Abstract
The pentacyclic triterpenoid ursolic acid (UA) and its isomer oleanolic acid (OA) are ubiquitous in food and plant medicine, and thus are easily exposed to the population through natural contact or intentional use. Although they have diverse health benefits, reported cardiovascular protective activity is contentious. In this study, the effect of UA and OA on platelet aggregation was examined on the basis that alteration of platelet activity is a potential process contributing to cardiovascular events. Treatment of UA enhanced platelet aggregation induced by thrombin or ADP, which was concentration-dependent in a range of 5–50 μM. Quite comparable results were obtained with OA, in which OA-treated platelets also exhibited an exaggerated response to either thrombin or ADP. UA treatment potentiated aggregation of whole blood, while OA failed to increase aggregation by thrombin. UA and OA did not affect plasma coagulation assessed by measuring prothrombin time and activated partial thromboplastin time. These results indicate that both UA and OA are capable of making platelets susceptible to aggregatory stimuli, and platelets rather than clotting factors are the primary target of them in proaggregatory activity. These compounds need to be used with caution, especially in the population with a predisposition to cardiovascular events.
Keywords: Ursolic acid, Oleanolic acid, Platelet aggregation
INTRODUCTION
Ursolic acid (UA) and its isomer oleanolic acid (OA) are pentacyclic triterpenoids that are common in the plant kingdom. They have similar structures (Fig. 1) and share distributional similarity in the natural world, often occurring together (Liu, 1995; Pollier and Goossens, 2012), especially in important medicinal plants such as Fructus Ligustrum lucidum (Xia et al., 2011a; Zha et al., 2010) and Fructus Forsythiae (Zha et al., 2010) used in traditional East Asian medicine, and Ocimum sanctum Linn (Holy Basil) used in Ayurvedic medicine (Sarkar et al., 2012). UA and OA are also abundant in food seasonings, such as rosemary (Jager et al., 2009), sage (Horiuchi et al., 2007), and olive oil (Andrikopoulos et al., 2002). Hence, people can be readily exposed to UA and OA naturally or intentionally.
Fig. 1.
Chemical structures of ursolic acid and oleanolic acid.
The similarity of UA and OA includes various biological activities (Liu, 1995; Pollier and Goossens, 2012). Initially, a hepatoprotective effect was identified for them. Diverse pharmacological activities reported since include anti-inflammatory, antioxidant, anticancer, and antimicrobial effects (Liu, 1995, 2005; Ikeda et al., 2008). Despite their benefits, UA and OA have some detrimental characteristics. UA exhibits anti-inflammatory effect in cells with established inflammation, but can be pro-inflammatory in normal cells (Ikeda et al., 2008). Similarly, OA is hepatoprotective at low doses, but it can induce cholestasis and hepatotoxicity at high doses (Liu, 2005).
Both UA and OA possess cardiovascular protective effects. They are effective against chemically-induced dysrhythmia (Somova et al., 2004) and protect cardiac cells from ischemic damage in a rat model of myocardial ischemia (Wang et al., 2009; Radhiga et al., 2012). They exhibit vasorelaxant activity in isolated blood vessels (Aguirre-Crespo et al., 2006; Rodriguez-Rodriguez et al., 2008) and are capable of reducing blood pressure in hypertension animal models (Somova et al., 2003). However, recent studies involving the circulatory system have been more equivocal. UA reportedly accelerates atherosclerotic plaque formation in vivo (Messner et al., 2011), contrary to another study showing the protective effect of UA against diabetes-accelerated atherosclerosis (Ullevig et al., 2011). OA directly elicits aggregation of isolated platelets (Liu et al., 2013), a process that is critically involved in the initiation of cardiovascular events (Liu et al., 2013), although other studies addressed the antiplatelet effect of UA and OA and anticoagulant activity of OA (Jin et al., 2004a; Jin et al., 2004b; Babalola et al., 2013). These inconsistent reports therefore prompted the present study.
Platelet aggregation is crucial in hemostasis under normal physiological conditions. Abnormal activation or hyperaggregation of platelets caused by xenobiotics including drugs, food and environmental toxicants, readily leads to thrombosis. Such altered platelet function is involved in the pathogenesis of atherosclerosis and inflammation, which ultimately contributes to the development of acute coronary syndrome (ACS), stroke, and the ischemic complications of peripheral vascular disease. Therefore, xenobiotics enhancing platelet activity are regarded as risk factors for cardiovascular diseases, and anti-platelet therapy is a general approach to prevent and manage them (Liu et al., 2013).
In the present study, the biological activity of UA and OA was examined in an isolated platelet system to clarify their effects on platelet function.
MATERIALS AND METHODS
Reagents
UA and OA were purchased from Abcam (Cambridge, UK). Adenosine diphosphate (ADP) and collagen were from Chrono-log (Havertown, PA, USA). Thrombin, sodium pyruvate, β-nicotinamide adenine dinucleotide reduced disodium salt hydrate (NADH), menadione and dipyridamole were obtained from Sigma-Aldrich (St. Louis, MO, USA). Hematologic reagents for prothrombin time (PT) and activated thromboplastin time (aPTT) assays were purchased from Fisher Diagnostics (Middletown, VA, USA). Heparin was obtained from Choongwae Pharma (Seoul, Korea). All other chemicals used were of the highest purity available and were purchased from standard suppliers.
Animals
All animal experiments were conducted in accordance with protocols approved by the Ethics Committee of Animal Service Center, Dongguk University. Male Sprague-Dawley rats (6–7 weeks of age) purchased from Daehan Biolink (Eumseong, Korea) were acclimated for 1 week before experiments. The laboratory animal facility was maintained at a constant temperature and humidity with a 12-h light/dark cycle. Food and water were provided ad libitum.
Preparation of washed platelets
Washed platelets (WP) were prepared as described previously (Liu et al., 2013). Briefly, blood was collected from the abdominal aorta of rats anesthetized with ether using acid-citrate-dextrose (ACD; 85 mM trisodium citrate, 66.6 mM citric acid, and 111 mM glucose) as an anticoagulant (ACD: blood =1:6). After centrifugation at 250×g for 15 min, platelet-rich plasma (PRP) was obtained from the supernatant. Platelets were centrifuged at 500×g for 10 min, and washed once with a buffer comprised of 138 mM NaCl, 2.8 mM KCl, 0.8 mM MgCl2, 0.8 mM NaH2PO4, 10 mM HEPES, and 5 mM EDTA (pH 7.4) by suspension and centrifugation. WP were prepared by resuspending the platelet pellets in suspension buffer solution (138 mM NaCl, 2.8 mM KCl, 0.8 mM MgCl2, 0.8 mM NaH2PO4, 10 mM HEPES, 5.6 mM dextrose, and 1mM CaCl2, pH 7.4). The number of cells was adjusted to 2×108 cells/ml.
Platelet aggregation
Platelet aggregation experiments were performed using a four-channel aggregometer (Chrono-log) as described previously (Liu et al., 2013). WP were treated with the indicated concentrations of UA or OA for 5 min, and were stimulated with either thrombin, ADP, or collagen. Aggregation was assessed by measuring the changes in light transmission with the aggregometer.
Whole blood aggregation
Blood was collected from the abdominal aorta of ether-anesthetized rats using 3.2% sodium citrate as an anticoagulant (sodium citrate:blood=1:9) and diluted with normal saline (1:1). Blood was incubated with UA or OA for 5 min and was stimulated with thrombin. Aggregation was assessed by measuring the impedance change with a whole blood aggregometer (Chrono-log).
Plasma coagulation
Plasma coagulability was tested by measuring prothrombin time (PT) and activated partial thromboplastin time (aPTT) using a Coagulator2 analyzer (Behnk Elektronik, Norderstedt, Germany) with thromboplastin-D or CaCl2 and APTT-XL re-agents (Fisher Diagnostics) respectively, according to the manufacturer’s instructions.
Lactate dehydrogenase (LDH) release assay
Cytotoxicity of UA and OA were evaluated by LDH release assay as previously described (Oh et al., 2014). WP were treated with 50 μM UA or OA, or 250 μM menadione, a positive control, and centrifuged at 13,000 g for 1 min. Resultant supernatant was used for LDH activity assay. Supernatant 20 μl was added to a 200 μl aliquot of working reagent containing 0.2 mM NADH and 2.5 mM sodium pyruvate in wells of 96-well plates. The decrease in absorbance at 340 nm was measured for 3 min with a SpectraMax M3 microplate reader (Molecular Devices, Sunnyvale, CA, USA). Relative LDH activity was calculated from the slope of decreasing absorbance. LDH activities measured from 1% Triton X-100-treated WP were regarded as 100% release.
Statistical analyses
The mean and standard error (SE) of the mean were calculated for all experimental groups. The data were subjected to one-way analysis of variance (ANOVA) or the Kruskal-Wallis test followed by Bonferroni correction to determine significant differences from the control. Statistical analyses were performed using SigmaStat Version 3.5 (Systat Software, San Jose, CA). In all cases, p<0.05 indicated significance.
RESULTS
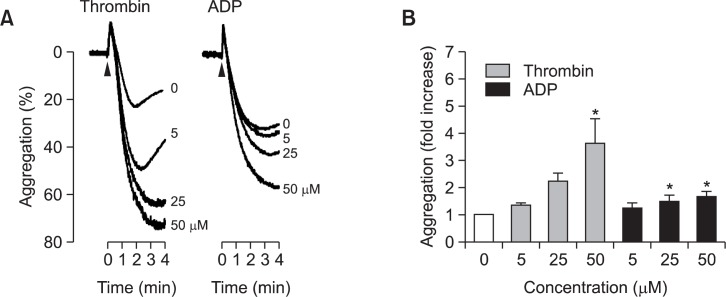
To test the effects of UA and OA on platelet aggregation, aggregatory responses to platelet agonists were assessed in UA-or OA-exposed WP. UA and OA were confirmed not to change platelet shape on an aggregometer, suggesting that they do not aggregate platelets directly (data not shown). Platelets were incubated with 5, 25, or 50 μM UA for 5 min and aggregation was elicited by the addition of 0.06–0.08 U/ml thrombin, which is a concentration that induced an intermediate extent of aggregation determined using an aggregometer. UA treatment enhanced platelet aggregation by thrombin, which was concentration-dependent and statistically significant in the tested range (Fig. 2A left panel and 2B gray bars). Similar results were obtained with ADP. UA also increased platelet aggregation by 4–10 μM ADP in the same concentration ranges, although the extent of potentiation was weaker than that in thrombin experiments (Fig. 2A, right panel; 2B, black bars). Quite comparable effects were observed with OA. OA potentiated the aggregation response of platelets to thrombin or ADP (Fig. 3). Such effect was concentration-dependent in the same range as UA and was statistically significant. In addition to thrombin and ADP, collagen was used to induce aggregation. However, UA and OA had no effect on collagen-induced platelet aggregation (data not shown), suggesting that a platelet effect may vary depending on aggregatory stimuli. To confirm whether the effect on platelet aggregation is related with cytotoxicity, LDH release, a marker of cytotoxicity, was assessed in WP treated with UA or OA at the highest concentration 50 μM. However, significant LDH release was not detected, suggesting that pro-aggregatory activity of them has nothing to do with cytotoxicity (Fig. 4).
Fig. 2.
Effect of ursolic acid (UA) on platelet aggregation. Platelets were incubated with UA for 5 min and aggregation was induced either by 0.06-0.08 U/ml thrombin or 4–10 μM ADP. Representative tracings of UA-treated platelet aggregation are presented in (A). The bar graph in (B) indicates the fold increase in aggregation compared to the control. All values are mean ± standard error (n=4 for thrombin experiment and 7 for ADP experiment). * indicates a statistically significant difference from control (p<0.05).
Fig. 3.
Effect of oleanolic acid (OA) on platelet aggregation. Following 5 min treatment of OA, platelet aggregation was induced by 0.06–0.08 U/ml thrombin or 4–10 μM ADP. (A) Representative tracings of OA-treated platelet aggregation. (B) The fold-increase of aggregation compared to the control. All values are mean ± standard error (n=3 for thrombin experiment and 4 for ADP experiment). * indicates a statistically significant difference from control (p<0.05).
Fig. 4.

Cytotoxicity of ursolic acid (UA) and oleanolic acid (OA) on platelets. Platelets were treated with 50 μM UA or OA, and the cytotoxicity was assessed by measuring the activity of lactate dehydrogenase (LDH) released. For a positive control, 250 μM menadione was treated to platelets. All values are mean ± standard error (n=3). *Indicates a statistically significant difference from control (p<0.05).
Aggregation-enhancing effects of UA and OA were tested in whole blood, which is more complicated, but which is a closer system to the in vivo condition than WP. UA or OA was used to pretreat whole blood for 5 min and whole blood aggregation was induced with 0.6–0.7 U/ml thrombin. Similar to WP, whole blood treated with 50 μM UA also exhibited an exaggerated response to thrombin which was statistically significant (Fig. 5). However, the effect of OA was minimal in whole blood. A positive control, dipyridamole effectively and significantly inhibited whole blood aggregation (Fig. 4 gray bar).
Fig. 5.
Effect of ursolic acid (UA) and oleanolic acid (OA) on thrombin-induced whole blood aggregation. After treatment with 50 μM UA or OA for 5 min, aggregation of whole blood was initiated by the addition of 0.6–0.7 U/ml thrombin. Dipyridamole (Dip) at 100 μM was used as a negative control. Values are mean ± standard error (n=4). *Indicates a statistically significant difference from control (p<0.05).
As another major player in blood clotting and thrombosis, coagulation factors were tested for their activities in the presence of UA or OA. PT and aPTT represented the activities of the extrinsic pathway and the intrinsic pathway in the coagulation cascade, respectively. Blood plasma was incubated with UA and OA for 5 min, and PT and aPTT were measured. No significant change was detected in PT and aPTT using up to 50 μM UA or OA, indicating little, if any, effect on plasma coagulation (Table 1).
Table 1.
Effect of ursolic acid (UA) and oleanolic acid (OA) on plasma coagulation
| PT | aPTT | |
|---|---|---|
| Control | 13.7 ± 0.2 | 20.3 ± 0.7 |
| UA 5 μM | 13.9 ± 0.3 | 18.7 ± 0.6 |
| UA 25 μM | 14.0 ± 0.3 | 19.8 ± 0.7 |
| UA 50 μM | 13.8 ± 0.3 | 20.2 ± 0.9 |
| OA 5 μM | 13.8 ± 0.2 | 19.6 ± 0.3 |
| OA 25 μM | 13.8 ± 0.3 | 19.5 ± 0.8 |
| OA 50 μM | 14.0 ± 0.0 | 19.8 ± 0.9 |
| Heparin | 27.1 ± 1.8* | > 120.0* |
Values are expressed as mean ± standard error (n=3). Heparin (0.4 IU/μl) was used as a negative control. PT: prothrombin time, aPTT: activated partial thromboplastin time.
Indicates a statistically significant difference from control (p<0.05).
DISCUSSION
This study investigated the effect of triterpenoids UA and OA on platelets. UA and OA made platelets susceptible to pro-aggregatory stimuli. Platelets play a primary role in a hemostasis through aggregation. However, primed platelets readily form thrombus and thereby contribute to the development of diverse cardiovascular diseases (Michelson, 2010). It is plausible that UA and OA may be causative factors in cardiovascular diseases by favoring platelet aggregation. Although the specific mechanism is elusive and clinical validation remains to be assessed, it is worth stressing that these compounds need to be used with caution, especially in the population with a predisposition to cardiovascular events.
Studies regarding the effect of UA and OA on the function of platelets have yielded equivocal findings. Jin et al. (2004a and 2004b) and Babalola et al. (2013) reported antiaggregatory effects of UA and OA, whereas Lee et al. (2007) observed aggregation-inducing activity of OA. The half-maximal inhibitory concentration (IC50) reported previously was unusually high in the studies reporting aggregation inhibitory effect. Babalola et al. (2013) reported IC50 at mM levels and Jin et al. (2004a and 2004b) reported values at high μM to mM levels. Most of all, it is hard to expect physiological relevancy if the effective concentration is high. Indeed, Babalola et al. (2013) also held the view that the antiplatelet activity of UA or OA was too weak to exert a practical effect. A comparably high concentration was used presently to reproduce previous studies. However, the solubility of UA or OA was too poor to be applied to platelets and such high concentrations were not feasible for measurements using the turbidimetric aggregometer (data not shown). UA or OA is practically insoluble in aqueous solution. Dimethylsulfoxide was used as a vehicle in our study. However, the solubility of UA and OA does not exceed several tens mM, even in organic solvent (Jin et al., 1997) and it is not possible to apply them in the mM range to WP considering the influence of organic solvent on platelet activity. In another study, OA supplied at 50 to 200 μM directly induced aggregation of rabbit platelets within 10 min without addition of any agonists (Lee et al., 2007). However, such direct aggregation was not observed in this study (data not shown). Instead, OA made platelets sensitive to stimuli. There seems to be no major difference in experimental methods except species difference of platelets source between the present and a previous (Lee et al., 2007) study. Lee et al. (2007) tested rabbit platelets, while rats were used for this study. Further study will be needed for a full understanding of the discrepancy.
UA and OA were effective at higher than 10 μM in this experiments. There are a limited number of studies reporting blood level of UA and OA. The peak plasma concentration (Cmax) was 3404.6 ± 748.8 ng/ml (= approximately 7.5 μM) in humans given an injection of UA at an average dose of 98 mg/m2 body surface area (an average is 1.7 m2 for adult males) (Xia et al., 2011b). In a study using an oral dose of 10 mg/kg bodyweight UA in rats, the Cmax was found to be 1.10 ± 0.31 μg/ml (= approximately 2.4 μM) (Chen et al., 2011). These levels in blood plasma are not quite different from the concentration used in this study, and therefore the effective concentration of UA may be a clinically relevant level. In case of OA, relatively lower levels were detected in blood plasma. The Cmax was 12.12 ± 6.84 ng/ml (= approximately 0.027 μM) in humans taking a single 40 mg dose of the OA capsule orally (Song et al., 2006). It was 132.0 ± 122.0 ng/ml (= approximately 0.29 μM) in rats orally administered at a dose of 50 mg/kg (Jeong et al., 2007). Plasma OA levels reported are lower than the concentration used in this study. However, clinical exposure to UA or OA can be more extensive than administered doses in these studies. UA and OA are very widely distributed in food products and herbs. There are a large number of rich sources for them including an apple and Ayurvedic herbs such as Holy Basil. For example, a common spice herb rosemary contains 2.95 g/100 g UA and the content of OA is 3.1 g/100 g dry weight in olive leaves (Jager et al., 2009). Such high content in food raises the possibility of longer and extensive exposure to UA or OA, which implies that a daily intake of UA or OA may be larger than doses in kinetic studies described above and thus the plasma concentration can be higher than the reported levels. Taken together, the concentrations of UA and OA in this study are not irrelevant clinically.
Currently, the mechanism how UA and OA contribute to platelet aggregation is elusive. Both UA and OA enhanced aggregation induced by thrombin or ADP, but not by collagen. Thrombin, ADP and collagen have their own distinct ways of activating platelets. The former two stimulate phospholipase Cβ through G protein-coupled receptors protease-activated receptors (PAR) and P2Y receptors respectively, while collagen triggers phospholipase Cγ2 activation via platelet receptor glycoprotein IV and generate downstream signals (Offermanns, 2006). Accordingly, UA and OA appear to affect specific signaling pathways rather than to exert non-specific activity, and G protein/phospholipase Cβ-mediated signals by thrombin and ADP receptors might be the targets for UA and OA.
The effects of UA and OA on coagulation were examined by measuring PT and aPTT. However, neither compound affected plasma clotting, suggesting that platelets, rather than clotting factors, are a major target in whole blood and that the hyperaggregation of whole blood is mainly caused by the influence on platelets (Fig. 4). This is consistent with previous studies reporting little or negligible effect of UA or OA (Lee and Han, 2003; Allouche et al., 2010), although a conflicting observation was reported (Lee et al., 2012).
Whole blood is generally regarded as being more similar to the in vivo situation than isolated platelets (Liu et al., 2013), although it is hard to interpret the results from whole blood because of the complexity of its composition. In a whole blood study, OA failed to potentiate aggregation unexpectedly, while UA was effective as was in WP experiments (Fig. 4). Ineffectiveness may be ascribed to a rapid metabolism in whole blood or uneven distribution in other cells than platelets and, thus, less exposure to platelets. Another possibility is the insensitivity of whole blood aggregometry. In general, whole blood aggregometry that relies on impedance measurement is less sensitive in detecting microaggregates compared with turbidimetric platelet aggregometer. Although both impedance and optical aggregometry are validated platelet function tests, these two methods do not always produce the same results (Ingerman-Wojenski et al., 1983; Dyszkiewicz-Korpanty et al., 2007). Impedance aggregometry is considered a less sensitive technique for detecting mild aggregation compared with optical method because formation of aggregates in the sample will have no effect on the measured impedance unless the aggregates attach to the electrodes, and a small number of attached platelets is unlikely to have a significant impact on the measured impedance of the circuit (Jarvis, 2004). Accordingly, aggregation in the whole blood aggregometer indicates the formation of substantial macroaggregates (Jarvis, 2004). Therefore, potentiation of platelet aggregation by OA may not be detected in whole blood, even though OA favors the formation of small aggregates. Along with structural similarity, UA and OA have diverse biological activities in common (Liu, 1995; Pollier and Goossens, 2012). Therefore, it is not strange that both UA and OA exhibit similar activity on platelet aggregation. However, detailed mechanism underlying such effect may be different each other, as shown in the whole blood experiments.
UA and OA have been generally considered to be safe and beneficial phytochemicals (Liu, 1995; Somova et al., 2003; Somova et al., 2004), but the results of our experiments, together with previous reports (Lee and Han, 2003; Lee et al., 2007; Allouche et al., 2010; Messner et al., 2011), raise the question regarding cardiovascular toxicity. The detailed mechanisms have not yet been revealed, nor have the conditions and the reasons for their opposing activities. Further study is warranted to ensure the safe use of UA, OA, and their derivatives.
Acknowledgments
This work was supported by the GRRC program of Gyeonggi province (GRRC-DONGGUK2013-B01, Development of new health supplements/therapeutics for neurodegenerative diseases).
REFERENCES
- Aguirre-Crespo F, Vergara-Galicia J, Villalobos-Molina R, Javier López-Guerrero J, Navarrete-Vázquez G, Estrada-Soto S. Ursolic acid mediates the vasorelaxant activity of Lepechinia caulescens via NO release in isolated rat thoracic aorta. Life Sci. 2006;79:1062–1068. doi: 10.1016/j.lfs.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Allouche Y, Beltrán G, Gaforio JJ, Uceda M, Mesa MD. Antioxidant and antiatherogenic activities of pentacyclic triterpenic diols and acids. Food Chem Toxicol. 2010;48:2885–2890. doi: 10.1016/j.fct.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Andrikopoulos NK, Kaliora AC, Assimopoulou AN, Papageorgiou VP. Inhibitory activity of minor polyphenolic and nonpolyphenolic constituents of olive oil against in vitro low-density lipoprotein oxidation. J. Med. Food. 2002;5:1–7. doi: 10.1089/109662002753723160. [DOI] [PubMed] [Google Scholar]
- Babalola IT, Shode FO, Adelakun EA, Opoku AR, Mosa RA. Platelet-aggregation inhibitory activity of oleanolic acid, ursolic acid, betulinic acid, and maslinic acid. J Pharmacogn Phytochem. 2013;1:54–60. [Google Scholar]
- Chen Q, Luo S, Zhang Y, Chen Z. Development of a liquid chromatography-mass spectrometry method for the determination of ursolic acid in rat plasma and tissue: application to the pharmacokinetic and tissue distribution study. Anal Bioanal Chem. 2011;399:2877–2884. doi: 10.1007/s00216-011-4651-x. [DOI] [PubMed] [Google Scholar]
- Dyszkiewicz-Korpanty A, Olteanu H, Frenkel EP, Sarode R. Clopidogrel anti-platelet effect: an evaluation by optical aggregometry, impedance aggregometry, and the platelet function analyzer (PFA-100TM) Platelets. 2007;18:491–496. doi: 10.1080/09537100701280654. [DOI] [PubMed] [Google Scholar]
- Horiuchi K, Shiota S, Hatano T, Yoshida T, Kuroda T, Tsuchiya T. Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococci (VRE) Biol Pharm Bull. 2007;30:1147–1149. doi: 10.1248/bpb.30.1147. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Murakami A, Ohigashi H. Ursolic acid: an anti-and pro-inflammatory triterpenoid. Mol Nutr Food Res. 2008;52:26–42. doi: 10.1002/mnfr.200700389. [DOI] [PubMed] [Google Scholar]
- Ingerman-Wojenski C, Smith JB, Silver MJ. Evaluation of electrical aggregometry: comparison with optical aggregometry, secretion of ATP, and accumulation of radiolabeled platelets. J Lab Clin Med. 1983;101:44–52. [PubMed] [Google Scholar]
- Jager S, Trojan H, Kopp T, Laszczyk MN, Scheffler A. Pentacyclic triterpene distribution in various plants - rich sources for a new group of multi-potent plant extracts. Molecules. 2009;14:2016–2031. doi: 10.3390/molecules14062016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis GE. Platelet aggregation in whole blood - Impedance and particle counting methods. In: Gibbins JM, Mahaut-Smith MP, editors. Platelets and megakaryocytes. Humana Press; Totowa, N.J: 2004. pp. 77–87. [DOI] [PubMed] [Google Scholar]
- Jeong DW, Kim YH, Kim HH, Ji HY, Yoo SD, Choi WR, Lee SM, Han CK, Lee HS. Dose-linear pharmacokinetics of oleanolic acid after intravenous and oral administration in rats. Biopharm Drug Dispos. 2007;28:51–57. doi: 10.1002/bdd.530. [DOI] [PubMed] [Google Scholar]
- Jin IJ, Ko YI, Kim YM, Han SK. Solubilization of oleanolic acid and ursolic acid by cosolvency. Arch Pharm Res. 1997;20:269–274. doi: 10.1007/BF02976156. [DOI] [PubMed] [Google Scholar]
- Jin JL, Lee S, Lee YY, Kim JM, Heo JE, Yun-Choi HS. Platelet anti-aggregating triterpenoids from the leaves of Acanthopanax senticosus and the fruits of A. sessiliflorus. Planta Med. 2004a;70:564–566. doi: 10.1055/s-2004-827159. [DOI] [PubMed] [Google Scholar]
- Jin JL, Lee YY, Heo JE, Lee S, Kim JM, Yun-Choi HS. Anti-platelet pentacyclic triterpenoids from leaves of. Campsis grandiflora Arch Pharm Res. 2004b;27:376–380. doi: 10.1007/BF02980076. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Jin YR, Lim Y, Yu JY, Kim TJ, Yoo HS, Shin HS, Yun YP. Oleanolic acid, a pentacyclic triterpenoid, induces rabbit platelet aggregation through a phospholipase C-calcium dependent signaling pathway. Arch Pharm Res. 2007;30:210–214. doi: 10.1007/BF02977696. [DOI] [PubMed] [Google Scholar]
- Lee MH, Han YN. A new in vitro tissue factor inhibitory triterpene from the fruits of Chaenomeles sinensis. Planta Med. 2003;69:327–331. doi: 10.1055/s-2003-38884. [DOI] [PubMed] [Google Scholar]
- Lee W, Yang EJ, Ku SK, Song KS, Bae JS. Anticoagulant activities of oleanolic acid via inhibition of tissue factor expressions. BMB Rep. 2012;45:390–395. doi: 10.5483/bmbrep.2012.45.7.065. [DOI] [PubMed] [Google Scholar]
- Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- Liu J. Oleanolic acid and ursolic acid: research perspectives. J Ethnopharmacol. 2005;100:92–94. doi: 10.1016/j.jep.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Liu Y, Oh SJ, Chang KH, Kim YG, Lee MY. Antiplatelet effect of AMP-activated protein kinase activator and its potentiation by the phosphodiesterase inhibitor dipyridamole. Biochem Pharmacol. 2013;86:914–925. doi: 10.1016/j.bcp.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Messner B, Zeller I, Ploner C, Frotschnig S, Ringer T, Steinacher-Nigisch A, Ritsch A, Laufer G, Huck C, Bernhard D. Ursolic acid causes DNA-damage, p53-mediated, mitochondria- and caspase-dependent human endothelial cell apoptosis, and accelerates atherosclerotic plaque formation in vivo. Atherosclerosis. 2011;219:402–408. doi: 10.1016/j.atherosclerosis.2011.05.025. [DOI] [PubMed] [Google Scholar]
- Michelson AD. Antiplatelet therapies for the treatment of cardiovascular disease. Nat Rev Drug Discov. 2010;9:154–169. doi: 10.1038/nrd2957. [DOI] [PubMed] [Google Scholar]
- Offermanns S. Activation of platelet function through G protein-coupled receptors. Circ Res. 2006;99:1293–1304. doi: 10.1161/01.RES.0000251742.71301.16. [DOI] [PubMed] [Google Scholar]
- Oh SJ, Kim H, Liu Y, Han HK, Kwon K, Chang KH, Park K, Kim Y, Shim K, An SS, Lee MY. Incompatibility of silver nanoparticles with lactate dehydrogenase leakage assay for cellular viability test is attributed to protein binding and reactive oxygen species generation. Toxicol Lett. 2014;225:422–432. doi: 10.1016/j.toxlet.2014.01.015. [DOI] [PubMed] [Google Scholar]
- Pollier J, Goossens A. Oleanolic acid. Phytochemistry. 2012;77:10–15. doi: 10.1016/j.phytochem.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Radhiga T, Rajamanickam C, Senthil S, Pugalendi KV. Effect of ursolic acid on cardiac marker enzymes, lipid profile and macroscopic enzyme mapping assay in isoproterenol-induced myocardial ischemic rats. Food Chem Toxicol. 2012;50:3971–3977. doi: 10.1016/j.fct.2012.07.067. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Rodriguez R, Stankevicius E, Herrera M, Østergaard L, Andersen MR, Ruiz-Gutierrez V, Simonsen U. Oleanolic acid induces relaxation and calcium-independent release of endothelium-derived nitric oxide. Br J Pharmacol. 2008;155:535–546. doi: 10.1038/bjp.2008.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Srimany A, Pradeep T. Rapid identification of molecular changes in tulsi (Ocimum sanctum Linn) upon ageing using leaf spray ionization mass spectrometry. Analyst. 2012;137:4559–4563. doi: 10.1039/c2an35655d. [DOI] [PubMed] [Google Scholar]
- Somova L, Shode F, Mipando M. Cardiotonic and anti-dysrhythmic effects of oleanolic and ursolic acids, methyl maslinate and uvaol. Phytomedicine. 2004;11:121–129. doi: 10.1078/0944-7113-00329. [DOI] [PubMed] [Google Scholar]
- Somova LO, Nadar A, Rammanan P, Shode FO. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine. 2003;10:115–121. doi: 10.1078/094471103321659807. [DOI] [PubMed] [Google Scholar]
- Song M, Hang TJ, Wang Y, Jiang L, Wu XL, Zhang Z, Shen J, Zhang Y. Determination of oleanolic acid in human plasma and study of its pharmacokinetics in Chinese healthy male volunteers by HPLC tandem mass spectrometry. J Pharm Biomed Anal. 2006;40:190–196. doi: 10.1016/j.jpba.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Ullevig SL, Zhao Q, Zamora D, Asmis R. Ursolic acid protects diabetic mice against monocyte dysfunction and accelerated atherosclerosis. Atherosclerosis. 2011;219:409–416. doi: 10.1016/j.atherosclerosis.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ma H, Zhang X, He L, Wu J, Gao X, Ren J, Li J. A novel AMPK activator from Chinese herb medicine and ischemia phosphorylate the cardiac transcription factor FOXO3. Int J Physiol Pathophysiol Pharmacol. 2009;1:116–126. [PMC free article] [PubMed] [Google Scholar]
- Xia EQ, Wang BW, Xu XR, Zhu L, Song Y, Li HB. Microwave-assisted extraction of oleanolic acid and ursolic acid from Ligustrum lucidum ait. Int J Mol Sci. 2011a;12:5319–5329. doi: 10.3390/ijms12085319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Wei G, Si D, Liu C. Quantitation of ursolic acid in human plasma by ultra performance liquid chromatography tandem mass spectrometry and its pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2011b;879:219–224. doi: 10.1016/j.jchromb.2010.11.037. [DOI] [PubMed] [Google Scholar]
- Zha XZ, Xie XM, Lu MH, Zhou A, Liu XH. Analysis of fruits from ten chinese medicinal herbs containing oleanolic acid and ursolic acid by HPLC. Chin J Exp Tradit Med Formulae. 2010;18:019. [Google Scholar]