Abstract
Chemotherapy has long been considered as one of useful strategies for cancer treatment. It is primarily based on the apoptosis that can selectively kill cancer cells. However, cancer cells can progressively develop an acquired resistance to apoptotic cell death, rendering refractory to chemo- and radiotherapies. Although the mechanism by which cells attained resistance to drug remains to be clarified, it might be caused by either pumping out of them or interfering with apoptotic signal cascades in response to cancer drugs. In case that cancer cells are defective in some part of apoptotic machinery by repeated exposure to anticancer drugs, alternative cell death mechanistically distinct from apoptosis could be adopted to remove cancer cells refractory to apoptosis-inducing agents. This review will mainly deal with harnessing of necrotic cell death, specifically, programmed necrosis and practical uses. Here, we begin with various defects of apoptotic death machinery in cancer cells, and then provide new perspective on programmed necrosis as an alternative anticancer approach.
Keywords: Necroptosis, Apoptosis, Autophagy, Programmed necrosis, Chemotherapy
OVERVIEW
Cells continuously strive to maintain the balance of survival and death from interior or exterior situations. Cells consume most energy to keep homeostasis, and its disturbance is more manifested in cancer cells (Buzea et al., 2007). Transformation of normal cell into cancer cells is characterized by loss of cell cycle and resistance to apoptosis as well as other phenotypic properties (Markert, 1968). These phenotypic alterations are related to malignant neoplasia. Therefore, the principal way for cancer treatment is to render cancer sensitive to chemotherapy, consequently causing programmed cell death apoptosis (Strasser et al., 2000; Dy and Adjei, 2002; Elmore, 2007). Apoptosis is a naturally occurring process in developing and adult tissues, but not for cancer. Moreover, dying cells by apoptosis are engulfed by neighboring cells or immune cells, leaving no danger debris that can provoke inflammation. Therefore, the apoptotic control of cancer cells with cancer drugs is considered as the most rational therapy. However, a large number of cancers have developed evasion mechanisms to chemotherapy (Gottesman, 1993, 2002). Since defects in cell death machinery limit the feasibility of clinical apoptosis inducer, novel regimens to improve clinical outcomes are being taken into consideration. Cell-based anticancer therapy has currently taken advantage of apoptosis, but another programmed cell death modes including autophagy and necroptosis have gained attention as potential therapeutic approaches. Since failures in the cell death process are frequently found in various cancers, key proteins governing cell death type could be used as therapeutic targets for a wide range of cancer. Therefore, molecular understanding of evasion mechanisms of cancer cells to death stresses could surely provide a customized strategy for choosing the cell death modality suitable for fighting cancer. This review will briefly begin with features of cell death types and crosstalk between cell death modes. Then, we particularly highlight state of the art reports on programmed necrosis, a specialized necrotic cell death and outlook of its clinical use for controlling cancers resistant to conventional chemotherapy.
CELL DEATH MODALITIES AND IMPAIRED CELL DEATH IN CANCERS
To begin with, general concepts and molecular machinery for cell death types were briefly described. Concomitantly, evasion mechanisms of cancer cells to cancer therapy based on cell death were dealt in this review. Regarding ineffective cancer treatment, defective death signals in various cancers were listed in Table 1.
Table 1.
Defective cell death found frequently in cancers
| Defective proteins | Death type | Cells | References |
|---|---|---|---|
| Bcl-2, Bcl-xL, Survivin, Bax, APAF-1 | Apoptosis | Various cancer cells | (Basu and Haldar, 1998; Lowe and Lin, 2000; Nelson et al., 2004; Strasser et al., 2000) |
| Caspase-9s, HSPs | Apoptosis | Non-small cell lung cancer/many cancers | (Speirs et al., 2011) |
| Caspase-8 | Apoptosis | Neuroblastoma/glioblastoma multiforme | (Hurst and Welch, 2011; Martinez et al., 2007) |
| Caspase | Apoptosis | CD34+ hematopoietic stem cells | (Mohr et al., 2005) |
| FLIP | Apoptosis | Jurkat lymphoma and MCF7 breast cancer cell lines | (Zobalova et al., 2008) |
| Beclin-1 | Autophagy | Various cancer cells | (Gozuacik and Kimchi, 2004; Mizushima, 2007; Muzes and Sipos, 2012; Yang et al., 2011) |
| Atg7 | Autophagy | A549-B480 | (Shen et al., 2010) |
| PARP | Necroptosis | Various cancer cells | (Wang and Weaver, 2011) |
| HMGB1 | Necroptosis | CT-26 | (Yamazaki et al., 2014) |
| MCA205 |
APOPTOSIS
Classical programmed cell death apoptosis has been well studied for four decades. Apoptosis can mainly be executed via intrinsic or extrinsic pathway according to the way that death signal initiates within or outside cell (Elmore, 2007; Jha et al., 2007). Intrinsic pathway is generally activated in response to diverse signals resulting from DNA damage, loss of cell-survival factors, or other types of severe cell stress. Normally, pro-apoptotic proteins are released from the mitochondria to activate a cascade of caspases, consequently leading to apoptosis. Its pathway is tightly regulated by a variety of other factors including Bcl-2 family proteins such as the mitochondrial anti-apoptotic proteins Bcl-2 and Bcl-xL, and pro-apoptotic proteins Bcl-2-associated X (Bax) and Bak. In various cancers, however, alteration and defects in the components of intrinsic pathway are identified (Elmore, 2007). Cancers themselves have developed refractory mechanisms to apoptotic stresses by overexpressing anti-apoptotic proteins or causing defects in pro-apoptotic proteins (Basu and Haldar, 1998; Strasser et al., 2000; Nelson et al., 2004). In fact, it has been reported that increased levels of Bcl-2, Bcl-xL or survivin correlate closely with drug resistance to chemotherapeutic agents in many cancer cell lines. Moreover, decreased expression or mutations of pro-apoptotic proteins such as Bax and APAF-1 contribute to chemotherapy resistance (Lowe and Lin, 2000). Besides, high expression of caspase-9S and heat shock proteins (HSPs), which can interfere with apoptotic signaling, has been observed in many malignant cancers (Speirs et al., 2011). Extrinsic pathway is triggered by ligand binding to death receptors, which can promote the formation of the death-inducing signaling complex (DISC). Sequentially, caspase-8 in turn activates effector caspases including caspase-3. Regulation of the extrinsic pathway can be achieved by cellular FADD-like interleukin-1 beta-converting enzyme (FLICE)-inhibitory protein (cFLIP), which prevents caspase-8 recruitment to the DISC. Upon DISC activation, the same effector caspases as used in the intrinsic pathway are activated for mediating apoptotic cell death. Additionally, inhibitors of apoptosis proteins (IAPs) and the second mitochondria-derived activator of caspases (Smac) affect negatively or positively apoptotic cell death (Deng et al., 2002). For instance, the x-linked IAP (XIAP) inhibits caspase-3 and -9 and is inversely inhibited by Smac. As deduced from extrinsic pathway, it is readily supposed that alterations of ligand binding confer the resistant mechanism to cells derived from multiple cancers. Both downregulation of receptor and the overexpression of decoy receptor can abolish the extrinsic signal pathway to apoptosis. Apart from aberrant expression of death receptor and decoy receptor, mutations and silencing in caspase-8 fail to convey death signals from ligation of death receptor to downstream signaling molecules. Such defects in caspase-8 are remarkable in neuroblastoma and glioblastoma multiforme (Martinez et al., 2007; Hurst and Welch, 2011). Also, deregulated apoptosis in hematopoietic system can cause the development of immune deficiencies and leukemia. CD34+ hematopoietic progenitor stem cells (HSCs) are constitutively refractory to CD95-mediated apoptosis. It is revealed that HSCs do not express caspase-8, a key player of the DISC of death receptors (Mohr et al., 2005). Moreover, it has been documented that cancer stem cells (CSCs) are resistant to apoptosis induction (Zobalova et al., 2008). Cell lines that express highly CD133 are more resistant than those harboring low CD133. It was concluded that FLIP conferred resistance to apoptosis mediated by the death ligand TRAIL.
AUTOPHAGY
Autophagy is regarded as the basic catabolic mechanism associated with cell degradation of unnecessary or dysfunctional cellular components through the lysosomal machinery. However, the physiological significances of autophagy present a wide range of spectra from cell survival, immunity to cell death. Largely, autophagy is classified into macroautophagy and compartment-specific autophagy (Amaravadi and Thompson, 2007; Yang and Klionsky, 2010). Macroautophagy is initiated by the formation of a double membrane to engulf the cytosol, followed by fusion with a lysosome to form an autophagolysome (Burman and Ktistakis, 2010; Levine et al., 2011). Subsequently, the contents within resulting vesicles are enzymatically degraded. Compartment-specific autophagy includes chaperone-mediated autophagy (CMA) and mitophagy (Speirs et al., 2011; Nordgren et al., 2013). CMA is responsible for the degradation of approximately 30% of cytosolic proteins in tissues. It is a very complex and specific pathway by which heat shock cognate protein of 70 kDa (hsc70)-containing complex recognizes and binds to substrate proteins. This resulting complex moves then to the lysosomal membrane-bound protein, allowing the substrate proteins into the lysosomal matrix, where those proteins are completely degraded by the proteases. Mitophagy is referred to as the autophagy of damaged mitochondria that specifically caused by starvation. Its process begins with the release of mitochondrial intermembrane proteins. In general, autophagy indicates macroautophagy unless otherwise specified. As for regulation, autophagy-related proteins are representatively mammalian target of rapamycin (mTOR) and Beclin-1. Atg13/Ulk1/FIP200 complex is prerequisite for autophagosome formation and can be inhibited by a cascade of PI3K/Akt/mTOR pathway through Atg13 phosphorylation. Beclin-1 is a tumor suppressor that activates autophagy together with UV irradiation resistance-associated gene (UVRAG) and endophilin B1 (Bif1). Autophagy was initially regarded as a prosurvival function due to its compensatory tendency to maintain ATP deficiency. Recent studies support that autophagy also plays a significant role in promoting cell death. In fact, not only excessive autophagy leads to cell death, but also autophagy activates apoptosis during HIV infection. There is another report that autophagy selectively degrades survival factors, reducing cell viability. Autophagy-related genes have been suggested to act as tumor suppressors, so that autophagy defects are found in a variety of tumors (Gozuacik and Kimchi, 2004; Mizushima, 2007; Yang et al., 2011; Muzes and Sipos, 2012). Deletions and mutations in Beclin-1 gene as well as its binding molecules were frequent identified in cancer patients. Meanwhile, once Bcl-2 bound to Beclin-1, autophagy is inhibited to increase cell survival concomitantly. Therefore, facilitating dissociation between Bcl-2 and Beclin-1 could be an attractive approach for inducing autophagic cell death in cancer patients. Additionally, it was documented that defective LC3 distribution was closely correlated with the capability of cells to be refractory to microtubule-stabilizing agent epothilone B. This consequence was attributed to complete loss of Atg7, essential for autophagy (Shen et al., 2010).
NECROPTOSIS
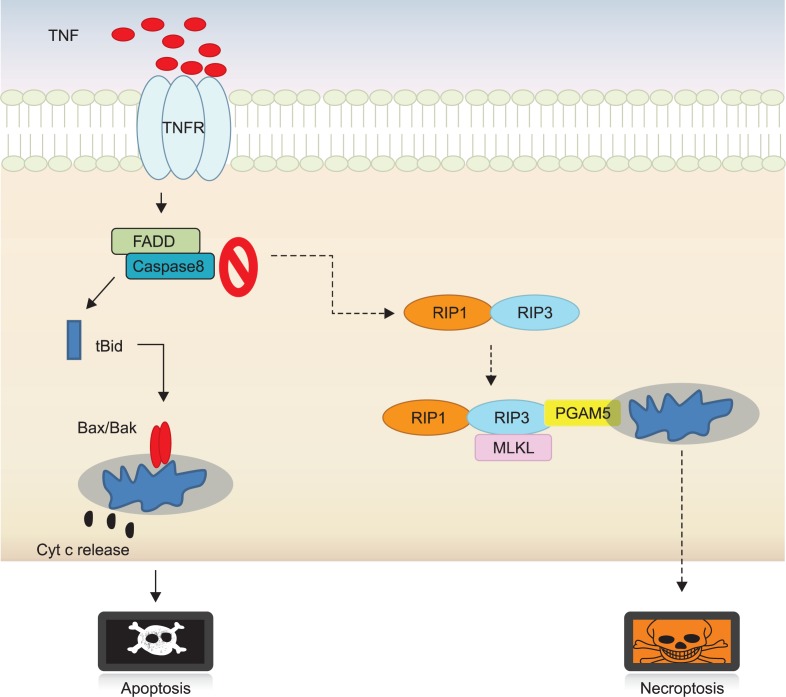
With typical features of necrotic cell death, cells are committed to die in an ordered and orchestrated manner when challenged with cytokines, infectious agents and chemicals. Such specialized cell death is referred to as type III programmed cell death, programmed necrosis or necroptosis. Necroptosis can be discriminated from other death types by caspase activation-defective and lysosome-independent characteristics (Kepp et al., 2011; Kaczmarek et al., 2013). Subcellular organ-elles including endosome, Golgi body and mitochondria are swollen at an early stage of necroptosis, later leading to functional loss of cell membrane. Receptor-mediated necroptosis and posttraumatic necrosis occur through a series of events: mitochondrial dysfunction, reactive oxygen species (ROS) generation, ATP depletion, calpains- and cathepsins-directed protein degradation. Finally, nuclear disintegration is accompanied by release of high mobility group box 1 (HMGB1), which invokes inflammation by recruiting inflammatory cells to damaged tissues. Necroptosis has been activated in an orchestrated manner via a cascade of signaling pathways (Fig. 1). Key mediators such as RIP1, RIP3, a mitochondrial phosphoglycerate mutase family member 5 (PGAM5) and mixed lineage kinase domain-like (MLKL) proteins for executing necroptosis have been identified through a genome-wide RNA interference screen. Necrotic cell death is commonly suppressed in cancer patients subjected to chemotherapy. Alkylating agent-induced poly (ADP-ribose) polymerase-1 (PARP-1) hyperactivation induces necrosis, although PARP-1 is ordinarily involved in repairing DNA damage. Accordingly, PARP inhibition in combination with standard chemotherapy can potentiate apoptotic cell death (Gaymes et al., 2009; Koster et al., 2011). By contrast, excessive activity of PARP-1 may be useful in the defective condition of apoptotic cell death machinery. In fact, patients with inactive retinoblastoma protein do not achieve clinical improvement from PARP inhibition. The primary screening for PARP mutations would be essential for decision-making of cancer therapy (Wang and Weaver, 2011). HMGB1 is released during necroptosis and at the late stage of other cell death. It can provoke an anticancer immune response as well, resulting in improvement of chemotherapy or radiotherapy. HMGB1-deficient tumors compromise chemotherapy-induced immunogenic cell death. To strengthen chemosensitivity, therefore, their immunogenicity could be successfully altered by providing artificial TLR4 ligands (Yamazaki et al., 2014). Ligation of death receptor with TNFα also induces necrosis through complex formation and activation of RIPs. More interestingly, it has been reported that RIP1 or RIP3 mediates necroptosis independently (Cho et al., 2009b; Galluzzi et al., 2009; Christofferson and Yuan, 2010; Vandenabeele et al., 2010; Linkermann et al., 2012). Therefore, mutational profiles in RIP1 and RIP3 may be the key issues for harnessing necrosis as the alternative therapeutic force to defective cell death machinery.
Fig. 1.
Molecular switching from apoptosis to necroptosis. Once cells are stimulated with TNFα, FADD and caspase-8 dissociated from membrane form of TNF receptor reconstitutes a cytoplasmic complex and active caspase-8 within complex cleaves Bid that cause to transform Bax/Bak into multimers in mitochondria. Afterward, a series of downstream events result in default apoptotic death. In contrast, under the circumstances that apoptosis is blocked or hindered by chemical or biological factors, cells activate a back-up cell death programmed necrosis in an active and ordered fashion by a cascade of signaling pathway. In fact, RIP1 interacts physically with RIP3 to trigger consecutive downstream signaling events including MLKL and PGAM5 recruitments, which transmit cytosolic death signals to mitochondria.
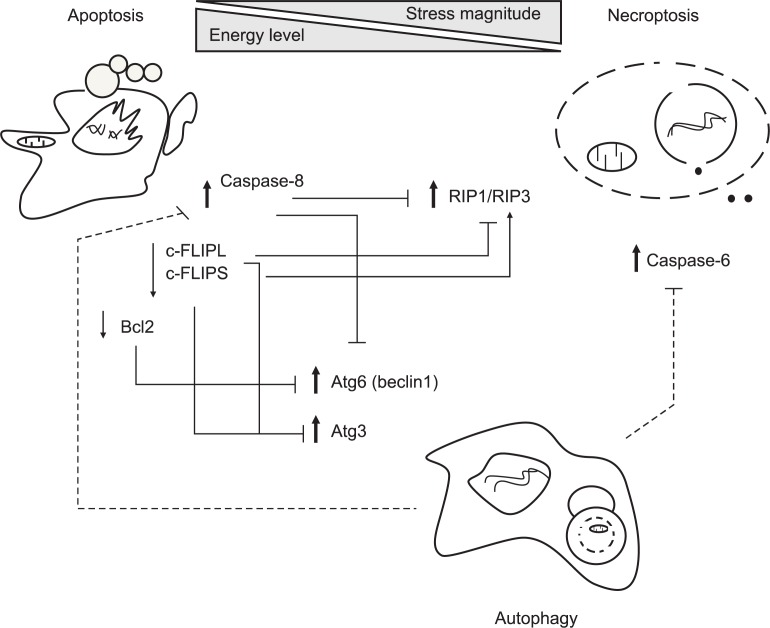
SWITCHING AND REGULATION BETWEEN CELL DEATH MODES
When cells are challenged by various death stimuli, they are typically doomed to undergo one form of 3 different death modalities such as apoptosis, necrosis and autophagy. Cell death modes are believed to cross-talk each other via a web of signaling networks in a tightly regulated way (Orrenius et al., 2011). Over the last 30 years, a cascade of signaling pathways leading to apoptosis has been well documented when compared with other death types like autophagy and necroptosis. Recently, proposed is a new notion that a specialized back-up cell death necroptosis is activated under pharmacological inhibition of apoptosis or defective apoptotic machinery in response to TNFα stimulation. This is a typical example of cell death switching between apoptosis and necroptosis. Regulatory crosstalk between apoptosis, necrosis and autophagy was schematically depicted in Fig. 2. Notably, novel necroptosis regulators RIP1 and RIP3 were identified, and their interacting complex RIP1-RIP3 was proposed to cause cells to die in a necroptotic way. Once caspase 8 activated, however, necroptosis is suppressed due to the cleavage of RIP1 and RIP3. c-FLIP functions as a master antiapoptotic regulator by binding to FADD and/or caspase-8 and caspase-10 (Nakajima et al., 2006; Safa, 2012). It is expressed as splice variants c-FLIPL and c-FLIPS, which are known to have multifunctional roles in various signaling pathways (Safa and Pollok, 2011; He and He, 2013). Interestingly, it is also involved in modulating necroptosis positively or negatively. When cells are killed by genotoxic stress, a large cell death-inducing signaling platform (∼2 MDa) referred to as ripoptosome is assembled. It consists of the core components such as RIP1, FADD and caspase-8/10. Specifically, c-FLIPL prevents formation of ripoptosome complex whereas c-FLIPS facilitates its assembly (Feoktistova et al., 2011). Moreover, the cellular inhibitor of apoptosis proteins (cIAPs) blocks ripoptosome formation by direct ubiquitination of its components (Feoktistova et al., 2011; Imre et al., 2011).
Fig. 2.
Crosstalk between apoptosis, necroptosis and autophagy. Cells determines actively cell death modes to external stresses through integrated decision of various factors including stress amplitude, cellular energy levels and induction of survival signals. Generally, controlled stimuli trigger default cell death apoptosis in a well-organized way while a specialized cell death necroptosis is activated under the condition when some death signals can induce apoptosis but a part of apoptotic machinery is defective. In aspects of energy crisis, apoptotic cell death occurs in the energy state sufficient to fuel caspase activity while necroptotic cell death prevails in the energy-deficient conditions, and autophagic suppression facilitates necroptosis. Occasionally, autophagy can be induced when default apoptosis is suppressed, but consequent effects depends on death contexts, leading to counteracting or promoting necroptosis. As for molecular regulation, apoptosis-associated proteins caspase-8 cleaves both RIP1 and RIP3 to abolish necroptotic downstream signals. Another antiapoptotic regulator c-FLIPL prevents formation of ripoptosome complex consisting of RIP, FADD and caspase-8/10 whereas c-FLIPS facilitates its assembly. Meanwhile, autophagy can also degrade caspase-8 to impede apoptosis although apoptosis reciprocally inhibits autophagy through Bcl-2 mediated sequestration and caspase-dependent degradation of Beclin-1. Autophagy can suppress necroptotic cell death by downregulation of C-6. Therefore, interplay between cell death modalities is so complicated that death fate will be determined depending on the different circumstances. Solid and dotted lines represent if associated proteins are identified or not. Also, bold and plain arrows indicate that specific proteins function positively and negatively on associated cell death, respectively.
Although autophagy is initially activated as a protective responses to stimuli, persistent autophagy can result in cell death by excessive degradation of essential cellular proteins. Therefore, autophagy and apoptosis play opposing or accompanying roles in cancers. The cytoprotective role of autophagy was supported by the finding that inhibition of autophagy by chloroquine sensitized apoptosis-resistant tumor cells to cancer therapy in chronic myelogenous leukemia (CML) cell lines (Carew et al., 2007). Another evidence was suggested by the report that overexpression of Beclin-1 diminished TNF-related apoptosis-inducing ligand (TRAIL)-mediated death (Cho et al., 2009a). Mechanism study revealed that active caspase-8 in apoptosis-resistant tumor cells are targeted to autophagosomes to be degraded in lysosomes, nullifying activation of downstream apoptotic signals. Since autophagy inhibition has been generally believed to promote cancer survival, a combined treatment of autophagy inhibitor and chemotherapy directs cell death toward apoptosis (Hou et al., 2011; Rikiishi, 2012). Clearance of ER stress-misfolded proteins by autophagy limited the induction of apoptosis. Evidently, crosstalk between apoptosis and autophagy is suggested from the report that caspase-8 degrades Atg6 during receptor activated cell death (Kang et al., 2011). Conversely, autophagic degradation of caspase-8 disconnects death signals to the downstream apoptotic machinery (Rikiishi, 2012). Accordingly, autophagic cell death become manifested under conditions that apoptosis is inhibited (Yu et al., 2004; Boya et al., 2005). cFLIP and Bcl-2 can physically interact with Atg3 and Atg6, respectively (Gordy and He, 2012). Notably, both isoforms of c-FLIP interfere with autophagic process by preventing Atg3 from binding LC3 while antiapoptotic protein Bcl-2 inhibits Atg6-dependent autophagy. On the contrary, autophagy can augment apoptosis by replenishing ATP levels which can drive ATP-dependent apoptotic processes. In contrast, autophagy induction leads to apoptotic cell death. Rottlerin causes autophagic and apoptotic cell death in breast and prostate cancers via AMP-activated protein kinase (AMPK) activation, suppression of Akt/mTOR and activation of caspases (Kumar et al., 2013). Additionally, compound K, an active metabolite of ginsenosides, induces apoptosis and autophagy via ROS generation and JNK activation in colon cancer cells (Kim et al., 2013). It was demonstrated that its dual roles in apoptosis and autophagy were caused from disruption of interaction between Bcl-2 and Atg6. Interconnections between apoptosis and autophagy are corroborated by the finding that both death modes can cooperatively induce cell death in parallel or in sequence (Wang et al., 2013). Not only apoptosis but also autophagy is induced in arsenic trioxide-treated T-lymphocytic leukemia and imatinib-treated Kaposi’s sarcoma, resulting in tumor remission (Qian et al., 2007; Jain et al., 2013). Under any circumstances, autophagy is thought to act upstream of apoptosis since cell death with autophagic features is found even in the inhibition of apoptosis. Suppression of autophagy by chemical inhibitors or siRNA knockdown of Beclin1 and Atg7 in turn leads to inhibition of apoptosis (Espert et al., 2006).
The interplay between autophagy and necroptosis appeared to be rather intricate and controversial. Its molecular interpretation remains elusive yet al though autophagy modulate necroptosis positively or negatively (Hammerova et al., 2012). That is, autophagy is required for necrotic cell death of acute neurodegenerative disorder such as ischemic stroke. By contrast, autophagy has been documented to antagonize TNFα-induced programmed necrosis (Tsuda et al., 2012). Moreover, zVAD mediates necroptosis via dual-inhibition of caspase and lysosomal cathepsins, which play key roles in undergoing apoptosis and autophagy, respectively (Wu et al., 2011). Inhibition of autophagy via the mTOR signaling pathway enhances zVAD-induced necroptosis while autophagic induction by starvation and chemical treatment protects cells from necroptosis (Chen et al., 2011). More recently, autophagy was reported to interplay with necroptosis via caspase-6 (Ye et al., 2013). Caspase-6 is required for TNFα-derived necroptosis, and autophagic inducer downregulates substantially caspase 6, resulting in suppression of necroptosis.
PRECLINICAL TEST OF NECROPTOSIS AGAINST CANCER
Most human cancers have mutations that disarm default cell death apoptosis to promise cancer survival. Accordingly, alternative cell death should be considered in order to effectively control apoptosis-resistant cancer cells. Various biological tests or preclinical trials of necroptosis for anticancer therapy were presented in Table 2. Okada group reported that leukemia cells with BCR-ABL positive cells were induced to cell death in a caspase-independent pathway when treated with an Abl kinase inhibitor Glivec (imatinib) (Okada et al., 2004). Imatinib-mediated necrosis is closely linked to release of HtrA2/Omi (serine protease) from mitochondria. Necrotic approaches have not been widely used to treat cancer cells due to inflammatory response. However, its clinical significance is increasing as a new emerging tool to overcome cancer with acquired anticancer drug-resistance. Harnessing of ordered necrotic death in clinical cancer therapy includes photodynamic treatment (PDT) and alkylating DNA-damaging agents. Primarily, selective localization of sensitization molecules to tumor sites is prerequisite for PDT. Upon illumination with light possessing specific wavelength spectra, ROS are generated by the photosensitizer, resulting in necrotic cell death. Therefore, its efficacy is dependent upon preferential accumulation of photosensitizer in tumor over normal and treatment of defined tumor area. PDT has the advantage that can selectively target the cancer cells resistant to apoptosis. In fact, apoptosis-resistant cells that overexpressing Bcl-2/Bcl-xL or xenografts of a breast cancer deficient in caspase-3 readily succumb to PDT (Bown et al., 2002; Castano et al., 2006). Also, given that PARP is suppressed, DNA damage-induced chemotherapy exhibits more outstanding efficacy. It could be inferred from the fact that PAPR is involved in repairing low levels of DNA damage. However, excessive activity of PARP-1 induces cell death through necrosis, so that it could be applicable when other cell death types are not activated properly. In fact, Treatment of cells with DNA alkylating agent causes PARP hyperactivation, leading to depletion of cellular NAD and ATP, and finally cell death with necroptotic characteristics (Zong et al., 2004). It was consequently concluded that DNA damage induced necroptosis in a PARP-dependent way distinct from the mitochondrial apoptotic pathway. Mechanistically, cleavage of PARP-1 induces JNK activation through RIP1 and TRAF2 and further depolarizes mitochondrial potential, eventually leading to cell death. Interestingly, patients with inactivated retinoblastoma protein were not treated if PARP was suppressed. It indicates that activation in PARP-1 signal transduction can affect feasibility of cell death. Accordingly, it is essential to detect mutations in PARP of cancer patients for anticancer control. Either inflammatory cytokine TNFα or other agonists mediate cell necrosis through activation of RIP1 phosphorylation (Christofferson et al., 2012; Moquin et al., 2013). RIP1 is a necroptosis mediator, but it not only activates NF-κB, but also induces apoptotic cell death. Physical interaction of RIP1 with RIP3 is prerequisite in determining a cell death route, but RIP3 overexpression is sufficient for inducing necroptosis (Moujalled et al., 2013). On the contrary, RIP3 deficiency facilitates RIP1-mediated apoptosis (Moriwaki and Chan, 2013). A recent in vivo research demonstrates that RIP3 potentiates necrotic cell death when infected with virus that is able to inhibit the apoptotic machinery, implying that RIP3 can play a key role in cancer with resistance to apoptosis-based therapy. Accordingly, mutation profiles in RIP1 and RIP3 could provide basis of decision-making that is able to take advantage of necroptosis for cancer control. Besides TNFα, some chemicals or agents such as lapachone, apoptolidin and honokil have been reported to kill cells through necrosis although their precise mechanisms remain elusive (Li et al., 1999; Salomon et al., 2000; Tagliarino et al., 2001; Bai et al., 2003; Sun et al., 2006). Furthermore, deoxynyboquinone, a NAD(P)H:quinone oxidoreductase 1(NQO1) substrate, exerts a potent antitumor activity with selectivity by PARP1 hyperactivation and concomitant ATP loss (Huang et al., 2012). More lately, shikonin induced necroptotic cell death of glioma cells by a dose-dependent upregulation of RIP1 (Huang et al., 2013). Perspective understanding of molecular mechanisms of necroptotic cell death caused from various sources could make it possible to adopt a strategy suitable for the tumor specific responses to necrotic stimuli.
Table 2.
Harnessing of necroptosis for controlling cancers
| Chemicals/Therapy | Target/Pathway | Stages | Comments | References |
|---|---|---|---|---|
| Imatinib | BCR-ABL | Clinical trial | Leukemia cell | (Okada et al., 2004) |
| PDT (photodynamic therapy) | ROS generation | Clinical application | Cancer cell defective of apoptosis | (Bown et al., 2002; Castano et al., 2006) |
| DNA alkylating agents | PARP-1 | Proof of concept | Cells deficient in p53 or Bax/Bak | (Zong et al., 2004) |
| Deoxynyboquinone | NAD(P)H:quinone oxidoreductase 1 (NQO1)/PARP-1 | Proof of concept | Pancreatic and lung cancers | (Huang et al., 2012) |
| Shikonin | Multitargets/RIP1-dependent | Proof of concept | Glioma | (Huang et al., 2013) |
OUTLOOKS AND CONCLUSIONS
Necroptosis, a form of backup cell death to apoptosis, is basically taken as the immunological concept of first defense mechanism against infection of virus with apoptosis evasion apparatus. Similar to virus’ survival tactic against host cell’s suicidal pressure, cancer cells have developed various ways to acquire resistance to anticancer drugs, perpetuating cancer growth and proliferation. Moreover, many cancers hold genetic aberration and deletion in specific genes that could be targeted by cancer drugs. The default programmed cell death apoptosis would not be generally activated in cancer cells refractory to chemotherapy. Therefore, alternative cell death modalities to fight cancers with defective apoptotic machinery should be pursued. Since there have been little information on interplay and regulation between cell death modes yet, however, other cell death modes such as autophagy and necroptosis have still limitation for clinical uses when compared with apoptosis.
Necroptosis has significant implications in the biological systems subjected to death stimuli. Principally, cells cope actively with a failure in conducting TNFα-mediated apoptosis, and consequently divert potential apoptotic force into alternative one. Switching of death modes like this has differential effects on physiological outcomes, depending on stimuli and tissue niche. Therefore, extensive studies on its underlying mechanism by which cell death switching determines cancer fate could provide treatment regimens suitable for chemotherapy-resistant cancers derived from diverse circumstances. Meanwhile, necroptosis itself is pathologically associated with a variety of diseases including inflammation and ischemic brain injury. Therefore, most studies have been focused on the development of pharmacological inhibitors targeting necroptosis-relevant proteins. Up till now, many efforts have made to discover necroptosis inhibitors, since the first identification of RIP1, a necroptosis regulator. As a result, Nec-1 is the first small molecule targeting a specific molecule RIP1. Thereafter, some necrotic proteins have been extensively explored and protein-signaling networks have been partly unveiled. Therefore, a few chemicals have been currently identified as chemical inhibitors of necroptosis. Nonetheless, little attention on chemicals to promote necroptosis has been gained yet in an attempt to make them feasible for anticancer therapy. Only a few chemicals have been studied as a means of understanding mechanisms of toxicity induced by chemicals. The combined treatment of TNFα with Hsp90 inhibitor geldanamycin (GA) has been reported to augment considerably cell death caused by TNFα stimulation alone, providing basis that small molecules be pursued for taking advantage of them to treat cancers (Vanden Berghe et al., 2003). Taken together, required are still comprehensive identification of necroptosis target molecules and construction of a network of signaling molecules associated with necroptosis. Optimistically, the pharmacological modulation of cell death by design will be feasible by customizing death therapy to cancer types.
REFERENCES
- Amaravadi RK, Thompson CB. The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin Cancer Res. 2007;13:7271–7279. doi: 10.1158/1078-0432.CCR-07-1595. [DOI] [PubMed] [Google Scholar]
- Bai X, Cerimele F, Ushio-Fukai M, Waqas M, Campbell PM, Govindarajan B, Der CJ, Battle T, Frank DA, Ye K, Murad E, Dubiel W, Soff G, Arbiser JL. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J Biol Chem. 2003;278:35501–35507. doi: 10.1074/jbc.M302967200. [DOI] [PubMed] [Google Scholar]
- Basu A, Haldar S. The relationship between BcI2, Bax and p53: consequences for cell cycle progression and cell death. Mol Hum Reprod. 1998;4:1099–1109. doi: 10.1093/molehr/4.12.1099. [DOI] [PubMed] [Google Scholar]
- Bown SG, Rogowska AZ, Whitelaw DE, Lees WR, Lovat LB, Ripley P, Jones L, Wyld P, Gillams A, Hatfield AW. Photodynamic therapy for cancer of the pancreas. Gut. 2002;50:549–557. doi: 10.1136/gut.50.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Metivier D, Meley D, Souquere S, Yoshimori T, Pierron G, Codogno P, Kroemer G. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman C, Ktistakis NT. Autophagosome formation in mammalian cells. Semin Immunopathol. 2010;32:397–413. doi: 10.1007/s00281-010-0222-z. [DOI] [PubMed] [Google Scholar]
- Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2:MR17–71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- Carew JS, Nawrocki ST, Kahue CN, Zhang H, Yang C, Chung L, Houghton JA, Huang P, Giles FJ, Cleveland JL. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood. 2007;110:313–322. doi: 10.1182/blood-2006-10-050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer. 2006;6:535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SY, Chiu LY, Maa MC, Wang JS, Chien CL, Lin WW. zVAD-induced autophagic cell death requires c-Src-dependent ERK and JNK activation and reactive oxygen species generation. Autophagy. 2011;7:217–228. doi: 10.4161/auto.7.2.14212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DH, Jo YK, Hwang JJ, Lee YM, Roh SA, Kim JC. Caspase-mediated cleavage of ATG6/Beclin-1 links apoptosis to autophagy in HeLa cells. Cancer Lett. 2009a;274:95–100. doi: 10.1016/j.canlet.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009b;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofferson DE, Li Y, Hitomi J, Zhou W, Upperman C, Zhu H, Gerber SA, Gygi S, Yuan J. A novel role for RIP1 kinase in mediating TNFalpha production. Cell Death Dis. 2012;3:e320. doi: 10.1038/cddis.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofferson DE, Yuan J. Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol. 2010;22:263–268. doi: 10.1016/j.ceb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Lin Y, Wu X. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 2002;16:33–45. doi: 10.1101/gad.949602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dy GK, Adjei AA. Novel targets for lung cancer therapy: part I. J Clin Oncol. 2002;20:2881–2894. doi: 10.1200/JCO.2002.11.145. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espert L, Denizot M, Grimaldi M, Robert-Hebmann V, Gay B, Varbanov M, Codogno P, Biard-Piechaczyk M. Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. J Clin Invest. 2006;116:2161–2172. doi: 10.1172/JCI26185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, Cain K, MacFarlane M, Hacker G, Leverkus M. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Kepp O, Kroemer G. RIP kinases initiate programmed necrosis. J Mol Cell Biol. 2009;1:8–10. doi: 10.1093/jmcb/mjp007. [DOI] [PubMed] [Google Scholar]
- Gaymes TJ, Shall S, MacPherson LJ, Twine NA, Lea NC, Farzaneh F, Mufti GJ. Inhibitors of poly ADP-ribose polymerase (PARP) induce apoptosis of myeloid leukemic cells: potential for therapy of myeloid leukemia and myelodysplastic syndromes. Haematologica. 2009;94:638–646. doi: 10.3324/haematol.2008.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordy C, He YW. The crosstalk between autophagy and apoptosis: where does this lead? Protein Cell. 2012;3:17–27. doi: 10.1007/s13238-011-1127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman MM. How cancer cells evade chemotherapy: sixteenth Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res. 1993;53:747–754. [PubMed] [Google Scholar]
- Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- Hammerova J, Uldrijan S, Taborska E, Vaculova AH, Slaninova I. Necroptosis modulated by autophagy is a predominant form of melanoma cell death induced by sanguilutine. Biol Chem. 2012;393:647–658. doi: 10.1515/hsz-2011-0279. [DOI] [PubMed] [Google Scholar]
- He MX, He YW. A role for c-FLIP(L) in the regulation of apoptosis, autophagy, and necroptosis in T lymphocytes. Cell Death Differ. 2013;20:188–197. doi: 10.1038/cdd.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou YJ, Dong LW, Tan YX, Yang GZ, Pan YF, Li Z, Tang L, Wang M, Wang Q, Wang HY. Inhibition of active autophagy induces apoptosis and increases chemosensitivity in cholangiocarcinoma. Lab Invest. 2011;91:1146–1157. doi: 10.1038/labinvest.2011.97. [DOI] [PubMed] [Google Scholar]
- Huang C, Luo Y, Zhao J, Yang F, Zhao H, Fan W, Ge P. Shikonin kills glioma cells through necroptosis mediated by RIP-1. PLoS One. 2013;8:e66326. doi: 10.1371/journal.pone.0066326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Dong Y, Bey EA, Kilgore JA, Bair JS, Li LS, Patel M, Parkinson EI, Wang Y, Williams NS, Gao J, Hergenrother PJ, Boothman DA. An NQO1 substrate with potent antitumor activity that selectively kills by PARP1-induced programmed necrosis. Cancer Res. 2012;72:3038–3047. doi: 10.1158/0008-5472.CAN-11-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst DR, Welch DR. Metastasis suppressor genes at the interface between the environment and tumor cell growth. Int Rev Cell Mol Biol. 2011;286:107–180. doi: 10.1016/B978-0-12-385859-7.00003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imre G, Larisch S, Rajalingam K. Ripoptosome: a novel IAP-regulated cell death-signalling platform. J Mol Cell Biol. 2011;3:324–326. doi: 10.1093/jmcb/mjr034. [DOI] [PubMed] [Google Scholar]
- Jain MV, Paczulla AM, Klonisch T, Dimgba FN, Rao SB, Roberg K, Schweizer F, Lengerke C, Davoodpour P, Palicharla VR, Maddika S, Los M. Interconnections between apoptotic, autophagic and necrotic pathways: implications for cancer therapy development. J Cell Mol Med. 2013;17:12–29. doi: 10.1111/jcmm.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha P, Matta B, Lyzogubov V, Tytarenko R, Bora PS, Bora NS. Crucial role of apoptosis in the resolution of experimental autoimmune anterior uveitis. Invest Ophthalmol Vis Sci. 2007;48:5091–5100. doi: 10.1167/iovs.07-0651. [DOI] [PubMed] [Google Scholar]
- Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepp O, Galluzzi L, Lipinski M, Yuan J, Kroemer G. Cell death assays for drug discovery. Nat Rev Drug Discov. 2011;10:221–237. doi: 10.1038/nrd3373. [DOI] [PubMed] [Google Scholar]
- Kim AD, Kang KA, Kim HS, Kim DH, Choi YH, Lee SJ, Kim HS, Hyun JW. A ginseng metabolite, compound K, induces autophagy and apoptosis via generation of reactive oxygen species and activation of JNK in human colon cancer cells. Cell Death Dis. 2013;4:e750. doi: 10.1038/cddis.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster R, Timmer-Bosscha H, Bischoff R, Gietema JA, de Jong S. Disruption of the MDM2-p53 interaction strongly potentiates p53-dependent apoptosis in cisplatin-resistant human testicular carcinoma cells via the Fas/FasL pathway. Cell Death Dis. 2011;2:e148. doi: 10.1038/cddis.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Shankar S, Srivastava RK. Rottlerin-induced autophagy leads to the apoptosis in breast cancer stem cells: molecular mechanisms. Mol. Cancer. 2013;12:171. doi: 10.1186/1476-4598-12-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YZ, Li CJ, Pinto AV, Pardee AB. Release of mitochondrial cytochrome C in both apoptosis and necrosis induced by beta-lapachone in human carcinoma cells. Mol Med. 1999;5:232–239. [PMC free article] [PubMed] [Google Scholar]
- Linkermann A, Brasen JH, De Zen F, Weinlich R, Schwendener RA, Green DR, Kunzendorf U, Krautwald S. Dichotomy between RIP1- and RIP3-mediated necroptosis in tumor necrosis factor-alpha-induced shock. Mol Med. 2012;18:577–586. doi: 10.2119/molmed.2011.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485–495. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- Markert CL. Neoplasia: a disease of cell differentiation. Cancer Res. 1968;28:1908–1914. [PubMed] [Google Scholar]
- Martinez R, Setien F, Voelter C, Casado S, Quesada MP, Schackert G, Esteller M. CpG island promoter hypermethylation of the pro-apoptotic gene caspase-8 is a common hallmark of relapsed glioblastoma multiforme. Carcinogenesis. 2007;28:1264–1268. doi: 10.1093/carcin/bgm014. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- Mohr A, Zwacka RM, Jarmy G, Buneker C, Schrezenmeier H, Dohner K, Beltinger C, Wiesneth M, Debatin KM, Stahnke K. Caspase-8L expression protects CD34+ hematopoietic progenitor cells and leukemic cells from CD95-mediated apoptosis. Oncogene. 2005;24:2421–2429. doi: 10.1038/sj.onc.1208432. [DOI] [PubMed] [Google Scholar]
- Moquin DM, McQuade T, Chan FK. CYLD deubiquitinates RIP1 in the TNFalpha-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS One. 2013;8:e76841. doi: 10.1371/journal.pone.0076841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki K, Chan FK. RIP3: a molecular switch for necrosis and inflammation. Genes Dev. 2013;27:1640–1649. doi: 10.1101/gad.223321.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moujalled DM, Cook WD, Okamoto T, Murphy J, Lawlor KE, Vince JE, Vaux DL. TNF can activate RIPK3 and cause programmed necrosis in the absence of RIPK1. Cell Death Dis. 2013;4:e465. doi: 10.1038/cddis.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzes G, Sipos F. Anti-tumor immunity, autophagy and chemotherapy. World J Gastroenterol. 2012;18:6537–6540. doi: 10.3748/wjg.v18.i45.6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima A, Komazawa-Sakon S, Takekawa M, Sasazuki T, Yeh WC, Yagita H, Okumura K, Nakano H. An antiapoptotic protein, c-FLIPL, directly binds to MKK7 and inhibits the JNK pathway. EMBO J. 2006;25:5549–5559. doi: 10.1038/sj.emboj.7601423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Ferguson LR, Denny WA. DNA and the chromosome - varied targets for chemotherapy. Cell Chromosome. 2004;3:2. doi: 10.1186/1475-9268-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordgren M, Wang B, Apanasets O, Fransen M. Peroxisome degradation in mammals: mechanisms of action, recent advances, and perspectives. Front Physiol. 2013;4:145. doi: 10.3389/fphys.2013.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Adachi S, Imai T, Watanabe K, Toyokuni SY, Ueno M, Zervos AS, Kroemer G, Nakahata T. A novel mechanism for imatinib mesylate-induced cell death of BCR-ABL-positive human leukemic cells: caspase-independent, necrosis-like programmed cell death mediated by serine protease activity. Blood. 2004;103:2299–2307. doi: 10.1182/blood-2003-05-1605. [DOI] [PubMed] [Google Scholar]
- Orrenius S, Nicotera P, Zhivotovsky B. Cell death mechanisms and their implications in toxicology. Toxicol Sci. 2011;119:3–19. doi: 10.1093/toxsci/kfq268. [DOI] [PubMed] [Google Scholar]
- Qian W, Liu J, Jin J, Ni W, Xu W. Arsenic trioxide induces not only apoptosis but also autophagic cell death in leukemia cell lines via up-regulation of Beclin-1. Leuk Res. 2007;31:329–339. doi: 10.1016/j.leukres.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Rikiishi H. Novel Insights into the Interplay between Apoptosis and Autophagy. Int J Cell Biol. 2012;2012:317645. doi: 10.1155/2012/317645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safa AR. c-FLIP, a master anti-apoptotic regulator. Exp Oncol. 2012;34:176–184. [PMC free article] [PubMed] [Google Scholar]
- Safa AR, Pollok KE. Targeting the anti-apoptotic protein c-FLIP for cancer therapy. Cancers (Basel) 2011;3:1639–1671. doi: 10.3390/cancers3021639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon AR, Voehringer DW, Herzenberg LA, Khosla C. Understanding and exploiting the mechanistic basis for selectivity of polyketide inhibitors of F(0)F(1)-ATPase. Proc Natl Acad Sci USA. 2000;97:14766–14771. doi: 10.1073/pnas.97.26.14766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Kepp O, Martins I, Vitale I, Souquere S, Castedo M, Pierron G, Kroemer G. Defective autophagy associated with LC3 puncta in epothilone-resistant cancer cells. Cell Cycle. 2010;9:377–383. doi: 10.4161/cc.9.2.10468. [DOI] [PubMed] [Google Scholar]
- Speirs CK, Hwang M, Kim S, Li W, Chang S, Varki V, Mitchell L, Schleicher S, Lu B. Harnessing the cell death pathway for targeted cancer treatment. Am J Cancer Res. 2011;1:43–61. [PMC free article] [PubMed] [Google Scholar]
- Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- Sun X, Li Y, Li W, Zhang B, Wang AJ, Sun J, Mikule K, Jiang Z, Li CJ. Selective induction of necrotic cell death in cancer cells by beta-lapachone through activation of DNA damage response pathway. Cell Cycle. 2006;5:2029–2035. doi: 10.4161/cc.5.17.3312. [DOI] [PubMed] [Google Scholar]
- Tagliarino C, Pink JJ, Dubyak GR, Nieminen AL, Boothman DA. Calcium is a key signaling molecule in beta-lapachone-mediated cell death. J Biol Chem. 2001;276:19150–19159. doi: 10.1074/jbc.M100730200. [DOI] [PubMed] [Google Scholar]
- Tsuda H, Ning Z, Yamaguchi Y, Suzuki N. Programmed cell death and its possible relationship with periodontal disease. J Oral Sci. 2012;54:137–149. doi: 10.2334/josnusd.54.137. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe T, Kalai M, van Loo G, Declercq W, Vandenabeele P. Disruption of HSP90 function reverts tumor necrosis factor-induced necrosis to apoptosis. J Biol Chem. 2003;278:5622–5629. doi: 10.1074/jbc.M208925200. [DOI] [PubMed] [Google Scholar]
- Vandenabeele P, Declercq W, Van Herreweghe F, Vanden Berghe T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal. 2010;3:re4. doi: 10.1126/scisignal.3115re4. [DOI] [PubMed] [Google Scholar]
- Wang H, Lu Q, Cheng S, Wang X, Zhang H. Autophagy activity contributes to programmed cell death in Caenorhabditis elegans. Autophagy. 2013;9:1975–1982. doi: 10.4161/auto.26152. [DOI] [PubMed] [Google Scholar]
- Wang X, Weaver DT. The ups and downs of DNA repair biomarkers for PARP inhibitor therapies. Am J Cancer Res. 2011;1:301–327. [PMC free article] [PubMed] [Google Scholar]
- Wu YT, Tan HL, Huang Q, Sun XJ, Zhu X, Shen HM. zVAD-induced necroptosis in L929 cells depends on auto-crine production of TNFalpha mediated by the PKC-MAPKs-AP-1 pathway. Cell Death Differ. 2011;18:26–37. doi: 10.1038/cdd.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Hannani D, Poirier-Colame V, Ladoire S, Locher C, Sistigu A, Prada N, Adjemian S, Catani JP, Freudenberg M, Galanos C, Andre F, Kroemer G, Zitvogel L. Defective immunogenic cell death of HMGB1-deficient tumors: compensatory therapy with TLR4 agonists. Cell Death Differ. 2014;21:69–78. doi: 10.1038/cdd.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZJ, Chee CE, Huang S, Sinicrope FA. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther. 2011;10:1533–1541. doi: 10.1158/1535-7163.MCT-11-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye YC, Wang HJ, Chen L, Liu WW, Tashiro S, Onodera S, Xia MY, Ikejima T. Negatively-regulated necroptosis by autophagy required caspase-6 activation in TNFalpha-treated murine fibrosarcoma L929 cells. Int Immunopharmacol. 2013;17:548–555. doi: 10.1016/j.intimp.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- Zobalova R, McDermott L, Stantic M, Prokopova K, Dong LF, Neuzil J. CD133-positive cells are resistant to TRAIL due to up-regulation of FLIP. Biochem Biophys Res Commun. 2008;373:567–571. doi: 10.1016/j.bbrc.2008.06.073. [DOI] [PubMed] [Google Scholar]
- Zong WX, Ditsworth D, Bauer DE, Wang ZQ, Thompson CB. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 2004;18:1272–1282. doi: 10.1101/gad.1199904. [DOI] [PMC free article] [PubMed] [Google Scholar]