Abstract
In this study, we prepared cordycepin-enriched (CE)-WIB801C, a n-butanol extract of Cordyceps militaris-hypha, and investigated the effect of CE-WIB801C on collagen-induced human platelet aggregation. CE-WIB801C dose-dependently inhibited collagen-induced platelet aggregation, and its IC50 value was 175 μg/ml. CE-WIB801C increased cAMP level more than cGMP level, but inhibited collagen-elevated [Ca2+]i mobilization and thromboxane A2 (TXA2) production. cAMP-dependent protein kinase (A-kinase) inhibitor Rp-8-Br-cAMPS increased the CE-WIB801C-downregulated [Ca2+]i level in a dose dependent manner, and strongly inhibited CE-WIB801C-induced inositol 1, 4, 5-trisphosphate receptor (IP3R) phosphorylation. These results suggest that the inhibition of [Ca2+]i mobilization by CE-WIB801C is resulted from the cAMP/A-kinase-dependent phosphorylation of IP3R. CE-WIB801C suppressed TXA2 production, but did not inhibit the activities of cyclooxygenase-1 (COX-1) and TXA2 synthase (TXAS). These results suggest that the inhibition of TXA2 production by WIB801C is not resulted from the direct inhibition of COX-1 and TXAS. In this study, we demonstrate that CE-WIB801C with cAMP-dependent Ca2+-antagonistic antiplatelet effects may have preventive or therapeutic potential for platelet aggregation-mediated diseases, such as thrombosis, myocardial infarction, atherosclerosis, and ischemic cerebrovascular disease.
Keywords: CE-WIB801C, cAMP, TXA2, Ca2+, IP3R
INTRODUCTION
Platelet aggregation is absolutely essential for the formation of a hemostatic plug when normal blood vessels are injured. However, the interactions between platelets and collagen can also cause circulatory disorders, such as thrombosis, atherosclerosis, and myocardial infarction (Schwartz et al., 1990). Accordingly, inhibition of the platelet-collagen interaction might be a promising approach for the prevention of thrombosis. It is known that collagen and its related peptide-induced stimulation of platelets activates tyrosine kinase-dependent mechanisms that involve the tyrosine phosphorylation of Syk and phospholipase C-r2 (PLC-r2) via collagen receptor glycoprotein (GP) VI (Wonerow et al., 2002). Phosphorylated PLC-r2 hydrolyzes phosphatidylinositol 4, 5-bisphosphate (PIP2) to inositol 1, 4, 5-trisphosphate (IP3) and diacylglycerol (DG). Moreover, IP3 mobilizes cytosol free Ca2+ ([Ca2+]i) from the endoplasmic reticulum via IP3 receptor (IP3R). An increase in the level of [Ca2+]i activates both the Ca2+/calmodulin-dependent phosphorylation of myosin light chain and the DG-dependent phosphorylation of pleckstrin to induce platelet aggregation (Nishikawa et al., 1980; Kaibuchi et al., 1982). In addition, DG can be hydrolyzed by DG- and monoacylglycerol-lipase to produce arachidonic acid (20:4), a precursor of thromboxane A2 (TXA2), which is a potent platelet aggregation agent. On the other hand, both intracellular cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) as antiplatelet regulators decrease the [Ca2+]i mobilization (Menshikov et al., 1993; Schwarz et al., 2001). The antiplatelet effects of cAMP and cGMP are mediated via cAMP-and cGMP-dependent protein kinases (A-kinase, G-kinase), which phosphorylate substrate protein IP3R (Halbrügge and Walter, 1989; Halbrügge et al., 1990; Butt et al., 1994). IP3R phosphorylation involves in inhibition of [Ca2+]i mobilization (Quinton and Dean, 1992; Cavallini et al., 1996; Schwarz et al., 2001) to inhibit platelet aggregation. Therefore, inhibiting the level of platelet aggregation-inducing molecules (i.e. [Ca2+]i and TXA2) or elevating the level of platelet aggregation-inhibiting molecules (i.e. cAMP and cGMP) is very useful for evaluating the antiplatelet effect of substances or compounds.
A species of the fungal genus Cordyceps is an ingredient of traditional Chinese medicine and is prescribed for inflammatory and cancer diseases (Cunningham et al., 1951; Ng and Wang, 2005). With regard to antiplatelet activity, cordycepin (3′-deoxyadenosine) is known to inhibit adenylate cyclase activity in platelets (Londos and Wolff, 1977; Haslam et al., 1978), and thus the elevation of cAMP would not be expected. A cordycepin analogue, 2′, 5′-dideoxyadenosine, does not affect on the inhibition of platelet aggregation, and the production of cGMP or cAMP is not altered by this analogue during collagen-induced platelet aggregation (Jang et al., 2002). In our previous report (Cho et al., 2007), we suggested that cordycepin (3′-deoxyadenosine, Fig. 1C) from Cordyceps militaris has an antiplatelet effect in a cAMP- and cGMP-dependent manner, which is associated with the down-regulation of [Ca2+]i. However, it is unknown how cordycepin involves in cAMP- and cGMP-downstream pathway (i.e. phosphorylation of IP3R and VASP) by cAMP/A-kinase or cGMP/G-kinase. In this present study, we prepared WIB801C (Compound from 2008 First Project of Bioteam, Whanin Pharm. Co., Ltd., Suwon, Korea), a n-butanol extract from Cordyceps militaris-hypha, and analyzed the composition of cordycepin in WIB801C with high performance liquid chromatography (HPLC). Cordyceps is known to contain nucleoside analogue such as cordycepin (3’-deoxyadenosine), adenine, and adenosine (Ng and Wang, 2005). Of these nucleoside analogue, we reported that cordycepin has an antiplatelet effect (Cho et al., 2007). Therefore, we investigated whether WIB801C contains cordycepin, adenine, and adenosine, and which, if any, adenine analogue has an antiplatelet effect. In this study, we found that WIB801C contains enough cordycepin, and investigated its antiplatelet effects to evaluate the efficacy that prevents or treats thrombotic disease.
Fig. 1.
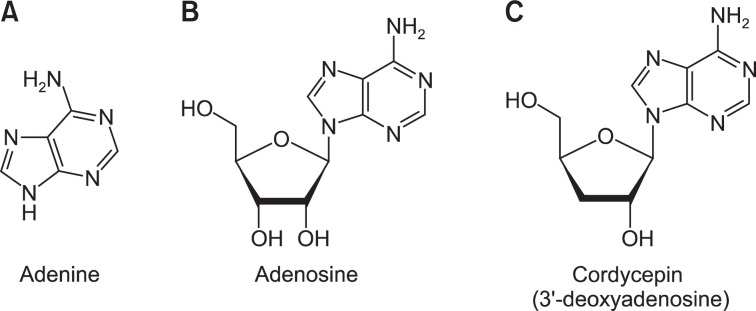
Chemical structures of adenine, adenosine, and cordycepin (3′-deoxyadenosine). (A) The structure of adenine. (B) The structure of adenosine. (C) The structure of cordycepin (3′-deoxyadenosine).
In addition, we investigated the effects of cordycepin-enriched (CE)-WIB801C on upregulation of aggregation-inhibiting molecules (i.e. cAMP, cGMP), and downregulation of aggregation-inducing molecules (Ca2+, TXA2). In special, we set out to investigate in this study whether CE-WIB801C has inhibitory effect on collagen-induced [Ca2+]i mobilization, and which, if any, cAMP and cGMP is responsible for the IP3R phosphorylation to exert Ca2+-antagonistic effect.
MATERIALS AND METHODS
Materials
Collagen was purchased from Chrono-Log Co. (Havertown, PA., USA). Fura 2-AM, and other reagents were obtained from Sigma Chemical Co. (St. Louis, MO., USA). TXB2, cAMP and cGMP enzyme immunoassay (EIA) kit, and cyclooxygenase (COX) fluorescent activity assay kit were purchased from Cayman Chemical Co. (Ann Arbor, MI., USA). Anti-phosphor-IP3-receptor, anti-rabbit IgG-horseradish peroxidase conjugate (HRP), and lysis buffer were obtained from Cell Signaling (Beverly, MA., USA). Polyvinylidene difluoride (PVDF) membrane was from GE Healthcare (Piseataway, NJ., USA). Enhanced chemiluminesence solution (ECL) was from GE Healthcare (Chalfont St, Giles, Buckinghamshire, UK).
Preparation of WIB801C
Culture-solution of Cordyceps militaris-hypha was concentrated with a rotary vacuum evaporator (Eyela N3000, Rikakikai Co. Ltd., Tokyo, Japan) at 60°C. The concentrate was extracted by extraction-shaker (Cosmos 660, Kyungseo Co. Ltd., Seoul, Korea) at 60°C two times with n-butanol, which was filtered two times using a filter paper (Advantec No.2). The filtrate was completely concentrated by an evaporator (Eyela N3000, Rikakikai Co. Ltd., Tokyo, Japan) under reduced pressure (40°C), and was lyophilized and stored at −20°C until used. This was named as cordycepin-enriched (CE)-WIB801C (Compound from 2008 First Project of Bioteam, Whanin Pharm. Co., Ltd., Suwon, Korea). CE-WIB801C was dissolved with distilled water to investigate the effects on platelet aggregation.
Detection of cordycepin in WIB801C with HPLC
WIB801C was dissolved in 50% methanol, for the first time, and then it was analyzed by high performance liquid chromatography (HPLC). An Agilent 1100 liquid chromatography system (Palo Alto, CA., USA), equipped with vacuum degasser, quaternary gradient pump, autosampler and diode array detector, connected to an Agilent ChemStation software. A Zorbax octadecylsilane (ODS) C18 column (250 mm×4.6 mm id, 5 μm) and a Zorbax ODS C18 guard column (12.5 mm×4.6 mm id, 5 μm) were used at a column temperature of 25°C. The mobile phase consisted of water (A) and methanol with 0.01M KH2PO4 (B) using the following program: 0-30 min, 15% B. The flow rate was at 1.0 ml/min and sample injection volume was 10 μL. The UV detection was operated at 254 nm. To detect and analyze the nucleoside analogue, we used various concentrations (cordycepin: 50, 100, 200, and 400 μg/ml; adenosine: 4, 20, 100, and 200 μg/ml; adenine: 10, 20, 50, 100, 200 μg/ml) of each authentic compounds (cordycepin, adenosine, and adenine) in duplicate with HPLC, then the calibration curves were constructed by plotting the peak area against the concentration of each analyte with regression analysis, and we calculated linear equation from the calibration curve (Table 1).
Table 1.
Calibration curves and contents of 2 peaks in CE-WIB801C
| RT (min) | Area (mAU×s) | Calibration curvea) | r2 | Test range (μg/ml) | χb) (μg/ml) | Contents (mg/g-CE-WIB801C) | |
|---|---|---|---|---|---|---|---|
| Authetic compound | |||||||
| Adenine | 6.6 | - | y=43.600χ+7.959 | 0.9996 | 10–200 | - | - |
| Cordycepin | 14.8 | - | y=27.618χ−64.657 | 0.9996 | 50–400 | - | - |
| CE-WIB801C | |||||||
| Peak 1 | 6.6 | 714 ± 11 | - | - | - | 16.21 ± 0.25 | 16.21 ± 0.25 |
| Peak 2 | 14.8 | 2210 ± 38 | - | - | - | 81.98 ± 1.37 | 81.98 ± 1.37 |
y, peak areas of analytes; x, concentrations of analytes in 1 mg/ml CE-WIB801C (μg/ml).
Preparation of washed human platelets
Human platelet-rich plasma (PRP) anti-coagulated with acid-citrate-dextrose solution (0.8% citric acid, 2.2% sodium citrate, 2.45% glucose) were obtained from Korean Red Cross Blood Center (Changwon, Korea). PRP was centrifuged for 10 min at 125×g to remove a little red blood cells, and was centrifuged for 10 min at 1,300 ×g to obtain the platelet pellets. The platelets were washed twice with washing buffer (138 mM NaCl, 2.7 mM KCl, 12 mM NaHCO3, 0.36 mM NaH2PO4, 5.5 mM glucose, and 1 mM EDTA, pH 6.5). The washed platelets were then resuspended in suspension buffer (138 mM NaCl, 2.7 mM KCl, 12 mM NaHCO3, 0.36 mM NaH2PO4, 0.49 mM MgCl2, 5.5 mM glucose, 0.25% gelatin, pH 6.9) to a final concentration of 5×108/ml. All of the above procedures were carried out at 25°C to avoid platelet aggregation from any effect of low temperature. The Korea National Institute for Bioethics Policy Public Institutional Review Board (Seoul, Korea) approved these experiments.
Measurement of platelet aggregation
Washed platelets (108/ml) were preincubated for 3 min at 37°C in the presence of 2 mM CaCl2 with or without substances, then stimulated with collagen (10 μg/ml) for 5 min. Aggregation was monitored using an aggregometer (Chrono-Log Corporation, Havertown, PA., USA) at a constant stirring speed of 1,000 rpm. Each aggregation rate was calculated as an increase in light transmission. The suspension buffer was used as the reference (transmission 0).
Measurement of cAMP and cGMP
Washed platelets (108/ml) were preincubated for 3 min at 37°C with or without substances in the presence of 2 mM CaCl2, and then stimulated with collagen (10 μg/ml) for 5 min for platelet aggregation. The aggregation was terminated by the addition of 80% ice-cold ethanol. cAMP and cGMP were measured with synergy HT multi-model microplate reader (BioTek Instruments, Winooski, VT., USA) using cAMP and cGMP EIA kits.
Determination of cytosolic-free Ca2+ ([Ca2+]i)
PRP was incubated with 5 μM Fura 2-AM at 37°C for 60 min. Because Fura 2-AM is light sensitive, the tube containing the PRP was covered with aluminum foil during loading. The Fura 2-loaded washed platelets were prepared using the procedure described above and 108 platelets/ml were preincubated for 3 min at 37°C with or without substances in the presence of 2 mM CaCl2, then stimulated with collagen (10 μg/ml) for 5 min for evaluation of [Ca2+]i. Fura 2 fluorescence was measured with a spectrofluorometer (SFM 25; Bio-Teck Instrument, Italy) with an excitation wavelength that was changed every 0.5 sec from 340 to 380 nm; the emission wavelength was set at 510 nm. The [Ca2+]i values were calculated using the method of Schaeffer (Schaeffer and Blaustein, 1989).
Western blot for analysis of IP3R phosphorylation
Washed platelets (108/ml) were preincubated with or without substances in the presence of 2 mM CaCl2 for 3 min and then stimulated with collagen (10 μg/ml) for 5 min at 37°C. The reactions were terminated by adding an equal volume (250 μl) of lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM serine/threonine phosphatase inhibitor β-gly cerophosphate, 1 mM ATPase, alkaline and acid phosphatase, and protein phosphotyrosine phosphatase inhibitor Na3VO4, 1 μg/ml serine and cysteine protease inhibitor leupeptin, and 1 mM serine protease and acetylcholinesterase inhibitor phenylmethanesulfonyl fluoride, pH 7.5). Platelet lysates containing the same protein (15 μg) were us ed for analysis. Protein concentrations were measured by using bicinchoninic acid protein assay kit (Pierce Biotechnology, USA). The effects of substances on IP3R phosphorylation were analyzed by western blotting. A 6–8% SDS-PAGE was used for electrophoresis and a PVDF membrane was used for protein transfer from the gel. The dilutions for anti-phosphor-IP3R and anti-rabbit IgG-HRP were 1:1000 and 1:10000, respectively. The membranes were visualized using ECL. Blots were analyzed by using the Quantity One, Ver. 4.5 (Bio-Rad, Hercules, CA., USA).
Measurement of TXB2
Washed platelets (108/ml) were preincubated with or without substances for 3 min in the presence of 2 mM CaCl2, and activated for 5 min with collagen (10 μg/ml). The reactions were terminated by the addition of ice-cold EDTA (5 mM) and indomethacin (0.2 mM). The amount of TXB2, a stable metabolite of TXA2, was determined with synergy HT multi-model microplate reader (BioTek Instruments, Winoosku, VT., USA) using a TXB2 EIA kit.
Measurement of cyclooxygenase-1 (COX-1) Activity
Washed platelets (108/ml) with 1% protease inhibitor cocktail (Sigma Chemical Co., St. Louis, MO., USA) were sonicated 10 times at sensitivity 100% for 20 seconds on ice with a model HD2070 sonicator (Bandelin Electronic, Bandelin, Germany) to obtain platelet lysates. The homogenates were centrifuged at 12,000×g for 15 min at 4°C to remove cell debris. The supernatant was used to measure COX-1 activity. The platelet lysates were pre-incubated with or without substances at 37°C for 30 min. COX-1 activity was measured with synergy HT multi-model microplate reader (BioTek Instruments, Winooski, VT., USA) using COX fluorescent activity assay kit.
Measurement of thromboxane A2 Synthase (TXAS) Activity
Washed platelets (108/ml) with 1% protease inhibitor cocktail (Sigma Chemical Co., St. Louis, MO., USA) were sonicated 10 times at sensitivity 100% for 20 seconds on ice with a model HD2070 sonicator (Bandelin Electronic, Bandelin, Germany) to obtain platelet lysates. Next, the homogenates were centrifuged at 12,000×g for 15 min at 4°C to remove cell debris. The platelet lysates were pre-incubated with or without substances at 37°C for 30 min. The reaction was initiated by the addition of prostaglandin H2 (PGH2) and allowed to proceed for 1 min at 37°C. The reaction was then terminated by the addition of 1M citric acid. After neutralization with 1N NaOH, the concentration of thromboxane B2 (TXB2), a stable metabolite of TXA2, was determined with synergy HT multi-model microplate reader (BioTek Instruments, Winoosku, VT., USA) using TXB2 EIA kit.
Statistical analyses
The experimental results are expressed as the mean ± S.E.M. accompanied by the number of observations. Data were assessed by analysis of variance (ANOVA). If this analysis indicated significant differences among the group means, then each group was compared by the Newman-Keuls method. p<0.05 was considered to be statistically significant.
RESULTS
Composition of cordycepin in WIB801C
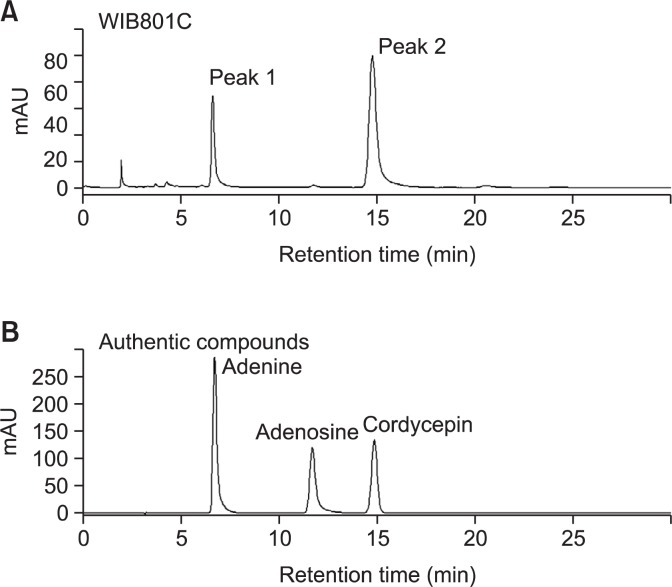
We analyzed the composition of cordycepin in WIB801C with HPLC, as shown in Fig. 2A, two peaks (peak 1, 2) mainly were observed. The retention time of peak 1 was 6.6 min, and peak 2 was 14.8 min (Fig. 2A, Table 1). We detected cordycepin and analyzed its composition. As shown in Fig. 2B, the retention times of authentic compounds, cordycepin, adenosine, and adenine, were 14.8, 11.7, and 6.6 min in order. The retention times of peak 1, and peak 2 were in accord with those of authentic adenine and cordycepin, but a certain peak corresponding authentic adenosine was not almost observed in WIB801C. Calibration curve were linear over the range of 50 to 400 μg/ml for cordycepin, and 10 to 200 μg/ml for adenine with r2>0.9996 (Table 1). With regard to contents of cordycepin and adenine calculated from calibration curve, as shown in Table 1, the content of peak 2 corresponding to cordycepin was 81.98 ± 1.37 mg/g-WIB801C (about 8.2%), and the content of peak 1 corresponding to adenine was 16.21 ± 0.25 mg/g-WIB801C (about 1.62%).
Fig. 2.
HPLC chromatograms of CE-WIB801C and authentic compounds (adenine, adenosine, and cordycepin). (A) The chromatogram of CE-WIB801C. (B) The chromatogram of authentic compounds (adenine, adenosine, and cordycepin). HPLC was performed on a Zorbax ODS C18 column (250 mm×4.6 mm id, 5 μm) and a Zorbax ODS C18 guard column (12.5 mm×4.6 mm id, 5 μm) were used at 25°C. The mobile phase consisted of water (a) and methanol with 0.01M KH2PO4 (b) using the following program: 0–30 min, 15% b. The flow rate was at 1.0 ml/min and sample injection volume was 10 μl. The UV detection was operated at 254 nm.
It is known that whole fruiting body myelia of Cordyceps militaris contains 0.16% of cordycepin, and whole fruiting body, stroma, and larva of Cordyceps sinensis does not contain cordycepin (Yue et al., 2008). Accordingly, the cordycepin level in WIB801C that we used in this study is very higher than those in whole fruiting body myelia of Cordyceps militaris, or in whole fruiting body, stroma, and larva of Cordyceps sinensis. Thus, WIB801C is named as cordycepin-enriched WIB801C (CE-WIB801C) in this report.
Effects of CE-WIB801C on collagen-induced platelet aggregation
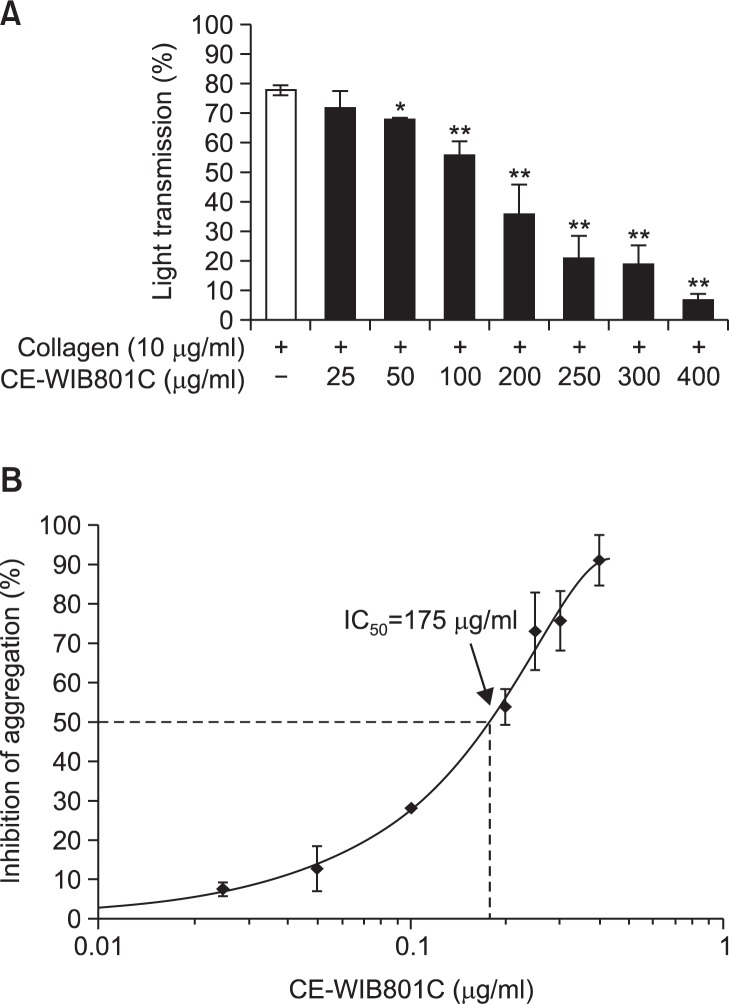
The concentration of collagen-induced maximal platelet aggregation was approximately 10 μg/ml (Lee et al., 2014). Therefore, collagen (10 μg/ml) was used as the platelet agonist in this study. When washed platelets (108/ml) were activated with collagen (10 μg/ml) in the presence of 2 mM CaCl2, the aggregation rate was increased up to 78.0 ± 1.7%. However, various concentrations of CE-WIB801C (25 to 400 μg/ml) significantly reduced collagen-stimulated platelet aggregation in a dose-dependent manner (Fig. 3A), and the half-maximal inhibitory concentration (IC50) value was approximately 175 μg/ml (Fig. 3B).
Fig. 3.
Effects of CE-WIB801C on collagen-induced platelet aggregation. (A) Effects of CE-WIB801C pretreatment on collagen-induced platelet aggregation. (B) IC50 value of CE-WIB801C on collagen-induced platelet aggregation. Washed platelets (108/ml) were preincubated with or without various concentrations of CE-WIB801C (25 to 400 μg/ml) in the presence of 2 mM CaCl2 for 3 min at 37°C, then stimulated with collagen (10 μg/ml) for 5 min in aggregometer. Platelet aggregation (%) was recorded as an increase in light transmission. Inhibition rate by CE-WIB801C was recorded as percentage of the collagen-induced aggregation rate. IC50 value of CE-WIB801C was calculated by 4-parameter log fit method. The data are expressed as the mean ± S.E.M. (n=4). *p<0.05, **p<0.001 versus the collagen-stimulated platelets.
Effects of CE-WIB801C on cAMP and cGMP production
As shown in Table 2, collagen decreased intracellular cAMP level from 5.2 ± 0.4 pmoL/109 platelets (basal level) to 2.8 ± 0.5 pmoL/109 platelets, which was reduced to 46.2% as compared with that of basal level (Table 2). When platelets, however, were incubated in the presence of both CE-WIB801C and collagen, 400 μg/ml of CE-WIB801C increased cAMP level from 2.8 ± 0.5 pmoL/109 platelets to 18.1 ± 1.0 pmoL/109 platelets (Table 2). This result suggests that CE-WIB801C (400 μg/ml) increased collagen-decreased cAMP level to 546.4% (Table 2). On the other hand, collagen decreased intracellular cGMP level from 4.0 ± 0.3 pmoL/109 platelets (basal level) to 3.0 ± 0.4 pmoL/109 platelets (Table 2). Collagen reduced basal cGMP level to 25.0% to aggregate platelets (Table 2). When platelets, however, were incubated in the presence of both CE-WIB801C (400 μg/ml) and collagen (10 μg/ml), the cGMP level was increased to 53.3% as compared with that (3.0 ± 0.4 pmoL/109 platelets) achieved by collagen (10 μg/ml) alone (Table 2).
Table 2.
Changes of cAMP and cGMP
| cAMP
|
cGMP
|
cAMP/cGMP
|
||||
|---|---|---|---|---|---|---|
| pmoL/109 platelets | Δ (%) | pmoL/109 platelets | Δ (%) | Ratio | Δ (%) | |
| Base | 5.2 ± 0.4 | - | 4.0 ± 0.3 | - | 1.3 | - |
| Collagen (10 μg/ml) | 2.8 ± 0.5a | − 46.21) | 3.0 ± 0.4a | − 252) | 0.9 | − 30.73) |
| CE-WIB801C (400 μg/ml) + Collagen (10 μg/ml) | 18.1 ± 1.0** | + 546.44) | 4.6 ± 0.7* | + 53.35) | 3.9 | + 3336) |
1) to 3) Δ (%)=(Collagen-Base)/Base×100,
4) to 6) Δ (%)=[(CE-WIB801C+Collagen)-Collagen]/Collagen×100.
The data are expressed as the mean ± S.E.M. (n=4).
p<0.05 versus non-stimulated platelets,
p<0.05,
p<0.001 versus the collagen-stimulated platelets.
Effect of CE-WIB801C on [Ca2+]i mobilization
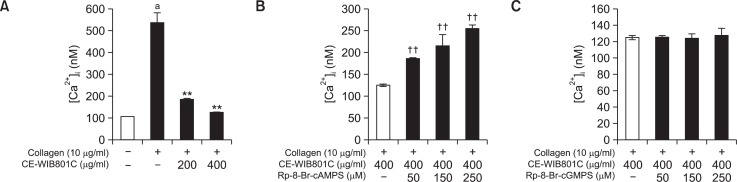
As shown in Fig. 4A, collagen increased [Ca2+]i level from 106.6 ± 2.1 nM (basal level) to 536.6 ± 45.0 nM. However, CEWIB801C (400 μg/ml) decreased collagen-elevated [Ca2+]i (536.6 ± 45.0 nM) to 124.9 ± 2.6 nM (Fig. 4A). This suggests that CEWIB801C decreased collagen-elevated [Ca2+]i level to 76.7% (Fig. 4A). The level of [Ca2+]i in the presence of both collagen and CE-WIB801C was 124.9 ± 2.6 nM, however, which level was dose dependently increased by A-kinase inhibitor Rp-8-Br-cAMPS (50 to 250 μM) and was increased to 254.9 ± 9.4 nM (104.1%) (Fig. 4B). On the other hand, the level of [Ca2+]i in the presence of both collagen and CE-WIB801C was not increased by G-kinase inhibitor Rp-8-Br-cGMPS (50 to 250 μM) (Fig. 4C). Because [Ca2+]i reduction is resulted from cAMP/A-kinase-phosphorylated IP3R, we next investigated whether CE-WIB801C involves in phosphorylation of IP3R.
Fig. 4.
Effects of CE-WIB801C on collagen-induced [Ca2+]i mobilization, and in the presence of A-kinase inhibitor (Rp-8-Br-cAMPS) or G-kinase inhibitor (Rp-8-Br-cGMPS). (A) Effect of CE-WIB801C on collagen-induced [Ca2+]i mobilization. (B) Effect of CE-WIB801C on collagen-induced [Ca2+]i mobilization in the presence of A-kinase inhibitor (Rp-8-Br-cAMPS). (C) Effect of CE-WIB801C on collagen-induced [Ca2+]i mobilization in the presence of G-kinase inhibitor (Rp-8-Br-cGMPS). Fura 2-loaded washed platelets (108/ml) were preincubated with or without CE-WIB801C, the A-kinase inhibitor Rp-8-Br-cAMPS or the G-kinase inhibitor Rp-8-Br-cGMPS in the presence of 2 mM CaCl2 for 3 min at 37°C, and then collagen (10 μg/ml) was added. [Ca2+]i was determined as described in “Materials and Methods”. The data are expressed as the mean ± S.E.M. (n=4). ap<0.05 versus non-stimulated platelets, **p<0.001 versus the collagen-stimulated platelets, ††p<0.001 versus each control: the collagen-stimulated platelets in the presence of CE-WIB801C (400 μg/ml).
Effect of CE-WIB801C on IP3R phosphorylation
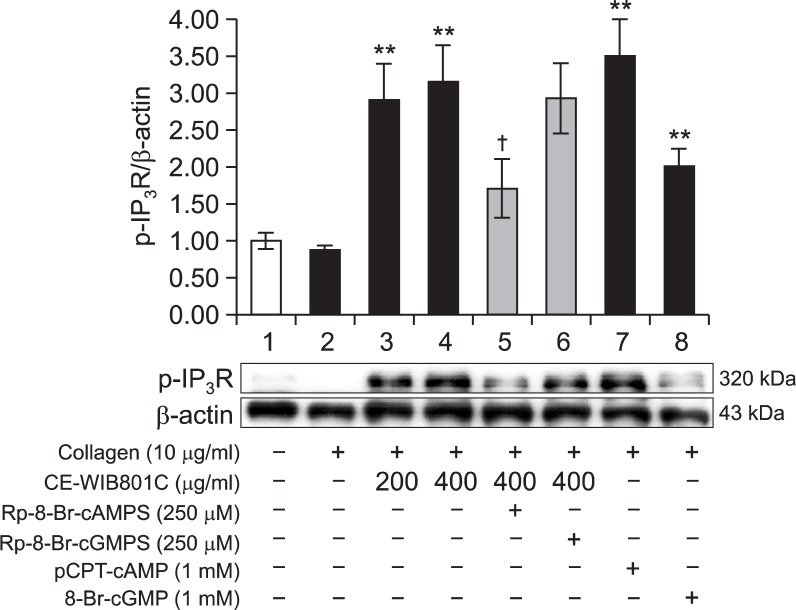
The phosphorylation (p-IP3R) of IP3R and the ratio of p-IP3R to β-actin were increased in the presence of A-kinase activator pCPT-cAMP (1 mM) (Fig. 5 lane 7), and G-kinase activator 8-Br-cGMP (1 mM) (Fig. 5 lane 8) as compared with collagen alone. These mean that cAMP/A-kinase and cGMP/G-kinase involve in IP3R phosphorylation. As shown in Fig. 5 lane 3 and 4, p-IP3R and the ratio of p-IP3R to β-actin were dose dependently increased in the presence of both collagen and CE-WIB801C (200 and 400 μg/ml). However, the ratio (3.15) of p-IP3R to β-actin by both collagen and CE-WIB801C (400 μg/ml) was decreased to 1.71 (45.7%) in the presence of A-kinase inhibitor Rp-8-Br-cAMPS (Fig. 5 lane 5, Table 3). On the other hand, the ratio (3.15, Fig. 5 lane 4) of p-IP3R to β-actin by both collagen and CE-WIB801C (400 μg/ml) was not almost decreased in the presence of G-kinase inhibitor Rp-8-Br-cGMPS (Fig. 5 lane 6, Table 3). Accordingly, cAMP/A-kinase-dependent IP3R phosphorylation exclusively contributed to the inhibitory effect of [Ca2+]i mobilization achieved by CE-WIB801C on collagen-activated platelets.
Fig. 5.
Effects of CE-WIB801C on inositol 1,4,5-trisphosphate receptor (IP3R) phosphorylation. Lane 1, Intact platelets (base); Lane 2, Collagen (10 μg/ml); Lane 3, Collagen (10 μg/ml)+CEWIB801C (200 μg/ml); Lane 4, Collagen (10 μg/ml)+CE-WIB801C (400 μg/ml); Lane 5, Collagen (10 μg/ml)+CE-WIB801C (400 μg/ml)+Rp-8-Br-cAMPS (250 μM); Lane 6, Collagen (10 μg/ml)+CE-WIB801C (400 μg/ml)+Rp-8-Br-cGMPS (250 μM); Lane 7, Collagen (10 μg/ml)+pCPT-cAMP (1 mM); Lane 8, Collagen (10 μg/ml)+8-BrcGMP (1 mM). Washed platelets (108/ml) were preincubatedwith or without W-cordycepin, the A-kinase inhibitor Rp-8-Br-cAMPS or the G-kinase inhibitor Rp-8-Br-cGMPS, and A-kinase activator pCPT-cAMP or the G-kinase activator 8-Br-cGMP in the presence of 2 mM CaCl2 for 3 min at 37°C, then stimulated with collagen (10 μg/ml) for 5 min at 37°C in an aggregometer. The reactions were terminated by adding an equal volume (250 μl) of lysis buffer. Proteins were separated by SDS-PAGE, transferred to PVDF, and immunoblotted with the indicated corresponding antibodies. The data are expressed as the mean ± S.E.M. (n=3). **p<0.001 versus the collagen-stimulated platelets, †p<0.05 versus the collagen-stimulated platelets in the presence of CE-WIB801C (400 μg/ml).
Table 3.
Changes of p-IP3R/β-actin ratio
| p-IP3R/β-actin | Δ (%) | ||
|---|---|---|---|
| Collagen (10 μg/ml) | 0.87 ± 0.07 | 0 | |
| CE-WIB801C (400 μg/ml) | 3.15 ± 0.50 | + 262.11) | 0 |
| + Collagen (10 μg/ml) | |||
| CE-WIB801C (400 μg/ml) | 1.71 ± 0.40 | - | −45.72) |
| + Rp-8-Br-cAMPS (250 μM) | |||
| + Collagen (10 μg/ml) | |||
| CE-WIB801C (400 μg/ml) | 2.93 ± 0.48 | - | −7.03) |
| + Rp-8-Br-cGMPS (250 μM) | |||
| + Collagen (10 μg/ml) | |||
Data were from Fig. 5.
Δ (%)=[(CE-WIB801C+Collagen)–Collagen]/Collagen×100,
Δ (%)=[(CE-WIB801C+Rp-8-Br-cAMPS +Collagen)–(CE-WIB801C+Collagen)]/(CE-WIB801C+Collagen) ×100,
Δ (%)=[(CE-WIB801C+Rp-8-Br-cGMPS+Collagen)–(CE-WIB801C+Collagen)]/(CE-WIB801C+Collagen)×100.
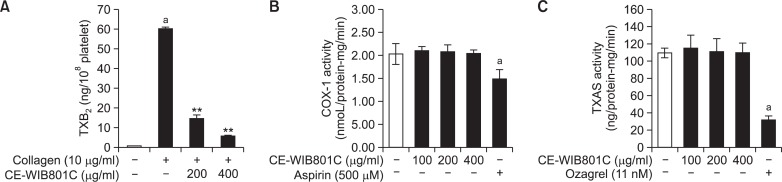
Effect of CE-WIB801C on TXA2 production, and it-associated enzymes (COX-1 and TXAS) activities
The TXA2 (determined as TXB2) level in intact platelets was 0.6 ± 0.1 ng/108 platelets, and collagen (10 μg/ml) ma rkedly increased TXA2 level to 60.1 ± 1.0 ng/108 platelets (Fig. 6A). This suggests that collagen increased TXA2 production to 9,917% (Fig. 6A). However, CE-WIB801C potently reduced TXA2 production to 5.5 ± 0.5 ng/108 platelets (90.8% inhibition at 400 μg/ml) (Fig. 6A). TXA2 production is concerned with COX-1 and TXAS, which convert 20:4 to TXA2 (Patrono, 1994; Cipollone et al., 1997). Therefore, we investigated whether CE-WIB801C inhibited COX-1 and TXAS activities to inhibit TXA2 production. As shown in Fig. 6B, aspirin (500 μM), a COX-1 inhibitor, significantly inhibited COX-1 activity from 2.03 ± 0.23 nmoL/protein-mg/min (basal level) to 1.49 ± 0.20 nmoL/protein-mg/min, however, CE-WIB801C did not inhibit COX-1 activity (Fig. 6B). In addition, ozagrel (11 nM), a TXAS inhibitor, significantly inhibited TXAS activity from 109.3 ± 5.6 ng/protein-mg/min (basal level) to 31.4 ± 4.8 ng/protein-mg/min, however, CE-WIB801C did not inhibit TXAS activity (Fig. 6C). These results mean that CE-WIB801C-reduced TXA2 was not resulted from inhibition of COX-1 and TXAS activities.
Fig. 6.
Effects of CE-WIB801C on collagen-induced TXA2 production, and TXA2-associated enzymes (COX-1 and TXAS) activities. (A) Effects of CE-WIB801C on TXB2 production. Washed platelets (108/ml) were preincubated with or without CE-WIB801C in the presence of 2 mM CaCl2 for 3 min at 37°C, then stimulated with collagen (10 μg/ml) for 5 min in an aggregometer. TXA2 was determined with the content of TXB2 using TXB2 EIA kit as described in “Materials and Methods”. (B) Effects of CE-WIB801C on COX-1 activity in platelet lysates. (C) Effects of CE-WIB801C on TXAS activity in platelet lysates. COX-1 and TXAS activity was determined with COX fluorescent activity assay and TXB2 EIA kit as described in “Materials and Methods”. The data are expressed as the mean ± S.E.M. (n=4). ap<0.05 versus non-stimulated platelets, **p<0.001 versus the collagen-stimulated platelets.
DISCUSSION
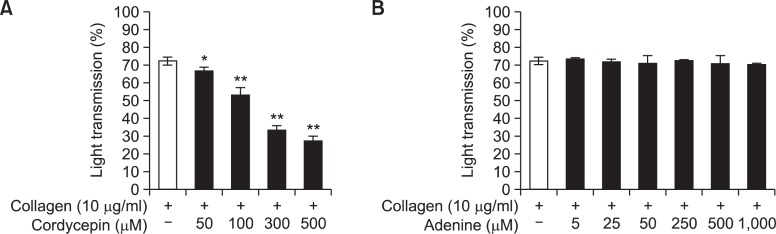
CE-WIB801C contained mainly adenine (Fig. 1A) and cordycepin (Fig. 1C), and inhibited collagen-induced platelet ag g regation, which is thought by cordycepin in WIB801C because authentic cordycepin inhibited collagen-induced platelet aggregation in a dose dependent manner (Fig. 7A), but authentic adenine did not inhibited collagen-induced platelet aggregation (Fig. 7B). It is established that cordycepin inhibits collagen-induced platelet aggregation in our previous report (Cho et al., 2007). CE-WIB801C significantly blocked both [Ca2+]i mobilization and TXA2 production, and increased the production of cAMP and cGMP in collagen-induced platelet aggregation, which are related with the inhibitory effect of collagen-induced platelet aggregation by CE-WIB801C. In collagen-activated platelets, TXA2 is produced from 20:4 via cyclooxygenase-1 (COX-1) and TXA2 synthase (TXAS) pathway.
Fig. 7.
Effects of cordycepin and adenine on collagen-induced platelet aggregation. (A) Effects of cordycepin pretreatment on collagen-induced platelet aggregation. (B) Effects of adenine pretreatment on collagen-induced platelet aggregation. Washed platelets (108/ml) were preincubated with or without various concentrations of cordycepin and adenine in the presence of 2 mM CaCl2 for 3 min at 37°C, then stimulated with collagen (10 μg/ml) for 5 min. Platelet aggregation (%) was recorded as an increase in light transmission. The data are expressed as the mean ± S.E.M. (n=4). *p<0.05, **p<0.001 versus the collagen-stimulated platelets.
Therefore, even though it is thought that CE-WIB801C might involve in inhibition of COX-1 or TXAS to suppress collagen-produced TXA2 level, because CE-WIB801C did not inhibit COX-1 and TXAS activities in a cell free system, it is inferred that CE-WIB801C would not directly involve in inhibition of COX-1 and TXAS activities to reduce TXA2 production in collagen-induced platelet aggregation.
In real, TXA2 precursor 20:4 is generated from DG/monoacylglycerol (MG) via DG-/MG-lipase pathway, and from phosphatidylinositol or phosphatidic acid via phospholipase A2 (PLA2), and these enzymes are activated by Ca2+ (Bell et al., 1979; Mauco et al., 1984; Moriyama et al., 1994). In the present study, although CE-WIB801C inhibited collagen-induced Ca2+ mobilization, it is unknown whether CE-WIB801C may involve in the indirect inhibition of DG-/MG-lipase or PLA2 to attenuate the supply of TXA2 precursor 20:4, which is remained elusive, and should be studied in the future.
CE-WIB801C more increased exclusively cAMP than cGMP on collagen-induced platelet aggregation, which trend is as well as those by phenolic compounds such as epigallocatechin-3-gallate (Ok et al., 2012), chlorogenic acid (Cho et al., 2012), and caffeic acid (Lee et al., 2014) that cAMP-dependently inhibited [Ca2+]i mobilization in collagen-activated platelets. The levels of intracellular cAMP and cGMP are regulated by the balance between cyclic nucleotide-producing enzymes, adenylate/guanylate cyclases, and hydrolyzing enzymes, cAMP/cGMP phosphodiesterases (PDEs). It is known that platelets have PDE2, PDE3, and PDE5 (Schwarz et al., 2001; Walter and Gambaryan, 2009). PDE2 hydrolyzes cAMP than cGMP as cGMP-stimulated PDE, PDE3 hydrolyzes cAMP rather than cGMP as cGMP-inhibited PDE, and PDE5 hydrolyzes cGMP only as cGMP-binding-cGMP specific PDE. Because CE-WIB801C increased cAMP than cGMP in collagen-induced platelet aggregation, it is thought that CE-WIB801C might involve in inhibition of PDE3 to increase both cAMP and cGMP. However, we have no obvious evidence how CE-WIB801C regulated the level of cAMP and cGMP. Further investigation along this line is underway.
The Ca2+-antagonistic reaction by cAMP or cGMP is mediated by A-kinase/IP3R phosphorylation or G-kinase/IP3R phosphorylation. CE-WIB801C elevated IP3R phosphorylation, and this was inhibited by A-kinase inhibitor Rp-8-Br-cAMPS only, not G-kinase inhibitor Rp-8-Br-cGMPS. In addition, CE-WIB801C-decreased [Ca2+]i was increased by A-kinase inhibitor Rp-8-Br-cAMPS in collagen-activated platelets. Accordingly, it is an evidence that the inhibition of [Ca2+]i mobilization by CEWIB801C is obviously due to cAMP/A-kinase-dependent IP3R phosphorylation. Otherwise, CE-WIB801C-reduced [Ca2+]i would not be increased by A-kinase inhibitor Rp-8-Br-cAMPS, and CE-WIB801C-elevated IP3R phosphorylation would not be decreased by A-kinase inhibitor Rp-8-Br-cAMPS.
Antiplatelet drugs such as thienopyridine derivatives (i.e. ticlopidine, clopidogrel) have characteristics that inhibit [Ca2+]i mobilization, which is mediated by cAMP or cGMP (Barragan et al., 2003). Therefore, it is thought that CE-WIB801C also may represent a useful tool in the therapy or prevention of vascular diseases (i.e. thrombosis, myocardial infarction, ischemic cerebrovascular disease, and atherosclerosis) associated w ith platelet aggregation.
Acknowledgments
This study was supported by a grant (NRF-2011-0012143 to Hwa-Jin Park) from Basic Science Research Program via the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology, Korea.
REFERENCES
- Barragan P, Bouvier JL, Roquebert PO, Macaluso G, Commeau P, Comet B, Lafont A, Camoin L, Walter U, Eigenthaler M. Resistance to thienopyridines: clinical detection of coronary stent thrombosis by monitoring of vasodilator-stimulated phosphoprotein phosphorylation. Catheter Cardiovasc Interv. 2003;59:295–302. doi: 10.1002/ccd.10497. [DOI] [PubMed] [Google Scholar]
- Bell RL, Kennerly DA, Stanford N, Majerus PW. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci USA. 1979;76:3238–3241. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, Walter U. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem. 1994;269:14509–14517. [PubMed] [Google Scholar]
- Cavallini L, Coassin M, Borean A, Alexandre A. Pros-tacyclin and sodium nitroprusside inhibit the activity of the platelet inositol 1,4,5-trisphosphate receptor and promote its phosphorylation. J Biol Chem. 1996;271:5545–5551. doi: 10.1074/jbc.271.10.5545. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Cho JY, Rhee MH, Park HJ. Cordycepin (3′-deoxyadenosine) inhibits human platelet aggregation in a cyclic AMP- and cyclic GMP-dependent manner. Eur J Pharmacol. 2007;558:43–51. doi: 10.1016/j.ejphar.2006.11.073. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Kang HJ, Kim YJ, Lee DH, Kwon HW, Kim YY, Park HJ. Inhibition of platelet aggregation by chlorogenic acid via cAMP and cGMP-dependent manner. Blood Coagul. Fibrinolysis. 2012;23:629–635. doi: 10.1097/MBC.0b013e3283570846. [DOI] [PubMed] [Google Scholar]
- Cipollone F, Patrignani P, Greco A, Panara MR, Padovano R, Cuccurullo F, Patrono C, Rebuzzi AG, Liuzzo G, Quaranta G, Maseri A. Differential suppression of thromboxane biosynthesis by indobufen and aspirin in patients with unstable angina. Circulation. 1997;6:1109–1116. doi: 10.1161/01.cir.96.4.1109. [DOI] [PubMed] [Google Scholar]
- Cunningham KG, Hutchinson SA, Manson W, Spring FS. Cordycepin: A metabolic product from cultures Cordyceps militaris Link. Part I. Isolation and characterization. J Chem Soc. 1951;2:2299–2300. [Google Scholar]
- Halbrügge M, Walter U. Purification of a vasodilator-regulated phosphoprotein from human platelets. Eur J Biochem. 1989;185:41–50. doi: 10.1111/j.1432-1033.1989.tb15079.x. [DOI] [PubMed] [Google Scholar]
- Halbrügge M, Friedrich C, Eigenthaler M, Schanzenbächer P, Walter U. Stoichiometric and reversible phosphorylation of a 46-kDa protein in human platelets in response to cGMP- and cAMP-elevating vasodilators. J Biol Chem. 1990;265:3088–3093. [PubMed] [Google Scholar]
- Haslam RJ, Davidson MM, Desjardins JV. Inhibition of adenylate cyclase by adenosine analogues in preparations of broken and intact human platelets. Evidence for the unidirectional control of platelet function by cyclic AMP. Biochem J. 1978;176:83–95. doi: 10.1042/bj1760083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang EK, Azzam JE, Dickinson NT, Davidson MM, Haslam RJ. Roles for both cyclic GMP and cyclic AMP in the inhibition of collagen-induced platelet aggregation by nitroprusside. Br J Haematol. 2002;117:664–675. doi: 10.1046/j.1365-2141.2002.03479.x. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K, Sano K, Hoshijima M, Takai Y, Nishizuka Y. Phosphatidylinositol turnover in platelet activation; calcium mobilization and protein phosphorylation. Cell Calcium. 1982;3:323–335. doi: 10.1016/0143-4160(82)90020-3. [DOI] [PubMed] [Google Scholar]
- Lee DH, Kim HH, Cho HJ, Bae JS, Yu YB, Park HJ. Antiplatelet effects of caffeic acid due to Ca2+ mobilization-inhibition via cAMP-dependent inositol-1, 4, 5-trisphosphate receptor phosphorylation. J Atheroscler Thromb. 2014;21:24–37. doi: 10.5551/jat.18994. [DOI] [PubMed] [Google Scholar]
- Londos C, Wolff J. Two distinct adenosine-sensitive sites on adenylate cyclase. Proc Natl Acad Sci USA. 1977;74:5482–5486. doi: 10.1073/pnas.74.12.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauco G, Fauvel J, Chap H, Douste-Blazy L. Studies on enzymes related to diacylglycerol production in activated platelets. II. Subcellular distribution, enzymatic properties and positional specificity of diacylglycerol- and monoacylglycerol-lipases. Biochim. Biophys. Acta. 1984;796:169–177. doi: 10.1016/0005-2760(84)90345-x. [DOI] [PubMed] [Google Scholar]
- Menshikov, Myu, Ivanova K, Schaefer M, Drummer C, Gerzer R. Influence of the cGMP analog 8-PCPT-cGMP on agonist-induced increases in cytosolic ionized Ca2+ and on aggregation of human platelets. Eur J Pharmacol. 1993;245:281–284. doi: 10.1016/0922-4106(93)90108-l. [DOI] [PubMed] [Google Scholar]
- Moriyama T, Wada K, Oki M, Matsuura T, Kitom M. The mechanism of arachidonic acid release in collagen-activated human platelets. Biosci Biotechnol Biochem. 1994;58:93–98. doi: 10.1271/bbb.58.93. [DOI] [PubMed] [Google Scholar]
- Ng TB, Wang HX. Pharmacological actions of Cordyceps, a prized folk medicine. J Pharm Pharmacol. 2005;57:1509–1519. doi: 10.1211/jpp.57.12.0001. [DOI] [PubMed] [Google Scholar]
- Nishikawa M, Tanaka T, Hidaka H. Ca2+-calmodulin dependent phosphorylation and platelet secretion. Nature. 1980;287:863–865. doi: 10.1038/287863a0. [DOI] [PubMed] [Google Scholar]
- Ok WJ, Cho HJ, Kim HH, Lee DH, Kang HY, Kwon HW, Rhee MH, Kim M, Park HJ. Epigallocatechin-3-gallate has an anti-platelet effect in a cyclic AMP-dependent manner. J Atheroscler Thromb. 2012;19:337–348. doi: 10.5551/jat.10363. [DOI] [PubMed] [Google Scholar]
- Quinton TM, Dean WL. Cyclic AMP-dependent phosphorylation of the inositol-1,4,5-trisphosphate receptor inhibits Ca2+ release from platelet membranes. Biochem Biophys Res Commun. 1992;184:893–899. doi: 10.1016/0006-291x(92)90675-b. [DOI] [PubMed] [Google Scholar]
- Patrono C. Aspirin as an antiplatelet drug. N Engl J Med. 1994;330:1287–1294. doi: 10.1056/NEJM199405053301808. [DOI] [PubMed] [Google Scholar]
- Schaeffer J, Blaustein MP. Platelet free calcium concentrations measured with fura-2 are influenced by the transmembrane sodium gradient. Cell Calcium. 1989;10:101–113. doi: 10.1016/0143-4160(89)90050-x. [DOI] [PubMed] [Google Scholar]
- Schwartz SM, Heinmark RL, Majesky MW. Developmental mechanisms underlying pathology of arteries. Physiol Rev. 1990;70:1177–1209. doi: 10.1152/physrev.1990.70.4.1177. [DOI] [PubMed] [Google Scholar]
- Schwarz UR, Walter U, Eigenthaler M. Taming platelets with cyclic nucleotides. Biochem Pharmacol. 2001;62:1153–1161. doi: 10.1016/s0006-2952(01)00760-2. [DOI] [PubMed] [Google Scholar]
- Walter U, Gambaryan S. cGMP and cGMP-dependent protein kinase in platelets and blood cells. Handb Exp Pharmacol. 2009;191:533–548. doi: 10.1007/978-3-540-68964-5_23. [DOI] [PubMed] [Google Scholar]
- Wonerow P, Obergfell A, Wilde JI, Bobe R, Asazuma N, Brdicka T, Leo A, Schraven B, Horejsí V, Shattil SJ, Watson SP. Differential role of glycolipid-enriched membrane domains in glycoprotein VI- and integrin-mediated phospholipase Cgamma2 regulation in platelets. Biochem J. 2002;364:755–765. doi: 10.1042/BJ20020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue GG, Lau CB, Fung KP, Leung PC, Ko WH. Effects of Cordyceps sinensis, Cordyceps militaris and their isolated compounds on ion transport in Calu-3 human airway epithelial cells. J Ethnopharmacol. 2008;117:92–101. doi: 10.1016/j.jep.2008.01.030. [DOI] [PubMed] [Google Scholar]