Abstract
Oxygen toxicity contributes to the pathogenesis of bronchopulmonary dysplasia (BPD). Neonatal mice exposed to hyperoxia develop a simplified lung structure that resembles BPD. Sustained activation of the transcription factor NF-κB and increased expression of protective target genes attenuate hyperoxia-induced mortality in adults. However, the effect of enhancing hyperoxia-induced NF-κB activity on lung injury and development in neonatal animals is unknown. We performed this study to determine whether sustained NF-κB activation, mediated through IκBβ overexpression, preserves lung development in neonatal animals exposed to hyperoxia. Newborn wild-type (WT) and IκBβ-overexpressing (AKBI) mice were exposed to hyperoxia (>95%) or room air from day of life (DOL) 0–14, after which all animals were kept in room air. Survival curves were generated through DOL 14. Lung development was assessed using radial alveolar count (RAC) and mean linear intercept (MLI) at DOL 3 and 28 and pulmonary vessel density at DOL 28. Lung tissue was collected, and NF-κB activity was assessed using Western blot for IκB degradation and NF-κB nuclear translocation. WT mice demonstrated 80% mortality through 14 days of exposure. In contrast, AKBI mice demonstrated 60% survival. Decreased RAC, increased MLI, and pulmonary vessel density caused by hyperoxia in WT mice were significantly attenuated in AKBI mice. These findings were associated with early and sustained NF-κB activation and expression of cytoprotective target genes, including vascular endothelial growth factor receptor 2. We conclude that sustained hyperoxia-induced NF-κB activation improves neonatal survival and preserves lung development. Potentiating early NF-κB activity after hyperoxic exposure may represent a therapeutic intervention to prevent BPD.
Keywords: NF-κB, IκBβ hyperoxic lung injury, bronchopulmonary dysplasia, lung development
despite advances in neonatal care, the incidence of bronchopulmonary dysplasia (BPD) in infants of 22–28 wk gestation age remains ∼40% (51). The hallmark of BPD is simplified distal airspaces, representing an arrest of lung development attributable to a combination of insults including hyperoxia (36). Recent studies have shown that limiting oxygen exposure can prevent BPD, but this does not appear to be without risk (27, 51a). A clearer understanding of the signaling pathways that protect the developing lung from hyperoxic injury may help identify therapeutic targets to prevent BPD.
Neonatal animals are resistant to the mortality caused by pulmonary oxygen toxicity observed in adults (24). Yang and colleagues (61) determined that activation of the transcription factor NF-κB mediated the resistance to hyperoxic lung injury in neonatal mice (61). The transcription factor NF-κB regulates the cellular response to oxidant and inflammatory stress and regulates the expression of many proinflammatory and apoptosis-related genes implicated in the pathogenesis of BPD (59). Neonatal mice exposed to hyperoxia exhibit robust pulmonary NF-κB activity, resulting in increased expression of antiapoptotic genes (61). Despite this relative resistance to hyperoxia, the newborn lung is not immune to injury. Hyperoxic exposure in the newborn period results in a simplified lung structure reminiscent of BPD (57). Although NF-κB activation prevents mortality in neonates exposed to hyperoxia, it is unknown whether further enhancing NF-κB activity would preserve lung development.
In quiescent cells, the inhibitory proteins IκBα and IκBβ sequester NF-κB dimers in the cytoplasm (46). Following exposure to oxidant or inflammatory stress, these proteins are degraded, which allows nuclear translocation of NF-κB dimers and subsequent target gene transcription. Importantly, IκBα and IκBβ have distinct functions following NF-κB activation. Following activation, both NF-κB isoforms enter the nucleus. A nuclear export sequence on IκBα allows for removal of activated dimers from the nucleus and facilitates termination of NF-κB activity (32). In contrast, IκBβ has no nuclear export sequence and, once in the nucleus, acts to stabilize NF-κB DNA binding and potentiate NF-κB activity and downstream gene expression (47). This sustained NF-κB activity protects cells from oxidant stress-induced cell death and prevents pulmonary oxygen toxicity in adult mice (44, 58).
Here, using IκBβ-overexpressing mice, we demonstrate that sustained NF-κB activity attenuates disrupted lung development secondary to hyperoxic injury. This manifests as improved survival and lung development. These findings are associated with enhanced expression of lung-protective factors including antiapoptotic proteins and vascular endothelial growth factor receptor 2 (VEGFR2). We speculate that interventions aimed at sustaining hyperoxia-induced NF-κB activity in the neonatal lung may prevent BPD.
MATERIALS AND METHODS
Animal model.
ICR mice were purchased from Taconic, and AKBI, or IκBβ knockin, mice were a gift of Richard Cohen (Harvard University). Wild-type (WT) mice have the normal IκBα gene. In the IκBβ knockin, or AKBI, mice, the IκBα gene has been replaced by IκBβ cDNA, and expression is driven by the IκBα promoter. Thus, these mice overexpress IκBβ and do not express IκBα. Neonatal mice (<4 h old) were exposed to room air or hyperoxia (O2 > 95%) conducted in an A-chamber (BioSpherix). Ambient carbon dioxide was maintained at <1,200 ppm. Dams were rotated (air to hyperoxia and vice versa) every 24 h to obviate the effects of hyperoxia on the mothers. Hyperoxic exposures continued for 14 days, which correlates with the alveolar stage of lung development in mice (63). At this point, surviving animals were placed into room air. All procedures were approved by the IACUC at the Children's Hospital of Philadelphia.
Preparation of cytosolic and nuclear extracts.
Whole lung homogenate and cytosolic and nuclear extracts were prepared from freshly collected whole lungs as previously described (61).
Assessment of lung development.
Assessment of lung development and pulmonary vessel density was performed after formalin fixation (61). Animals were euthanized, and the trachea was cannulated with a 26-gauge angiocatheter; the lungs were then inflation fixed at 25 cm H2O pressure for 1 min with 10% formalin and paraffin embedded. Radial alveolar counts (RAC), an objective measure of alveolar number, were assessed as previously described (16, 21). Counts were performed on four separate WT and AKBI mice at each time point (3 and 28 days) and for each condition (room air and hyperoxia exposure). Because of the technical difficulty of uniform inflation of the neonatal mouse lung, a thorough evaluation of pulmonary morphometry was performed using two separate sections of lung per mouse. The average RAC was obtained from a minimum of 30 perpendicular lines obtained from photomicrographs of 5–10 high-powered fields of two separate sections of lung per animal. Measurements of mean linear intercept (MLI), surface area, airspace area, and average alveolar size were performed using a computer-assisted image-analysis program on images captured on a MicroPublisher digital camera using a Zeiss Axioscope2 microscope (×20 objective). Images were processed using ImageJ (public domain Java image, processing program created by Wayne Rasband at the Research Services Branch, National Institute of Mental Health, Bethesda, MD) and a plug-in developed by Drs. V. Balasubramaniam and C. Coulon (1, 2). The MLI was determined by averaging six randomly chosen images per section, with three sections evaluated per mouse. Four mice per genotype per time point and exposure were used.
Assessment of pulmonary vessel density.
Paraffin-embedded lung sections were stained for the presence of von Willebrand Factor (vWF). Following incubation with biotinylated anti-VWF primary antibody (F3520, Sigma), staining was completed according to the TSA kit protocol (NEL701A001KT, Perkin-Elmer). Images of the vWF-stained slides were captured with the ×10 objective. Pulmonary vessel density was determined by counting the number of VWF-stained vessels with external diameter <100 μM per high-powered field. Five high-powered fields from the lungs from three separate animals per exposure were assessed.
Immunoblot analysis.
Cytosolic and nuclear extracts were electrophoresed on a 4–12% polyacrylamide gel (Invitrogen), and proteins were transferred to a HyBond PVDF membrane (Amersham). Membranes were blotted with antibodies against the following proteins: IκBα (sc-371, Santa Cruz Biotechnology), IκBβ (sc-9130, Santa Cruz Biotechnology), p65 (ab7970, Abcam), calnexin (ADI-SPA-860-D, Enzo Life Sciences), VEGFR2 (2479, Cell Signaling), poly(ADP-ribose) polymerase (PARP) (9542, Cell Signaling), lamin B (sc-6216, Santa Cruz Biotechnology), Bax (sc-493, Santa Cruz), and Bcl-xL (2762, Cell Signaling). Densitometric analysis was performed using ImageLab. All densitometric values were first normalized to a loading control (cytosolic, calnexin; nuclear, lamin b) and expressed as a ratio of the unexposed appropriate genotype control (set at 1).
Evaluation of nuclear NF-κB binding by EMSA.
A 32P-labeled oligonucleotide with the consensus sequence for NF-κB (5′-AGTTGAGGGGACTTTCCCAGGC-3′) (Promega) was used as a probe to evaluate NF-κB binding ability as described previously (60, 62). To identify nonspecific binding of nuclear proteins, competition reactions were performed by addition of either 50-fold excess of the nonradiolabeled NF-κB consensus sequence or 50-fold excess of nonradiolabeled mutated NF-κB consensus sequence (5′-AGTTGAGGCGACTTTCCCAGGC-3′) (Santa Cruz Biotechnology) to the reaction mixtures before electrophoresis. To identify the NF-κB subunit proteins in the binding complex, 2.5 μl of p50 (ab7917, Abcam; pc136, Calbiochem; or sc7178, Santa Cruz Biotechnology), p65 antibodies (ab7970, Abcam; pc137, Calbiochem), or p65-phosphorylated serine 276 (3037, Cell Signaling) was incubated with nuclear proteins for 1 h at 37°C before addition of the radiolabeled probe. As an additional control for binding conditions, binding buffer lacking DTT was used.
qPCR.
Relative mRNA levels were evaluated by quantitative real-time PCR using the TaqMan gene expression system (Applied Biosystems). Total lung RNA was extracted with TRIzol Plus RNA Purification System (Ambion). RNA was assessed for purity and concentration using NanoDrop (Thermo Scientific), and cDNA was synthesized using the Verso cDNA Synthesis Kit (Thermo Scientific). Gene expression was assessed using predesigned exon-spanning primers [manganese superoxide dismutase (MnSOD), Mm00449726_m1; VEGFR2, Mm01222421_m1; VEGFA, Mm01281449_m1; IL-1β, Mm01336189_m1; IL-6, Mm00446190_m1; IL-11, Mm00434162_m1; TNF-α, Mm00443258_m1; regulated on activation, normal T cell expressed, and presumably secreted (RANTES), Mm01302428_m1; ICAM, Mm00516023_m1; matrix metalloproteinase (MMP)-12, Mm00500554_m1; Bcl-2, Mm00477631_m1; Bcl-xL, Mm00437783_m1; bacloviral IAP repeat-containing (BIRC)2 Mm00431811_m1; BIRC3 Mm01168413_m1; and Bax, Mm00432050_m1; Applied Biosystems] using the StepOnePlus Real Time PCR System (Applied Biosystems). Relative quantitation was performed via normalization to the endogenous control B2M (Mm00437762_m1, Applied Biosystems) using cycle threshold (ΔΔCT) method. All values are expressed as a ratio of the unexposed appropriate genotype control (set at 1).
Statistical analysis.
For comparison between treatment groups, the null hypothesis that no difference existed between treatment means was tested by Student's t-test for two groups and two-way ANOVA for multiple groups with potentially interacting variables (genotype, hyperoxia exposure), with statistical significance between and within groups determined by means of Bonferroni method of multiple comparisons (InStat, GraphPad Software). Statistical significance was defined as P < 0.05.
RESULTS
Neonatal AKBI mice demonstrate improved survival in hyperoxia.
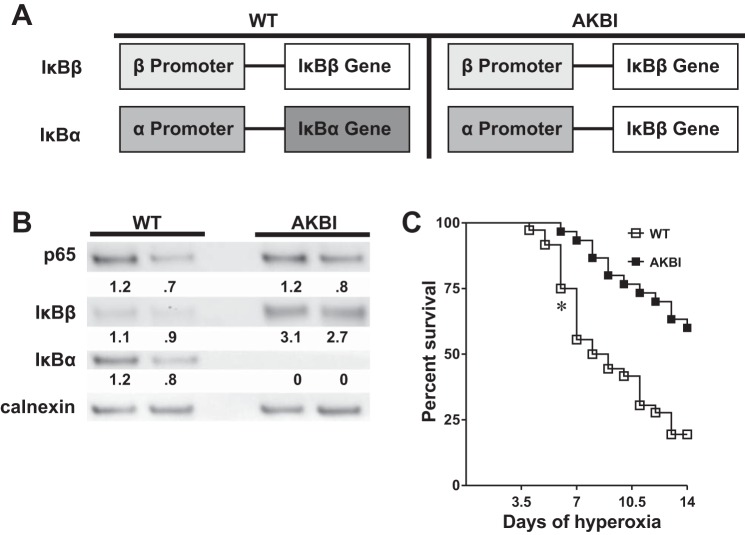
In this study, we used IκBβ knockin, or AKBI, mice to evaluate the specific role of the IκB family of proteins in mediating hyperoxic lung injury. The AKBI mice have IκBβ cDNA inserted in place of the IκBα gene (Fig. 1A). These phenotypically normal mice are bred on an ICR background, overexpress IκBβ, and do not express IκBα (14). Western blots performed on whole lung lysates confirmed that AKBI mice did not express IκBα and overexpressed IκBβ compared with WT mice (Fig. 1B). Furthermore, expression of the NF-κB subunit p65 was not different between WT and AKBI mice (Fig. 1B). WT mice began to experience significant mortality at 6 days of hyperoxic exposure and by 14 days had >80% mortality (Fig. 1C). In contrast, AKBI mice had significantly improved survival in hyperoxia, with significant differences from WT mice starting from 6 days through 14 days of exposure. Of note, AKBI neonatal mice demonstrated remarkable resistance to hyperoxic injury, with only 40% mortality through 14 days of exposure. These results demonstrate that IκBβ overexpression provides a protective advantage against hyperoxic lung injury in newborn mice.
Fig. 1.
Neonatal AKBI mice are resistant to hyperoxia-induced mortality. A: schematic of IκB expression patterns in wild-type (WT) and AKBI mice. The IκBα gene has been replaced by IκBβ cDNA. The IκBα promoter controls the expression of the IκBβ transgenic loci. Thus AKBI overexpress IκBβ, without expressing IκBα. B: representative Western analysis of the NF-κB subunit p65 and NF-κB inhibitory proteins IκBα and IκBβ from WT and AKBI whole lung homogenate. Calnexin is shown as loading control. Densitometric evaluation of protein expression normalized to loading control (calnexin) and expressed as a ratio to WT is provided. C: Kaplan-Meier survival analysis of WT and AKBI neonatal mice exposed to hyperoxia (>95%, 14 days). Values are expressed as the percentage of surviving animals. n = 30/group. *P < 0.05 vs. WT.
Hyperoxia-induced disruption of lung development is attenuated in AKBI mice.
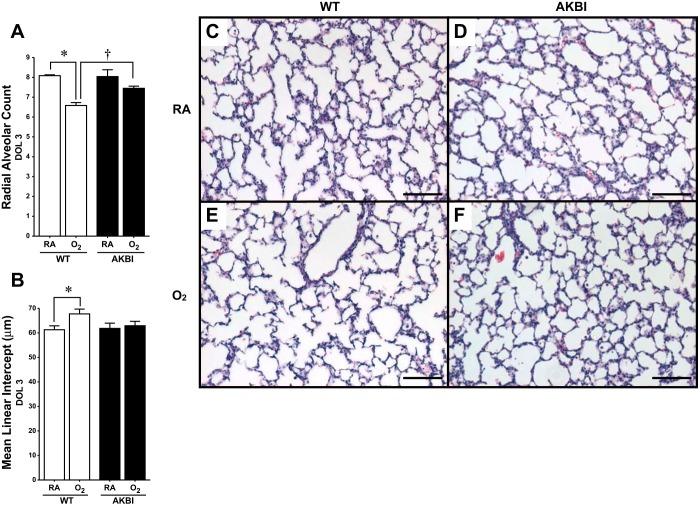
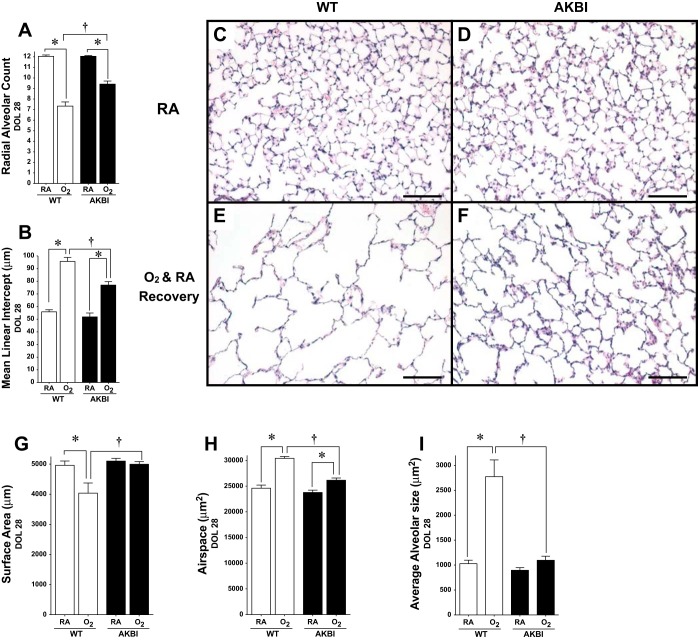
Neonatal mice exposed to prolonged hyperoxia demonstrate decreased alveolarization (57). To assess the effect of hyperoxia on lung development in AKBI mice, assessment of RAC and MLI was performed. Neither RAC nor MLI were different between WT and AKBI mice reared in room air at 3 or 28 days (Fig. 2, A–D, and Fig. 3, A–D). However, a significant reduction in RAC and an increase in MLI were evident after 3 days of hyperoxia in WT but not AKBI mice (Fig. 2, A, B, E, and F). Additional mice were exposed to hyperoxia for 14 days to ensure hyperoxic stress during the early alveolar stage of lung development in mice (day of life, DOL, 5–30) (63). Following this exposure, mice were allowed to recover in room air for an additional 14 days. In this model, a significant reduction in RAC and even greater difference in MLI existed at 28 days in WT mice (Fig. 3, A, B, and E). Noting these differences, we performed further assessments of lung morphology. Significantly decreased surface area and increased airspace area and average alveolar size developed in WT mice exposed to hyperoxia (Fig. 3, G–I). Importantly, all morphological changes observed in hyperoxia-exposed WT mice were attenuated in similarly exposed AKBI mice. (Fig. 3, A, B, G–I).
Fig. 2.
Impaired alveolarization induced by short-term exposure to hyperoxia is attenuated in AKBI mice. Radial alveolar counts (A) and mean linear intercept (B) at 3 days in WT and AKBI mice in room air (RA) or 95% O2. *P < 0.05 vs. genotype control, †P < 0.05 vs. paired WT exposure. C–F: representative hematoxylin and eosin-stained photomicrographs of lung tissue from WT exposed to RA (C). AKBI exposed to RA (D), WT exposed to O2 (E), and AKBI exposed to O2 (F). All images were obtained using the ×10 objective lens, internal scale bar 100 = μM. DOL, days of life.
Fig. 3.
Impaired alveolarization caused by prolonged exposure to hyperoxia is attenuated in AKBI mice. Radial alveolar counts (A) and mean linear intercept (B) at 28 days in WT and AKBI mice in RA or 95% O2 for 14 days followed by 14 days of RA (O2). *P < 0.05 vs. air exposed genotype control, †P <0.05 vs. paired WT exposure. C–F: representative hematoxylin and eosin-stained photomicrographs of lung tissue from WT exposed to RA (C). AKBI exposed to RA (D), WT exposed to O2 and RA recovery (E), and AKBI exposed to O2 and RA recovery (F). All images were obtained using the ×10 objective lens, internal scale bar = 100 μM. Pulmonary surface area per high-powered field (in μm) (G), airspace volume (in μm2) (H), and average alveolar size (in μm2) (I) at 28 days in WT and AKBI mice in RA or 95% O2 for 14 days followed by 14 days of RA (O2). *P < 0.05 vs. air exposed genotype control, †P < 0.05 vs. paired WT exposure.
AKBI mice demonstrate sustained hyperoxia-induced NF-κB activation.
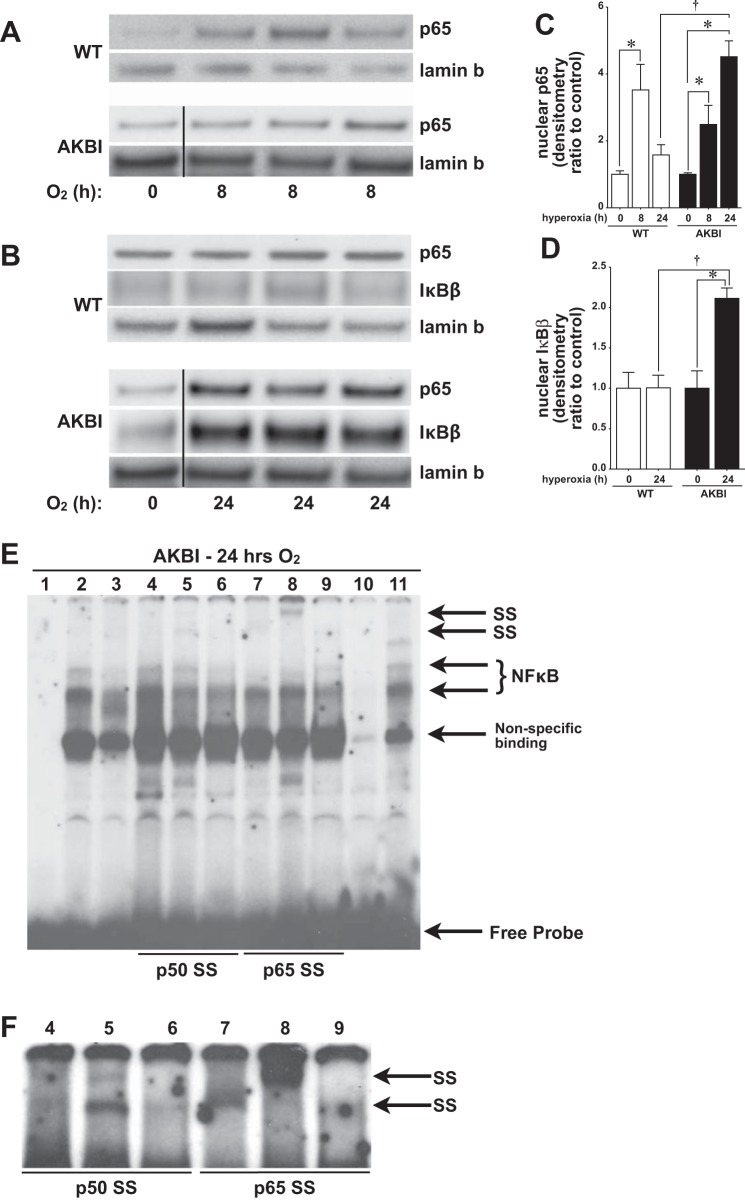
To understand what dictated the improved survival and improved lung development observed in AKBI mice exposed to hyperoxia, we evaluated pulmonary NF-κB activity before the differences seen in lung development on DOL 3 (Fig. 2). In the current study, we used nuclear translocation of p65 as a read-out of NF-κB activity for two reasons. First, previous studies have demonstrated that hyperoxia induces nuclear translocation of p65, both in vitro and in vivo and in neonatal and adult animals (12, 20, 23, 25, 28, 33, 40). Second, the NF-κB inhibitory proteins IκBα and IκBβ preferentially bind unique NF-κB dimer combinations (31, 53, 55), and the p65-cRel heterodimer is a primary target of IκBβ (29, 47). Consistent with previous reports, both WT and AKBI mice demonstrated nuclear translocation of the NF-κB subunit p65 after 8 h of hyperoxia (Fig. 4, A and C). By 24 h of hyperoxic exposure, nuclear p65 remained significantly elevated only in AKBI mice (Fig. 4, B and C). The sustained NF-κB nuclear translocation observed in AKBI mice was associated with significantly increased nuclear IκBβ compared with similarly exposed WT mice (Fig. 4, B and D). To confirm the presence of p65-DNA binding after 24 h in hyperoxia-exposed AKBI mice, EMSA with supershift was performed on nuclear extracts (Fig. 4, E and F). Given the differential affinity of commercially available antibodies for nondenatured proteins, we used three separate p50 and p65 antibodies to assess NF-κB-DNA binding. Consistent with increased nuclear translocation of p65 in AKBI mice exposed to 24 h of hyperoxia demonstrated by Western blot (Fig. 4B), supershift analysis revealed presence of both p50 (Fig. 4F, lane 5) and p65 (Fig. 4, E and F, lanes 7 and 8). These findings confirm sustained hyperoxia-induced nuclear NF-κB translocation in AKBI mice.
Fig. 4.
Hyperoxia-induced nuclear NF-κB translocation is sustained in AKBI mice. A: representative Western blot showing p65 in lung nuclear extracts from WT and AKBI mice exposed to RA or 8 h of hyperoxia (O2 >95%). B: representative Western blot showing p65 and IκBβ in lung nuclear extracts from WT and AKBI mice exposed to RA or 24 h of hyperoxia (O2 >95%, 24 h), with lamin B as loading control. Densitometric evaluation of nuclear p65 at 8 and 24 h of exposure (C) and nuclear IκBβ at 24 h of exposure (D). Values are means ± SE (n = 3/time point); *P < 0.05 vs. unexposed control; †P < 0.05 vs. paired WT exposure. Dividing lines in A and B indicate that lanes are noncontiguous. Time point 0 (AKBI) presented in the p65 and lamin B blots is the same control for both the 8-h (A) and 24 h (B) time points. E: representative EMSA of lung nuclear extracts from neonatal AKBI mice exposed to hyperoxia (24 h). F: darker contrast of lanes 4–9. Bands representing NF-κB consensus sequence binding, nonspecific binding, free probe, and super shift (SS) bands are labeled. Lane 1, dye; lane 2, hyperoxia; lane 3, hyperoxia, no DTT; lanes 4–6, hyperoxia with p50 supershift; lane 4, Abcam; lane 5, Calbiochem; lane 6, Santa Cruz Biotechnology; lanes 7–8, hyperoxia with p65 supershift; lane 7, Abcam; lane 8, Calbiochem; lane 9, hyperoxia with p65-phosphorylated serine 276, Cell Signaling; lane 10, cold, 50-fold excess of unlabeled oligonucleotide added to hyperoxia sample; lane 11, mutant, 50-fold excess of mutated oligonucleotide added to hyperoxia sample.
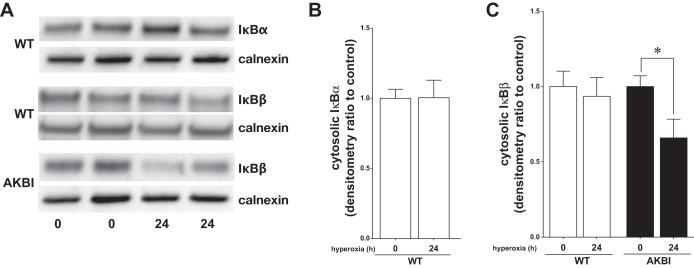
To define the signaling events upstream of sustained hyperoxia-induced NF-κB nuclear translocation observed in AKBI mice at 24 h of exposure, levels of immunoreactive IκBα and IκBβ were evaluated in lung cytosolic extracts. After 24 h of hyperoxia, levels of IκBα and IκBβ in WT were similar to unexposed controls (Fig. 5, A–C). In contrast, in AKBI mice, cytosolic IκBβ decreased significantly throughout the exposure (Fig. 5, A and C). These data suggest that hyperoxia promotes IκBβ degradation and that AKBI mice have sustained NF-κB signaling associated with nuclear IκBβ accumulation. Furthermore, these findings support that sustained IκBβ-mediated NF-κB activation dictates a protective response to hyperoxia given the difference in survival and lung development observed in AKBI mice.
Fig. 5.
Hyperoxia-induced IκBβ degradation is sustained in AKBI mice. A: representative Western blot showing IκBα and IκBβ in lung cytosolic extracts from WT and AKBI mice exposed to RA or 24 h of hyperoxia (O2 >95%), with calnexin as loading control. Densitometric evaluation of cytosolic IκBα (B) and cytosolic IκBβ (C) at 24 h of exposure. Values are means ± SE (n = 4/time point); *P < 0.05 vs. unexposed genotype control.
Sustained hyperoxia-induced NF-κB nuclear translocation is associated with increased expression of NF-κB target genes.
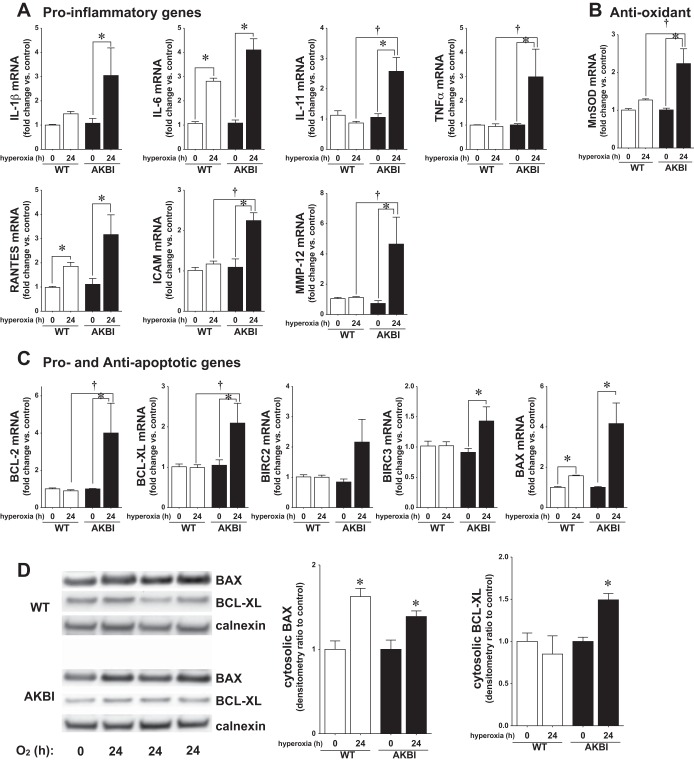
To understand what events downstream of NF-κB nuclear translocation contributed to improved survival and preserved lung development in the AKBI mice, we investigated the expression of NF-κB-regulated proteins previously implicated in hyperoxic lung injury and/or the pathogenesis of BPD (4, 6–9, 15, 34, 38, 56). These were broadly categorized into inflammation (IL-1β, IL-6, IL-11, TNF-α, RANTES, ICAM, and MMP-12), apoptosis (Bcl-2, Bcl-xL, BIRC2, BICR3, and Bax), antioxidant (MnSOD), and vascular growth related (VEGFR2, VEGFA) (59). Expression of all proinflammatory targets and MnSOD was significantly increased in AKBI mice exposed to 24 h of hyperoxia (Fig. 6, A and B). In contrast, only IL-6 was significantly increased in hyperoxia-exposed WT mice. Of the apoptosis-related genes, only proapoptotic Bax was significantly increased in WT mice, whereas Bax and the antiapoptotic proteins Bcl-2, Bcl-xL, and BIRC3 were increased in AKBI mice (Fig. 6C). Additionally, Western blot analysis revealed increased Bax in both WT and AKBI mice exposed to hyperoxia, whereas only AKBI mice demonstrated increased Bcl-xL (Fig. 6D).
Fig. 6.
Sustained NF-κB activation induces NF-κB target gene expression in AKBI mice. A: pulmonary proinflammatory mRNA expression. B: pulmonary antioxidant mRNA expression. MnSOD, manganese superoxide dismutase; RANTES, regulated on activation, normal T cell expressed, and presumably secreted; MMP, matrix metalloproteinase. C: pulmonary pro- and antiapoptotic mRNA expression. Values are means ± SE (n = 3–4/time point); *P < 0.05 vs. unexposed genotype control; †P <0.05 vs. paired WT exposure. BIRC, bacloviral IAP repeat-containing. D: representative Western blot showing Bax and Bcl-xL from lung cytosolic extracts from WT and AKBI mice exposed to RA or hyperoxia (O2 >95%, 0–24 h), with calnexin as a loading control. Densitometric evaluation of Bax and Bcl-xL is provided. Values are means ± SE (n = 3–4/time point); *P < 0.05 vs. unexposed genotype control.
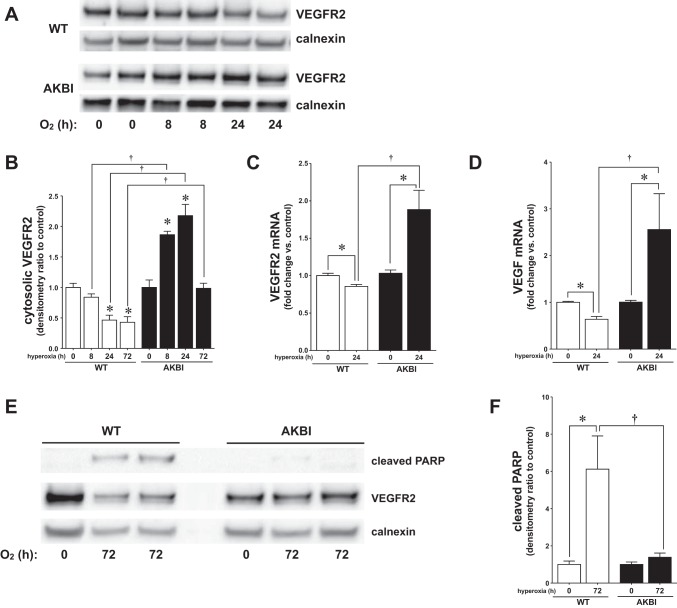
Previous studies have demonstrated that VEGF signaling through VEGFR2 attenuates hyperoxic lung injury (17, 30, 54). Furthermore, constitutive NF-κB activity plays a critical role in lung development, in part because of regulating VEGFR2 expression (34). WT mice exposed to hyperoxia demonstrated a gradual decrease in VEGFR2 protein levels over 72 h of exposure (Fig. 7, A, B, and E). In contrast, AKBI mice demonstrate a gradual and significant increase in VEGFR2 protein levels over the first 24 h of exposure, with a return to baseline by 72 h (Fig. 7, A, B, and E). Noting these differences in protein expression at 24 h of exposure, we assessed VEGFR2 gene expression in WT and AKBI mice. At 24 h of exposure, WT mice demonstrated a significant decrease in VEGFR2 mRNA expression (Fig. 7C). In contrast, VEGFR2 gene expression was significantly increased in hyperoxia-exposed AKBI mice (Fig. 7C). A similar pattern of expression was seen with the VEGFR2 ligand VEGF (Fig. 7D). These results suggest that sustained hyperoxia-induced NF-κB activation in the newborn lung prevents injury and preserves lung development through the sustained expression of cytoprotective genes including VEGFR2.
Fig. 7.
Sustained NF-κB activation induces protective gene expression in AKBI mice. A: representative Western blot showing VEGFR2 from lung cytosolic extracts from WT and AKBI mice exposed to RA or hyperoxia (O2 >95%, 0–72 h), with calnexin as a loading control. B: densitometric evaluation of VEGFR2. C: pulmonary VEGFR2 mRNA expression. D: pulmonary VEGF mRNA expression. E: representative Western blot showing cleaved poly(ADP-ribose) polymerase (PARP) from lung cytosolic extracts from WT and AKBI mice exposed to RA or hyperoxia (O2 >95%, 0–72 h), with calnexin as a loading control. F: densitometric evaluation of cleaved PARP. Values are means ± SE (n = 3–4/time point); *P < 0.05 vs. unexposed genotype control; †P <0.05 vs. paired WT exposure.
To assess the effect of these changes on apoptosis, cleaved PARP was evaluated at 72 h of hyperoxia, after changes in gene expression noted above. Consistent with increased NF-κB-regulated antiapoptotic target gene expression, cleaved PARP was not detected in AKBI mice exposed to hyperoxia (Fig. 7, E and F). In contrast, WT mice exposed to hyperoxia demonstrated significantly increased cleaved PARP at 72 h of hyperoxic exposure (Fig. 7, E and F). Together, these data suggest that sustained NF-κB activation attenuates apoptosis induced by hyperoxia.
Preservation of protective NF-κB target gene expression preserves pulmonary vessel density.
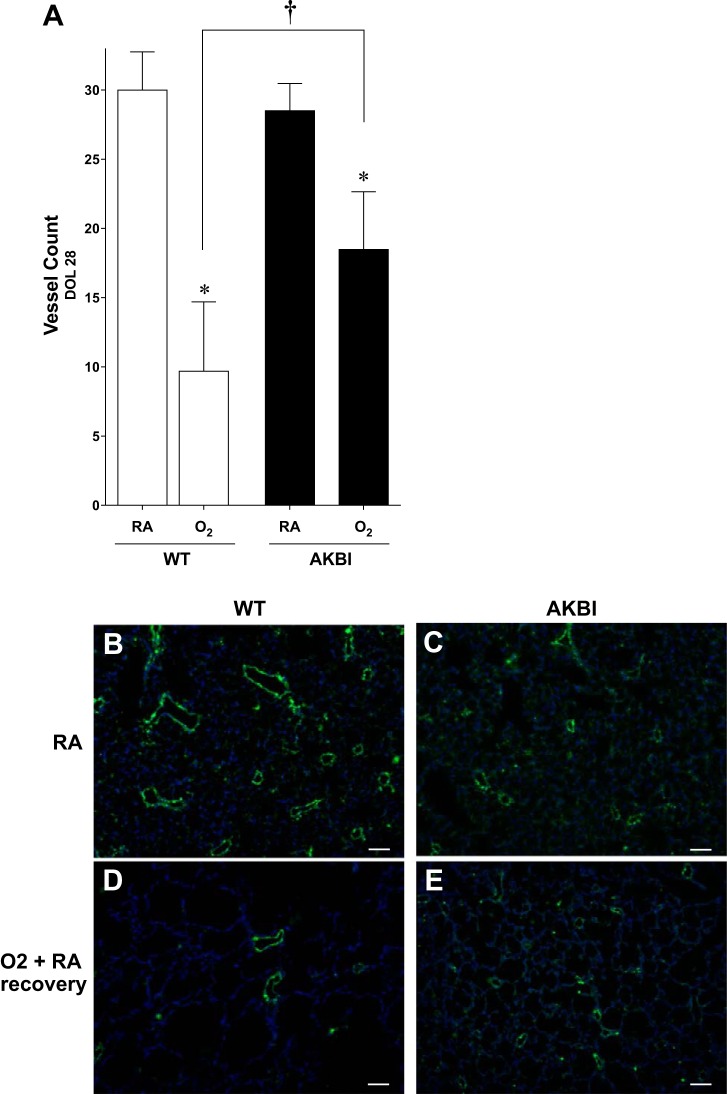
Previous studies have demonstrated that endothelial dysfunction contributes to the alveolar simplification and reduced lung surface area that characterizes BPD (50). We then determined the effect of sustained hyperoxia-induced NF-κB activation, and protective target gene expression on blood vessel density was assessed at DOL 28. Pulmonary vessel density was not different between WT and AKBI mice raised in room air (Fig. 8, A–C). Blood vessel density in WT mice exposed to 14 days of hyperoxia followed by 14 days in room air was reduced by 66% (Fig. 8, A, B, and D). In contrast, blood vessel density was reduced by only 32% in similarly exposed AKBI mice (Fig. 8, A, C, and E). Importantly, pulmonary vessel density in oxygen-exposed AKBI mice was significantly higher compared with similarly exposed WT mice (P < 0.05, Fig. 8, A, D, and E). These results suggest that sustained hyperoxia-induced NF-κB activation and target gene expression preserve pulmonary vascular development.
Fig. 8.
Pulmonary vessel density is preserved in AKBI mice exposed to hyperoxia followed by RA recovery. A: pulmonary vessel density at 28 days in WT and AKBI mice in RA or 95% O2 for 14 days followed by 14 days of RA (O2). *P < 0.05 vs. air exposed control, †P < 0.05 vs. paired WT exposure. Number represents an average in 5 high-power fields in 3 separate animals per condition. B–E: representative images of on Willebrand Factor -stained lung tissue from WT exposed to RA (B), AKBI exposed to RA (C), WT exposed to O2 and RA recovery (D), and AKBI exposed to O2 and RA recovery (E). All images were obtained using the ×10 objective lens, internal scale bar = 100 μM.
DISCUSSION
We demonstrated that hyperoxia-induced NF-κB activation occurs in neonatal WT mice and that IκBβ overexpression is associated with sustained NF-κB nuclear translocation. This in turn was associated with improved survival and enhanced lung development in neonatal mice exposed to hyperoxia for 14 days. Importantly, attenuation of lung injury and preservation of lung development, as assessed by RAC and MLI, could be detected early (3 days) and persisted after a prolonged (14 days) hyperoxic exposure. Enhanced expression of known NF-κB target genes, including VEGFR2 and antiapoptotic factors, may explain these findings and are associated with preserved pulmonary vascular growth following 14 days of hyperoxic exposure. Although previous studies have demonstrated that NF-κB activity mediates neonatal resistance to hyperoxia, before this report, the effect sustained NF-κB activity on lung development and ongoing lung injury had not been investigated. These results suggest that enhancing NF-κB activity in the newborn lung exposed to hyperoxia represents a potential therapeutic target to limit lung injury.
Recent clinical studies have demonstrated that limiting oxygen exposure in an attempt to minimize pulmonary and ophthalmological complications in the premature infant does not appear to be without risk (27, 51a). Thus it is likely that we will not reduce hyperoxic exposure in clinical practice in the near future. Furthermore, the pulmonary epithelium and endothelium of premature infants will continue endure injury secondary to hyperoxia. Therefore, the approach may need to be developing interventions to prevent hyperoxic injury while preserving lung development. Our findings show that enhancing NF-κB activity represents one such potential therapeutic intervention.
Previous studies have demonstrated that NF-κB activity plays an important role in lung development. Epithelial overexpression of p65 enhances lung development and is associated with decreased apoptosis and increased expression of antiapoptotic genes (42). Supporting this finding, both genetic (IKK-β−/−) and pharmacological (BAY 11–7082) inhibitions of NF-κB activity severely compromise lung development (34, 41). Interestingly, treating neonatal mice with the NF-κB inhibitor BAY 11–7082 inhibits VEGFR2 expression and results in decreased RAC and simplified alveolar structure (34), similar to what is seen with VEGFR2 inhibition (26, 35, 39). Previous reports have shown that hyperoxia disrupts VEGF and VEGFR2 expression in the neonatal lung and that VEGF treatment attenuates these findings (54). These studies clearly demonstrate the detrimental effect of NF-κB inhibition on lung development. Our study compliments this literature by demonstrating that sustained NF-κB activity during and following hyperoxic exposure maintains VEGFR2 expression and is associated with preservation of lung development.
In the current study, we demonstrate hyperoxia-induced NF-κB activity in the newborn lung of WT mice. Previous studies have demonstrated that NF-κB activity mediates neonatal resistance to hyperoxic lung injury (61). Despite this relative resistance, neonatal mice exposed to hyperoxia demonstrate disrupted lung development (57). Previous studies have not evaluated the effect of sustained NF-κB activity following exposure to hyperoxia. This report provides evidence that nuclear IκBβ is associated with a NF-κB response to hyperoxic stress. Specifically, nuclear retention of p65-containing NF-κB dimers protects the lung from ongoing injury and preserves lung development. We speculate that, in WT mice, hyperoxia acutely induces NF-κB activation, thus improving neonatal survival compared with similarly exposed adults. However, when this activity wanes, the developmentally appropriate constitutive NF-κB activity is disrupted, leading to decreased VEGFR2 expression and abnormal lung development. In contrast, sustained NF-κB activation in hyperoxia-exposed AKBI neonatal mice is associated with enhanced VEGFR2 expression, decreased apoptosis, and preserved pulmonary vessel density. The current study is supported by recent reports demonstrating that inhibiting apoptosis in the neonatal lung exposed to hyperoxia preserves lung development (48). We speculate that preservation of lung development after hyperoxic exposure could be achieved by sustaining NF-κB-regulated gene expression, including VEGFR2 and antiapoptotic proteins.
Possible mechanisms underlying our findings are enhanced NF-κB-DNA binding mediated by nuclear IκBβ. It is well established that IκBβ acts to enhance inflammatory stress-induced NF-κB-DNA binding and potentiates transcriptional activity and downstream gene expression (47, 55). Interestingly, sustained LPS-induced NF-κB activation mediated by IκBβ increases proinflammatory gene expression and increases mortality in adult mice (47, 49). Furthermore, previous studies have shown that inflammatory stress-induced NF-κB activation disrupts lung branching and lung morphogenesis (5, 10). In contrast, multiple studies have demonstrated that NF-κB activity is protective against oxidative stress, demonstrated by increased injury with NF-κB inhibition (13, 22, 43, 52, 58). Our results demonstrate that sustained NF-κB activation is associated with increased expression of multiple protective target genes, including Bcl-2, Bcl-xL, MnSOD, and VEGFR2. We have recently demonstrated that adult mice overexpressing IκBβ and exhibiting sustained hyperoxia-induced NF-κB activation have improved survival attributable to enhanced expression of NF-κB regulated antiapoptotic genes (44). Of note, neonatal AKBI mice exposed to hyperoxia demonstrate early and enhanced expression of proinflammatory NF-κB-regulated target genes compared with similarly exposed WT mice and previous reports (4, 15, 19, 37, 56, 57). This would suggest that hyperoxic lung injury can be attenuated without specifically targeting proinflammatory pathways. Despite this, enhanced NF-κB activation mediated by nuclear IκBβ in neonatal mice affords protection against hyperoxic lung injury and preserves lung development.
The study has some limitations. Although we demonstrate that sustained hyperoxia-induced NF-κB nuclear translocation and enhanced target gene expression in the AKBI mice, these determinations were made on whole lung homogenate and in mice with global, not lung or specific cell type, IκBβ overexpression. It is well known that lung cell types are differentially susceptible to oxygen toxicity, with the most damaging effects seen in the pulmonary endothelium (18). Furthermore, the AKBI mice overexpress IκBβ, but, in addition, they do not express IκBα. Our results indicate that sustained hyperoxia-induced nuclear NF-κB translocation is associated with nuclear IκBβ. However, the current model does not allow us to determine the relative contribution of IκBβ overexpression vs. deficiency of IκBα. Early studies showed that IκBα−/− mice died early in the perinatal period (3). To overcome this problem, an IκBβ knockin (AKBI) mouse was generated and described as phenotypically indistinct from their WT controls (14). Undoubtedly, the combination lacking IκBα-driven nuclear export of NF-κB and enhanced IκBβ-NF-κB-DNA binding contributes to our findings. Further studies are necessary to determine the roles of these unique functions of IκBα and IκBβ. Furthermore, it is not known whether our findings are attributable to enhanced NF-κB activation in all cells, a subset of cells, or in cells recruited to the lung following exposure to hyperoxia. Additionally, although sustained NF-κB activation may explain the increased expression of VEGFR2 demonstrated in AKBI mice, it is possible that enhanced endothelial survival explains these findings. It remains unknown whether targeting enhanced NF-κB activation to cells most susceptible to oxygen toxicity would provide the same or additional benefit. The use of conditional IκBβ-overexpressing mice could help answer this question. We have not fully interrogated the mechanisms to explain the disparate role played by sustained NF-κB activation in mediating the response to inflammatory vs. oxidant stress. Determining these mechanisms remains critically important to determine potential therapies aimed at preserving lung development in neonates exposed to oxidants and/or inflammatory stress. Finally, we chose to evaluate NF-κB activity early in the course of hyperoxic exposure, before the effect of hyperoxia on lung development seen in WT mice at DOL 3 (Fig. 2). We were interested in the early effects of hyperoxia, as early studies of neonatal mice suggested that the initial pulmonary response to hyperoxia (3 days) was critical in determining survival (11). These studies were designed to help understand whether intervening early in the course of oxygen exposure would have an effect on lung development. Although we demonstrate clear differences in hyperoxia-induced NF-κB activation between WT and AKBI mice, it is likely that there are differences that extend beyond 24 h of exposure, and this needs to be further verified.
We conclude that sustained NF-κB activation improves survival and preserves lung development in neonatal mice exposed to hyperoxia. Sustained hyperoxia-induced NF-κB activation is associated with enhanced expression of antiapoptotic factors and VEGFR2. We speculate that enhancing NF-κB activation may represent a therapeutic intervention to attenuate hyperoxia-induced lung injury.
GRANTS
This work was supported by NIH grant K08 HL098562 to C. Wright and HL058752-11 to P. Dennery.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.M., G.Y., P.A.D., and C.J.W. conception and design of research; S.M., K.A.M., F.A., T.L., K.H., and C.J.W. performed experiments; S.M., K.A.M., F.A., T.L., K.H., G.Y., P.A.D., and C.J.W. analyzed data; S.M., G.Y., P.A.D., and C.J.W. interpreted results of experiments; S.M., G.Y., P.A.D., and C.J.W. edited and revised manuscript; S.M., K.A.M., F.A., T.L., K.H., G.Y., P.A.D., and C.J.W. approved final version of manuscript; C.J.W. prepared figures; C.J.W. drafted manuscript.
REFERENCES
- 1.Balasubramaniam V, Maxey AM, Morgan DB, Markham NE, Abman SH. Inhaled NO restores lung structure in eNOS-deficient mice recovering from neonatal hypoxia. Am J Physiol Lung Cell Mol Physiol 291: L119–L127, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Balasubramaniam V, Ryan SL, Seedorf GJ, Roth EV, Heumann TR, Yoder MC, Ingram DA, Hogan CJ, Markham NE, Abman SH. Bone marrow-derived angiogenic cells restore lung alveolar and vascular structure after neonatal hyperoxia in infant mice. Am J Physiol Lung Cell Mol Physiol 298: L315–L323, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science 274: 782–784, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Ben-Ari J, Makhoul IR, Dorio RJ, Buckley S, Warburton D, Walker SM. Cytokine response during hyperoxia: sequential production of pulmonary tumor necrosis factor and interleukin-6 in neonatal rats. Isr Med Assoc J 2: 365–369, 2000 [PubMed] [Google Scholar]
- 5.Benjamin JT, Carver BJ, Plosa EJ, Yamamoto Y, Miller JD, Liu JH, van der Meer R, Blackwell TS, Prince LS. NF-kappaB activation limits airway branching through inhibition of Sp1-mediated fibroblast growth factor-10 expression. J Immunol 185: 4896–4903, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhandari A, Bhandari V. Biomarkers in bronchopulmonary dysplasia. Paediatr Respir Rev 14: 173–179, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Bhandari A, Bhandari V. Pitfalls, problems, and progress in bronchopulmonary dysplasia. Pediatrics 123: 1562–1573, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Bhandari V. Hyperoxia-derived lung damage in preterm infants. Semin Fetal Neonatal Med 15: 223–229, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhandari V, Elias JA. Cytokines in tolerance to hyperoxia-induced injury in the developing and adult lung. Free Radic Biol Med 41: 4–18, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Blackwell TS, Hipps AN, Yamamoto Y, Han W, Barham WJ, Ostrowski MC, Yull FE, Prince LS. NF-kappaB signaling in fetal lung macrophages disrupts airway morphogenesis. J Immunol 187: 2740–2747, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonikos DS, Bensch KG, Ludwin SK, Northway WH., Jr Oxygen toxicity in the newborn. The effect of prolonged 100 per cent O2 exposure on the lungs of newborn mice. Lab Invest 32: 619–635, 1975 [PubMed] [Google Scholar]
- 12.Cazals V, Nabeyrat E, Corroyer S, de Keyzer Y, Clement A. Role for NF-kappa B in mediating the effects of hyperoxia on IGF-binding protein 2 promoter activity in lung alveolar epithelial cells. Biochim Biophys Acta 1448: 349–362, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Chen LW, Egan L, Li ZW, Greten FR, Kagnoff MF, Karin M. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med 9: 575–581, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Cheng JD, Ryseck RP, Attar RM, Dambach D, Bravo R. Functional redundancy of the nuclear factor kappa B inhibitors I kappa B alpha and I kappa B beta. J Exp Med 188: 1055–1062, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choo-Wing R, Nedrelow JH, Homer RJ, Elias JA, Bhandari V. Developmental differences in the responses of IL-6 and IL-13 transgenic mice exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol 293: L142–L150, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 2–intrauterine and early postnatal lung growth. Thorax 37: 580–583, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corne J, Chupp G, Lee CG, Homer RJ, Zhu Z, Chen Q, Ma B, Du Y, Roux F, McArdle J, Waxman AB, Elias JA. IL-13 stimulates vascular endothelial cell growth factor and protects against hyperoxic acute lung injury. J Clin Invest 106: 783–791, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crapo JD. Morphologic changes in pulmonary oxygen toxicity. Annu Rev Physiol 48: 721–731, 1986 [DOI] [PubMed] [Google Scholar]
- 19.D'Angio CT, Johnston CJ, Wright TW, Reed CK, Finkelstein JN. Chemokine mRNA alterations in newborn and adult mouse lung during acute hyperoxia. Exp Lung Res 24: 685–702, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Dieperink HI, Blackwell TS, Prince LS. Hyperoxia and apoptosis in developing mouse lung mesenchyme. Pediatr Res 59: 185–190, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Emery JL, Mithal A. The number of alveoli in the terminal respiratory unit of man during late intrauterine life and childhood. Arch Dis Child 35: 544–547, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franek WR, Horowitz S, Stansberry L, Kazzaz JA, Koo HC, Li Y, Arita Y, Davis JM, Mantell AS, Scott W, Mantell LL. Hyperoxia inhibits oxidant-induced apoptosis in lung epithelial cells. J Biol Chem 276: 569–575, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Franek WR, Morrow DM, Zhu H, Vancurova I, Miskolci V, Darley-Usmar K, Simms HH, Mantell LL. NF-kappaB protects lung epithelium against hyperoxia-induced nonapoptotic cell death-oncosis. Free Radic Biol Med 37: 1670–1679, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Frank L, Bucher JR, Roberts RJ. Oxygen toxicity in neonatal and adult animals of various species. J Appl Physiol 45: 699–704, 1978 [DOI] [PubMed] [Google Scholar]
- 25.George CL, Fantuzzi G, Bursten S, Leer L, Abraham E. Effects of lisofylline on hyperoxia-induced lung injury. Am J Physiol 276: L776–L785, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development 126: 1149–1159, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Group BIUKC, Group BIAC, Group BINZC, Stenson BJ, Tarnow-Mordi WO, Darlow BA, Simes J, Juszczak E, Askie L, Battin M, Bowler U, Broadbent R, Cairns P, Davis PG, Deshpande S, Donoghoe M, Doyle L, Fleck BW, Ghadge A, Hague W, Halliday HL, Hewson M, King A, Kirby A, Marlow N, Meyer M, Morley C, Simmer K, Tin W, Wardle SP, Brocklehurst P. Oxygen saturation and outcomes in preterm infants. N Engl J Med 368: 2094–2104, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Haddad JJ, Land SC. O2-evoked regulation of HIF-1α and NF-κB in perinatal lung epithelium requires glutathione biosynthesis. Am J Physiol Lung Cell Mol Physiol 278: L492–L503, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Hayden M, Ghosh S. Shared principles in NF-kappaB signaling. Cell 132: 344–362, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Ho YS, Vincent R, Dey MS, Slot JW, Crapo JD. Transgenic models for the study of lung antioxidant defense: enhanced manganese-containing superoxide dismutase activity gives partial protection to B6C3 hybrid mice exposed to hyperoxia. Am J Respir Cell Mol Biol 18: 538–547, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann A, Levchenko A, Scott M, Baltimore D. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science 298: 1241–1245, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Huang TT, Kudo N, Yoshida M, Miyamoto S. A nuclear export signal in the N-terminal regulatory domain of IkappaBalpha controls cytoplasmic localization of inactive NF-kappaB/IkappaBalpha complexes. Proc Natl Acad Sci USA 97: 1014–1019, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hummler SC, Rong M, Chen S, Hehre D, Alapati D, Wu S. Targeting glycogen synthase kinase-3beta to prevent hyperoxia-induced lung injury in neonatal rats. Am J Respir Cell Mol Biol 48: 578–588, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Iosef C, Alastalo TP, Hou Y, Chen C, Adams ES, Lyu SC, Cornfield DN, Alvira CM. Inhibiting NF-κB in the developing lung disrupts angiogenesis and alveolarization. Am J Physiol Lung Cell Mol Physiol 302: L1023–L1036, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol 279: L600–L607, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res 46: 641–643, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Johnston CJ, Wright TW, Reed CK, Finkelstein JN. Comparison of adult and newborn pulmonary cytokine mRNA expression after hyperoxia. Exp Lung Res 23: 537–552, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Kojima T, Sasai M, Kobayashi Y. Increased soluble ICAM-1 in tracheal aspirates of infants with bronchopulmonary dysplasia. Lancet 342: 1023–1024, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Le Cras TD, Markham NE, Tuder RM, Voelkel NF, Abman SH. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am J Physiol Lung Cell Mol Physiol 283: L555–L562, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Zhang W, Mantell LL, Kazzaz JA, Fein AM, Horowitz S. Nuclear factor-kappaB is activated by hyperoxia but does not protect from cell death. J Biol Chem 272: 20646–20649, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Londhe VA, Maisonet TM, Lopez B, Jeng JM, Xiao J, Li C, Minoo P. Conditional deletion of epithelial IKKbeta impairs alveolar formation through apoptosis and decreased VEGF expression during early mouse lung morphogenesis. Respir Res 12: 134, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Londhe VA, Nguyen HT, Jeng JM, Li X, Li C, Tiozzo C, Zhu N, Minoo P. NF-kB induces lung maturation during mouse lung morphogenesis. Dev Dyn 237: 328–338, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Lu X, Liu H, Wang L, Schaefer S. Activation of NF-κB is a critical element in the antiapoptotic effect of anesthetic preconditioning. Am J Physiol Heart Circ Physiol 296: H1296–H1304, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Michaelis KA, Agboke F, Liu T, Han K, Muthu M, Galambos C, Yang G, Dennery PA, Wright CJ. IkappaBbeta-mediated NF-kappaB activation confers protection against hyperoxic lung injury. Am J Respir Cell Mol Biol 50: 429–438, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol 8: 49–62, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Rao P, Hayden MS, Long M, Scott ML, West AP, Zhang D, Oeckinghaus A, Lynch C, Hoffmann A, Baltimore D, Ghosh S. IkappaBbeta acts to inhibit and activate gene expression during the inflammatory response. Nature 466: 1115–1119, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakurai R, Villarreal P, Husain S, Liu J, Sakurai T, Tou E, Torday JS, Rehan VK. Curcumin protects the developing lung against long-term hyperoxic injury. Am J Physiol Lung Cell Mol Physiol 305: L301–L311, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheibel M, Klein B, Merkle H, Schulz M, Fritsch R, Greten FR, Arkan MC, Schneider G, Schmid RM. IkappaBbeta is an essential co-activator for LPS-induced IL-1beta transcription in vivo. J Exp Med 207: 2621–2630, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol 67: 623–661, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sanchez PJ, O'Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID, 3rd, Watterberg KL, Saha S, Das A, Higgins RD, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126: 443–456, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51a.SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Carlo WA, Finer NN, Walsh MC, Rich W, Gantz MG, Laptook AR, Yoder BA, Faix RG, Das A, Poole WK, Schibler K, Newman NS, Ambalavanan N, Frantz ID, 3rd, Piazza AJ, Sánchez PJ, Morris BH, Laroia N, Phelps DL, Poindexter BB, Cotten CM, Van Meurs KP, Duara S, Narendran V, Sood BG, O'Shea TM, Bell EF, Ehrenkranz RA, Watterberg KL, Higgins RD. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med 362: 1959–1969, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tahepold P, Vaage J, Starkopf J, Valen G. Hyperoxia elicits myocardial protection through a nuclear factor kappaB-dependent mechanism in the rat heart. J Thorac Cardiovasc Surg 125: 650–660, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Tergaonkar V, Correa R, Ikawa M, Verma I. Distinct roles of IkappaB proteins in regulating constitutive NF-kappaB activity. Nat Cell Biol 7: 921–923, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Thebaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, Archer SL. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation 112: 2477–2486, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Thompson J, Phillips R, Erdjument-Bromage H, Tempst P, Ghosh S. I kappa B-beta regulates the persistent response in a biphasic activation of NF-kappa B. Cell 80: 573–582, 1995 [DOI] [PubMed] [Google Scholar]
- 56.Wagenaar GT, ter Horst SA, van Gastelen MA, Leijser LM, Mauad T, van der Velden PA, de Heer E, Hiemstra PS, Poorthuis BJ, Walther FJ. Gene expression profile and histopathology of experimental bronchopulmonary dysplasia induced by prolonged oxidative stress. Free Radic Biol Med 36: 782–801, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol 275: L110–117, 1998 [DOI] [PubMed] [Google Scholar]
- 58.Wright CJ, Agboke F, Muthu M, Michaelis KA, Mundy MA, La P, Yang G, Dennery PA. Nuclear factor-kappaB (NF-kappaB) inhibitory protein IkappaBbeta determines apoptotic cell death following exposure to oxidative stress. J Biol Chem 287: 6230–6239, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wright CJ, Kirpalani H. Targeting inflammation to prevent bronchopulmonary dysplasia: can new insights be translated into therapies? Pediatrics 128: 111–126, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wright CJ, Zhuang T, La P, Yang G, Dennery PA. Hyperoxia-induced NF-κB activation occurs via a maturationally sensitive atypical pathway. Am J Physiol Lung Cell Mol Physiol 296: L296–L306, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang G, Abate A, George AG, Weng YH, Dennery PA. Maturational differences in lung NF-kappaB activation and their role in tolerance to hyperoxia. J Clin Invest 114: 669–678, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang G, Madan A, Dennery PA. Maturational differences in hyperoxic AP-1 activation in rat lung. Am J Physiol Lung Cell Mol Physiol 278: L393–L398, 2000 [DOI] [PubMed] [Google Scholar]
- 63.Zoetis T, Hurtt ME. Species comparison of lung development. Birth Defects Res B Dev Reprod Toxicol 68: 121–124, 2003 [DOI] [PubMed] [Google Scholar]