Abstract
About a decade ago everyone who read, at least occasionally, the science part of the local newspapers or followed popular science broadcasts “knew” that reactive oxygen species (ROS) are dangerous. They were thought to be bad, really bad, and many looking for a healthier lifestyle took action against these nasty small molecules accused of harming lipids and proteins by taking antioxidants like vitamin E. Food industry teamed up with pharmaceutical companies and brought up functional food enriched or supplemented with trustful antioxidants in the fight against ROS, which seemed to be produced under pathological conditions with just one mission: to damage the body. Things have changed during recent years, and there is emerging evidence that ROS are not only continuously produced even in healthy individuals but also exert important physiological functions. While we are still in-between a period that provides exciting new findings on the physiology of ROS, one can already summarize our present knowledge rather accurately by citing Paracelsus’ adage of “Dosis (sola) facit venenum” (tertio defensio, 1538).
The present work of Edwards et al1 is one of these exciting new reports that open our eyes to recognize the good side of the previously dammed ROS. This article deals with the effect of hydrogen peroxide (H2O2) on endothelium-derived hyperpolarization and is quite remarkable in several ways:
First, the article is focused on EDHF (formerly termed endothelium-derived hyperpolarizing factor), a nitric oxide (NO) and prostacyclin-independent phenomenon of endothelium-dependent relaxation, of which the molecular mechanism and nature are still somewhat mysterious. Thus, this endothelium-dependent smooth muscle hyperpolarization has been attributed to either the formation and release of diffusible factors from the endothelium2 such as potassium ions (K+),3 cytochrome P450-derived arachidonic acid metabolites (epoxyeicosatrienoic acids),4,5 anandamide,6 or even H2O27 but also to a direct electric coupling between endothelial and smooth muscle cells.8 The latter work was largely guided and conducted by Dr Griffith and his team, who convincingly showed that gap junctions between endothelial and smooth muscle cells are a prerequisite to spread EDHF-type relaxations in many vascular beds.9 In this context, it is interesting that despite the ongoing debate on the nature of EDHF—most probably there is more than one such “factor”—the authors used EDHF-type relaxation of isolated ring segments of the rat iliac artery as readout for endothelial cell EDHF generation in their study. For scientists who were involved in or witnessed the early days of NO research, such procedure provides a déjà vu of the bioassay experiments performed at the time when the nature of endothelium-derived relaxing factor (EDRF or NO) was still unknown.10
Second, the article addresses the rather unusual beneficial effect of H2O2—formerly denounced as one of the worst ROS—on the generation of EDHF in endothelial cells. H2O2 represents the end product of the “detoxification” of superoxide anions (O2−) by 1 of 3 different superoxide dismutases (CuZn-SOD, SOD1; Mn-SOD, SOD2; EC-SOD, SOD3) in endothelial cells, so that its accumulation in the cell reflects an increase in O2− formation, which is most likely accompanied by a decreased bioavailability of NO because of its ultra fast reaction with O2−.11 This observation, along with previous findings demonstrating that EDHF-type relaxations frequently become detectable only or are accentuated in conditions of “endothelial dysfunction”, ie, a reduced bioavailability of NO,12 reinforces the view that at least 2 endothelium-derived vasodilator principles coexist whereby NO limits the generation of EDHF.13 EDHF-mediated relaxation may thus serve as a backup system and substitute for a diminished NO-mediated relaxation in conditions of “oxidative stress”, ie, an increased vascular O2− formation, such as in, eg, diabetes mellitus.14
Surprisingly, just about two decades ago or so it was realized that molecular oxygen not only is essential for aerobic metabolism but also gives rise to the formation of ROS which can act both as powerful defensive arms, eg, against invading microorganisms, and as intercellular or intracellular signaling molecules. Most prominent among these ROS is O2−, generated by Nox-2– or Nox-4–containing NADPH oxidase in endothelial cells,15 and its primary SOD-derived or nonenzymatic dismutation product H2O2.16 Both molecules, either directly or indirectly, eg, by way of secondary peroxynitrite formation with NO (O2−) and through metal-catalyzed generation of hydroxyl radicals (H2O2), are capable of altering proteins chemically thus influencing their function. To date many signal transduction pathways have been characterized that operate at least in part through enzymatic ROS formation and consecutive protein modification, thereby eliciting changes in gene expression, cell migration, and proliferation, but also in ion channel activity.17 Chief modifications comprise a direct oxidation of the target protein, namely that of amino acids with a thiol group such as cysteine, oxidative glycation, and carbonylation.18-20 In this context, it is noteworthy that not the oxidation of cysteine residues, as favored by the authors in the discussion, but oxidative protein carbonylation may represent the most frequent type of protein modification in conditions of oxidative stress.
Oxidative carbonylation preferentially occurs at the amino acids proline, threonine, lysine, and arginine, presumably through a metal-catalyzed activation of hydrogen peroxide to a reactive intermediate, and according to a recent study conducted with mammalian vascular smooth muscle cells the carbonylated proteins can—comparable with the reduction of oxidized thiols—be decarbonylated as well, thus offering a whole host of novel possibilities for intracellular signal transduction.18,20 In this context, it would be quite interesting to find out whether the sensitivity of the inositol-1,4,5-trisphosphate (inositol triphosphate [IP3])–stimulated calcium ion (Ca2+) release channel in the endoplasmic reticulum (ER) of endothelial cells can in fact be altered by oxidation of critical sulfhydryl groups, as suggested by the authors, or by (reversible) oxidative carbonylation. In this way, the potentially harmful ROS may be transformed into physiologically important second messengers that, eg, help to maintain or even improve endothelial cell Ca2+ homeostasis.
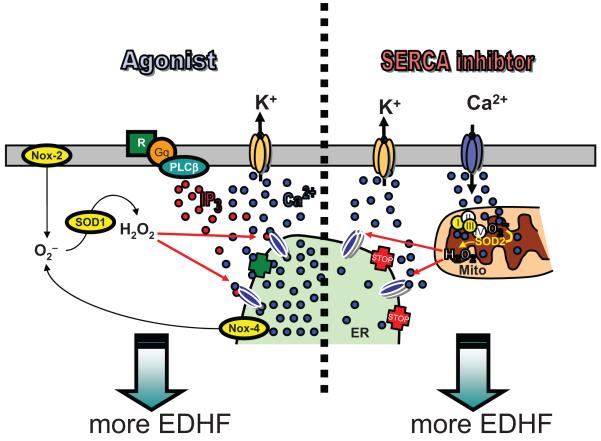
Third, and perhaps most importantly, the article provides evidence that H2O2 enhances EDHF formation through its stimulatory effect on endothelial Ca2+ homeostasis. While an effect of ROS on endothelial Ca2+ signaling per se is not unprecedented,17 the study by Edwards et al is insofar a surprise that the SERCA inhibitor cyclopiazonic acid (CPA) was used.1 In most studies dealing with an impact of ROS on endothelial Ca2+ homeostasis so far, an increase in intracellular Ca2+ was evoked by IP3-generating agonists such as bradykinin, histamine, or ATP, whereas SERCA inhibitors were frequently used as receptor-independent control stimulus that is insensitive to ROS, at least in terms of intracellular Ca2+ mobilization. The data obtained by the authors argue for a sensitization by H2O2 of IP3 receptors resulting in an accelerated Ca2+ leakage from the ER that becomes evident when SERCA activity is blocked (Figure). Although this hypothesis seems conclusive, the observation that H2O2 augments CPA-triggered intracellular Ca2+ mobilization only at concentrations of the SERCA inhibitor below or equal to 10 μmol/L may deserve further attention. In fact, passive leakage of Ca2+ should be even more pronounced when SERCA activity is blocked more effectively. On the other hand, as demonstrated by the authors, higher concentrations of CPA are also more effective in depleting the intracellular Ca2+ store so that no additional effect by H2O2 might be detectable. ER IP3 receptors, however, have been shown to be closely linked, on a functional level, with mitochondrial Ca2+ uniporters resulting in the establishment of a concerted interorganelle Ca2+ cycling21 for which some SERCA activity would seem to be essential, eg, by affecting mitochondrial Ca2+ handling. Another important aspect to be solved is whether H2O2, besides its effect on basal IP3 receptor activity (ie, Ca2+ leakage through the channel), also affects its IP3-induced opening.
Figure.
Schematic illustration of the interplay between the intracellular generation of H2O2 and intracellular Ca2+ mobilization from the endoplasmic reticulum (ER) as a consequence of the effect of H2O2 on inositol-1,4,5 trisphosphate (IP3) receptors that was described by Edwards et al.
Fourth, calcium ions probably represent the most versatile (second) messengers in living nature. The physiological consequences of the observed augmentation by H2O2 of the mobilization of intracellular Ca2+ most likely exceed those for the formation of EDHF by far. A more pronounced rise in endothelial Ca2+, for example, has been associated with an elevated synthesis/release of the (potent) vasoconstrictor endothelin-1,22 which might counteract the vasodilator effect of EDHF on the vasculature, more precisely the smooth muscle cells of the media. Moreover, a continuously elevated release by H2O2 of Ca2+ from the ER that is accompanied by an accumulation of Ca2+ in the mitochondria may on the one hand trigger an increased biosynthesis of ATP23 but then, if the worst comes to the worst, may also result in mitochondria-induced apoptosis.24 Furthermore, the cytosolic Ca2+ level governs the activity of numerous signaling pathways and transcription factors, and maintenance of the Ca2+ concentration in the ER is a prerequisite for correct protein folding therein. In this respect, cellular Ca2+ homeostasis needs to be tightly regulated to avoid any uncontrolled activation of Ca2+-senstive processes. Despite its beneficial effect on EDHF formation, the physiological or pathophysiological consequences of the enhanced leakage of the intracellular Ca2+ store in the presence of H2O2 can thus not be properly surveyed at present and require further investigation.
So there is yet another solid piece of evidence that our judgment on the contribution of ROS in physiology and pathophysiology needs to be reconsidered. Moreover, the current work by Edwards et al1 again points at Ca2+ as one of the major second messengers, particularly in endothelial cells, and supports the concept of EDHF as an important principle of vasodilatation and not just an epiphenomenon of the NO-mediated control of blood flow. One of several questions that nonetheless needs to be answered is where or what the endogenous source of H2O2 in the native endothelial cells is. It may the mitochondria reacting to an increase in intracellular Ca2+, the agonist-dependent NADPH oxidase assembled at the plasma membrane (Nox-2), or the constitutively active NADPH oxidase situated in the ER (Nox-4). In each case, effective enzymatic dismutation of the primary reaction product O2− to H2O2 seems to be a must.
Acknowledgments
Sources of Funding
Research in the laboratories of Drs Graier and Hecker is supported by the Austrian Science Funds (FWF; P20181-B05, F 3010-B05), the German Research Foundation (DFG; SFB/TR 23-C5, SFB 405-B17, GRK 880, HE 1587/9-1), and the Federal Ministry of Education and Research (BMBF; 01GR0822), respectively.
Footnotes
Disclosures
None.
Contributor Information
Wolfgang F. Graier, Institute of Molecular Biology and Biochemistry, Center of Molecular Medicine, Medical University Graz, Austria
Markus Hecker, Institute of Physiology and Pathophysiology, Division of Cardiovascular Physiology, University of Heidelberg, Germany.
References
- 1.Edwards D, Li Y, Griffith T. Hydrogene peroxide potentiates the EDHF phenomenon by promoting endothelial Ca2+ mobilization. Arterioscler Thromb Vasc Biol. 2008;28:1774–1781. doi: 10.1161/ATVBAHA.108.172692. [DOI] [PubMed] [Google Scholar]
- 2.Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 3.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 4.Hayabuchi Y, Nakaya Y, Matsuoka S, Kuroda Y. Endothelium-derived hyperpolarizing factor activates Ca2+-activated K+ channels in porcine coronary artery smooth muscle cells. J Cardiovasc Pharmacol. 1998;32:642–649. doi: 10.1097/00005344-199810000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- 6.Randall MD, Kendall DA. Involvement of a cannabinoid in endothelium-derived hyperpolarizing factor-mediated coronary vasorelaxation. Eur J Pharmacol. 1997;335:205–209. doi: 10.1016/s0014-2999(97)01237-5. [DOI] [PubMed] [Google Scholar]
- 7.Shimokawa H, Morikawa K. Hydrogen peroxide is an endotheliumderived hyperpolarizing factor in animals and humans. J Mol Cell Cardiol. 2005;39:725–732. doi: 10.1016/j.yjmcc.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Griffith TM. Endothelium-dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis? Br J Pharmacol. 2004;141:881–903. doi: 10.1038/sj.bjp.0705698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris D, Martin PE, Evans WH, Kendall DA, Griffith TM, Randall MD. Role of gap junctions in endothelium-derived hyperpolarizing factor responses and mechanisms of K+-relaxation. Eur J Pharmacol. 2000;402:119–128. doi: 10.1016/s0014-2999(00)00512-4. [DOI] [PubMed] [Google Scholar]
- 10.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 11.Graier WF, Posch K, Wascher TC, Kostner GM. Role of superoxide anions in changes of endothelial vasoactive response during acute hyperglycemia. Horm Metab Res. 1997;29:622–626. doi: 10.1055/s-2007-979113. [DOI] [PubMed] [Google Scholar]
- 12.Bauersachs J, Popp R, Hecker M, Sauer E, Fleming I, Busse R. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation. 1996;94:3341–3347. doi: 10.1161/01.cir.94.12.3341. [DOI] [PubMed] [Google Scholar]
- 13.Fleming I, Busse R. Endothelium-derived epoxyeicosatrienoic acids and vascular function. Hypertension. 2006;47:629–633. doi: 10.1161/01.HYP.0000208597.87957.89. [DOI] [PubMed] [Google Scholar]
- 14.Fleischhacker E, Esenabhalu VE, Spitaler M, Holzmann S, Skrabal F, Koidl B, Kostner GM, Graier WF. Human diabetes is associated with hyperreactivity of vascular smooth muscle cells due to altered subcellular Ca2+ distribution. Diabetes. 1999;48:1323–1330. doi: 10.2337/diabetes.48.6.1323. [DOI] [PubMed] [Google Scholar]
- 15.Brandes RP, Schröder K. Composition and functions of vascular nicotinamide adenine dinucleotide phosphate oxidases. Trends Cardiovasc Med. 2008;18:15–19. doi: 10.1016/j.tcm.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Cai H. NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ Res. 2005;96:818–822. doi: 10.1161/01.RES.0000163631.07205.fb. [DOI] [PubMed] [Google Scholar]
- 17.Spitaler MM, Graier WF. Vascular targets of redox signalling in diabetes mellitus. Diabetologia. 2002;45:476–494. doi: 10.1007/s00125-002-0782-0. [DOI] [PubMed] [Google Scholar]
- 18.Dalle-Donne I, Aldini G, Carini M, Colombo R, Rossi R, Milzani A. Protein carbonylation, cellular dysfunction, and disease progression. J Cell Mol Med. 2006;10:389–406. doi: 10.1111/j.1582-4934.2006.tb00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.England K, Cotter TG. Direct oxidative modifications of signalling proteins in mammalian cells and their effects on apoptosis. Redox Rep. 2005;10:237–245. doi: 10.1179/135100005X70224. [DOI] [PubMed] [Google Scholar]
- 20.Wong CM, Cheema AK, Zhang L, Suzuki YJ. Protein carbonylation as a novel mechanism in redox signaling. Circ Res. 2008;102:310–318. doi: 10.1161/CIRCRESAHA.107.159814. [DOI] [PubMed] [Google Scholar]
- 21.Szabadkai G, Simoni AM, Rizzuto R. Mitochondrial Ca2+ uptake requires sustained Ca2+ release from the endoplasmic reticulum. J Biol Chem. 2003;278:15153–15161. doi: 10.1074/jbc.M300180200. [DOI] [PubMed] [Google Scholar]
- 22.Brunner F, Stessel H, Simecek S, Graier W, Kukovetz WR. Effect of intracellular Ca2+ concentration on endothelin-1 secretion. FEBS Lett. 1994;350:33–36. doi: 10.1016/0014-5793(94)00727-6. [DOI] [PubMed] [Google Scholar]
- 23.Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci U S A. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demaurex N, Distelhorst C. Apoptosis - the calcium connection. Science. 2003;300:65–67. doi: 10.1126/science.1083628. [DOI] [PubMed] [Google Scholar]