Abstract
β-lapachone is a naturally occurring quinone that selectively induces apoptotic cell death in a variety of human cancer cells in vitro and in vivo; however, its mechanism of action needs to be further elaborated. In this study, we investigated the effects of β-lapachone on the induction of apoptosis in human gastric carcinoma AGS cells. β-lapachone significantly inhibited cellular proliferation, and some typical apoptotic characteristics such as chromatin condensation and an increase in the population of sub-G1 hypodiploid cells were observed in β-lapachone-treated AGS cells. Treatment with β-lapachone caused mitochondrial transmembrane potential dissipation, stimulated the mitochondria-mediated intrinsic apoptotic pathway, as indicated by caspase-9 activation, cytochrome c release, Bcl-2 downregulation and Bax upregulation, as well as death receptor-mediated extrinsic apoptotic pathway, as indicated by activation of caspase-8 and truncation of Bid. This process was accompanied by activation of caspase-3 and concomitant with cleavage of poly(ADP-ribose) polymerase. The general caspase inhibitor, z-VAD-fmk, significantly abolished β-lapachone-induced cell death and inhibited growth. Further analysis demonstrated that the induction of apoptosis by β-lapachone was accompanied by inactivation of the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway. The PI3K inhibitor LY29004 significantly increased β-lapachone-induced apoptosis and growth inhibition. Taken together, these findings indicate that the apoptotic activity of β-lapachone is probably regulated by a caspase-dependent cascade through activation of both intrinsic and extrinsic signaling pathways, and that inhibition of the PI3K/Akt signaling may contribute to β-lapachone-mediated AGS cell growth inhibition and apoptosis induction.
Keywords: β-lapachone, Apoptosis, Caspase, PI3K/Akt
INTRODUCTION
β-lapachone (3,4-dihydro-2,2-dimethyl-2H-naphtho[1,2-b] pyran-5,6-dione), a naturally occurring quinone, was originally obtained from the bark of the South American lapacho tree (Tabebuia vellanedae) and has been used as herbal medicine for various diseases. β-lapachone exerts divergent therapeutic activities such as anti-bacterial, anti-fungal, anti-viral, anti-psoriasis, anti-arthritic, and anti-inflammatory effects (Schuerch and Wehrli, 1978; Binutu et al., 1996; Müller et al., 1999; Tzeng et al., 2003; Moon et al., 2007; Sitônio et al., 2013). In recent years, this quinone compound has attracted considerable attention particularly in the cancer research community due to its topoisomerase inhibitory and apoptosis induction activities (Boothman et al., 1989; Li et al., 1993; Krishnan and Bastow, 2000). β-lapachone also promotes apoptotic cell death by sensitizing human tumor cells to ionizing radiation and DNA-damaging agents (Frydman et al., 1997; Lee et al., 2006b). In addition, several researchers have shown that increased generation of reactive oxygen species (ROS) by β-lapachone is mainly attributed to apoptosis (Chau et al., 1998; Shiah et al., 1999; Bey et al., 2007; Wu et al., 2011; He et al., 2013). We demonstrated previously that β-lapachone-induced apoptosis is associated with phosphorylation of tumor suppressor p53, upregulation of cyclin-dependent kinase inhibitor p21 and pro-apoptotic Bax, activation of caspases, and inactivation of telomerase and the NF-κB signaling pathway in some cancer cell lines (Choi et al., 2002, 2003; Lee et al., 2005, 2006a; Woo and Choi, 2005; Woo et al., 2006; Moon et al., 2010). Our previous studies also indicate that β-lapachone induces apoptosis by interrupting multiple cell cycle checkpoints (Choi et al., 2003) and inhibits invasive ability by upregulating expression of the early growth response gene-1 and throbospondin-1 (Kim et al., 2007). Lien et al. (2008) suggested that β-lapachone-induced endoplasmic reticulum stress and mitogen-activated protein kinase activation are novel signaling pathways underlying the molecular mechanism of the anti-cancer effect of β-lapachone.
Many of the intracellular signaling pathways involved in cellular transformation have been elucidated, and efforts are underway to develop treatment strategies that target these specific signaling molecules or their downstream effectors. Among them, the phosphoinositide 3-kinase (PI3K)/Akt pathway has achieved major importance as a target for cancer therapy, as its signaling axis plays an important role in cancer (Fresno Vara et al., 2004; Tokunaga et al., 2008; Yea and Fruman, 2013). PI3K is a lipid kinase that plays a central role in control of cell fate, and drives the progression of cancer by activating a phosphoinositide-dependent serine/threonine kinase called Akt (also known as protein kinase B). Activated Akt enhances cell survival by inhibiting pro-apoptotic proteins and activating anti-apoptotic proteins thereby promoting cell survival. PI3K/Akt signaling pathway components are frequently altered in human cancers (Fresno Vara et al., 2004; Tokunaga et al., 2008). Moreover, survival signals induced by several receptors are also mediated mainly by PI3K/Akt; thus, development of PI3K inhibitors could both prevent cancer cell proliferation and induce programmed cell death (apoptosis) by fully suppressing Akt activation (Carnero et al., 2008; Hixon et al., 2010). However, whether the PI3K/Akt pathway is involved in β-lapachone-mediated apoptosis and the pathway involved have not been elucidated. In this study, we used the AGS human gastric carcinoma cell line as a model to examine whether the PI3K/Akt pathway and its associated signals are involved in β-lapachone-induced apoptosis.
MATERIALS AND METHODS
Reagents and antibodies
β-lapachone was purchased from Biomol (Plymouth Meeting, PA, USA). A stock solution of β-lapachone (10 mM) was prepared in ice-cold absolute alcohol and diluted with fresh complete medium immediately before use. RPMI 1640 medium, fetal bovine serum (FBS) and penicillin/streptomycin were purchased from Gibco-BRL (Gaithersburg, MD, USA). 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphnyl-2H-tetrazolium bromide (MTT), 4,6-dianmidino-2-phenylindole (DAPI), propidium iodide (PI), 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazol carbocyanine iodide (JC-1) and bovine serum albumin (BSA) were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). All primary antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA), Cell Signaling Technology (Danvers, MA, USA) and Calbiochem (Cambridge, MA, USA). Peroxidase-labeled donkey anti-rabbit immunoglobulin, and peroxidase-labeled sheep anti-mouse immunoglobulin were purchased from Amersham Co. (Arlington Heights, IL, USA).
Cell culture and viability assay
The human gastric carcinoma AGS cells (American Type Culture Collection, Manassas, VA, USA) were cultured in RPMI 1640 medium containing 10% heat inactivated FBS and 100 U/mL penicillin/streptomycin in a humidified incubator with a 5% CO2 atmosphere at 37°C. The effect of β-lapachone on cell viability was determined by the MTT colorimetric method. In brief, cells were cultured in each well of 96-well plates with varying concentrations of β-lapachone for 24 h. After adding MTT solution (0.5 mg/ml), the plates were incubated for 3 h, the media was removed, and then the formazan crystals were solubilized in dimethyl sulfoxide (DMSO). The optical density (OD) of each culture well was measured using an enzyme-linked immunosorbent assay (ELISA) reader at 570 nm. The OD570 in control cells was taken as 100% viability.
Apoptosis assay
Apoptotic cells were evaluated by DAPI staining and flow cytometry. For the DAPI staining assay, cells treated with or without β-lapachone for 24 h were washed twice in ice-cold phosphate buffered saline (PBS), fixed in 4% paraformalde-hyde for 15 min at room temperature, and then stained with DAPI for 5 min. The cells were dropped on a glass slide and covered with a coverslip, then observed under a florescent microscope (Carl Zeiss, Oberkochen, Germany). For flow cytometry, the cells were fixed in 70% ethanol at 4°C for 30 min, washed once with PBS, resuspended in PI staining reagent containing RNase, and incubated in the dark for 30 min. Flow cytometry was performed on a FACScan flow-cytometry system (Becton Dickinson, San Jose, CA, USA) and the sub-G1 fraction was measured as apoptotic cells (Hwang et al., 2013).
Protein extraction and Western blot analysis
Whole-cell protein extracts were prepared in cell lysis buffer (20 μM sucrose, 1 mM EDTA, 20 mM Tris-HCl, pH 7.2, 1 mM DTT, 10 mM KCl, 1.5 mM MgCl2, and 5 μg/ml aprotinin) for 30 min. In a parallel experiment, the mitochondrial and cytosolic fractions were isolated using a mitochondrial fractionation kit (Active Motif, Carlsbad, CA, USA) according to the manufacturer’s instructions. The protein extracts were quantified using a Bio-Rad kit (Pierce Biotechnology). Equal amounts of protein were resolved electrophoretically on 8–12% SDS-polyacrylamide gels under denatured reducing conditions and transferred to nitrocellulose membranes (Schleicher & Schuell, Keene, NH, USA). The membranes were soaked in blocking buffer (5% skimmed milk) and incubated overnight with primary antibodies, followed by horseradish peroxidase-conjugated antibodies, and immune complexes were then visualized by the enhanced chemiluminescence (ECL, Amersham) detection system according to the recommended procedure.
Detection of mitochondrial membrane potential (MMP)
After a 24 h incubation with β-lapachone, the cells were washed with PBS and incubated with the mitochondrial potential sensor JC-1 under dark conditions for 15 min at 37°C, immediately centrifuged to remove the supernatant, resuspended in PBS, and then analyzed by flow cytometry. Loss of the MMP was quantified as the percentage of cells expressing JC-1 monomer fluorescence.
Caspase activity assay
The enzymatic activity of caspases was assayed in cell lysates using commercial colorimetric assay kits (Calbiochem) following the manufacturer’s instructions. Briefly, cells were cultured in the presence or absence of β-lapachone, and then 2×106 cells were incubated with specific colorimetric peptide substrates (acetyl (Ac)-Ile-Glu-Thr-Asp (IETD)-p-nitroaniline (pNA) for caspase-8, Ac-Leu-Glu-His (LEHD)-Asp-pNA for caspase-9, and Ac-Asp-Glu-Val-Asp (DEVD)-pNA for caspase-3, respectively) in the presence of dithiothreitol for 60 min at 37°C. The reaction was measured by changes in absorbance at 405 nm using a microplate reader
Statistical analysis
Results are expressed as mean ± standard deviation (SD) of data obtained from triplicate experiments. The statistical analysis was performed with the paired Student’s t-test. Differences at p<0.05 were considered significant.
RESULTS
β-lapachone inhibits cell growth and induces apoptosis in AGS cells
To determine the effect of β-lapachone on AGS cell proliferation, the cells were treated with different concentrations of β-lapachone for 24 h, and cell viability was examined with the MTT assay. As shown in Fig. 1, β-lapachone inhibited AGS cell proliferation significantly at concentrations >3 μM in a dose-dependent manner. We further examined the DAPI staining and flow cytometry assays to determine whether the cytotoxic effect of β-lapachone was mediated by apoptosis. A morphological analysis with DAPI staining revealed nuclei with dose-dependent chromatin condensation and the formation of apoptotic bodies, characteristic morphological changes of apoptosis, in cells cultured with β-lapachone. In contrast, very few apoptotic cells were observed in the control culture (Fig. 2A). The results of flow cytometric analysis after PI staining showed that β-lapachone increased the percentage of cells in the hypodiploid sub-G1 phase in a dose-dependent manner (Fig. 2B), which was consistent with the cytotoxic effect of β-lapachone shown in Fig. 1. These results demonstrate that the cytotoxic effects observed in response to β-lapachone are associated with the induction of apoptosis in AGS cells.
Fig. 1.

β-lapachone inhibits cell viability in AGS cells. Cells were seeded at 2×105 cell per well onto 96-well culture plates overnight and then treated with the indicated concentrations of β-lapachone for 24 h. Cell viability was determined by the MTT assay. Data are mean ± SD of three independent experiments (*p<0.05 vs. untreated control).
Fig. 2.
β-lapachone induces apoptosis in AGS cells. (A) The cells were treated with the indicated concentrations of β-lapachone for 24 h, sampled, fixed, and stained with DAPI solution. The stained nuclei were observed under a fluorescent microscope (original magnification, 400×). (B) To quantify the degree of apoptosis induced by β-lapachone, cells grown under the same conditions as (A) were evaluated by a flow cytometry for sub-G1 DNA content, which represents the cells undergoing apoptotic DNA degradation. Data are mean ± SD of three independent experiments (*p<0.05 vs. untreated control).
β-lapachone induces the caspase-dependent apoptotic pathway in AGS cells
Recent evidence indicates that caspases play important roles denaturing the cellular infrastructure during apoptosis, and that many chemotherapeutic agents activate caspases to kill cancer cells. Therefore, we examined the effect of β-lapachone on caspase activity to determine whether caspase activation occurs during β-lapachone-induced apoptosis. The immunoblotting data indicated that treating the cells with β-lapachone increased the levels of active caspase-8 and -9, which are initiator caspases of the extrinsic (death receptor-mediated) and intrinsic (mitochondria-mediated) apoptotic pathways, respectively (Fig. 3A). Although we did not observe the active form of caspase-3, an effector caspase, β-lapachone decreased the levels of pro-caspase-3. Furthermore, activation of caspases by β-lapachone was also confirmed by measuring enzyme activity using the specific synthetic substrates for each caspase. As indicated in Fig. 3B, we observed a dose-dependent gradual increase in caspase-3, -8 and -9 activities in β-lapachone-treated AGS cells similar to the proteolytic processing of pro-caspases. Activation of caspase-3 was further evidenced by cleavage of poly(ADP-ri-bose) polymerase (PARP) from an 116 kDa band to an 89 kDa fragment, which is a substrate of active caspase-3, and also serves as a marker of cells undergoing apoptosis (Kaufmann et al., 1993).
Fig. 3.
Activation of caspases and degradation of PARP by β-lapachone in AGS cells. (A) Cells were treated with the indicated concentrations of β-lapachone for 24 h. The cells were lysed, and cellular proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The proteins were visualized using the indicated antibodies and an ECL detection system. Proteolytic cleavage of PARP is indicated by the arrow. Actin was used as the internal control. (B) After a 24 h incubation with the indicated concentrations of β-lapachone, the cells were lysed, and aliquots were assayed for in vitro caspase-3, -8, and -9 activity using Ac-DEVD-pNA, Ac-IETD-pNA, and Ac-LEHD-pNA as substrates, respectively, at 37°C. After incubation of 1 h, the amount of pNA released was measured at 405 nm using an ELISA microplate reader. Each point represents the mean ± SD of three independent experiments (*p<0.05 vs. untreated control).
To further address the significance of caspase activation in β-lapachone-induced apoptosis, we examined the effects of the general caspase inhibitor, z-VAD-fmk. As shown in Fig. 4A, pretreatment of cells with z-VAD-fmk significantly inhibited downregulation of pro-caspase-3 and cleavage of PARP induced by β-lapachone, and completely abrogated chromatin concentration (Fig. 4B). The flow cytometric analysis and MTT assay indicated that pretreating cells with z-VAD-fmk prevented β-lapachone-induced accumulation of the sub-G1 population and growth inhibition (Fig. 4C and D). These results show that β-lapachone-induced apoptosis in AGS cells occurs via a caspase-dependent pathway.
Fig. 4.
Caspase-mediated apoptosis induced by β-lapachone in AGS cells. (A) Cells were incubated with 4 μM β-lapachone for 24 h after 1 h pretreatment with the pan-caspase inhibitor, z-VAD-fmk (50 μM). Equal amounts of cell lysates isolated from cells were subjected to SDS-polyacrylamide gel electrophoresis and analyzed by Western blot for caspase-3 and PARP. (B) Cells grown under the same conditions as (A) were fixed and stained with DAPI solution. Stained nuclei were observed under a fluorescent microscope. (C) Cells were evaluated by flow cytometry for sub-G1 DNA content, suggestive of apoptotic cell death. (D) Cell viability was determined by the MTT assay. Results are mean ± SD of three independent experiments (*p<0.05 vs. β-lapachone treated cells).
β-lapachone induces mitochondrial dysfunction by regulating the expression of Bcl-2 family proteins in AGS cells
The disturbance of MMP is an early but crucial process in the intrinsic pathways of apoptosis. The cytosolic release of mitochondrial pro-apoptotic proteins such as cytochrome c is also one of the key events in the mitochondria-dependent apoptotic death process (Martinou and Youle, 2011). To investigate whether mitochondrial dysfunction contributes to β-lapachone-induced apoptosis in AGS cells, MMP was measured by the JC-1 staining assay. After treatment with β-lapachone for 24 h, the loss of MMP was increased markedly, which was demonstrated by the change in JC-1-derived fluorescence from red to green in a dose-dependent manner, demonstrating disruption of the MMP during β-lapachone-induced apoptosis in AGS cells (Fig. 5A). This result demonstrated that disruption of mitochondrial function originated from expression of Bcl-2 family protein members. It was observed that β-lapachone decreased the expression of anti-apoptotic Bcl-2 in a dose-dependent manner with concomitant enhanced expression of pro-apoptotic Bax protein (Fig. 5B). These results indicate that β-lapachone may rearrange the ratio of Bcl-2 to Bax and therefore may augment apoptotic cell death by activating the intrinsic signaling pathway.
Fig. 5.
Effect of β-lapachone on levels of MMP, Bcl-2 family proteins, and cytochrome c in AGS cells. (A) Cells were treated with indicated concentrations of β-lapachone for 24 h. They were collected and incubated with 10 μM JC-1 for 15 min at 37°C in the dark. The cells were washed once with PBS and analyzed by a DNA flow cytometer. Results are presented as the mean of two independent experiments. (B) Equal amounts of cell lysates was resolved by SDS-polyacrylamide gel electrophoresis, transferred to membranes, and probed with specific antibodies. The anti-actin antibody was a protein loading control. (C) Cytosolic and mitochondrial proteins were extracted from cells grown under the same conditions and analyzed by Western blotting using anti-cytochrome c antibody. Actin and cytochrome oxidase subunit IV (COX IV) were used as internal controls for the cytosolic and mitochondrial fractions, respectively.
As β-lapachone activated caspase-9 and induced MMP loss, cytochrome c might possibly be released from the mitochondria into the cytosol. As suspected, a significant fraction of the mitochondrial cytochrome c was released into the cytosol following treatment with β-lapachone in a dose-dependent manner (Fig. 5C). Another important BH-3 protein, Bid, which belongs to the Bcl-2 family of proteins is involved in maintaining the potential of outer mitochondrial membrane integrity. Bid cleaved by activated caspase-8 in the death extrinsic pathway directly triggers the release of cytochrome c from mitochondria (Singh et al., 2002). Our Western blot results revealed that Bid was present as a 22 kDa protein in intact AGS cells; however, incubating the cells with β-lapachone resulted in the formation of 15 kDa fragments (tBid) from Bid (Fig. 5B). This result indicates that β-lapachone-induced activation of caspase-8 and subsequent processing of Bid occurs downstream of cytochrome c release to aid in activation of the intrinsic pathway of apoptosis.
β-lapachone induces apoptosis in AGA cells by inhibiting the PI3K/Akt signaling pathway
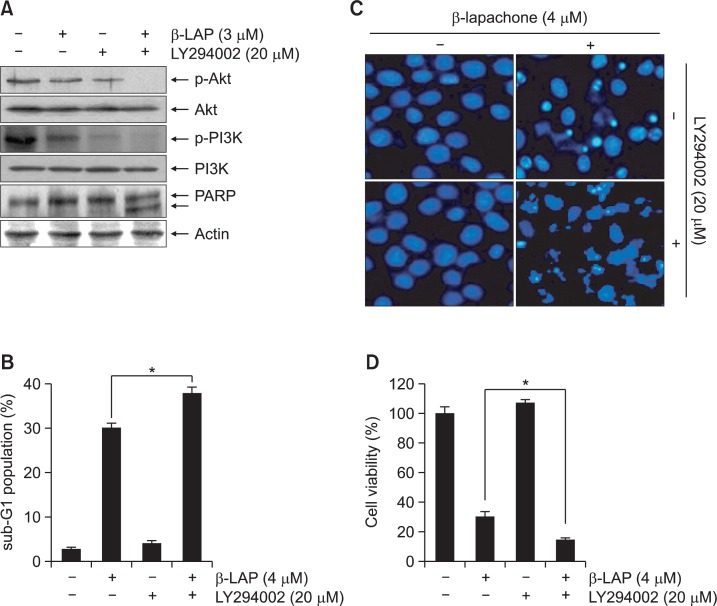
Previous studies have demonstrated that constitutive activation of PI3K/Akt is considerably high in cancer cells and regulates their survival and proliferation by triggering downstream expression of a cascade of responses (Carnero et al., 2008; Hixon et al., 2010). Therefore, we postulated that β-lapachone inhibits PI3K/Akt activity and consequently leads to apoptosis. To examine whether β-lapachone inhibits PI3K/Akt activity, AGS cells were treated with β-lapachone and the level of PI3K/Akt was measured. As shown in Fig. 6, β-lapachone significantly downregulated the expression of activated forms of PI3K and Akt, as determined by decreasing levels of phosphorylation of PI3K and Akt proteins, without altering their total levels. Thus, involvement of the PI3K/Akt signaling pathway in β-lapachone-induced apoptosis was examined using the PI3K/Akt inhibitor LY294002 to determine if inhibition of PI3K/Akt signaling was responsible for the induction of apoptosis. Treatment with LY294002 alone did not modify PI3K and Akt levels; however, it produced potent effects on level of phosphorylated PI3K and Akt, whose levels remarkably decreased compared with those in the untreated control group and became undetectable after co-treatment with 3 mM β-lapachone (Fig. 7A). Our results also indicate that LY294002 significantly enhanced PARP cleavage and encouraged the apoptotic events and inhibited growth following β-lapachone treatment (Fig. 7), indicating close involvement of inactivation of the PI3K/Akt pathway in β-lapachone-induced apoptosis in AGS cells.
Fig. 6.

Effect of β-lapachone on the levels of phosphorylation of PI3K and Akt in AGS cells. The cells were incubated with the indicated concentrations of β-lapachone for 24 h. The cells were lysed, and the cellular proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were probed with specific antibodies to p-Akt (Ser 473), total Akt, p-PI3K (p85 Tyr 458/p55 Tyr 199), and total PI3K. The proteins were visualized using an ECL detection system. Actin was used as the internal control.
Fig. 7.
The role of PI3K/Akt signaling in β-lapachone-induced apoptosis in AGS cells. (A) Cells were stimulated with 3 μM β-lapachone for 24 h after pretreatment with 20 μM LY290042 for 1 h. Equal amounts of cell lysates were resolved by SDS-polyacrylamide gel electrophoresis, transferred to membranes, and probed with the indicated antibodies. Anti-actin antibody was a protein loading control. (B and D) The percentage of the sub-G1 population (B) and cell viability (D) were assessed by flow cytometry and the MTT assay, respectively. Results are mean ± SD of three independent experiments (*p<0.05 vs. β-lapachone treated cells). (C) The cells were fixed and stained with DAPI solution. Stained nuclei were observed under a fluorescent microscope.
DISCUSSION
Although numerous studies on β-lapachone-induced apoptosis have been carried out in many cancer cell lines, the cytotoxic mechanism of β-lapachone in human gastric carcinoma cells remains conflicting. In our study, we investigated the molecular mechanism of apoptosis by β-lapachone in AGS cells and propose that the possible causal sequence is PI3K/Akt inactivation, caspase-8 activation, Bid cleavage, cytochrome c release, caspase-9 and -3 activation, PARP cleavage, and DNA fragmentation.
Apoptosis is a complex activity that mobilizes a wide variety of death signals, and the induction of apoptosis in malignant cells is a critical feature for the development of anti-cancer agents. Therefore, signal transduction pathways involved in inhibiting growth and apoptosis are potential targets for chemopreventive and chemotherapeutic agents. Apoptosis is classified into caspase-dependent or independent mechanisms (Kroemer and Reed, 2000; Jin and El-Deiry, 2005). Caspase-dependent apoptosis can be further divided into the death receptor-mediated extrinsic pathway and the mitochondria-mediated intrinsic pathway, as determined by involvement of caspase-8 or -9, as initiator capases, respectively. Both the extrinsic and intrinsic pathways involve activation of executioner caspases (-3 and -7), which play a pivotal role in the terminal execution phase of apoptosis (Kiechle and Zhang, 2002; Chowdhury et al., 2006). Here, our data indicate that β-lapachone activated caspase-8, -9, and -3, and induced PARP cleavage in a dose-dependent manner (Fig. 3) suggesting that activating caspase-8 indicated that the extrinsic pathway may be involved in β-lapachone-induced apoptosis, as β-lapachone activated the initiator caspase-9, which in turn activated the downstream effector caspase-3 leading to PARP cleavage. However, pretreatment with the pan-caspase inhibitor, z-VAD-fmk, significantly attenuated the cleavage of caspase-3 and PARP induced by β-lapachone, and reduced β-lapachone-induced cell death and growth inhibition (Fig. 4), confirming that β-lapachone-induced apoptosis is caspase-dependent which was similar to other reports (Shiah et al., 1999; Gupta et al., 2002; Woo et al., 2006; Kung et al., 2007; Moon et al., 2010).
Mitochondria play a critical role in caspase-dependent apoptosis by disrupting of MMP and releasing proapoptotic proteins such as cytochrome c that normally reside in the intermembrane space and activate the caspase-9-mediated intrinsic apoptosis pathway (Chowdhury et al., 2006; Martinou and Youle, 2011). The Bcl-2 family proteins also act as critical regulators of mitochondrial permeability and consist of pro- and anti-apoptotic members, which form heterodimers to inhibit or activate each other. The anti-apoptotic members Bcl-2 and Bcl-xL protect against apoptotic stimuli. In contrast, it is generally accepted that Bax and cleaved Bid trigger the release of cytochrome c and that activation of caspase-8 leads to cleavage of Bid, thereby inducing mitochondria-mediated downstream events (Singh et al., 2002; Martinou and Youle 2011). Our Western blotting results indicated that β-lapachone significantly and dose-dependently downregulated Bcl-2 and upregulated Bax thereby increasing the Bax/Bcl-2 expression ratio, which is consistent with our previous reports (Choi et al., 2002; Lee et al., 2006a; Woo et al., 2006). Apoptosis induction by β-lapachone was also accompanied by depletion of the MMP and cytochrome c release (Fig. 5), showing that β-lapachone induces mitochondria-mediated intrinsic apoptosis in AGS cells. Furthermore, the results demonstrate that β-lapachone caused a dose-dependent decrease in Bid expression, which corresponded with increased expression of tBid and enzymatic activity of caspase-8 (Fig. 3, 5). These data suggest that the increased expression of tBid may be related to its truncation by activated caspase-8, which forms a central point in the cross-talk between caspase-8 and -9 activation. Although it is unclear whether there is a direct link between the intrinsic and extrinsic apoptosis pathways by β-lapachone, our results indicate the possibility that caspase-8 activation by β-lapachone is a death receptor-mediated extrinsic event.
An increasing number of studies have shown that the PI3K/Akt signaling pathway, as a critical survival factor in signal transduction pathways, plays an important role in many physiological processes, such as cell growth and proliferation, apoptosis, cell motility, and invasion (Fresno Vara et al., 2004; Tokunaga et al., 2008; Yea and Fruman, 2013). This pathway is overactive in many cancers to reduce apoptosis and allow tumor cell proliferation, and is thereby considered a possible target for cancer therapy. Several studies have indicated that the majority of patients with gastric cancer exhibit increased expression and activation of the PI3K/Akt pathway (Ang et al., 2005; Kobayashi et al., 2006; Liu et al., 2010). Furthermore, over activation of this pathway is associated with poor overall survival, disease-free survival, and high tumor recurrence in patients with gastric cancer (Kobayashi et al., 2006). It also promotes cell growth, survival, invasion, and metastasis of gastric cancer cells (Kobayashi et al., 2006; Liu et al., 2010). Therefore, blocking the constitutively active PI3K/Akt signaling pathway may provide a novel strategy for targeted cancer therapy. Our results demonstrate that β-lapachone inhibited the constitutive activity of PI3K and its downstream target Akt (Fig. 6), and that β-lapachone action was further enhanced by LY294002, a specific PI3K inhibitor (Fig. 7A). Our data also indicate that LY294002 promoted β-lapachone-induced growth inhibition and cell apoptosis in AGS cells (Fig. 7B–D), suggesting that LY294002 enhanced the chemotherapeutic sensitivity to β-lapachone in gastric cancer cells.
In conclusion, our results indicate that β-lapachone-induced apoptosis in AGS cells is mediated via both the intrinsic and extrinsic apoptotic pathways and is predominantly associated with a reduction in the Bcl-2/Bax ratio, release of cytochrome c, loss of the MMP, and truncation of Bid, consistent with caspase-8 activation. Additionally, β-lapachone inactivated PI3K/Akt signaling, leading to an inhibition of survival signals, presenting a novel therapeutic approach for treatment of gastric cancer cells using the combined β-lapachone and the PI3K/Akt inhibitor. Although more in vivo studies are needed to elucidate the exact mechanism and therapeutic effects, these findings pave the way for future investigations on the potential of β-lapachone as a targeted therapy agent for treating human gastric cancer.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2008-0062611 and 2013 041811).
REFERENCES
- Ang KL, Shi DL, Keong WW, Epstein RJ. Up-regulated Akt signaling adjacent to gastric cancers: implications for screening and chemoprevention. Cancer Lett. 2005;225:53–59. doi: 10.1016/j.canlet.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Bey EA, Bentle MS, Reinicke KE, Dong Y, Yang CR, Girard L, Minna JD, Bornmann WG, Gao J, Boothman DA. An NQO1- and PARP-1-mediated cell death pathway induced in non-small-cell lung cancer cells by β-lapachone. Proc Natl Acad Sci USA. 2007;104:11832–11837. doi: 10.1073/pnas.0702176104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binutu OA, Adesogan KE, Okogun JI. Antibacterial and antifungal compounds from Kigelia pinnata. Planta Med. 1996;62:352–353. doi: 10.1055/s-2006-957900. [DOI] [PubMed] [Google Scholar]
- Boothman DA, Trask DK, Pardee AB. Inhibition of potentially lethal DNA damage repair in human tumor cells by β-lapachone, an activator of topoisomerase I. Cancer Res. 1989;49:605–612. [PubMed] [Google Scholar]
- Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr. Cancer Drug Targets. 2008;8:187–198. doi: 10.2174/156800908784293659. [DOI] [PubMed] [Google Scholar]
- Chau YP, Shiah SG, Don MJ, Kuo ML. Involvement of hydrogen peroxide in topoisomerase inhibitor β-lapachone-induced apoptosis and differentiation in human leukemia cells. Free Radic Biol Med. 1998;24:660–670. doi: 10.1016/s0891-5849(97)00337-7. [DOI] [PubMed] [Google Scholar]
- Choi YH, Kang HS, Yoo MA. Suppression of human prostate cancer cell growth by β-lapachone via down-regulation of pRB phosphorylation and induction of Cdk inhibitor p21(WAF1/CIP1) J Biochem Mol Biol. 2003;36:223–229. doi: 10.5483/bmbrep.2003.36.2.223. [DOI] [PubMed] [Google Scholar]
- Choi YH, Kim MJ, Lee SY, Lee YN, Chi GY, Eom HS, Kim ND, Choi BT. Phosphorylation of p53, induction of Bax and activation of caspases during β-lapachone-mediated apoptosis in human prostate epithelial cells. Int J Oncol. 2002;21:1293–1299. [PubMed] [Google Scholar]
- Chowdhury I, Tharakan B, Bhat GK. Current concepts in apoptosis: the physiological suicide program revisited. Cell Mol Biol Lett. 2006;11:506–525. doi: 10.2478/s11658-006-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Frydman B, Marton LJ, Sun JS, Neder K, Witiak DT, Liu AA, Wang HM, Mao Y, Wu HV, Sanders MM, Liu LF. Induction of DNA topoisomerase II-mediated DNA cleavage by β-lapachone and related naphthoquinones. Cancer Res. 1997;57:620–627. [PubMed] [Google Scholar]
- Gupta D, Podar K, Tai YT, Lin B, Hideshima T, Akiyama M, LeBlanc R, Catley L, Mitsiades N, Mitsiades C, Chauhan D, Munshi NC, Anderson KC. β-lapachone, a novel plant product, overcomes drug resistance in human multiple myeloma cells. Exp Hematol. 2002;30:711–720. doi: 10.1016/s0301-472x(02)00839-1. [DOI] [PubMed] [Google Scholar]
- He T, Banach-Latapy A, Vernis L, Dardalhon M, Chanet R, Huang ME. Peroxiredoxin 1 knockdown potentiates β-lapachone cytotoxicity through modulation of reactive oxygen species and mitogen-activated protein kinase signals. Carcinogenesis. 2013;34:760–769. doi: 10.1093/carcin/bgs389. [DOI] [PubMed] [Google Scholar]
- Hixon ML, Paccagnella L, Millham R, Perez-Olle R, Gualberto A. Development of inhibitors of the IGF-IR/PI3K/Akt/mTOR pathway. Rev. Recent Clin. Trials. 2010;5:189–208. doi: 10.2174/157488710792007329. [DOI] [PubMed] [Google Scholar]
- Hwang YJ, Lee EJ, Kim HR, Hwang KA. Molecular mechanisms of luteolin-7-O-glucoside-induced growth inhibition on human liver cancer cells: G2/M cell cycle arrest and caspase-independent apoptotic signaling pathways. BMB Rep. 2013;46:611–616. doi: 10.5483/BMBRep.2013.46.12.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4:139–163. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993;53:3976–3985. [PubMed] [Google Scholar]
- Kiechle FL, Zhang X. Apoptosis: biochemical aspects and clinical implications. Clin. Chim. Acta. 2002;326:27–45. doi: 10.1016/s0009-8981(02)00297-8. [DOI] [PubMed] [Google Scholar]
- Kim SO, Kwon JI, Jeong YK, Kim GY, Kim ND, Choi YH. Induction of Egr-1 is associated with anti-metastatic and anti-invasive ability of β-lapachone in human hepatocarcinoma cells. Biosci Biotechnol Biochem. 2007;71:2169–2176. doi: 10.1271/bbb.70103. [DOI] [PubMed] [Google Scholar]
- Kobayashi I, Semba S, Matsuda Y, Kuroda Y, Yokozaki H. Significance of Akt phosphorylation on tumor growth and vascular endothelial growth factor expression in human gastric carcinoma. Pathobiology. 2006;73:8–17. doi: 10.1159/000093087. [DOI] [PubMed] [Google Scholar]
- Krishnan P, Bastow KF. Novel mechanisms of DNA topoisomerase II inhibition by pyranonaphthoquinone derivatives-eleutherin, α lapachone, and β lapachone. Biochem Pharmacol. 2000;60:1367–1379. doi: 10.1016/s0006-2952(00)00437-8. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- Kung HN, Chien CL, Chau GY, Don MJ, Lu KS, Chau YP. Involvement of NO/cGMP signaling in the apoptotic and anti-angiogenic effects of β-lapachone on endothelial cells in vitro. J Cell Physiol. 2007;211:522–532. doi: 10.1002/jcp.20963. [DOI] [PubMed] [Google Scholar]
- Lee JH, Cheong J, Park YM, Choi YH. Down-regulation of cyclooxygenase-2 and telomerase activity by β–lapachone in human prostate carcinoma cells. Pharmacol Res. 2005;51:553–560. doi: 10.1016/j.phrs.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Lee JI, Choi DY, Chung HS, Seo HG, Woo HJ, Choi BT, Choi YH. β-lapachone induces growth inhibition and apoptosis in bladder cancer cells by modulation of Bcl-2 family and activation of caspases. Exp Oncol. 2006a;28:30–35. [PubMed] [Google Scholar]
- Lee YJ, Park SJ, Ciccone SL, Kim CR, Lee SH. An in vivo analysis of MMC-induced DNA damage and its repair. Carcinogenesis. 2006b;27:446–453. doi: 10.1093/carcin/bgi254. [DOI] [PubMed] [Google Scholar]
- Li CJ, Averboukh L, Pardee AB. β-lapachone, a novel DNA topoisomerase I inhibitor with a mode of action different from camptothecin. J Biol Chem. 1993;268:22463–22468. [PubMed] [Google Scholar]
- Lien YC, Kung HN, Lu KS, Jeng CJ, Chau YP. Involvement of endoplasmic reticulum stress and activation of MAP kinases in β-lapachone-induced human prostate cancer cell apoptosis. Histol Histopathol. 2008;23:1299–1308. doi: 10.14670/HH-23.1299. [DOI] [PubMed] [Google Scholar]
- Liu JF, Zhou XK, Chen JH, Yi G, Chen HG, Ba MC, Lin SQ, Qi YC. Up-regulation of PIK3CA promotes metastasis in gastric carcinoma. World J Gastroenterol. 2010;16:4986–4991. doi: 10.3748/wjg.v16.i39.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon DO, Choi YH, Kim ND, Park YM, Kim GY. Anti-inflammatory effects of β-lapachone in lipopolysaccharide-stimulated BV2 microglia. Int Immunopharmacol. 2007;7:506–514. doi: 10.1016/j.intimp.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Moon DO, Kang CH, Kim MO, Jeon YJ, Lee JD, Choi YH, Kim GY. β-lapachone (LAPA) decreases cell viability and telomerase activity in leukemia cells: suppression of telomerase activity by LAPA. J. Med. Food. 2010;13:481–488. doi: 10.1089/jmf.2008.1219. [DOI] [PubMed] [Google Scholar]
- Müller K, Sellmer A, Wiegrebe W. Potential antipsoriatic agents: lapacho compounds as potent inhibitors of HaCaT cell growth. J Nat Prod. 1999;62:1134–1136. doi: 10.1021/np990139r. [DOI] [PubMed] [Google Scholar]
- Schuerch AR, Wehrli W. β-lapachone, an inhibitor of oncornavirus reverse transcriptase and eukaryotic DNA polymerase-alpha. Inhibitory effect, thiol dependence and specificity. Eur J Biochem. 1978;84:197–205. doi: 10.1111/j.1432-1033.1978.tb12157.x. [DOI] [PubMed] [Google Scholar]
- Shiah SG, Chuang SE, Chau YP, Shen SC, Kuo ML. Activation of c-Jun NH2-terminal kinase and subsequent CPP32/Yama during topoisomerase inhibitor β-lapachone-induced apoptosis through an oxidation-dependent pathway. Cancer Res. 1999;59:391–398. [PubMed] [Google Scholar]
- Singh R, Pervin S, Chaudhuri G. Caspase-8-mediated BID cleavage and release of mitochondrial cytochrome c during Nomega-hydroxy-L-arginine-induced apoptosis in MDA-MB-468 cells. Antagonistic effects of L-ornithine. J Biol Chem. 2002;277:37630–37636. doi: 10.1074/jbc.M203648200. [DOI] [PubMed] [Google Scholar]
- Sitônio MM, Carvalho Júnior CH, Campos Ide A, Silva JB, Lima Mdo C, Góes AJ, Maia MB, Rolim Neto PJ, Silva TG. Anti-inflammatory and anti-arthritic activities of 3,4-di-hydro-2,2-dimethyl-2H-naphthol[1,2-b]pyran-5,6-dione (β-lapa chone) Inflamm Res. 2013;62:107–113. doi: 10.1007/s00011-012-0557-0. [DOI] [PubMed] [Google Scholar]
- Tokunaga E, Oki E, Egashira A, Sadanaga N, Morita M, Kakeji Y, Maehara Y. Deregulation of the Akt pathway in human cancer. Curr. Cancer Drug Targets. 2008;8:27–36. doi: 10.2174/156800908783497140. [DOI] [PubMed] [Google Scholar]
- Tzeng HP, Ho FM, Chao KF, Kuo ML, Lin-Shiau SY, Liu SH. β-Lapachone reduces endotoxin-induced macrophage activation and lung edema and mortality. Am J Respir Crit Care Med. 2003;168:85–91. doi: 10.1164/rccm.200209-1051OC. [DOI] [PubMed] [Google Scholar]
- Woo HJ, Choi YH. Growth inhibition of A549 human lung carcinoma cells by β-lapachone through induction of apoptosis and inhibition of telomerase activity. Int J Oncol. 2005;26:1017–1023. [PubMed] [Google Scholar]
- Woo HJ, Park KY, Rhu CH, Lee WH, Choi BT, Kim GY, Park YM, Choi YH. β-lapachone, a quinone isolated from Tabebuia avellanedae, induces apoptosis in HepG2 hepatoma cell line through induction of Bax and activation of caspase. J. Med. Food. 2006;9:161–168. doi: 10.1089/jmf.2006.9.161. [DOI] [PubMed] [Google Scholar]
- Wu YT, Lin CY, Tsai MY, Chen YH, Lu YF, Huang CJ, Cheng CM, Hwang SP. β-Lapachone induces heart morphogenetic and functional defects by promoting the death of erythrocytes and the endocardium in zebrafish embryos. J Biomed Sci. 2011;18:70. doi: 10.1186/1423-0127-18-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yea SS, Fruman DA. Achieving cancer cell death with PI3K/mTOR-targeted therapies. Ann N Y Acad Sci. 2013;1280:15–18. doi: 10.1111/nyas.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]