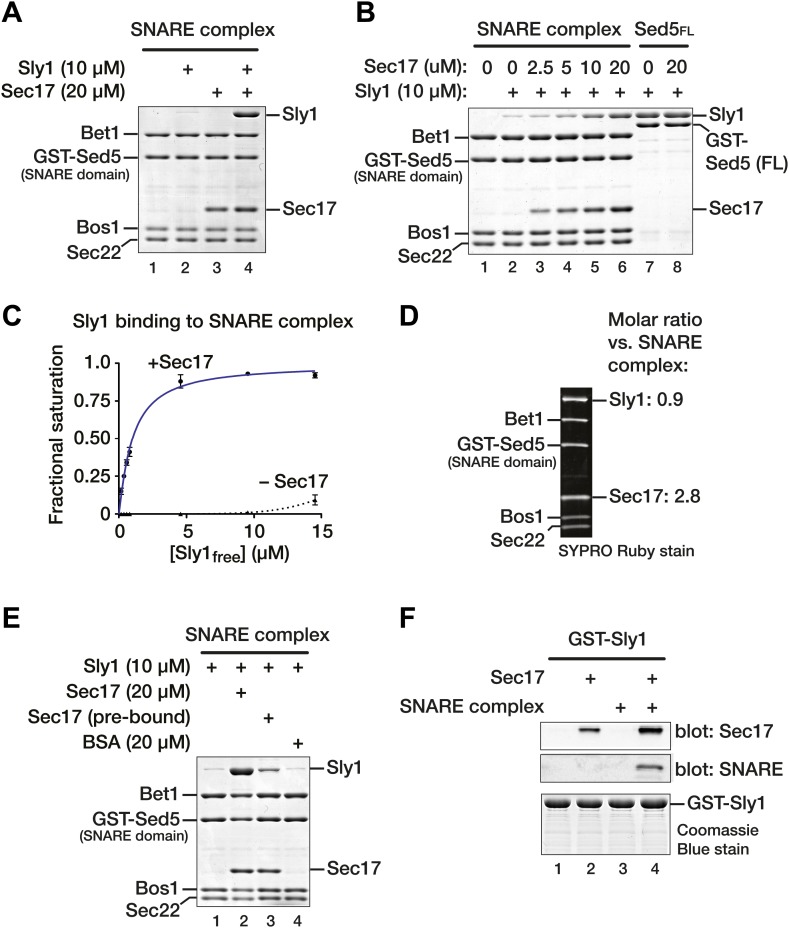
Figure 6. Sec17 promotes Sly1 binding to Golgi SNARE complex.
(A) Golgi SNARE complex (500 nM) was assembled on immobilized Sed5 SNARE domain (GST-Sed5SNARE domain) lacking the N-peptide and Habc segments. SNARE complexes were incubated with Sec17 (20 μM), Sly1 (10 μM), or both for 60 min at 30°C. Unbound proteins were washed out, and bound proteins were separated by SDS-PAGE and stained with Coomassie blue. (B) The dose–response for Sec17 stimulation of Sly1 binding to SNARE complexes was assayed as in A, but Sec17 concentration was varied (2.5–20 μM) while Sly1 was held constant (10 μM). In lanes 7 and 8, the full cytoplasmic domain of Sed5 (Sed5FL, including the Habc and N-peptide segments; 500 nM) was immobilized to test for direct binding of Sec17 to Sed5 and Sly1 in the absence of assembled SNARE complex. (C) Sly1 binding to SNARE complex with or without Sec17 was assayed as in A and B, except that Sly1 concentration was varied and protein bands were stained and quantified using SYPRO Ruby. The fractional saturation of total Sly1-SNARE complex binding was plotted vs free (total minus bound) Sly1. Fits of a one-site binding model yielded with an apparent Kdobs = 1.0 ± 0.1 µM for Sly1 binding to the SNARE complex in the presence of Sec17. It was not possible to fit the no-Sec17 condition. Two-site or cooperative binding models did not substantially improve the fits. (D) Stoichiometry of SNARE-Sec17-Sly1 complexes assembled under saturation conditions was estimated using standard curves of purified proteins of known concentrations. (E) Sly1 binding is stimulated by SNARE-associated Sec17. Binding was assayed in lanes 1 and 2 as in A. In lane 3, Sec17 was pre-bound to SNARE complexes for 60 min at 30°C. Unbound Sec17 was then washed out and Sly1 (10 μM) was added for an additional 60 min at 30°C. In lane, 4 BSA (20 µM) was substituted for Sec17. (F) Cooperativity of assembly. GST-Sly1 (500 nM) was immobilized and incubated with 20 μM Sec17, SNARE complex, or both. Bound material was separated by SDS-PAGE and stained with Coomassie blue or analyzed by immunoblot.