardioprotective pharmacology. J Mol Cell Cardiol 32: 677–695, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Halestrap AP, Connern CP, Griffiths EJ, Kerr PM. Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Mol Cell Biochem 174: 167–172, 1997 [PubMed] [Google Scholar]
- 27.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem 278: 5557–5563, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Hausenloy DJ, Yellon DM. The mitochondrial permeability transition pore: its fundamental role in mediating cell death during ischaemia and reperfusion. J Mol Cell Cardiol 35: 339–341, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Hernando V, Inserte J, Sartorio CL, Parra VM, Poncelas-Nozal M, Garcia-Dorado D. Calpain translocation and activation as pharmacological targets during myocardial ischemia/reperfusion. J Mol Cell Cardiol 49: 271–279, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Hoppel CL, Kerr DS, Dahms B, Roessmann U. Deficiency of the reduced nicotinamide adenine dinucleotide dehydrogenase component of complex I of mitochondrial electron transport. Fatal infantile lactic acidosis and hypermetabolism with skeletal-cardiac myopathy and encephalopathy. J Clin Invest 80: 71–77, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inserte J, Barba I, Hernando V, Abellan A, Ruiz-Meana M, Rodriguez-Sinovas A, Garcia-Dorado D. Effect of acidic reperfusion on prolongation of intracellular acidosis and myocardial salvage. Cardiovasc Res 77: 782–790, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Inserte J, Barba I, Hernando V, Garcia-Dorado D. Delayed recovery of intracellular acidosis during reperfusion prevents calpain activation and determines protection in postconditioned myocardium. Cardiovasc Res 81: 116–122, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Inserte J, Barba I, Poncelas-Nozal M, Hernando V, Agullo L, Ruiz-Meana M, Garcia-Dorado D. cGMP/PKG pathway mediates myocardial postconditioning protection in rat hearts by delaying normalization of intracellular acidosis during reperfusion. J Mol Cell Cardiol 50: 903–909, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 38: 474–478, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol 6: 343–350, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krahenbuhl S, Chang M, Brass EP, Hoppel CL. Decreased activities of ubiquinol:ferricytochrome c oxidoreductase (complex III) and ferrocytochrome c:oxygen oxidoreductase (complex IV) in liver mitochondria from rats with hydroxycobalamin[c-lactam]-induced methylmalonic aciduria. J Biol Chem 266: 20998–21003, 1991 [PubMed] [Google Scholar]
- 37.Lesnefsky EJ, Chen Q, Moghaddas S, Hassan MO, Tandler B, Hoppel CL. Blockade of electron transport during Ischemia protects cardiac mitochondria. J Biol Chem 279: 47961–47967, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia-reperfusion, aging, and heart failure. J Mol Cell Cardiol 33: 1065–1089, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Lesnefsky EJ, Slabe TJ, Stoll MS, Minkler PE, Hoppel CL. Myocardial ischemia selectively depletes cardiolipin in rabbit heart subsarcolemmal mitochondria. Am J Physiol Heart Circ Physiol 280: H2770–H2778, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Lesnefsky EJ, Tandler B, Ye J, Slabe TJ, Turkaly J, Hoppel CL. Myocardial ischemia decreases oxidative phosphorylation through cytochrome oxidase in subsarcolemmal mitochondria. Am J Physiol Heart Circ Physiol 273: H1544–H1554, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Li B, Chauvin C, De Paulis D, De Oliveira F, Gharib A, Vial G, Lablanche S, Leverve X, Bernardi P, Ovize M, Fontaine E. Inhibition of complex I regulates the mitochondrial permeability transition through a phosphate-sensitive inhibitory site masked by cyclophilin D. Biochim Biophys Acta 1817: 1628–1634, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflügers Arch 447: 710–721, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev 88: 581–609, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nadtochiy SM, Burwell LS, Brookes PS. Cardioprotection and mitochondrial S-nitrosation: effects of S-nitroso-2-mercaptopropionyl glycine (SNO-MPG) in cardiac ischemia-reperfusion injury. J Mol Cell Cardiol 42: 812–825, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohnishi ST, Ohnishi T, Muranaka S, Fujita H, Kimura H, Uemura K, Yoshida K, Utsumi K. A possible site of superoxide generation in the complex I segment of rat heart mitochondria. J Bioenerg Biomembr 37: 1–15, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Ortiz-Ramirez C, Mora SI, Trejo J, Pantoja O. PvAMT1;1, a highly selective ammonium transporter that functions as H+/NH4(+) symporter. J Biol Chem 286: 31113–31122, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paillard M, Gomez L, Augeul L, Loufouat J, Lesnefsky EJ, Ovize M. Postconditioning inhibits mPTP opening independent of oxidative phosphorylation and membrane potential. J Mol Cell Cardiol 46: 902–909, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Pasdois P, Beauvoit B, Costa AD, Vinassa B, Tariosse L, Bonoron-Adele S, Garlid KD, Dos Santos P. Sarcoplasmic ATP-sensitive potassium channel blocker HMR1098 protects the ischemic heart: implication of calcium, complex I, reactive oxygen species and mitochondrial ATP-sensitive potassium channel. J Mol Cell Cardiol 42: 631–642, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, Andre-Fouet X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med 359: 473–481, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Prime TA, Blaikie FH, Evans C, Nadtochiy SM, James AM, Dahm CC, Vitturi DA, Patel RP, Hiley CR, Abakumova I, Requejo R, Chouchani ET, Hurd TR, Garvey JF, Taylor CT, Brookes PS, Smith RA, Murphy MP. A mitochondria-targeted S-nitrosothiol modulates respiration, nitrosates thiols, and protects against ischemia-reperfusion injury. Proc Natl Acad Sci USA 106: 10764–10769, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rose IA, O'Connell EL. Mechanism of aconitase action. I. The hydrogen transfer reaction. J Biol Chem 242: 1870–1879, 1967 [PubMed] [Google Scholar]
- 52.Scaduto RC, Jr, Grotyohann LW. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J 76: 469–477, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schild L, Reiser G. Oxidative stress is involved in the permeabilization of the inner membrane of brain mitochondria exposed to hypoxia/reoxygenation and low micromolar Ca2+. FEBS J 272: 3593–3601, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med 204: 2089–2102, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277: 44784–44790, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Steel R, Torrie J. Principles and Procedures of Statistics. New York: McGraw-Hill, 1960 [Google Scholar]
- 57.Stewart S, Lesnefsky EJ, Chen Q. Reversible blockade of electron transport with amobarbital at the onset of reperfusion attenuates cardiac injury. Transl Res 153: 224–231, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Stowe DF, Aldakkak M, Camara AK, Riess ML, Heinen A, Varadarajan SG, Jiang MT. Cardiac mitochondrial preconditioning by Big Ca2+-sensitive K+ channel opening requires superoxide radical generation. Am J Physiol Heart Circ Physiol 290: H434–H440, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Strub GM, Paillard M, Liang J, Gomez L, Allegood JC, Hait NC, Maceyka M, Price MM, Chen Q, Simpson DC, Kordula T, Milstien S, Lesnefsky EJ, Spiegel S. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J 25: 600–612, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Szczepanek K, Chen Q, Derecka M, Salloum FN, Zhang Q, Szelag M, Cichy J, Kukreja RC, Dulak J, Lesnefsky EJ, Larner AC. Mitochondrial-targeted Signal transducer and activator of transcription 3 (STAT3) protects against ischemia-induced changes in the electron transport chain and the generation of reactive oxygen species. J Biol Chem 286: 29610–29620, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teixeira G, Abrial M, Portier K, Chiari P, Couture-Lepetit E, Tourneur Y, Ovize M, Gharib A. Synergistic protective effect of cyclosporin A and rotenone against hypoxia-reoxygenation in cardiomyocytes. J Mol Cell Cardiol 56: 55–62, 2013 [DOI] [PubMed] [Google Scholar]
- 62.Titz S, Hormuzdi S, Lewen A, Monyer H, Misgeld U. Intracellular acidification in neurons induced by ammonium depends on KCC2 function. Eur J Neurosci 23: 454–464, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Treberg JR, Quinlan CL, Brand MD. Evidence for two sites of superoxide production by mitochondrial NADH-ubiquinone oxidoreductase (complex I). J Biol Chem 286: 27103–27110, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol 552: 335–344, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res 93: 292–301, 2003 [DOI] [PubMed] [Google Scholar]
- 66.Xu A, Szczepanek K, Hu Y, Lesnefsky EJ, Chen Q. Cardioprotection by modulation of mitochondrial respiration during ischemia-reperfusion: role of apoptosis-inducing factor. Biochem Biophys Res Commun 435: 627–633, 2013 [DOI] [PubMed] [Google Scholar]
- 67.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 285: H579–H588, 2003 [DOI] [PubMed] [Google Scholar]