Abstract
Postnatal lung development requires coordination of three processes (surface area expansion, microvascular growth, and matrix remodeling). Because normal elastin structure is important for lung morphogenesis, because physiological remodeling of lung elastin has never been defined, and because elastin remodeling is angiogenic, we sought to test the hypothesis that, during lung development, elastin is remodeled in a defined temporal-spatial pattern, that a novel protease is associated with this remodeling, and that angiogenesis is associated with elastin remodeling. By elastin in situ zymography, lung elastin remodeling increased 24-fold between embryonic day (E) 15.5 and postnatal day (PND) 14. Remodeling was restricted to major vessels and airways on PND1 with a sevenfold increase in alveolar wall elastin remodeling from PND1 to PND14. By inhibition assays and literature review, we identified chymotrypsin-like elastase 1 (CELA1) as a potential mediator of elastin remodeling. CELA1 mRNA levels increased 12-fold from E15.5 to PND9, and protein levels increased 3.4-fold from E18.5 to PND9. By costaining experiments, the temporal-spatial pattern of CELA1 expression matched that of elastin remodeling, and 58–85% of CELA1+ cells were <10 μm from an elastase signal. An association between elastin remodeling and angiogenesis was tested by similar methods. At PND7 and PND14, 60–95% of angiogenin+ cells were associated with elastin remodeling. Both elastase inhibition and CELA1 silencing impaired angiogenesis in vitro. Our data defines the temporal-spatial pattern of elastin remodeling during lung development, demonstrates an association of this remodeling with CELA1, and supports a role for elastin remodeling in regulating angiogenesis.
Keywords: matrix remodeling, elastin, pulmonary vascular development
three coordinated processes occur during the saccular and alveolar stages of lung development that are critical to meeting the metabolic demands of ex utero life. Airway branching and septation dramatically increase gas exchange surface area, expansion of the pulmonary microvasculature increases perfusion capacity to match this increased surface area, and matrix remodeling and epithelial thinning increase gas diffusion capacity. Multiple studies have demonstrated that these three processes are intertwined. Microvascular development is necessary for alveolar septation (21), and models of impaired alveolar septation have marked pruning of the pulmonary vasculature (24). Reduced matrix metalloproteinase (MMP)-2 and MMP14 activity impairs alveolar septation (4, 30), and pulmonary epithelial cells (30), vascular cells (37), and macrophages (26) all remodel lung matrix during saccular and alveolar lung development. Because elastin plays a critical mechano-developmental role in the lung (1), and remodeling of elastin is angiogenic (31–33), we asked whether elastin remodeling might provide a mechanistic link between matrix remodeling, alveolar growth, and microvascular expansion.
Elastin is a uniquely deformable multimeric protein with highly evolutionarily conserved domains among all chordates (13), and its elastic properties are indispensable for normal lung function. Thick elastin bands colocalize with collagen-I at the developing alveolar septal tip (29), and thinner, higher-compliance elastin fibers are located throughout alveolar walls and bases permitting elastic recoil during passive exhalation (41). Elastin fibers in pulmonary arterial walls act as a windkessel providing continuous pulmonary arterial blood flow during diastole (23). In addition to its role in alveolar septation (36, 40), elastin may also play a role in pulmonary microvascular development by one of three mechanisms. First, elastin degradation products containing the valine-glycine-valine-alanine-glycine-proline motif may directly stimulate angiogenesis as they do in cancers of some elastin-rich tissues (31, 33). Second, elastin remodeling may release elastin-bound vascular endothelial growth factor as occurs in proximal airways (32). Third, elastin fibers may serve as a scaffold for microvascular growth as they do in the mesentery during repair following direct injury (5). None of these three mechanisms of elastin-mediated angiogenesis have been investigated in the lung.
While a physiological level of lung elastin remodeling appears necessary for normal lung development, excessive lung elastin remodeling, particularly by nonphysiological proteases, impairs normal development. In humans, prematurely born infants with higher levels of elastin turnover after the first postnatal week (as assessed by urinary desmosine) are less likely to go on to develop bronchopulmonary dysplasia (11); however, the presence of neutrophil elastase in endotracheal aspirates of prematurely born infants is associated with the development of bronchopulmonary dysplasia (10). In a mouse pup mechanical ventilation model, inhibition of neutrophil elastase improves alveolar septation (18). While investigating the effect of glucocorticoid-receptor signaling on lung cell proliferation during embryonic lung development, Bird et al. found a 25-fold reduction in mRNA levels of the pancreatic serine protease chymotrypsin-like elastase 1 (CELA1) (7). Published microarray data of normally developing mouse lung demonstrated increased CELA1 mRNA starting at embryonic day 14 (22). Although it is a pancreatic enzyme, protein-level expression of CELA1 has been demonstrated in the basal skin layer (39), but never in the lung. Whether CELA1 may have a physiological role in lung development is unknown.
Given the importance of elastin in lung development, the fact that elastin remodeling regulates angiogenesis, and data supporting developmental regulation of CELA1 expression, we hypothesized that lung elastin remodeling changes in a developmentally regulated manner, that elastin remodeling is associated with both angiogenesis and CELA1, and that both elastase activity and CELA1 regulate in vitro angiogenesis.
MATERIALS AND METHODS
Mouse model of lung development.
C57BL/6J mice from Jackson Laboratories were used at gestational ages 15.5 days [embryonic day (E) 15.5], E18.5, postnatal day (PND) 0, PND1, 3, 5, 9, 14, and 8 wk. For mesenchymal cell lineage-tracing experiments, PND9 pups or PN 8 wk males from B6.Twist2tm1.1(cre)Dor/J (Dermo1-cre) crossed to B6.ROSA26Sortm4(ACTB−tdTomato,−EGFP)Luo/J (Tomato) matings were used. Animals were housed in a barrier facility with purified air and provided purified water and autoclaved food ad libitum. Animal use was approved by the Cincinnati Children's Hospital Medical Center Animal Use and Care Committee.
Posttranslational CELA1 silencing using vivo-morpholino.
Beginning at PND14, C57BL/6J mice were intraperitoneally injected with 12.5 μg/g body wt of CELA1 vivo-morpholino (Gene Tools, Philomath, OR). Lungs were collected after 1 and 2 wk of administration.
Tissue procurement and processing.
Mice were killed by intraperitoneal injection of ketamine, xylazine, and acepromazine (100, 6, and 2 mg/kg, respectively) and severing of the left renal artery. After exsanguination, the trachea was cannulated, and lungs were isovolumetrically inflated with 4% paraformaldehyde in PBS at a pressure of 25 cmH2O. Lung inflation was maintained by securing a silk ligature around the trachea, and the lungs were then removed and fixed overnight at 4°C. After fixation, the lung lobes were removed from the bronchi and dehydrated by serial passage into 70% ethanol and paraffinized. Five-micrometer sections were obtained at random angles through all five lobes.
Generation and validation of guinea pig anti-CELA1 antibody.
Guinea pig use was approved by the animal use and care committee. A polyclonal antibody was generated as previously described (25) using the peptide sequence GEHNLSQNDGTEQYVNVQKIVSHPY, which was purchased from Genscript. For validation studies, CELA1 antibody was incubated with 500 μg/ml of the peptide for 1 h before use.
Immunohistochemistry.
Paraffin-embedded sections were passaged back to water, blocked with donkey serum, and immunostained with a generated guinea pig CELA1 antibody at 1:1,000 dilution and the ABC Vectastain kit (Vector Labs). Images were obtained using a Zeiss Axio ImagerA.2.
Elastin in situ zymography.
The tracheas of PND1, PND3, PND7, PND 14, and PN 8 wk mice were cannulated, and the lungs were hand-inflated with a 50:50 mixture of 50% sucrose solution and OCT compound. The tracheas were secured, and the lungs were snap-frozen in liquid nitrogen. The lungs were then embedded in OCT and again snap-frozen. Ten-micrometer sections were placed on glass slides, lightly fixed in 2% PFA in PBS for 5 min, and incubated overnight in MEM with 10% fetal bovine serum and 10 μg/ml of DQ-elastin (Invitrogen), which emits a green fluorescent signal when cleaved. The following morning, slides were stained with DAPI, rinsed in PBS, and mounted in Prolong Gold. Confocal images were obtained with a Nikon A1Rsi microscope. CELA1 (1:2,000), angiogenin (1:1,000; Abcam), and tropoelastin (1:1,000; Abcam) immunostaining were performed on elastin in situ zymography specimens. Serine proteases were inhibited by incubation with 5 μM 3,4-dichloisocoumarin, MMPs were inhibited with 100 μM GM-6001 and 10 mM EDTA, and cysteine proteases were inhibited with 1 μg/ml E-64. Control slides were incubated in MEM with 10% FBS without DQ-elastin.
Quantitation of elastin in situ zymography signal.
For all quantitative in situ zymography experiments, specimens were cut and processed identically on the same day. The following day, 3 × 3 tiled 10-μm z-stacks were obtained using identical laser and microscope settings for all sections. Images were quantitated using Imaris (Bitplane, South Windsor, CT). For elastase surfaces, a threshold intensity of 500 was applied to all images, and total elastase signal for each image was calculated. Each time point or experiment was assayed in triplicate from three different mice from at least two different litters.
To localize elastase signal to major airways and major vessels, elastin tips, or nontip elastin, three masks were applied to elastin in situ zymography sections costained for tropoelastin. A major airway and vessel mask was hand drawn for each image. A tropoelastin surface was created with a threshold intensity of 350. The elastin tip mask was defined as elastin particles with sphericity of 0.8 or greater and the nontip mask as particles with sphericity <0.8.
To localize elastase signal to major vessels, major airways, or distal lung parenchyma, zymography sections were costained for Clara cell-specific protein (CCSP, 1:1,000; Seven Hills Bioreagents, Cincinnati, OH) and tropoelastin. Masks were hand drawn for major airways (tubular structures with multiple CCSP+ cells) and major vessels (CCSP-negative tubular structures). Total lung elastase signal was subtracted from elastase signal within each of these masks to calculate distal lung parenchymal signal. Elastase signal was normalized to tropoelastin signal.
Immunofluorescent staining and confocal image analysis.
To localize CELA1+ cells with cell markers, PND7 and PND14 frozen lung sections were stained for CELA1, and angiogenin, surfactant protein B (1:1,000; Seven Hills Bioreagents), CD31 (1:1,000; Abcam), desmin (1:1,000; Abcam), platelet-derived growth factor receptor-β (PDGFRβ, 1:200; R&D), adipocyte differentiation-related peptide (ADRP, 1:200; Santa Cruz), and α-smooth muscle actin (α-SMA, 1:1,000; Sigma). CELA1 cells were defined as colocalized if they were within 5 μm of the corresponding spot or surface.
Flow cytometry.
Single cell suspensions of PN 8 wk Dermo1-cre/Tomato mouse lungs were sorted for green fluorescent protein (GFP) expression using a FACSAria II cytometer (BD biosciences).
Elastase assay.
Lungs were snap-frozen and homogenized in RIPA buffer without protease inhibitors using a TissueLyser II (Qiagen). Protein was quantitated, and 10 μg of protein were diluted to 75 μl with RIPA buffer and assayed in duplicate using the Enzcheck Elastase assay per the manufacturer's protocol (Invitrogen). At 0 and 60 min of incubation at 37°C, emission at 515 nm with excitation at 485 nm was determined using a BioTek SynergyHT II plate reader. Change in emission was used for determination of lung protease activity. For protease inhibitor assays, GM-6001 (100 μM) with EDTA (10 mM), E-64 (1 μg/ml), 3,4-dichloroisocoumarin (5 μM), N-methoxysuccinyl-Ala-Ala-Pro-Val-chloromethyl ketone (1 μg/ml; Invitrogen), or DMSO were added to lung homogenates and analyzed as above.
Desmosine ELISA.
Desmosine ELISA was performed on these same lung homogenates per the manufacturer's protocol (Antibodies-online).
mRNA quantitation by PCR.
Lung or cell RNA was isolated using the RNEasy kit (Qiagen), cDNA was synthesized using the high-capacity RNA to cDNA kit (Applied Biosystems), and CELA1, CELA2B, and CELA3A mRNA was quantitated using a proprietary Taqman primer and the Rodent GAPDH control kit (Applied Biosystems).
Western blot.
Lung homogenate samples in RIPA buffer with 1:1,000 protease inhibitor cocktail (Sigma) were electrophoretically separated and transferred to nitrocellulose. Western blot for β-actin (1:1,000 dilution; Santa Cruz) and CELA1 (1:1,000) was performed using fluorescent-conjugated secondary antibodies and an Odyssey CLx Imaging System (Licor). Band density was quantitated using Image Studio. Nonspecific binding of secondary antibody was excluded by using anti-guinea pig secondary alone.
Cell culture.
A mouse fetal mesenchymal cell line from E14.5 mice (MFLM4) was grown and differentiated using reagents as originally described (3). All MFLM4 experiments were performed at cell passages 17–20, and endothelial identity was confirmed by Western blot for CD31 (1:1,000; Abcam). A fetal lung fibroblast cell line obtained from E18.5 rats (RFL6, ATCC CCL-192) was used for CELA1 transfection studies at passage 12.
Matrigel-based tubulogenesis assay.
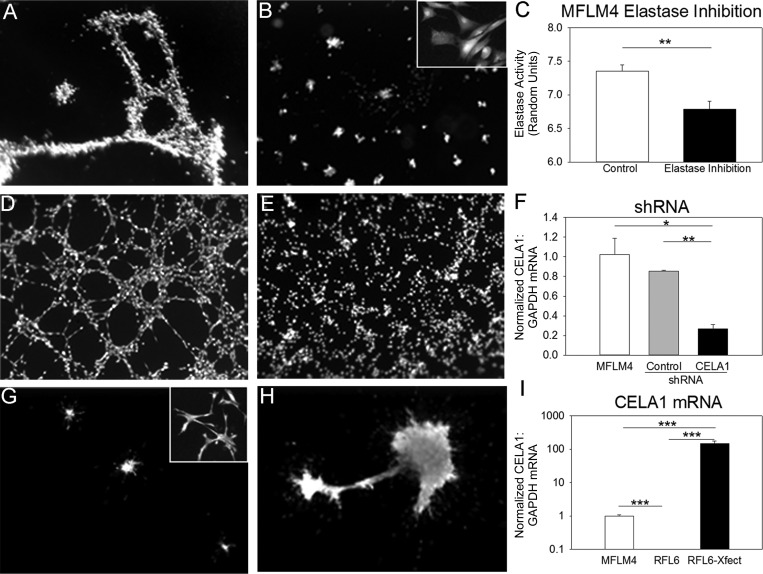
MFLM4 cells were differentiated and used for tubulogenesis assays as previously described (3) using 200 μl of Matrigel in 48-well plates with 50,000 differentiated MFLM4 cells in 200 μl of differentiation media. Cells were incubated at 37°C with 5% CO2 and 100% humidity overnight. At 18 h, media was removed, and the vital dye calcein AM 0.1 μM diluted in HBSS was added and incubated for 1 h at 37°C. Fluorescence microscopy of entire wells was performed using a dissecting microscope with a mercury lamp and FITC cube. Tubulogenesis was quantitated by counting the number of tubules and nodes in each well. Higher-magnification fluorescence images were obtained using an Olympus IX70 microscope.
In vitro CELA1 silencing, elastase inhibition, and CELA1 expression.
CELA1 mRNA was silenced in MFLM4 cells using short-hairpin RNA (shRNA). Ten micrograms of CELA1 shRNA plasmid or control plasmid (Origene) were transfected into a T75 flask of MFML4 cells using 40 μl of Fugene 6 (Roche) per the manufacturer's protocol. Cells were incubated overnight with shRNA in Optimem media with 10% FBS. The following morning, cells were differentiated for 48 h as above, and a tubulogenesis assay was performed. Elastase inhibition was performed by adding N-methoxysuccinyl-Ala-Ala-Pro-Val-chloromethyl ketone to media at a concentration of 1 μg/ml. An equivalent volume of DMSO was used as a control.
CELA1 overexpression was achieved by transfecting a murine CELA1 expression plasmid (Origene) into RFL6 cells. CELA1 mRNA and protein were confirmed by PCR and immunofluorescence. Tubulogenesis assays were performed as above but without the differentiation step.
Statistical analysis.
Statistical comparisons between groups were performed using a two-tailed Student's t-test (two groups) or one-way ANOVA (>2 groups). For ANOVA, pairwise comparisons were performed using the Holm-Sidak test. P values of <0.05 were considered significant. All error bars depict SEs.
RESULTS
Temporal-spatial changes in elastin remodeling during saccular and alveolar lung development.
Since human (10, 11) and animal (8, 9) data suggested a physiological role for elastin remodeling in lung development, we sought to quantitate and localize elastin remodeling during pseudoglandular, saccular, and alveolar stages. To do so, we used elastin in situ zymography, desmosine ELISA, and a fluorometric elastase assay to quantify and localize lung elastin remodeling. By elastin in situ zymography, lung elastase activity increased throughout lung development, increasing 24-fold from E15.5 to PND14. The elastin in situ zymography signal decreased 33-fold between PND14 and PN 8 wk (Fig. 1A). Desmosine is a biomarker for elastin degradation (19). Whole lung desmosine was increased 1.8- to 2.8-fold from PND1 to PND14 homogenates compared with E15.5 (P < 0.05 by 1-way ANOVA). By fluorometric elastase assay, lung elastase activity was increased 1.5- to 2-fold (not statistically significant). By way of comparison, the fluorometric elastase signal of PN 8 wk pancreas homogenate was >600-fold higher than the elastase signal of PND14 lung homogenate. These data demonstrate that lung elastase activity is increased during the saccular and alveolar stages of lung development compared with the pseudoglandular stage and the adult lung.
Fig. 1.
Elastin remodeling during lung development. A: quantitation of total elastin in situ zymography signal demonstrated increasing lung elastase activity starting on postnatal day (PND) 1 and increasing activity at PND7 and PND14 compared with earlier time points. Adult mouse lung had very little elastin remodeling. B: elastin remodeling localized to major airways and vessels (white bars) on PND1. Nonseptal tip elastin remodeling (gray bars) increased during late saccular and alveolar development (PND3-PND14), with relatively consistent septal tip elastin remodeling (black bars). For clarity, only statistical comparisons between nonseptal tip elastin are noted. C: normalizing elastase signal to tropoelastin signal demonstrated that increased nonseptal tip elastin remodeling is not due to increased tropoelastin content. D: in E15.5 lung sections, tropoelastin (red) was localized to the mesenchyme underneath the airway epithelium, and the small amount of elastase activity (green) present was located within the epithelial layer. DAPI staining is blue. Scale bar = 150 μm. E: in PND1, lung section elastin remodeling was most pronounced in major vessels (white arrows), with relatively less activity in major airways, although major airways accounted for a larger percentage of total lung volume. F: PND3 lung sections demonstrated continued arterial elastin remodeling and increased remodeling of airspace walls. G: at PND7, early in the alveolar stage of lung development, elastin remodeling in arteries and major airways was relatively diminished with increased elastase activity in airspace walls (*) and appreciable activity in septal tips (white arrow). H: this remodeling of alveolar walls was accentuated in PND14 lung. I: in the adult lung (PN 8 wk), little elastin remodeling was detected. All presented images were processed identically and obtained on the same day with the same microscope and laser settings. J: 3X3 stitched X20 confocal z-stacks stained as above were used to quantitate and localize elastase activity. Images from three mice from different litters provided the quantitative data used for analysis. Scale bar = 500 μm. K: masks were created to define elastase signal (green) with manual masks defining major airways and vessels (mustard-colored shapes on right half of image). L: the tropoelastin signal (red) was masked based on intensity and sphericity to define tropoelastin structures as elastin tips (magenta) or nontips (mustard color). * P < 0.05, **P < 0.01, and ***P < 0.001 by one-way ANOVA.
To define the location of elastase activity during the saccular and alveolar stages of lung development, we immunostained elastin in situ zymography sections for tropoelastin and localized elastase activity. In the PND1 lung, the zymography signal was equally distributed between nonseptal tip elastin and major airway and vascular structures. In PND3, PND7, and PND14 lung sections, elastase activity was predominantly located within nonseptal tip elastin. Remodeling of nonseptal tip elastin increased sevenfold between PND1 and PND14 (Fig. 1B). The increased elastase signal persisted after normalization to elastin signal (Fig. 1C). Structural differences between uninflated embryonic lung and inflated postnatal lung prevented quantitative comparison of E15.5 lungs with postnatal lungs. Representative images of each time point analyzed demonstrate that, in the E15.5 lung, scant elastase signal localized to the mesenchyme underlying the airway epithelium (Fig. 1D). Elastase activity was largely restricted to major airways and vessels in PND1 lung (Fig. 1E), with progressively increasing elastase activity associated with nonseptal tip elastin from PND3 to PND14 (Fig. 1, F–H). Adult lung had very little elastase activity (Fig. 1I). These quantitative data demonstrate that lung elastin remodeling increases, postnatally peaking late in the alveolar stage of lung development. Elastin remodeling is associated with major vessels and airways during early saccular development, with increasing levels of remodeling in airspace walls during late saccular and alveolar stages of lung development.
Identification of CELA1 as a potential mediator of elastin remodeling during lung development.
To identify the protease responsible for elastin remodeling, we first performed elastin in situ zymography and fluorometric elastase assays using protease inhibitors to identify protease class. Negative in situ zymography controls demonstrated that the elastase signal was not due to elastin autofluorescence. Serine protease inhibition reduced the in situ zymography signal by 57%, whereas neither MMP nor cysteine protease inhibition altered elastase activity (Fig. 2A). Serine protease inhibition reduced PND14 lung homogenate elastase signal by 40% in a fluorometric elastase assay (not significant). To identify the specific serine protease responsible for this elastase activity, we queried published array data in normal (22) and impaired (7, 12) lung development. In normal lung array data, the serine protease CELA1 increased threefold between E12 and PND1, and gene expression remained at that level through adulthood (22). In glucocorticoid receptor-null mouse lung array data, at E18.5, expression of CELA1 was reduced 9.4-fold compared with wild type, and PCR confirmation demonstrated a 25-fold decrease in CELA1 mRNA (7). Microarray data demonstrated no differences in CELA1 expression in the hyperoxia model of impaired lung development (12). These data demonstrate that serine proteases are responsible for elastin remodeling during the saccular and alveolar stages of lung development and identify CELA1 as a candidate protease.
Fig. 2.
Chymotrypsin-like elastase 1 (CELA1) in lung development. A: quantitation of elastin in situ zymography signal of PND14 lung sections assayed in triplicate demonstrated reduced signal upon incubation with 3,4-dichloroisocoumarin (Dichloro) when compared with control (Zymo). Neither the matrix metalloproteinase inhibitor GM-6001 nor the cysteine protease inhibitor E-64 altered elastase signal. Sections incubated in media but without substrate (Neg) had no elastase signal. B: lung CELA1 mRNA increased throughout the saccular and early alveolar stages of lung development and decreased in the late alveolar stage of development. Lung samples were obtained from three mice from different litters. For clarity purposes, only one-way ANOVA comparisons to E15.5 are noted. C: CELA1 protein concentrations increased throughout postnatal lung development. D: Western blot densitometric analysis of lung homogenates from three mice from at least two different litters demonstrated significant increases in CELA1 lung protein concentrations with increasing age. E: in CELA1 and Clara cell-specific protein (CCSP) costained 3X3 X20 tiled z-stacks, CELA1+ cells were located principally in major airways (gray bars) and vessels (black bars) at PND1. At subsequent postnatal time points, CELA1+ cells were located predominantly in the distal lung (white bars). Inset numbers denote mean number of CELA1+ cells/image. One-way ANOVA statistical comparisons were made between identical compartments at different time points. For clarity, notations of differences between compartments at the same time period are omitted. F: the majority of CELA1+ cells were within 10 μm of an elastase signal at all developmental time points assessed. G: a X20 elastin in situ zymography image of PND14 lung demonstrated elastase signal (green) localizing with CELA1+ cells (red) in airspace walls. DAPI is blue. Scale bar = 150 μm. H: the same image with masking demonstrates how quantitative analysis was performed. The elastase mask is green with CELA1+ cells within 10 μm of this signal being red and CELA1+ cells further than 10 μm being aqua. I: to account for differences in CELA1 mRNA and protein levels, CELA1+ cells were masked by sphericity <0.7 or ≥0.7. A higher percentage of CELA1+ cells was spherical PN 8 wk lung compared with earlier time points. Examples of nonspherical and spherical CELA1+ cells from PND14 lung are demonstrated. Scale bar = 10 μm. J: vivo-morpholino targeted against CELA1 achieved a reduction of lung CELA1 protein levels after both 1 and 2 wk of administration compared with control and vehicle (Veh). Protein levels were quantitated by Western blot. Each bar represents one mouse. K: Western blot of lung homogenate after CELA1 morpholino administration. L: 2 wk of CELA1 vivo-morpholino administration (Morph) inhibited lung elastase activity half as much as incubation of control lung homogenate with 3,4-disochlorocoumarin (Dichloro). On this chart only, SE bars for control and Dichloro represent lung homogenates from three mice, but the error bar for Morph represents six replicates of the single morpholino-treated mouse lung homogenate. No statistical comparisons were made. *P < 0.05, **P < 0.01, and ***P < 0.001 by one-way ANOVA.
Temporal-spatial changes in CELA1 expression correlate with changes in elastin remodeling.
To define the temporal pattern of CELA1 expression during lung development, we quantitated lung mRNA and protein levels at sequential stages of development. Compared with the canalicular stage of lung development, CELA1 mRNA levels were 12-fold higher during the saccular and early alveolar stages of lung development, and mRNA levels decreased at PND14 and PN 8 wk (Fig. 2B). Lung CELA1 protein levels were 12-fold higher at PND9 compared with E18.5 and 3.4-fold higher at PN 8 wk compared with PND9 (Fig. 2, C and D). We detected neither CELA2A nor CELA3B mRNA in PND9 or PN 8 wk lung (data not shown). These data confirm the expression of CELA1 protein in the lung and demonstrate higher levels of expression during the saccular and alveolar stages of lung development. CELA1 mRNA and protein levels were discrepant in the adult lung.
To define the location of CELA1+ cells and to demonstrate association with areas of elastin remodeling, we performed immunofluorescent costaining of elastin in situ zymography sections. In CELA1 and CCSP costained sections, the majority of CELA1+ cells was associated with major vessels and major airways on PND1, but located in the distal lung at subsequent time points (Fig. 2E). To demonstrate an association between CELA1 and lung elastase activity, the fraction of CELA1+ cells <10 μm from an elastase surface was calculated. CELA1+ cells (58–85%) were associated with elastase activity from PND1 to PND14, and 33% of CELA1+ cells were associated with elastase activity at PN 8 wk (Fig. 2, F–H). These data demonstrate that CELA1+ cells localize to elastin remodeling in major airways, major vessels, and distal lung parenchyma during the saccular and alveolar stages of lung development and that the temporal-spatial pattern of CELA1 expression mirrors that of elastin remodeling.
To rationalize the discrepancy between CELA1 mRNA levels and CELA1 protein levels, we applied a sphericity mask to CELA1+ cells as a rough surrogate for quiescent (spherical) vs. activated or infiltrative (nonspherical) cells. We found that the majority of CELA1 particles had a sphericity coefficient of <0.7 before PND14, but that fraction of cells with a high sphericity coefficient increased at both PND14 and PN 8 wk (Fig. 2I). These data suggest that CELA1+ cells are more activated or infiltrative during saccular and early alveolar stages of development and more quiescent during later stages, thus accounting for the discrepancy between mRNA and protein levels at these later time points.
To demonstrate the contribution of CELA1 to lung elastase activity, CELA1 protein was posttranslationally knocked down using CELA1 “vivo-morpholino,” and lung elastase activity was quantitated. Starting at PND14, a 53% knockdown of lung CELA1 protein was achieved after 1 wk of treatment and a 72% knockdown after 2 wk (Fig. 2, J and K). Mouse lung treated for 2 wk with CELA1 morpholino had 53% as much elastase inhibition as control lung homogenate incubated with 3,4-dichloroisocoumarin (Fig. 2L). Collection of lung for protein and elastase assay precluded histological or in situ zymography analysis. These data support the assertion that CELA1 is a major contributor to physiological elastin remodeling during lung development.
CELA1 is expressed by a unique subset of lung fibroblasts or fibroblast-like cells.
We performed immunohistochemistry, immunofluorescent colocalization, and flow cytometry experiments to determine the cellular source of CELA1 expression. Consistent with previous quantitative data, by immunohistochemistry, CELA1+ cells were located predominantly around major airways and vessels on PND1 (Fig. 3A) and in the distal lung parenchyma on subsequent days (Fig. 3, B and C). To verify that CELA1+ cells were mesenchymal in origin, PND9 Dermo1-cre/Tomato heterozygote lungs were immunostained for CELA1. CELA1+ cells colocalized with eGFP, denoting a mesenchymal origin (Fig. 3D). By flow cytometry of Dermo1-cre/Tomato lungs, only eGFP-expressing cells contained CELA1 mRNA (Fig. 3E). By immunofluorescent colocalization, 71% of CELA1+ cells were strongly desmin positive (Fig. 3F). CELA1 did not localize with the alveolar type II cell marker surfactant protein B nor with the endothelial marker CD31. CELA1 did not localize with the pericyte marker NG2, with the vascular-associated fibroblast marker PDGFRβ, with the myofibroblast marker α-SMA, or with the lipofibroblast marker ADRP (data not shown). Thus, while CELA1 was expressed in fibroblasts or fibroblast-like cells, it was not expressed in pericytes, perivascular fibroblasts, myofibroblasts, or lipofibroblasts.
Fig. 3.
CELA1 is expressed in lung fibroblasts or fibroblast-like cells. A: immunohistochemistry for CELA1 demonstrated CELA1+ cells near the pulmonary artery (art) of PND1 mice. Scale bar = 50 μm. B: in PND5 lung, CELA1+ cells were predominantly located in alveolar walls and bases. Scale bar = 20 μm. C: CELA1+ cells remained in the adventitia of the adult lung. Scale bar = 50 μm. D: Dermo1-cre/Tomato heterozygous mice express green fluorescent protein (GFP) in mesenchymal cells and red fluorescent protein in nonmesenchymal cells. PND9 lung sections costained for CELA1 (purple) demonstrated expression only in green mesenchymal cells (white arrows). Scale bar = 20 μm. E: by flow sorting of PN 8 wk Dermo1-cre/Tomato lung cell suspension, CELA1 mRNA was present only in GFP+ cells. Cells were from one mouse. F: in PN 8 wk frozen sections, CELA1 (green) localized to a subset of cells expressing the fibroblast marker desmin (red). Red arrows identify cells only positive for desmin, and yellow arrows identify cells strongly positive for desmin and CELA1. Some CELA1+ cells (green arrows) stained only weakly for desmin; 71% of CELA1+ cells were strongly positive for desmin. Scale bar = 50 μm.
Proliferating endothelial cells localize to areas of elastin remodeling during alveolar lung development.
Elastin remodeling may be angiogenic by one of several mechanisms (20, 32, 33). To define whether lung elastase activity was associated with angiogenesis, we stained in situ zymography sections for angiogenin. Angiogenin is a cystolic protein upstream of several angiogenic processes (2) and important for angiogenesis in many organs (38), including the lung (42). During the saccular stage of lung development, ∼20% of angiogenin+ cells were within 10 μm of an elastase signal; however, during the alveolar stage of lung development, 60–95% of angiogenin+ cells were associated with elastin remodeling. In the adult lung, the fewer number of angiogenin+ cells was only rarely associated with elastin remodeling (Fig. 4, A and B). To determine whether CELA1 was expressed in angiogenin-expressing cells, we quantified colocalization in PND7 and PND14 sections. CELA1 and angiogenin did not colocalize, with only 7 and 12% of CELA1+ cells being within 10 μm of angiogenin+ cells at PND7 and PND14, respectively. These data demonstrate an association between lung elastin remodeling and angiogenesis and that CELA1 is only rarely expressed adjacent to proliferating endothelial cells.
Fig. 4.

Elastin remodeling is associated with proliferating endothelial cells. A: >70% of angiogenin+ cells were located within 10 μm of an elastase signal during the alveolar stage of lung development (PND7 and PND14). The average number of angiogenin+ cells (inset numbers) was highest in PND14 lung. For analysis, 3X3 X20 tiled z-stacks from three different PND7 and PND14 mice were used. B: in PND14 lung, angiogenin+ cells (red) were associated with elastase signal (green). DAPI is blue. Scale bar = 150 μm. **P < 0.01 and ***P < 0.001 by one-way ANOVA.
Validation of CELA1 antibody.
Several experiments demonstrated antibody specificity. First, CELA1 Western blot demonstrated no nonspecific bands (Fig. 5A). Second, no bands were present when the membrane was incubated with secondary antibody alone (data not shown). Third, by preadsorbing the anti-CELA1 antibody with peptide used in antibody generation, the lung CELA1 band became difficult to detect. Notably, the molecular mass of lung CELA1 was approximately double expected (56 kDa), but pancreatic homogenate had the expected band at 28 kDa (Fig. 5B). Also, secondary-alone staining of PND14 lung sections with analysis for number of CELA1+ cells did not yield any CELA1+ cells in the negative-stained section (Fig. 5, C and D). These data demonstrate antibody specificity and an unexpectedly high molecular mass for lung CELA1.
Fig. 5.
CELA1 antibody validation. A: an immunoblot for CELA1 (green) and β-actin (red) demonstrated the lack of nonspecific bands but an unexpectedly high molecular mass for CELA1, approximately double the expected molecular mass of 28 kDa. B: with the use of PN 8 wk lung homogenate and pancreatic homogenate, adsorption of the generated antibody with the antigenic peptide prevented antibody-antigen interaction with loss of the band. Pancreatic homogenate generated a CELA1 band at the expected 28 kDa, and this band was also lost with adsorption. The protein concentration of pancreatic homogenate was 0.1% that of the lung homogenates; thus, there is no visible β-actin band. C: there was no signal apparent after incubating PND14 elastin in situ zymography lung sections with fluorophore-conjugated anti-guinea pig secondary antibody. D: in contrast, CELA1+ cells were visualized with the use of the generated anti-CELA1 antibody before secondary antibody incubation.
CELA1 regulates angiogenesis in vitro.
Because elastin remodeling was associated with proliferating endothelial cells during lung development, CELA1 was also associated with this remodeling, and CELA1 was expressed in fibroblasts or fibroblast-like cells, we tested whether CELA1 might be expressed in a mouse lung vascular-mesenchymal cell line. The MFLM4 cell line was derived from mouse E14.5 mesenchyme and expresses both fibroblast (vimentin) and endothelial (CD31, CD34, Tie2) markers. MFLM4 cells form tubules when seeded on Matrigel after “differentiation” with fibroblast growth factor 2 and leukemia inhibitory factor (3). Western blot of the MFLM4 cells demonstrated expression of CD31 at both the RNA and protein levels (data not shown), and expression of CELA1 mRNA as assessed by serial dilution assay. MFLM4 cells stained positive for CELA1 by immunofluorescence, but protein levels were not sufficient for detection by Western blot (data not shown). Addition of the elastase inhibitor methoxysuccinyl-Ala-Ala-Pro-Val-chloromethyl ketone reduced MFLM4 tubulogenesis 2.1-fold (P < 0.001, Fig. 6, A–C). shRNA silencing of MFLM4 CELA1 to nearly undetectable levels reduced tubule formation 17-fold (P < 0.001, Fig. 6, D–F). In gain-of-function experiments, we transfected murine CELA1 in a rat lung fibroblast cell line (RFL6) that expressed neither CELA1 nor the endothelial marker CD31. CELA1-transfected RFL6 cells formed tubules between cell clusters, whereas control RFL6 cells did not (Fig. 6, G–I). Cell viability and health were determined by these assays by uptake of the vital dye calcein AM and by demonstrating normal cell morphology at higher magnification. These in vitro loss and gain of function experiments support a role for CELA1 in lung angiogenesis.
Fig. 6.
CELA1 regulates tubule formation in an in vitro angiogenesis assay. A: differentiated MFLM4 cells formed tubules when seeded on Matrigel under control conditions. B: addition of the specific elastase inhibitor N-methoxysuccinyl-Ala-Ala-Pro-Val-chloromethyl ketone largely inhibited tubule formation. At higher magnification, no cytotoxicity was appreciable. C: with the use of a fluorometric assay, addition of the specific elastase inhibitor to MFLM4 cell culture reduced MFLM4 elastase activity. D: control short-hairpin RNA (shRNA) did not inhibit tubule formation. E: shRNA silencing of CELA1 inhibited MFLM4 tubule formation. F: by PCR, shRNA silencing of CELA1 in MFLM4 cells reduced mRNA levels. G: RFL6 cells did not form tubules when transfected with a control vector and seeded on Matrigel. H: transfection of murine CELA1 into RFL6 cells induced tubule formation between cell clusters. I: whereas RLF6 cells did not contain CELA1 mRNA, transfection of CELA1 induced CELA1 mRNA expression 149-fold higher than seen in MFLM4 cells. *P < 0.05, **P < 0.01, and ***P < 0.001 by t-test (two groups) or one-way ANOVA (three groups).
DISCUSSION
We have for the first time described the temporal-spatial pattern of elastin remodeling during lung development, demonstrated that this remodeling is mediated by serine proteases, and shown an association between lung elastin remodeling and CELA1 expression. Elastin remodeling localized to major arteries and airways on PND1 with increasing remodeling of distal airspace walls during late saccular and alveolar stages of lung development. The temporal-spatial pattern of CELA1 expression mirrored that of elastin remodeling. During the alveolar stage of lung development, lung elastin remodeling was associated with proliferating endothelial cells, but CELA1 was not associated with these cells. Both elastase inhibition and CELA1 silencing impaired angiogenesis in vitro. Our findings suggest a model of lung development in which CELA1 remodels distal airspace elastin with subsequent microvascular growth in response to this remodeling.
Despite the near absence of elastin remodeling in the adult lung, CELA1 expression remained high, bringing into question the role of CELA1 in lung development. Three possible explanations could account for this discrepancy and would continue to support a role for CELA1 in lung development. First, CELA1 may be bound to an as-of-yet-unidentified inhibitor, resulting in a discrepancy between active and total CELA1. Second, it may be that CELA1+ cells are more active and are secreting CELA1 during the saccular and alveolar stages of lung development and are more quiescent in the adult lung. The observation that CELA1+ cells were less spherical during the saccular and alveolar stages of lung development compared with the adult lung supports this assertion. If extracellular CELA1 is inactivated and degraded as are other proteases, this would also explain the discrepancy between CELA1 mRNA and protein levels at PND14 and PN 8 wk. Third, CELA family members require cleavage of a targeting sequence to form active enzyme. Our antibody cannot distinguish active from inactive CELA1, so it may be that the increased CELA1 concentration in adult lung is inactive, accounting for differences between CELA1 quantities and elastase activity.
We did not investigate why the molecular mass of CELA1 in the lung is approximately double the expected weight. With the presented mRNA data, Western blot data of lung and pancreas, competitive inhibition data, and CELA1 morpholino data, we are confident that we are indeed detecting lung CELA1. To the best of our knowledge, dimerization of CELA1 or any other CELA family member has never been reported. If we are detecting CELA1 dimer, then covalent modification would be required; otherwise, at least some small amount of monomer would be expected under denaturing conditions. Posttranslational modifications are unlikely to account for this notable weight difference. The serpin family of antiproteases covalently binds target proteases, and serpins have molecular masses in the 45- to 55-kDa range. Our observed molecular mass is lower than what would be predicted if CELA1 were bound to a serpin-family molecule; however, it may be that we are detecting CELA1 bound to another antiprotease. A third possibility is that CELA1 is alternatively spliced. The epitope for our antibody is entirely contained within exon 4. Understanding the unexpectedly large molecular mass of CELA1 will be important in defining its molecular biology in the lung.
Our conclusions are largely dependent upon the quantitative analysis of masked in situ zymography images. With regards to acquisition of the images, all tissues were processed identically on the same day, and all the images were obtained on the same day with identical confocal microscope settings. With regard to quantitation of the in situ zymography signal, the low intragroup variability, a consistent pattern over the course of development, and consistency with both a fluorometric plate-based elastase assay and desmosine ELISA support technique validity. With regards to masking, it was necessary to manually draw airway and vessel masks, and we acknowledge that the passage of major vessels or airways parallel to the plane of sectioning could have falsely reduced our measurement of airway or vessel-associated elastase signal or CELA1+ cells. With regard to the masking of septal tip and nonseptal tip elastin, the masking was completely algorithm-driven, and we acknowledge that the empirical sphericity mask of 0.8 may have incorrectly classified longitudinally sectioned elastin tips as nontips, but these events should be relatively rare and would not affect our conclusions given the large differences in elastase signal between the two groups. With regard to colocalization measurements, we acknowledge that sectioning may underreport the frequency with which one signal was located within 10 μm of another; however, such a confounder would apply equally to all groups and not affect our conclusions. Finally, we applied a sphericity mask of 0.7 to CELA1+ cells to quantitate the observation that CELA1+ cells were more rounded in the adult lung compared with CELA1+ cells in the lungs of younger mice. Whereas a high degree of sphericity by no means definitively indicates quiescence, we believe it is reasonable to believe that elongated fibroblasts are more likely to be activated or in the process of migrating. A criticism of quantitative image analysis is that parameters and image selection can be manipulated to demonstrate false associations. Our use of large (1.5 mm × 1.5 mm × 10 μm) lung sections, automation of most masking algorithms, and validation of findings with non-image-based quantitative techniques allay these concerns.
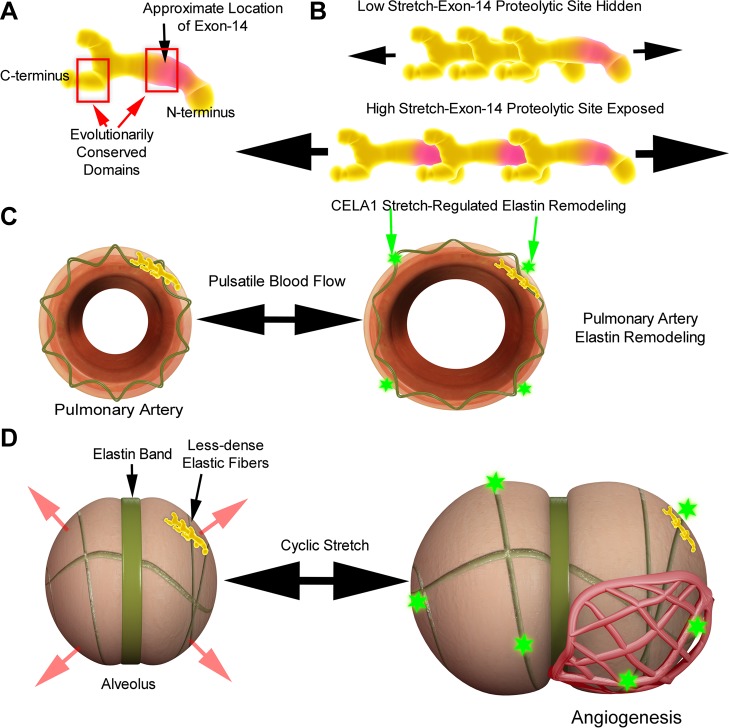
To understand how CELA1 may link lung elastin remodeling and microvascular growth, one must understand how elastin monomers are arranged in the elastin fibril, how these monomers interact during conditions of stretch, and how chymotrypsin-like elastase family members bind and cleave elastin fibers. The tropoelastin molecule contains two highly evolutionarily conserved hydrophobic domains at both the COOH-terminus and at exons 10–14 (Fig. 7A) (13). Although the precise location of these tropoelastin exons in the tertiary structure of tropoelastin has not been definitely defined, it has been shown that hydrophobic domains in these approximate locations align head to tail in the tropoelastin polymer (6). These hydrophobic interactions are key to the polymer's elastic properties. Chymotrypsin-like elastase family members are the principal components of pancreatic elastase, and pancreatic elastases preferentially cleave tropoelastin at one of these two conserved domains, specifically exon 14 (15, 35). As demonstrated in Fig. 7B, under static conditions, exon 14 is likely hidden by the COOH-terminal hydrophobic domain of an adjacent tropoelastin monomer, an assertion supported by elastase-binding kinetic studies. Using fluorophore-labeled pancreatic elastase, Hamakawa et al. demonstrated that CELA family elastases bind to lung elastin fibers only in the vector of stretch and that binding increases proportionate to the degree of stretch (17). Thus, the association of CELA1 with areas of lung elastin remodeling during postnatal lung development and the molecular biology of both tropoelastin and CELA family members support a potential role for CELA1 in stretch-regulated elastin remodeling.
Fig. 7.
Schematic of how cyclic stretch and protease activity may regulate elastin remodeling during alveolar and pulmonary arterial growth. A: the tropoelastin monomer contains two evolutionally conserved domains at the COOH-terminus and at exons 10–14. Both of these domains contain a high percentage of hydrophobic residues. B: the hydrophobic domains align head-to-tail and under low-stretch states, and the CELA1 proteolytic site at exon 14 is hidden. However, once a critical stretch threshold is exceeded, the preferred CELA1 proteolytic site is exposed and CELA1 cleaves the tropoelastin molecule, leading to elastin remodeling. C: following closure of the ductus arteriosus, stretch of the pulmonary arterial elastic fiber is increased due to increased pulmonary blood flow. This stretch induces stretch-regulated elastin remodeling (green star) and increases pulmonary arterial compliance. Remodeling ceases once the tropoelastin exon 14 is no longer exposed. D: a low-compliance elastin band exists at the site of a nascent secondary alveolar septum. The cyclic stretch of the respiratory cycle expands the relatively lower-compliance alveolar walls while the elastin band remains relatively fixed. The degree of stretch gradually increases as the thorax grows. Once a certain stretch threshold is exceeded, tropoelastin exon 14 is exposed, leading to CELA1-mediated elastin remodeling and subsequent alveolar growth. This elastin remodeling then leads to angiogenesis, which increases perfusion to the expanding lung surface area.
On a tissue level, the temporal-spatial pattern of elastin remodeling and CELA1 expression also supports a role for CELA1 in stretch-regulated elastin remodeling. On PND1, the majority of elastin remodeling was present in major airways and vessels. Shortly after birth, the ductus arteriosus closes, resulting in a 10-fold increase in pulmonary blood flow with increased stretch of the pulmonary vasculature. Additionally, lung expansion places radial traction on conducting airways and blood vessels to an extent not seen with fetal breathing of denser amniotic fluid. The association of both CELA1 and elastase activity with these structures on PND1 supports the assertion that stretch induces elastin remodeling in these structures (Fig. 7C). During later saccular and alveolar development, there was increasing elastase activity in alveolar walls and bases, but elastase activity did not increase at alveolar septal tips (although it was present). During the respiratory cycle, higher-compliance alveolar walls stretch to a greater degree than lower-compliance septal tips (Fig. 7D). In our conceptual model, elastin in the higher-compliance alveolar walls is remodeled when stretch exposes tropoelastin exon 14, permitting cleavage by CELA1. If our in vitro findings of a role for elastin remodeling and CELA1 in angiogenesis are also true in vivo, then our findings would also provide a mechanism by which alveolar and microvascular growth are coordinated in a ventilation- and perfusion-matched manner.
Our study underscores the importance of protease/antiprotease balance in lung health and disease (26). While the collagen remodeling enzymes MMP14 (30) and MMP2 (4) have both been shown to positively regulate alveolar growth, other MMPs are deleterious to lung development (16, 27). Interestingly, elastin degradation products induce MMP14 transcription (33). In a mouse mechanical ventilation model, neutrophil elastase is deleterious to lung development, and its inhibition partially restores lung development in a mouse pup mechanical ventilation model (18), and neutrophil elastase is an important mediator of elastin destruction in chronic obstructive pulmonary disease (34). Our proposed model accounts for differences between the elastolytic activity of neutrophil elastase and the elastin-remodeling activity of CELA1. In our model, CELA1-mediated elastin remodeling is stretch-dependent, predicting cell autonomous regulation of CELA1-mediated elastolysis. If our model is correct, then elastin remodeling and alveolar growth would cease once alveolar stretch no longer exposes the CELA1 cleavage site.
We chose angiogenin as a marker for endothelial cell proliferation for several reasons. It was downregulated in the lungs of ventilated preterm infants (14), has been implicated in the pathogenesis of lung adenocarcinoma, a carcinoma of the distal lung epithelium (42), and is a potent activator of angiogenesis in multiple organs (38). Angiogenin also increases MMP2 expression (28). In our model, CELA1 remodels lung elastin with subsequent angiogenesis, which explains why CELA1 and angiogenin are only rarely colocalized during the alveolar stage of lung development.
In conclusion, we have defined the pattern of postnatal elastin remodeling in murine lung development, identified an association of both CELA1+ and angiogenin+ cells with this remodeling, and provided in vitro gain and loss of function data supporting a role for CELA1 in angiogenesis. Future studies defining the importance of CELA1 in vivo, the mechanism of CELA1-regulated angiogenesis, and the molecular biology of CELA1-mediated tropoelastin cleavage are needed.
GRANTS
This research was funded with support from the National Institute for Child Health and Human Development Grant K12-HD-028827 and from the Proctor Scholar Program at Cincinnati Children's Hospital Research Foundation.
DISCLOSURES
The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Author contributions: S.L., S.M.Y., and B.M.V. performed experiments; S.L. and B.M.V. analyzed data; S.L., S.M.Y., and B.M.V. interpreted results of experiments; S.L., S.M.Y., and B.M.V. approved final version of manuscript; S.M.Y. and B.M.V. edited and revised manuscript; B.M.V. conception and design of research; B.M.V. prepared figures; B.M.V. drafted manuscript.
ACKNOWLEDGMENTS
Dr. Jeffrey Whitsett provided outstanding mentorship and guidance. Dr. Matt Kofron provided technical assistance with confocal microscopy. Angela Keiser synthesized the anti-CELA1 guinea pig antibody. Dr. Lei Wei provided statistical support. Jeff Cimprich provided computer animation support.
REFERENCES
- 1.Ad Hoc Statement Committee of the American Thoracic Society. Mechanisms and limits of induced postnatal lung growth. Am J Respir Crit Care Med 170: 319–343, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Adams SA, Subramanian V. The angiogenins: an emerging family of ribonuclease related proteins with diverse cellular functions. Angiogenesis 3: 189–199, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Akeson AL, Wetzel B, Thompson FY, Brooks SK, Paradis H, Gendron RL, Greenberg JM. Embryonic vasculogenesis by endothelial precursor cells derived from lung mesenchyme. Dev Dyn 217: 11–23, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Ambalavanan N, Nicola T, Li P, Bulger A, Murphy-Ullrich J, Oparil S, Chen YF. Role of matrix metalloproteinase-2 in newborn mouse lungs under hypoxic conditions. Pediatr Res 63: 26–32, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson CR, Ponce AM, Price RJ. Immunohistochemical identification of an extracellular matrix scaffold that microguides capillary sprouting in vivo. J Histochem Cytochem 52: 1063–1072, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Baldock C, Oberhauser AF, Ma L, Lammie D, Siegler V, Mithieux SM, Tu Y, Chow JY, Suleman F, Malfois M, Rogers S, Guo L, Irving TC, Wess TJ, Weiss AS. Shape of tropoelastin, the highly extensible protein that controls human tissue elasticity. Proc Natl Acad Sci USA 108: 4322–4327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bird AD, Tan KH, Olsson PF, Zieba M, Flecknoe SJ, Liddicoat DR, Mollard R, Hooper SB, Cole TJ. Identification of glucocorticoid-regulated genes that control cell proliferation during murine respiratory development. J Physiol 585: 187–201, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bland RD, Albertine KH, Pierce RA, Starcher BC, Carlton DP. Impaired alveolar development and abnormal lung elastin in preterm lambs with chronic lung injury: potential benefits of retinol treatment. Biol Neonate 84: 101–102, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Bland RD, Xu L, Ertsey R, Rabinovitch M, Albertine KH, Wynn KA, Kumar VH, Ryan RM, Swartz DD, Csiszar K, Fong KS. Dysregulation of pulmonary elastin synthesis and assembly in preterm lambs with chronic lung disease. Am J Physiol Lung Cell Mol Physiol 292: L1370–L1384, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Bruce MC, Schuyler M, Martin RJ, Starcher BC, Tomashefski JF, Jr, Wedig KE. Risk factors for the degradation of lung elastic fibers in the ventilated neonate Implications for impaired lung development in bronchopulmonary dysplasia. Am Rev Respir Dis 146: 204–212, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Bruce MC, Wedig KE, Jentoft N, Martin RJ, Cheng PW, Boat TF, Fanaroff AA. Altered urinary excretion of elastin cross-links in premature infants who develop bronchopulmonary dysplasia. Am Rev Respir Dis 131: 568–572, 1985 [DOI] [PubMed] [Google Scholar]
- 12.Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol 26: 175–182, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Chung MI, Miao M, Stahl RJ, Chan E, Parkinson J, Keeley FW. Sequences and domain structures of mammalian, avian, amphibian and teleost tropoelastins: clues to the evolutionary history of elastins. Matrix Biology 25: 492–504, 2006 [DOI] [PubMed] [Google Scholar]
- 14.De Paepe ME, Greco D, Mao Q. Angiogenesis-related gene expression profiling in ventilated preterm human lungs. Exp Lung Res 36: 399–410, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Getie M, Schmelzer CE, Neubert RH. Characterization of peptides resulting from digestion of human skin elastin with elastase. Proteins 61: 649–657, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev 87: 69–98, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamakawa H, Bartolak-Suki E, Parameswaran H, Majumdar A, Lutchen KR, Suki B. Structure-function relations in an elastase-induced mouse model of emphysema. Am J Respir Cell Mol Biol 45: 517–524, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilgendorff A, Parai K, Ertsey R, Jain N, Navarro EF, Peterson JL, Tamosiuniene R, Nicolls MR, Starcher BC, Rabinovitch M, Bland RD. Inhibiting lung elastase activity enables lung growth in mechanically ventilated newborn mice. Am J Respir Crit Care Med 184: 537–546, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang JT, Chaudhuri R, Albarbarawi O, Barton A, Grierson C, Rauchhaus P, Weir CJ, Messow M, Stevens N, McSharry C, Feuerstein G, Mukhopadhyay S, Brady J, Palmer CN, Miller D, Thomson NC. Clinical validity of plasma and urinary desmosine as biomarkers for chronic obstructive pulmonary disease. Thorax 67: 502–508, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin E, Fujiwara M, Pan X, Ghazizadeh M, Arai S, Ohaki Y, Kajiwara K, Takemura T, Kawanami O. Protease-activated receptor (PAR)-1 and PAR-2 participate in the cell growth of alveolar capillary endothelium in primary lung adenocarcinomas. Cancer 97: 703–713, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 106: 1311–1319, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kho AT, Bhattacharya S, Mecham BH, Hong J, Kohane IS, Mariani TJ. Expression profiles of the mouse lung identify a molecular signature of time-to-birth. Am J Respir Cell Mol Biol 40: 47–57, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ku DN. Blood flow in arteries. Annu Rev Fluid Mech 29: 399–434, 1997 [Google Scholar]
- 24.Kunig AM, Balasubramaniam V, Markham NE, Seedorf G, Gien J, Abman SH. Recombinant human VEGF treatment transiently increases lung edema but enhances lung structure after neonatal hyperoxia. Am J Physiol Lung Cell Mol Physiol 291: L1068–L1078, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Lange AW, Keiser AR, Wells JM, Zorn AM, Whitsett JA. Sox17 promotes cell cycle progression and inhibits TGF-beta/Smad3 signaling to initiate progenitor cell behavior in the respiratory epithelium. PLoS One 4: e5711, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loffek S, Schilling O, Franzke CW. Series “matrix metalloproteinases in lung health and disease”: biological role of matrix metalloproteinases: a critical balance. Eur Respir J 38: 191–208, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Lukkarinen H, Hogmalm A, Lappalainen U, Bry K. Matrix metalloproteinase-9 deficiency worsens lung injury in a model of bronchopulmonary dysplasia. Am J Respir Cell Mol Biol 41: 59–68, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Miyake M, Goodison S, Lawton A, Gomes-Giacoia E, Rosser CJ. Angiogenin promotes tumoral growth and angiogenesis by regulating matrix metallopeptidase-2 expression via the ERK1/2 pathway. Oncogene In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicola T, Hagood JS, James ML, Macewen MW, Williams TA, Hewitt MM, Schwiebert L, Bulger A, Oparil S, Chen YF, Ambalavanan N. Loss of Thy-1 inhibits alveolar development in the newborn mouse lung. Am J Physiol Lung Cell Mol Physiol 296: L738–L750, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oblander SA, Zhou Z, Galvez BG, Starcher B, Shannon JM, Durbeej M, Arroyo AG, Tryggvason K, Apte SS. Distinctive functions of membrane type 1 matrix-metalloprotease (MT1-MMP or MMP-14) in lung and submandibular gland development are independent of its role in pro-MMP-2 activation. Dev Biol 277: 255–269, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Pocza P, Suli-Vargha H, Darvas Z, Falus A. Locally generated VGVAPG and VAPG elastin-derived peptides amplify melanoma invasion via the galectin-3 receptor. Int J Cancer 122: 1972–1980, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Reddel CJ, Cultrone D, Rnjak-Kovacina J, Weiss AS, Burgess JK. Tropoelastin modulates TGF-beta1-induced expression of VEGF and CTGF in airway smooth muscle cells. Matrix Biology 32: 407–413, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinet A, Fahem A, Cauchard JH, Huet E, Vincent L, Lorimier S, Antonicelli F, Soria C, Crepin M, Hornebeck W, Bellon G. Elastin-derived peptides enhance angiogenesis by promoting endothelial cell migration and tubulogenesis through upregulation of MT1-MMP. J Cell Sci 118: 343–356, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Sandhaus RA, Turino G. Neutrophil elastase-mediated lung disease. Copd 10, Suppl 1: 60–63, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Senior RM, Bielefeld DR, Starcher BC. Comparison of the elastolytic effects of human leukocyte elastase and porcine pancreatic elastase. Adv Exp Med Biol 79: 249–261, 1977 [DOI] [PubMed] [Google Scholar]
- 36.Srisuma S, Bhattacharya S, Simon DM, Solleti SK, Tyagi S, Starcher B, Mariani TJ. Fibroblast growth factor receptors control epithelial-mesenchymal interactions necessary for alveolar elastogenesis. Am J Respir Crit Care Med 181: 838–850, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Strydom DJ. The angiogenins. Cell Mol Life Sci 54: 811–824, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talas U, Dunlop J, Khalaf S, Leigh IM, Kelsell DP. Human elastase 1: evidence for expression in the skin and the identification of a frequent frameshift polymorphism. J Invest Dermatol 114: 165–170, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Thibeault DW, Mabry SM, Ekekezie II, Truog WE. Lung elastic tissue maturation and perturbations during the evolution of chronic lung disease. Pediatrics 106: 1452–1459, 2000 [DOI] [PubMed] [Google Scholar]
- 41.West JB. Respiratory Physiology-The Essentials. Baltimore, MD: Williams & Wilkins, 1973, p. x, 185 p [Google Scholar]
- 42.Yuan Y, Wang F, Liu XH, Gong DJ, Cheng HZ, Huang SD. Angiogenin is involved in lung adenocarcinoma cell proliferation and angiogenesis. Lung Cancer 66: 28–36, 2009 [DOI] [PubMed] [Google Scholar]