Abstract
Abnormal adaptation of the stress-response system following traumatic stress can lead to alterations in the hypothalamic-pituitary-adrenal (HPA) axis that may contribute to the development of post-traumatic stress disorder (PTSD). The present study used several behavioral tests to investigate the anxiolytic-like and antidepressant activity of L-tetrahydropalmatine (L-THP) in an experimental rat model of anxiety and depression induced by single prolonged stress (SPS), an animal model of PTSD. Male rats were treated intraperitoneally (i.p.) with vehicle or varied doses of THP 30 min prior to SPS for 8 consecutive days. Daily THP (50 mg/kg) administration significantly increased the number and duration of open arm visits in the elevated plus maze (EPM) test, reduced the anxiety index, increased the risk assessment, and increased the number of head dips over the borders of the open arms after SPS. THP was also associated with increased time spent at the center of the open field, reduced grooming behaviors in the EPM test, and reduced time spent immobile in the forced swimming test (FST). It also blocked the decrease in neuropeptide Y (NPY) and the increase in corticotrophin-releasing factor (CRF) expression in the hypothalamus. This is the first study to determine that THP exerts pronounced anxiolytic-like and antidepressant effects on the development of the behavioral and biochemical symptoms associated with PTSD, indicating its prophylactic potential. Thus, THP reversed several behavioral impairments triggered by the traumatic stress of SPS and is a potential non-invasive therapeutic intervention for PTSD.
Keywords: Post-traumatic stress disorder, Single prolonged stress, Anxiety, Neuropeptide Y, L-tetrahydropalmatine
INTRODUCTION
Anxiety, an aversive emotional state, is a common psychological condition involving the central nervous system (CNS) that contributes to the increasing health burden worldwide. It includes both a powerful emotional component associated with fearful thoughts as well as a physiological response (Jindal et al., 2013). Several studies have reported that anxiety disorders are the most frequent psychiatric conditions, closely followed by mood disorders (Márquez et al., 2006). Despite a steady increase in the development of anxiolytics for the treatment of anxiety, the prevalence of these disorders remains stable, and this may be due to our incomplete neurobiological understanding of its pathophysiology or the inconsistent efficacy of current pharmacological treatments (Jindal et al., 2013). Anxiety results from exposure to stressful life events that evoke fear, helplessness, and horror (Colman and Ataullahjan et al., 2010; Uliaszek et al., 2010).
Post-traumatic stress disorder (PTSD) is a debilitating neuropsychiatric or psychological disorder that develops in a subset of individuals following severe, life-threatening traumatic stress (Ceremuqa et al., 2013). It is characterized by intrusive recollections of the traumatic event, avoidance of reminders of the event, hyperarousal, and as being outside the realm of a normal human experience (Serova et al., 2014). PTSD is often co-morbid with anxiety and major depression, as well as with substance abuse, sleep disturbances, and marked psychosocial and occupational impairment (Serova et al., 2014). The symptoms of PTSD are believed to reflect trauma-induced changes that lead to long-term dysfunctional stress regulation and inappropriate adaptation of the hypothalamic-pituitary-adrenal (HPA) axis (George et al., 2013a, 2013b). Many studies have shown that hyperarousal and dysregulation of the HPA axis are closely associated with the development of PTSD, which produces serious changes in affective behavior that are indicative of or consistent with anxiety- and depression-like symptoms (Shea et al., 2005). Single prolonged stress (SPS) has been found to reproduce the cardinal symptoms of PTSD (Serova et al., 2014). SPS causing HPA axis dysregulation may lead to profound maladaptive changes manifested in behaviors that resemble anxiety and depression, thereby mimicking human mental disorders involving these states (Serova et al., 2013a).
Antidepressants, anxiolytics, and antipsychotics are the most widely prescribed drugs for the treatment of PTSD, despite the fact that as many as 40-50% of patients do not respond to current treatments and suffer from a chronic form of the condition (Kozlovsky et al., 2009). Given the importance of developing pharmacological treatments that can reduce the suffering experienced by those with PTSD. Much attention has been devoted to the use of naturally occurring compounds and their formulations as alternative therapeutic agents for the treatment of different psychiatric symptoms, including anxiety and depression (Deligiannidis and Freeman, 2010).
L-tetrahydropalmatine (THP) is extracted from the Corydalis yanhusuo plant, a traditional Korean analgesic herb. (Ceremuga et al., 2013). This compound improves multiple physiological symptoms and produces a variety of biological effects in the central nervous and immune systems. Indeed, several studies have investigated its anticoagulant, antinociceptive, antihyperalgesic, antioxidant, antiviral, and anti-inflammatory activities (Han et al., 2012). THP has beneficial cerebral and excellent analgesic effects (Han et al., 2012). Recent studies have demonstrated the ability of THP to attenuate anxiety and the locomotor-stimulating effects induced by oxycodone in mice (Liu et al., 2005). Several studies have shown that THP significantly inhibits cocaine or methamphetamine-induced locomotor activity and activation of extracellular-regulated kinase (ERK) in the nucleus accumbens (NAc) and caudate putamen (CPu) of mice (Zhao et al., 2014), as well as the cocaine-enhanced locomotor activity and changes in extracellular dopamine (DA) in the NAc of rats (Xi et al., 2007). Additionally, THP has been reported to attenuate PTSD-induced changes in gene expression, such as those involving DA, serotonin, and acetylcholine, in the amygdala and hippocampus of rats (Ceremuga et al., 2013).
Thus, THP may alleviate anxiety- and depression-like behaviors induced by trauma in stress-related animal models. However, it remains unknown whether the therapeutic efficacy of THP in alleviating the anxiety- and depression-like behaviors that mimic PTSD and follow SPS in rats is based on the regulation of neuropeptide Y (NPY) and corticotrophin-releasing factor (CRF) in the hypothalamus. These observations have led to the hypothesis that THP may also be effective for improving PTSD-associated psychiatric symptoms, such as anxiety and depression.
Based on these observations, the present study investigated the efficacy of THP for alleviating the PTSD-associated symptoms of anxiety- and depression-like behaviors in rats exposed to SPS, a rat model of PTSD. Anxiety- and depression-like behaviors were assessed using the elevated plus maze (EPM) test, open field test (OFT), and the forced swimming test (FST). Additionally, the underlying neurobiological mechanism of these behaviors was investigated by evaluating NPY and CRF expression in the hypothalamus of rats following SPS.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley (SD) rats weighing 200-220 g (6 weeks-old) were obtained from Samtako Animal Co. (Seoul, Korea). The rats were housed in a limited access rodent facility with up to five rats per polycarbonate cage. The room controls were set to maintain the temperature at 22 ± 2°C and the relative humidity at 55 ± 15%. Cages were lit by artificial light for 12 h each day. Sterilized drinking water and standard chow diet were supplied ad libitum to each cage during the experiments. The animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23), revised in 1996, and were approved by the Kyung Hee University Institutional Animal Care and Use Committee. All animal experiments began at least 7 days after the animals arrived. The effects were made to minimize the number of animals used and their discomfort.
THP administration
Different groups of rats, 7 animals per group, were used for drugs treatment and tests. All the experimental animals including control and drug-treatment groups were administration. The aim of food withdrawn prior to drug administration was just to ensure the bioavailability of drug. In other time periods, all animals had free access to food. The standard doses of THP in the rat and considering the long-term treatment used in the present study was based on previous study (Ceremuga et al., 2013). THP (10, 20, 50 mg/kg body weight, Sigma-Aldrich Chemical Co. St. Louise, MO, USA) and the positive drug fluoxetine (15 mg/kg, FLX, Fluoxetine hydrochloride; Eli Lilly and Company, Basingstoke, Hampshire) were administered by intraperitoneally (i.p.) in a volume of 1 ml/kg for 8 days. All drugs were freshly prepared right before every experiment.
Single prolonged stress (SPS)
SPS model of PTSD was performed as previously described (Serova et al., 2013b). Briefly, rats were first immobilized for 2 h on restraint tubes (20 cm height, 7 cm diameter). Following immobilization, rats were immediately subjected to forced swim for 20 min in a plexiglass cylinder (50 cm height, 20 cm diameter) filled to two-thirds with 24°C fresh water. The animals were dried and allowed to recuperate for 15 min and then exposed to ether vapor until loss of consciousness. The SPS procedure was performed between 10 AM and 3 PM. Following SPS stressors, animals were housed two per cage and left undisturbed for 7 days to allow PTSD-like symptoms to manifest (Serova et al., 2013b). The following parameters were measured to monitor the effects of the development of psychiatric disorders by SPS model of PTSD: changes of body weight gains, serum CORT levels, and sucrose intake. Behavioral testing for anxiety- and depression-like behaviors were done rightly after the end of the SPS protocol. The entire experimental schedules of SPS and behavioral examinations are shown in Fig. 1. To avoid carryover form one test to another, each behavioral test was performed on separate or interval between each behavioral test.
Fig. 1.
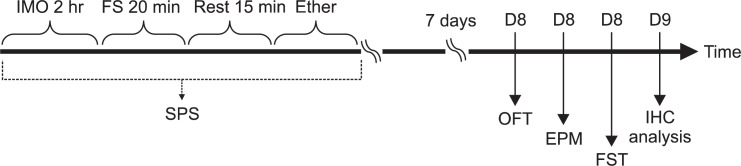
Experimental schedules of developing SPS-induced depression- and anxiety-like behaviors, and the THP treatment in the rats. Different groups of rats, 7 animals per group, were used for all experiments. IHC: immunohistochemistry.
Measurement of sucrose intake
The sucrose intake test was performed as described previously with minor modifications (Mao et al., 2009). For this test, rats were trained to consume 1% sucrose solution prior to the start of the experiment. Briefly, 48 hours before the test, the rats were trained to adapt to 1% sucrose solution (w/v): two bottle of 1% sucrose solution were placed in each cage, and 24 hours later 1% sucrose solution in one bottle was replaced with tap water for 24 hours. After the adaption, rats were deprived of water and food for 10 hours. Sucrose preference test was conducted at 9:00 a.m. in which rats were housed in individual cages and were free to access to two bottles containing 100 mL of sucrose solution (1%, w/v) and 100 mL of water, respectively. After 3 hours, the volumes of consumed sucrose solution and water were measured, and the sucrose preference was calculated by the following formula: sucrose preference=sucrose consumption/(water consumption+sucrose consumption)×100% (Mao et al., 2009, Zhu et al., 2012).
Elevated plus maze test (EPM)
Anxiety-like behavior was tested on the EPM. Animals conduct anxiety-like behaviors usually show the reductions both in the number of entries and in the time spent in the open arms, along with an increase in the amount of time spent in the closed arms in the EPM. The elevated plus test was conducted. This apparatus consisted of two open arms (50×10 cm each), two closed arms (50×10×20 cm each) and a central platform (10×10 cm), arranged in a way such that the two arms of each type were opposite to each other. The maze was made from black Plexiglas and elevated 50 cm above the floor. Exploration of the open arms was encouraged by testing under indirect dim light (2×60W). At the beginning of each trial, animals were placed at the centre of the maze, facing a closed arm. During a 5-min test period, the following parameters were recorded: 1) number of open arm entries, b) number of closed arm entries, c) time spend in open arms, d) time spent in closed arms, e) time and frequency of risk assessment, and f) track length. Entry by an animal into an arm was defined as the condition in which the animal has placed its four paws in that arm. The maze was cleaned with alcohol after each rat had been tested. The behavior in the maze was recorded using a video camera mounted on the ceiling above the center of the maze and relayed to the S-MART program (PanLab, Barcelona, Spain). Anxiety reduction, indicated by open arm exploration in the EPM, was defined as an increase in the numbers of entries into the open arms relative to total entries into either open or closed arm, and an increase in the proportion of time spent in the open arms relative to total spending time in either open or closed arm. Total arm entries were also used as indicators of changes in locomotor activities of the rats. Risk assessment was defined as the rat poking its head or trunk into an open arm while its hind quarters were located in one of the closed arms (Serova et al., 2013a). Anxiety index was calculated as follows:
Anxiety index values range from 0 to 1 where an increase in the index expresses increased anxiety-like behavior (Serova et al., 2013a). Unprotected head dips were defined as peering over the edge of an open arm with head, neck, and shoulders. Increase in this parameter expresses decreased anxiety-like behavior (Serova et al., 2013a). Grooming behavior has been reported to be a response to novelty or other stressors and might vary in accordance with stress intensity (Serova et al., 2013a). Because appropriate examination grooming behavior in EPM requires longer than 5 min session, duration (total time spent for grooming) and frequency (number of grooming bouts) were scored during 10 min in the EPM (Serova et al., 2013a). Intervals greater than 5 s were considered to separate two different bouts. Duration of each single bout was calculated as duration of grooming divided by frequency (Serova et al., 2013a).
Open field test
Several parameters, such as defecation and time in a center within the first 5 min, likely gauge some aspects of emotionality including anxiety (Hsiao et al., 2012). Animals were treated in an open field apparatus in a room. The rats were individually housed in a rectangular container that was made of dark polyethylene (60×60×30 cm) to provide best contrast to the white rats in a dimly lit room equipped with a video camera above the center of the room, and their locomotor activities (animal’s movements) were then measured. The locomotor activity indicated by the speed and the distance of movements was monitored by a computerized video-tracking system using S-MART program (PanLab Co., Barcelona, Spain). After 5 min adaptation, the distance they traveled in the container was recorded for another 5 min.
Forced swimming test (FST)
To evaluate effect of THP on depression-like behavior induced by SPS, FST was carrying out for 5 min in the same conditions as above. Briefly, the test was done by placing a rat in a transparent Plexiglas cylinder (20 cm diameter ×50 cm height) was filled up to a depth of 30 cm with water at 25°C. Two swimming sessions were conducted: an initial 15 min pre-test, followed by a 5 min test 24 h later. Animals were subjected to 5 min of forced swim, and escape behaviors (climbing and swimming) were determined. The animals’ behavior was continuously recorded by experimenter-manual scoring during the testing session with an overhead video camera to tape behavior for later manual scoring. Immobility behavior was calculated as the length of time in which the animal did not show escape responses (e.g., total time of the test minus time spent in climbing and swimming behaviors). The rats were judged to be immobile when it remained in the water without struggling and was making only those movements necessary to keep its head above water. Climbing behavior was defined as upward-directed movements of the forepaws alone the side of the swim chamber and swimming behavior was considered as movements throughout the swim chamber including crossing into another quadrant.
CORT analysis
Animals were killed by decapitation one day after behavioral measurement. For this, the unanesthetized rats were rapidly decapitated, and blood was quickly collected via the abdominal aorta. The blood samples were centrifuged at 4000 g for 10 min, and serum was collected and stored at −20°C until use. The CORT concentration was measured by a competitive enzyme-linked immunoassay (ELISA) using a rabbit polyclonal CORT antibody (Novus Biologicals Corticosterone kit; Novus Biologicals, LLC., Littleton, CO, USA) according to the manufacturer’s protocol. Samples (or standard) and conjugate were added to each well, and the plate was incubated for 1 h at room temperature without blocking. After wells were washed several times with buffers and proper color developed, the optical density was measured at 450 nm using an ELISA reader (MutiRead 400; Authos Co., Vienna, Austria).
Immunohistochemistry
For immunohistochemical studies, the primary antibodies against the following specific antigen were used: CRF (goat polyclonal CRF, 1:500 dilution, Santa Cruz Biotechnology Inc., California, CA, USA) and NPY (rabbit polyclonal NPY, 1:2000 dilution, Immunostar, Hudson, WI, USA). Briefly, the sections were incubated with primary antiserum in PBST (PBS plus 0.3% Triton X-100) for 72 h at 4°C. The sections were incubated for 120 min at room temperature with secondary antibody. The secondary antibodies were obtained from Vector Laboratories Co. (Burlingame, CA, USA) and diluted 1:200 in PBST containing 2% normal serum. To visualize immunore-activity, the sections were incubated for 90 min in avidin-biotin-peroxidase complex (ABC) reagent (Vectastain Elite ABC kit; Vector Labs. Co., Burlingame, CA, USA), and incubated in a solution containing 3,3’-diaminobenzidine (DAB; Sigma-Aldrich Chemical Co., St. Louis, MO, USA) and 0.01% H2O2 for 1 min. Finally, the tissues were washed in PBS, followed by a brief rinse in distilled water, and mounted individually onto slides. Images were captured using the DP2-BSW imaging system (Olympus, CA, USA) and processed using Adobe Photoshop (Adobe Systems, Inc., San Jose, CA, USA). The sections were viewed at 100× magnification, and the numbers of CRF and NPY labeled cells was quantified in the hypothalamus. CRF-, and NPY-labeled cells were counted by an observer blinded to the experimental groups. Counting the immunopositive cells were performed within the square (200 ×200 μm2), anatomically localized in at least three different sections per rat brain according to the stereotactic rat brain atlas of Paxinos and Watson (Paxinos and Watson, 1986). The stained cells for which intensities to a defined value above the background were only considered as immunopositive cells. The differences of brightness and contrast among raw images were not adjusted, in order to exclude any possibility of subjective selection of the immunoreactive cells.
Statistical analysis
All measurements were performed by an independent investigator blinded to the experimental conditions. Results in figures are expressed as mean ± standard error of means (SE). Differences within or between normally distributed data were analyzed by analysis of variance (ANOVA) using SPSS (Version 13.0; SPSS, Inc., Chicago, IL, USA) followed by Tukey’s post-hoc test. Statistical significance was set at p<0.05.
RESULTS
Effect of THP on body weight and serum CORT level following single prolonged stress
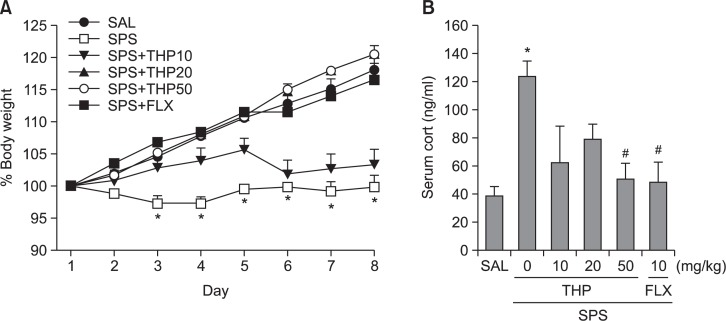
We measured the body weight of each rat in each group for 8 days. Different groups of rats, 7 animals per group, were used for all experiments. In the present study, body weight was evaluated daily for 8 days to identify whether the single prolonged stress would result in body weight loss (difference between daily weights and starting weight; Fig. 2A). Analysis of the body weight values revealed a significant gradual reduction of body weight gain over 8 days in the SPS group relative to control rats (SAL group)[F(5,36)=22.048, p<0.001]. During this period, 20 mg/kg or 50 mg/kg THP-treated rats showed slightly inhibitions of reductions in body weight gains, as compared to SPS group, although the findings were only minimally significance.
Fig. 2.
Effect of THP administration on body weights gain (A) and blood levels of corticosterone (B) of the rats under SPS. *p<0.05, vs. SAL group, #p<0.05 vs. SPS group.
Additionally, the serum CORT levels were measured in each group following the single prolonged stress for 8 days (Fig. 2B). ELISA analysis revealed that single prolonged stress treatment over 8 days significantly increased serum CORT concentration by 321.71% compared to saline-treated rats [F(5,17)=4.597, p<0.05]. Daily administration of THP significantly inhibited the single prolonged stress-induced increase of serum CORT levels compared to the SPS group (p<0.05).
Effect of THP on consumed sucrose intake following single prolonged stress
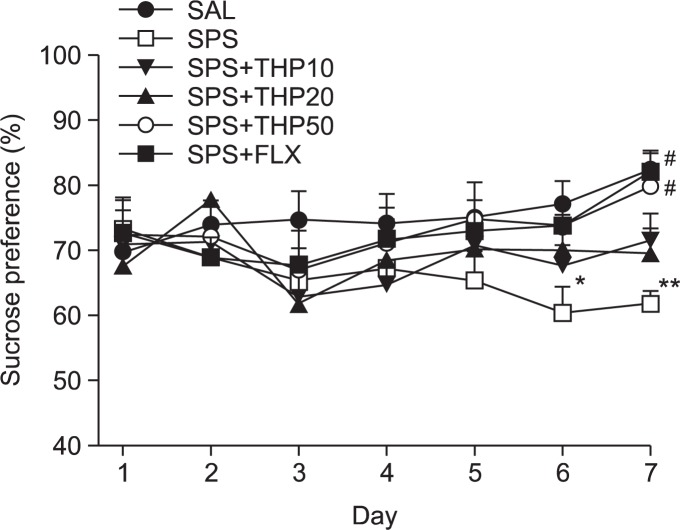
In the present study, we examined sucrose intake once daily for 7 days to indentify whether single prolonged stress (SPS group) caused consumed sucrose solution much less than the SAL group, as seen in Fig. 3 [F(5,12)=5.349, p<0.01]. Analysis of the sucrose intake values revealed a significant gradually reduction in consumed sucrose intake gain for 7 day in the SPS group, as compared to the control rats (SAL) group (p<0.05 on day 6; p<0.01 on day 7). During this period, 50 mg/kg THP-treated rats showed significant inhibitions of reductions in consumed sucrose intake, as compared to SPS group (p<0.05 on day 7). The results also showed that the recovery of consumed sucrose intake in the SPS+THP50 group was almost comparable to that in the SPS+FLX group.
Fig. 3.
Effect of THP administration on sucrose intake of rats subjected to SPS. *p<0.05, **p<0.01 vs. the SAL group, #p<0.05 vs. the SPS group.
Effect of THP on anxiety-like behavior following single prolonged stress
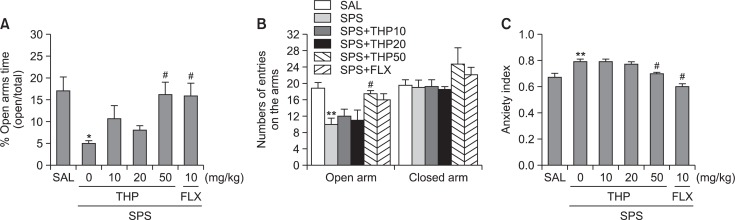
The impact of single prolonged stress on different parameters of anxiety-like behaviors is shown in Fig. 4. Different groups of rats, 7 animals per group, were used for all behavioral test. Eight days after single prolonged stress stressors were applied rats demonstrated lower exploration behavior, anxiety index, risk assessment and higher grooming activity compared to the control rats (SAL group). The effects of THP administration on anxiety-like behaviors, characterized by decreases in open-arm exploration in the EPM test, were also investigated. Statistical analyses of EPM behavioral data showed that the percentage of open-arm time differed significantly among the six groups [5,41]=6.843, p<0.01]. Post hoc comparisons revealed a significant decrease in the percentage of time spent by rats in the open arms of the maze following the single prolonged stress compared to the saline-treated rats (p<0.05; Fig. 4A). However, the rats in the SPS+THP50 group showed a significant restoration of the percentage of time spent, formerly decreased by single prolonged stress, in open-arm of the maze, as compared to that in the SPS group (p<0.05). Statistical analyses of EPM behavioral data showed that the number of entries into the open arms of the maze differed significantly among the six groups [5,41]=0.443, p>0.05]. Similarly, post hoc comparisons revealed a significant decrease in the number of entries into the open arms of the maze after the single prolonged stress compared to the SAL group (p<0.01; Fig. 4B). Rats in the SPS+THP50 group also exhibited a significant restoration in the number of entries into the open arms of the maze compared to the SPS group (p<0.05). Because no significant differences appeared in the number of closed-arm entries between groups in the EPM test, the observed anxiety-like behaviors of the rats receiving single prolonged stress are likely not attributable to differences in their locomotor activities (Fig. 4B). THP administration prior single prolonged stress did elicit anxiolytic or anxiogenic behavior in this study. These results reveal that the increase in the number of entries into the open arms of the maze by the SPS+THP50 group was almost comparable to those of the SPS+FLX group. Overall anxiety index calculated based on the number of visits and duration in open and closed arms was also different in these six groups of rats with lower values in the THP treated rats (p<0.05; Fig. 4C).
Fig. 4.
Effect of THP administration on the percentage of time spent on open arm (A), the numbers of entries into open and closed arms (B), and anxiety index (C) in the EPM test during SPS. *p<0.05, **p<0.01 vs. SAL group, #p<0.05 vs. SPS group.
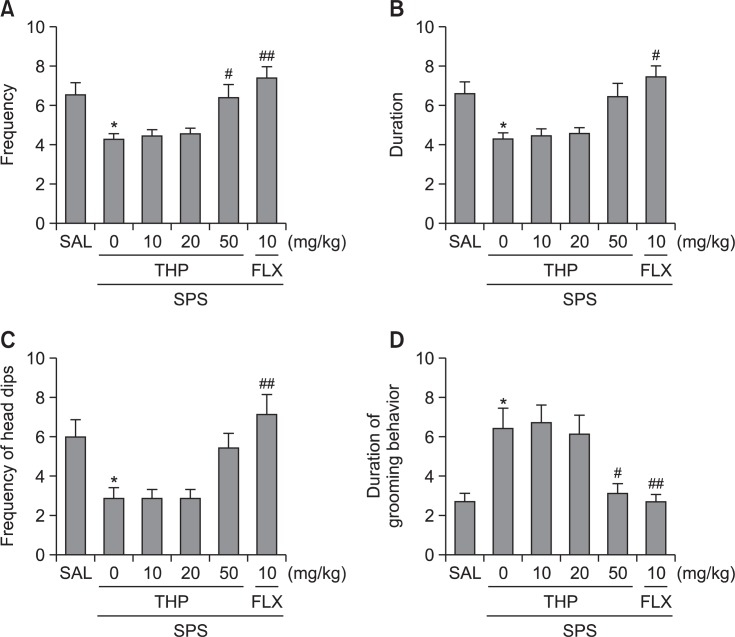
Administration of THP significantly increased frequency of risk assessment (p<0.05; Fig. 5A), but slightly increased duration of risk assessment, and frequency of unprotected head dips compared to the SPS group, although the findings were only minimally significance (Fig. 5B, C).
Fig. 5.
Effect of THP administration on the frequency (A) and duration (B) of risk assessment, unprotected head dips (C) and Grooming behavior (D) in the EPM test during SPS. *p<0.05 vs. SAL group, #p<0.05, ##p<0.01 vs. SPS group.
Grooming behavior was observed predominantly in the closed arms and was manifested as washing of snout and head. Total time, frequency and duration of a single bout of grooming behavior in EPM test are shown in Fig. 5D. Frequency of grooming behavior were modified only by 50 mg of THP given prior to SPS (p<0.05).
Effect of THP on motor functions or exploratory behaviors following single prolonged stress
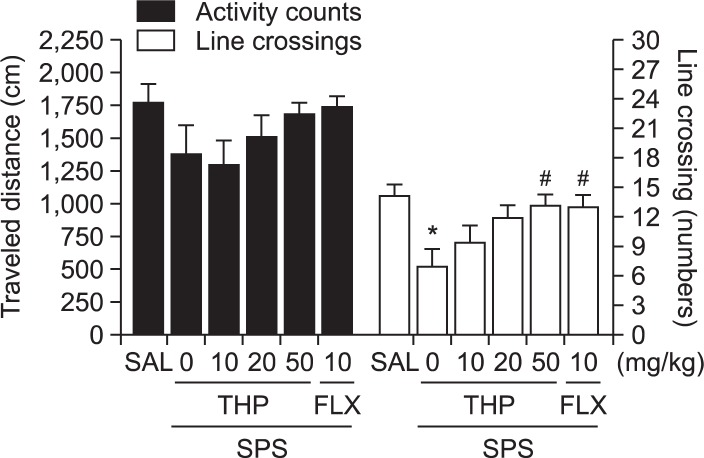
In addition to EPM test, an open field test was used to examine anxiety-like behavior, exploration behavior, and locomotion among the rats receiving single prolonged stress. As shown in Fig. 6, rats exposed to single prolonged stress spent a significant less time in the center, and decrease in the total number of line crossings compared to the SAL group (p<0.05). This finding suggested that single prolonged stress-treated rats subsequently produce exploration activities that are closely associated with anxiety-like behaviors in the OFT. However, THP-treated rats (50 mg/kg) revealed a significant influence of treatment on time spent in the center of OFT, and increase in the total number of line crossings compared to the SPS group (p<0.05), indicating lower anxiety. However, no significant differences appeared in locomotion in the OFT among groups.
Fig. 6.
Effect of THP administration on activity counts of locomotor activity and total number of line crossing in the OFT during SPS. *p<0.05 vs. SAL group, #p<0.05 vs. SPS group.
Long-term effect of THP on depression-like behavior following single prolonged stress
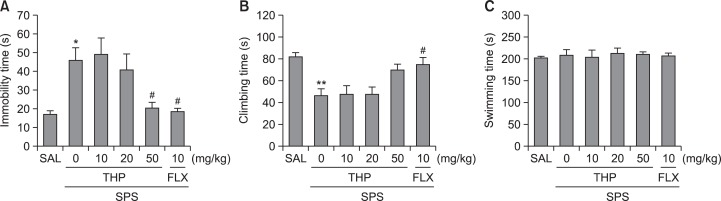
To examine effects of THP or response to traumatic stress, rats were treated with THP or saline 30 min before single prolonged stress. Rats subjected to the single prolonged stress for 8 days exhibited a significant depression-like phenotype, characterized by increased an increased duration of immobility during the FST compared to saline-treated controls (Fig. 7). One-way ANOVA indicated that THP administration prior SPS had a significant effect on immobility score [5,41]=6.338, p<0.001]. Rats in the SPS group exhibited more immobility during the FST compared to the SAL group (p<0.05; Fig. 7A). However, rats in the SPS+THP50 group displayed a significant decrease in durations of immobility during 5 min in the FST compared to SPS group (p<0.05), indicating that the administration of 50 of mg/kg THP decreases depression-like behaviors. Another key behavior, climbing behavior was also analyzed. Rats in the SPS group exhibited a significant decrease in climbing behavior during the FST relative to the SAL group (p<0.01; Fig. 7B). Furthermore, compared with the SPS group, rats in the SPS+THP50 group exhibited slightly a restoration of climbing behavior time during 5 min in the FST, although the findings were only minimally significance. However, single prolonged stress over 8 days did not induce significant differences in swimming behaviors among the groups during the FST (Fig. 7C). These results reveal that the reduction in immobility during depression-like behaviors in the SPS+THP50 group was almost comparable to those of the SPS+FLX group.
Fig. 7.
Effect of THP administration on immobility time (A), climbing behavior (B), and swimming behavior (C) in the FST during SPS. *p<0.05, **p<0.01 vs. the SAL group, #p<0.05 vs. the SPS group.
Effect of THP on CRF- and NPY-like immunoreactivities following single prolonged stress
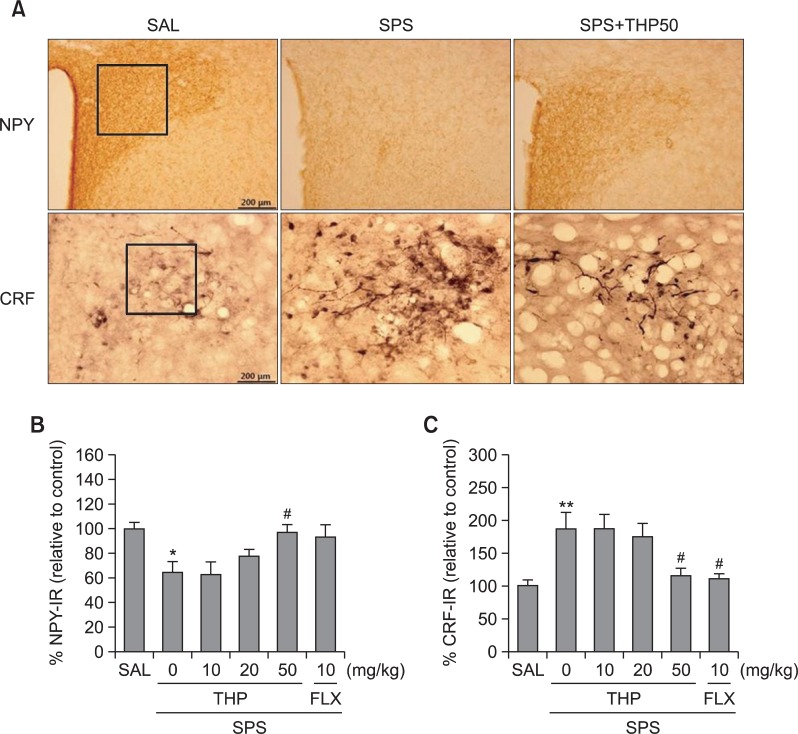
Following the behavioral tasks, NPY- and CRF-like immunoreactivities were analyzed in the cell bodies of various hypothalamic regions including the paraventricular nucleus (PVN; Fig. 8). NPY-like immunoreactivity was also analyzed in the PVN (Fig. 8A). The numbers of NPY-immunoreactive fibers in the PVN of the SPS group decreased to 64.14%. Analysis of the numbers of NPY-immunoreactive neurons values revealed that rats receiving singled prolonged stress exhibit a significant decrease of NPY expression compared to the SAL group (p<0.05; Fig. 8B). The number of NPY-immunoreactive neurons significantly increased in PVN of the SPS+THP50 group relative to the SPS group (p<0.05). The numbers of CRF-immunoreactive fibers in the PVN of the SPS group were increased by 187.32%. Analysis of the numbers of CRF-immunoreactive neurons values revealed that rats receiving single prolonged stress exhibited a significant increase of CRF expression compared to the SAL group (p<0.01; Fig. 8C). The number of CRF-immunoreactive neurons was significantly decreased in the PVN region of the SPS+THP50 group compared to the SPS group (p<0.05). This finding suggested that the increased CRF-immunoreactivity induced by the single prolonged stress was significantly restored by THP administration and that the number of CRF-immunopositive neurons in the SPS+THP50 group was closely associated with that in the SPS+FLX group (p<0.05). This finding indicated that the number of CRF- and NPY-immunoreactive neurons in rats receiving single prolonged stress was significantly restored by THP administration.
Fig. 8.
Effect of THP administration on the mean number of NPY and CRF expression in the PVN of the hypothalamus. Representative photographs and the relative percentage values are indicated in (A) and (B and C), respectively. *p<0.05, **p<0.01 vs. SAL group, #p<0.05 vs. SPS group.
DISCUSSION
These results clearly demonstrate that repeated administration of THP prior to SPS significantly increased the number and duration of open-arm visits in the EPM test, reduced the anxiety index, increased the higher risk assessment, and increased the number of head dips over the borders of the open arms after SPS. THP was also associated with increased time spent at the center of the open field, and reduced time spent immobile in the FST. It is likely that these behavioral effects were based on a modulation of hypothalamic NPY and CRF activity in paraventricular nucleus of the hypothalamus (PVN), which is the primary projection area that underlies the development of anxiety and depression. Thus, the current results support the possibility that THP has anxiolytic and antidepressant effects.
PTSD is unique among mental health disorders, as it requires that exposure to a life-threatening or traumatic event precede and precipitate the emergence of symptoms; common comorbid psychiatric disorders include major depression, anxiety, and substance use disorders (Serova et al., 2014). We chose SPS as the traumatic stress, because it is among the most reliable models of PTSD, characterized by high face validity and core etiological factors of this disease, such as reduced sensorimotor gating of the startle response, anxiety- and depression-like behaviors, and enhanced negative feedback of the HPA axis (Serova et al., 2013a). Our slightly modified model of SPS, clearly increased the anxiety- and depression-like behaviors of the stressed animals compared with the unstressed controls and, consistent with previous studies (Serova et al., 2013b), these animals more successfully managed the stressful situations. A THP inhibitor administered before the traumatic stress suggested THP to be a new candidate for reducing the long-term impact of traumatic stress (Ceremuga et al., 2013). This is also the first study to show the pronounced resiliency-promoting effects of THP with regard to the development of the behavioral symptoms associated with PTSD.
Furthermore, we observed a gradual decrease in body weight gain, an increase in serum CORT levels, and a decrease in sucrose preference compared with immediately before testing, indicating that SPS was sufficiently stressful (Castro et al., 2012). Many studies have shown that, consistent with PTSD models, SPS increased serum CORT concentrations, and decreased the sucrose preference of rats (Kozlovsky et al., 2009; Roth et al., 2012; Meyer et al., 2013). Accordingly, the forced maintenance of high CORT levels and the decrease in sucrose preference associated with animal models can affect anxiety- and depression-like symptoms under experimental conditions, and this may be related to the progression or exacerbation of PTSD in humans (Serova et al., 2013a). The administration of THP produced anxiolytic and antidepressant activities, suggesting that this therapy inhibited the HPA axis-associated psychological dysfunction induced by decreased serum CORT levels and increased sucrose preference, thereby normalizing behavioral and neuro-chemical responses.
The results of these behavioral investigations revealed the anxiolytic-like effects of THP in animal models of anxiety. Many studies have suggested that stressed rats decrease in the proportion of time spent in and the number of entries into the open arms of the EPM (Cohen et al., 2012). In the present study, the administration of a higher dose of THP (50 mg/kg) prior to SPS significantly reduced anxiety-like behaviors in the EPM test, as indicated by an increase in the number of entries and exploratory behaviors, and a reduction in avoidance of the open arms. THP-treated rats had lower anxiety indices, visited the open arms more frequently, spent much more time in the open arms, and spent much less time in the closed arms of the EPM. Our results revealed that a higher dose, which is usually used for studying anxiety-like behaviors, is also effective for minimizing depression-like behaviors. However, in this study, lower doses of THP (10 and 20 mg/kg) prior to SPS did not produce anxiolytic effects. Thus, these results suggest that the decreased anxiety-related behaviors following inhalation of THP may be related an attenuation in the activity of the HPA axis.
An OFT was also performed to rule out any confounding motor impairments that may have influenced the outcomes of the behavioral anxiety tests (Kokras et al., 2012). No significant individual differences in locomotor activity were observed between groups, suggesting that the administration of THP had no effect on sensorimotor performance. However, the administration of THP prior to SPS significantly reduced anxiety-like behaviors, as indicated by an increase in the total number of line crossings in the OFT and in the amount of time spent at the center of the field, reflecting less activation of the CNS by novelty and fear (Lee et al., 2013). The changes in anxiety-like behaviors appear to have been specific and not the result of impaired locomotor activity, as the track length in the EPM test and OFT was similar for all groups. Accordingly, these results suggest that the observed increase in the total number of line crossings in the OFT is similar to the changes in the behavioral performance observed in the EPM test, which were likely due to anxiolytic activity.
However, based on grooming behavior, only rats treated with THP showed better adaptation to novelty stress, such as the EPM test (Serova et al., 2013a). These results suggest that animals not treated before SPS were more vulnerable than were those who received THP before SPS to the novel stress of the EPM as a consequence of their unpredicted exposure to trauma. Thus, the current results support the possibility that THP has anxiolytic effects, reflected by changes in grooming behavior.
Furthermore, the current results are consistent with previous findings showing that SPS decreased immobility during the FST (Takahashi et al., 2006). In this study, the administration of THP significantly decreased immobility and also increased climbing behaviors during the FST, but we observed no effect on swimming, which confirms an antidepressant-like activity that does not result in deficits in motor functioning (Serova et al., 2013b). The FST is a valuable and reliable behavioral research model of depression in rodents and is also an important tool with which to study the neurobiological mechanisms involved in antidepressant responses (Cryan and Holmes, 2005). The observed immobility behavior in the FST is similar to a state of depressed mood or helplessness and may be considered analogous to depression in humans (Cryan and Holmes, 2005). Therefore, these data support the possibility that THP may have antidepressant effects.
NPY is widely distributed throughout the CNS and is one of the most evolutionarily conserved peptides, with varying concentrations detected throughout the limbic system, which has been repeatedly implicated in the modulation of emotional processing and in the pathogenesis of anxiety and depressive disorders (Serova et al., 2014). Several studies have indicated that NPY levels are correlated with anxiety in an animal model of depression, suggesting a possible link between low NPY expression and a predisposition to anxiety or stress-induced depression (Serova et al., 2013b). Decreased NPY expression was detected after SPS in rats, indicating that stress-induced NPY expression in the PVN is independent of HPA activation (Cui et al., 2008). The neuropeptide CRF plays an important role in the central regulation of stress and anxiety (Fox and Lowry, 2013). In addition to its well-characterized ability to activate the HPA axis, CRF functions as a neurotransmitter at extrahypothalamic brain sites to mediate physiological and behavioral responses to stress (Kohda et al., 2007). Previous studies have shown that i.c.v. injections of CRF induced anxiety and depression-like behaviors and increased plasma CORT via regulation of HPA axis hyperactivity (Kupferschmidt et al., 2012). The current data suggest that the CRF circuits in the PVN of the hypothalamus are activated by SPS, leading to the observed anxiety and depression-like activity in the behavioral tests (Dabrowska et al., 2013). These results show that the administration of THP significantly blocked the decrease in NPY and the increase in CRF immunoreactivity in the PVN. This suggests that the anxiolytic and antidepressant effects following the administration of THP are closely associated with NPY and CRF modulation in the PVN in the hypothalamus and with activation of the HPA axis.
The anxiolytic and anti-depressant effects of THP blockade observed in this study may be mediated by several possible mechanisms. First, they may have regulatory effects on the activation of the HPA axis. Thus, the behavioral observations and extensive staining of NPY and CRF in the PVN in this study seem to be related to the activation of the HPA axis, a widely used model of anxiety and depression and one of the main mechanisms implicated in manifestation of anxiety and depressive behaviors. In human and “PTSD-specific” animal models, dysregulation of the HPA axis is considered one of the core symptoms of PTSD (Shea et al., 2005). As discussed above, behavioral, psychogenic and neurochemical changes may be induced by administration of THP. Additionally, a new hypothesis or the mechanisms underpinning the operation of THP can be proposed based on the present observations, which elucidate the anxiety- and depression-like behaviors induced by HPA axis dysregulation and the neurochemical interactions between NPY and CRF in the hypothalamus.
In summary, the present study demonstrated that administration of THP is associated with anxiolytic- and antidepressant-like profiles in the EPM test, the OFT, and the FST, possibly via modification of NPY and CRF expression in the hypothalamus. These findings indicate that THP is capable of ameliorating the complex behaviors and neurochemical responses involved in anxiety and depression via modulation of HPA activity. Future studies should focus on the mechanisms of action of THP that underpin these anxiolytic and antidepressant properties.
Acknowledgments
This research was supported by a Grant from the National Research Foundation of Korea funded by the Korean government (MEST)(2013R1A1A2063051).
REFERENCES
- Castro JE, Diessler S, Varea E, Márquez C, Larsen MH, Cordero MI, Sandi C. Personality traits in rats predict vulnerability and resilience to developing stress-induced depression-like behaviors, HPA axis hyper-reactivity and brain changes in pERK1/2 activity. Psychoneuroendocrinology. 2012;37:1209–1223. doi: 10.1016/j.psyneuen.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Ceremuga TE, Shellabarger P, Persson T, Fanning M, Galey P, Robinson D, Bertsch S, Ceremuga GA, Bentley M. Effects of tetrahydropalmatine on post-traumatic stress disorder-induced changes in rat brain gene expression. J Integr Neurosci. 2013;12:513–528. doi: 10.1142/S0219635213500313. [DOI] [PubMed] [Google Scholar]
- Cohen H, Liu T, Kozlovsky N, Kaplan Z, Zohar J, Mathé AA. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology. 2012;37:350–363. doi: 10.1038/npp.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman I, Ataullahjan A. Life course perspectives on the epidemiology of depression. Can. J. Psychiatry. 2010;55:622–632. doi: 10.1177/070674371005501002. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Cui H, Sakamoto H, Higashi S, Kawata M. Effects of sing le-prolonged stress on neurons and their afferent inputs in the amygdala. Neuroscience. 2008;152:703–712. doi: 10.1016/j.neuroscience.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Guo JD, Li C, Dewitt S, Xu J, Lombroso PJ, Rainnie DG. Striatal-enriched protein tyrosine phosphatase-STEPs toward understanding chronic stress-induced activation of corticotrophin releasing factor neurons in the rat bed nucleus of the stria terminalis. Biol. Psychiatry. 2013;74:817–826. doi: 10.1016/j.biopsych.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deligiannidis KM, Freeman MP. Complementary and alternative medicine for the treatment of depressive disorders in women. Psychiatr Clin North Am. 2010;33:441–463. doi: 10.1016/j.psc.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Fox JH, Lowry CA. Corticotropin-releasing factor-related peptides, serotonergic systems, and emotional behavior. Front Neurosci. 2013;7:169–173. doi: 10.3389/fnins.2013.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SA, Knox D, Curtis AL, Aldridge JW, Valentino RJ, Liberzon I. Altered locus coeruleus-norepinephrine function following single prolonged stress. Eur J Neurosci. 2013a;37:901–909. doi: 10.1111/ejn.12095. [DOI] [PubMed] [Google Scholar]
- George SA, Stout SA, Tan M, Knox D, Liberzon I. Early handling attenuates enhancement of glucocorticoid receptors in the prefrontal cortex in an animal model of post-traumatic stress disorder. Biol Mood Anxiety Disord. 2013b;3:22–23. doi: 10.1186/2045-5380-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Zhang W, Tang Y, Bai W, Yang F, Xie L, Li X, Zhou S, Pan S, Chen Q, Ferro A, Ji Y. l-Tetrahydropalmatine, an active component of Corydalis yanhusuo W.T. Wang, protects against myocardial ischaemia-reperfusion injury in rats. PLoS One. 2012;7:e38627. doi: 10.1371/journal.pone.0038627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao YT, Yi PL, Li CL, Chang FC. Effect of cannabidiol on sleep disruption induced by the repeated combination tests consisting of open field and elevated plus-maze in rats. Neuropharmacology. 2012;62:373–384. doi: 10.1016/j.neuropharm.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Jindal A, Mahesh R, Kumar B. Anxiolytic-like effect of linezolid in experimental mouse models of anxiety. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;40:47–53. doi: 10.1016/j.pnpbp.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Kohda K, Harada K, Kato K, Hoshino A, Motohashi J, Yamaji T, Morinobu S, Matsuoka N, Kato N. Glucocorticoid receptor activation is involved in producing abnormal phenotypes of single-prolonged stress rats: a putative post-traumatic stress disorder model. Neuroscience. 2007;148:22–33. doi: 10.1016/j.neuroscience.2007.05.041. [DOI] [PubMed] [Google Scholar]
- Kokras N, Dalla C, Sideris AC, Dendi A, Mikail HG, Antoniou K, Papadopoulou-Daifoti Z. Behavioral sexual dimorphism in models of anxiety and depression due to changes in HPA axis activity. Neuropharmacology. 2012;62:436–445. doi: 10.1016/j.neuropharm.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Kozlovsky N, Matar MA, Kaplan Z, Zohar J, Cohen H. The role of the galaninergic system in modulating stress-related responses in an animal model of posttraumatic stress disorder. Biol. Psychiatry. 2009;65:383–391. doi: 10.1016/j.biopsych.2008.10.034. [DOI] [PubMed] [Google Scholar]
- Kupferschmidt DA, Newman AE, Boonstra R, Erb S. Antagonism of cannabinoid 1 receptors reverses the anxiety-like behavior induced by central injections of corticotropin-releasing factor and cocaine withdrawal. Neuroscience. 2012;204:125–133. doi: 10.1016/j.neuroscience.2011.07.022. [DOI] [PubMed] [Google Scholar]
- Lee B, Sur B, Kwon S, Yeom M, Shim I, Lee H, Hahm DH. Chronic administration of catechin decreases depression and anxiety-like behaviors in a rat model using chronic corticosterone injections. Biomol Ther. 2013;21:313–322. doi: 10.4062/biomolther.2013.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YL, Liang JH, Yan LD, Su RB, Wu CF, Gong ZH. Effects of l-tetrahydropalmatine on locomotor sensitization to oxycodone in mice. Acta Pharmacol Sin. 2005;26:533–538. doi: 10.1111/j.1745-7254.2005.00101.x. [DOI] [PubMed] [Google Scholar]
- Mao QQ, Ip SP, Ko KM, Tsai SH, Che CT. Peony glycosides produce antidepressant-like action in mice exposed to chronic unpredictable mild stress: effects on hypothalamic-pituitary-adrenal function and brain-derived neurotrophic factor. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33:1211–1216. doi: 10.1016/j.pnpbp.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Márquez C, Nadal R, Armario A. Influence of reactivity to novelty and anxiety on hypothalamic-pituitary-adrenal and prolactin responses to two different novel environments in adult male rats. Behav Brain Res. 2006;168:13–22. doi: 10.1016/j.bbr.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Meyer EM, Long V, Fanselow MS, Spigelman I. Stress increases voluntary alcohol intake, but does not alter established drinking habits in a rat model of posttraumatic stress disorder. Alcohol Clin Exp Res. 2013;37:566–574. doi: 10.1111/acer.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3th ed. Academic Press; New York: 1986. pp. 54–85. [Google Scholar]
- Roth MK, Bingham B, Shah A, Joshi A, Frazer A, Strong R, Morilak DA. Effects of chronic plus acute prolonged stress on measures of coping style, anxiety, and evoked HPA-axis reactivity. Neuropharmacology. 2012;63:1118–1126. doi: 10.1016/j.neuropharm.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serova LI, Laukova M, Alaluf LG, Pucillo L, Sabban EL. Intranasal neuropeptide Y reverses anxiety and depressive-like behavior impaired by single prolonged stress PTSD model. Eur. Neuropsychopharmacol. 2014;24:142–147. doi: 10.1016/j.euroneuro.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Serova LI, Laukova M, Alaluf LG, Sabban EL. Intranasal infusion of melanocortin receptor four (MC4R) antagonist to rats ameliorates development of depression and anxiety related symptoms induced by single prolonged stress. Behav Brain Res. 2013a;250:139–147. doi: 10.1016/j.bbr.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Serova LI, Tillinger A, Alaluf LG, Laukova M, Keegan K, Sabban EL. Single intranasal neuropeptide Y infusion attenuates development of PTSD-like symptoms to traumatic stress in rats. Neuroscience. 2013b;236:298–312. doi: 10.1016/j.neuroscience.2013.01.040. [DOI] [PubMed] [Google Scholar]
- Shea A, Walsh C, Macmillan H, Steiner M. Child mal-treatment and HPA axis dysregulation: relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinology. 2005;30:162–178. doi: 10.1016/j.psyneuen.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Morinobu S, Iwamoto Y, Yamawaki S. Effect of paroxetine on enhanced contextual fear induced by single prolonged stress in rats. Psychopharmacology (Berl) 2006;189:165–173. doi: 10.1007/s00213-006-0545-6. [DOI] [PubMed] [Google Scholar]
- Uliaszek AA, Zinbarg RE, Mineka S, Craske MG, Sutton JM, Griffith JW, Rose R, Waters A, Hammen C. The role of neuroticism and extraversion in the stress-anxiety and stress-depression relationships. Anxiety Stress Coping. 2010;23:363–381. doi: 10.1080/10615800903377264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Yang Z, Li SJ, Li X, Dillon C, Peng XQ, Spiller K, Gardner EL. Levo-tetrahydropalmatine inhibits cocaine's rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropharmacology. 2007;53:771–782. doi: 10.1016/j.neuropharm.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Chen Y, Zhu J, Wang L, Cao G, Dang Y, Yan C, Wang J, Chen T. Levo-tetrahydropalmatine attenuates the development and expression of methamphetamine-induced locomotor sensitization and the accompanying activation of ERK in the nucleus accumbens and caudate putamen in mice. Neuroscience. 2014;258:101–110. doi: 10.1016/j.neuroscience.2013.11.025. [DOI] [PubMed] [Google Scholar]
- Zhu KY, Mao QQ, Ip SP, Choi RC, Dong TT, Lau DT, Tsim KW. A standardized chinese herbal decoction, kai-xin-san, restores decreased levels of neurotransmitters and neurotrophic factors in the brain of chronic stress-induced depressive rats. Evid Based Complement Alternat Med. 2012;2012:149256. doi: 10.1155/2012/149256. [DOI] [PMC free article] [PubMed] [Google Scholar]