Abstract
Skin hyperpigmentation is one of the most common skin disorders caused by abnormal melanogenesis. The mechanism and key factors at play are not fully understood. Previous reports have indicated that cystamine (CTM) inhibits melanin synthesis, though its molecular mechanism in melanogenesis remains unclear. In the present study, we investigated the effect of CTM on melanin production using ELISA reader and the expression of proteins involved in melanogenesis by Western blotting, and examined the involvement of transglutaminase-2 (Tgase-2) in SK-MEL-2 human melanoma cells by gene silencing. In the results, CTM dose-dependently suppressed melanin production and dendrite extension in α-MSH-induced melanogenesis of SK-MEL-2 human melanoma cells. CTM also suppressed α-MSH-induced chemotactic migration as well as the expressions of melanogenesis factors TRP-1, TRP-2 and MITF in α-MSH-treated SK-MEL-2 cells. Meanwhile, gene silencing of Tgase-2 suppressed dendrite extension and the expressions of TRP-1 and TRP-2 in α-MSH-treated SK-MEL-2 cells. Overall, these findings suggested that CTM suppresses α-MSH-induced melanogenesis via Tgase-2 inhibition and that therefore, Tgase-2 might be a new target in hyperpigmentation disorder therapy.
Keywords: Cystamine, Melanogenesis, Transglutaminase-2, TRP-1, TRP-2, SK-MEL-2 melanoma cells
INTRODUCTION
Skin pigmentation consists of the synthesis and transfer of melanin by epidermal melanocytes to the governing keratinocytes (Yamaguchi et al., 2007). This skin pigmentation may result from several steps including melanocyte proliferation, differentiation, melanogenesis, migration or increases in dendricity (Park et al., 2009). In melanocytes, melanins are synthesized within melanosomes which contain three major pigment enzymes: tyrosinase, tyrosinase-related protein (TRP-1), and TRP-2 (Lee et al., 2012b). The expression of these proteins is regulated transcriptionally by the microphthalmia associated transcription factor (MITF). MITF is a unique, important transcription factor involved in all aspects of melanocyte survival and function (Lee et al., 2011).
Skin hyperpigmentation is the most common skin disorder caused by sun damage, inflammation, or other skin injuries, including those related to acne vulgaris (Lim et al., 2009; Chang and Chen, 2012). Disorders of hypopigmentation include albinism, piebaldism, tuberous sclerosis, hypomelanosis of Ito, vitiligo, pityriasis alba, tinea versicolor and post-inflammatory hypopigmentation (Plensdorf and Martinez, 2009). Although various compounds and physical therapies have been developed over the past decades for the treatment of skin pigmentation disorder, none is completely satisfactory.
α-melanocyte-stimulating hormone (α-MSH) is a naturally-occurring endogenous peptide of the melanocortin family that stimulates melanin synthesis and its distribution in melanocytes (in mammals) and melanophores (in lower vertebrates) (Brzoska et al., 2008). α-MSH exerts its effect on pigmentation by binding the melanocortin-1 receptr (MC1R) at the cell membrane of melanocytes (Yang et al., 1997).
Cystamine (CTM) is an organic disulfide known to inhibit transglutaminase 2 (Tgase-2) activity via a disulfide exchange process (Borrell-Pages et al., 2006). CTM is involved in melanin synthesis via the reduction of the tyrosinase (TYR) activity of pigmented melanoma cells (Qiu et al., 2000). However, the exact molecular mechanism of CTM in melanogenesis is currently unknown.
Tgase-2 is a multifunctional protein having both intracellular and extracellular functions (Lee et al., 2012a). In addition to catalyzing Ca2+-dependent transamidation reactions, Tgase-2 can bind and hydrolyze GTP/GDP with an affinity and catalytic rate similar to those of the α subunit of large heterotrimeric G proteins and small Ras-type G proteins (Lorand and Graham, 2003; Mhaouty-Kodja, 2004; Lee et al., 2012a). Interestingly, it has been shown that metastatic melanoma cell lines express Tgase-2 levels up to 24-fold higher than do primary melanoma cell lines in the radial growth phase (Fok et al., 2006). The involvement of Tgase-2 in human melanogenesis, however, has not been reported. Therefore, in the present study, using gene silencing and CTM, we investigated the involvement of Tgase-2 in melanogenesis. In this report, we found that transglutaminase-2 is involved in α-MSH-induced melanogenesis of SK-MEL-2 melanoma cells.
MATERIALS AND METHODS
Materials
Fetal bovine serum (FBS) and culture media were obtained from WelGENE Inc (Daegu, South Korea). CTM and α-melanocyte-stimulating hormone (α-MSH) were purchased from Sigma Aldrich (St. Louis, MO, USA). All other chemicals used were of standard analytical grade. TRP-1, TRP-2, and β-actin antibodies were acquired from Santa Cruz Biotechnology Inc (Santa Cruz, CA, USA). Transglutaminase 2 (Tgase-2) monoclonal antibody (clone CUB 7402) was purchased from NeoMarkers (Fremont, CA, USA). siRNAs and control siRNAs were obtained from Invitrogen (Carlsbad, CA, USA).
Cell culture
SK-MEL-2 cells were purchased from Korean Cell Line Bank (KCLB). The cells were grown and maintained at 37°C in a 95% air, 5% CO2 atmosphere in RPMI-1640 supplemented to a final concentration of 10% heat-inactivated FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were passaged every 3 days with a maximal passage number of 33. Confluent monolayers of melanocytes were harvested with a mixture of 0.05% trypsin and 0.53 mM EDTA (Bibco BRL, Grand Island, NY, USA).
Melanin measurement
The melanin content of the cultured melanoma was determined as described previously (Kim et al., 2010). Preparatory to cellular melanin measurement, cells were seeded in a 6-well plate at an appropriate density, and, after 24 hrs of cultivation, were treated with α-MSH and various concentrations of CTM for 48 hrs. The cells were then harvested, washed twice with PBS, resuspended in 1 N NaOH containing 10% DMSO, and heated at 80°C for 1 hr. Subsequently, the absorbance of extracted melanin was read at 405 nm using an ELISA microplate reader.
Western blot analysis
After incubation, cells were collected and washed twice with cold PBS. The cells were then lysed in a lysis buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% triton X-100, 2 mM EDTA, 1% DOC (Deoxycholic acid), 0.1% SDS, 1 mM NaVO3, 10 mM NaF, 1 mM DTT] and centrifuged to yield whole-cell lysates, the protein concentration of which was measured using the Bradford method. Aliquots of the lysates (20–30 μg of protein) were separated on 4–12% SDS-polyacrylamide gel and transferred onto a polyvinylidene fluoride (PVDF) membrane (Invitrogen) with a glycine transfer buffer [192 mM glycine, 25 mM Tris-HCl (pH 8.8), 10% MeOH (v/v)]. After blocking the non-specific site with 3% non-fat dry milk, the membrane was then incubated with specific primary antibody in 3% BSA at 4°C overnight, and then further incubated with peroxidaseconjugated secondary antibody (1:5000, Santa Cruz, CA, USA) at room temperature for 60 min. Immunoactive proteins were detected using the PowerOpti-ECL Western blotting detection reagent (Animal Genetics Inc., Gyeonggi, Korea).
Chemotactic migration assay
Migration assays were performed using multiwell chambers (Neuroprobe Inc. Gaithersburg, MD, USA) coated with 10 μg/ml fibronectin as a chemoattractant. Briefly, the cells were suspended at 1×106 cells/ml in DMEM (2% FBS), a 25 μl aliquot of which was poured into the upper well of a chamber. Next, the aliquot was separated from the sample-containing lower well by means of an 8 μm polyhydrocarbon filter. After incubation for 5 hrs at 37°C, non-migrated cells on the upper surface of the membrane were scraped off, and the migrated cells on lower surface were stained by Diff-quick and subsequently counted under four randomly chosen high-power fields (400×).
Cell proliferation assay
The cell proliferation was measured using the EZ-Cytox Cell viability assay kit (Daeillab Service, Seoul, Korea). Briefly, 100 μl of cell suspension (3000 cells per well) was added to each well of a 96-well plate. After the required incubation with stimulants for 48 hrs, 10 μl of EZ-Cytox solution was added to each well of the plate and incubated at 37°C for 2–4 hrs. The absorbance was measured by spectrophotometry (Multscan, Thermo, USA) at 450 nm. The cell proliferation (%) was calculated using the formula [As/Ac]×%, where As is the absorbance of the well-contained cells, culture medium, EZ-Cytox solution and stimulants, and Ac is the absorbance of the well-contained cell, culture medium and EZ-Cytox solution.
Tgase-2 gene silencing by small-interfering RNA
A small-interfering RNA (siRNA) duplex-targeting human Tgase-2, 5′-AAG AGC GAG AUG AUC UGG AAC-3′ (Invitrogen), was introduced into the cells using Lipofectamine 2000 Reagent (Invitrogen) according to the manufacturer's instructions. The cells were then cultured with or without α-MSH (100 nM). Universal negative siRNA (Invitrogen) was employed as a negative control.
Statistical analysis
An analysis of variance (ANOVA) in conjunction with Tukey’s post-hoc test was used to determine the statistical significance of the differences between the values for a variety of experimental and control groups. The data are expressed as mean ± s. d. of at least three independent experiments performed in triplicate. p<0.05 was considered statistically significant.
RESULTS
CTM suppressed melanin production, cell proliferation and dendrite extension of SK-MEL-2 cells
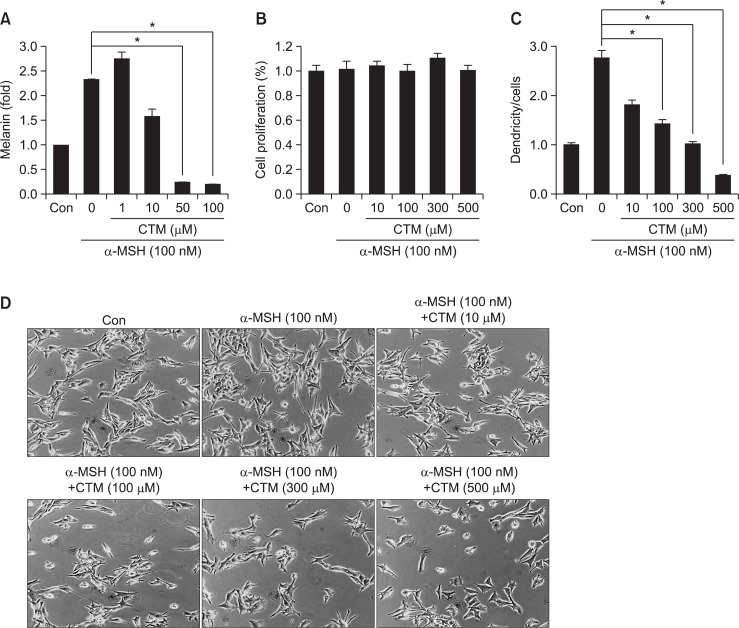
To confirm CTM’s antimelanogenic functionality, we examined its effect on SK-MEL-2 cell melanogenesis. Cells were incubated with the indicated concentrations of CTM for 24 hrs, and, 2 hrs before CTM treatment, melanogenesis was stimulated by α-MSH (100 nM). As a result of this α-MSH stimulation, the cells’ melanin content was increased considerably (Fig. 1A), subsequent to which, CTM dose-dependently decreased it (Fig. 1A). Notably, CTM showed no cytotoxic activity in the doses used (Fig. 1B). We also examined the effects of CTM on the SK-MEL-2 cells’ dendricity. Whereas α-MSH-induced well-developed dendricity, dendrite outgrowth was suppressed by CTM (Fig. 1C, D). These results suggest that CTM suppresses α-MSH-stimulated melanogenesis of SK-MEL-2.
Fig. 1.
Effects of CTM on pigmentation in SK-MEL-2 cells. (A) Effects of CTM on melanin production. Melanoma cells were cultured in RPMI media. The cells were seeded in a 6-well plate with or without CTM (1, 10, 50, 100 μM) and α-MSH (100 nM). After 2 days of incubation, the cells were washed twice with PBS, resuspended in 1 N NaOH containing 10% DMSO, and heated at 80°C for 1 hr. The absorbance of the extracted melanin was read at 405/450 nm using an ELISA microplate reader. (B) Effects of CTM on cell proliferation. SK-MEL-2 cells were cultured in a 96-well plate with or without CTM (10, 100, 300, 500 μM) and α-MSH (100 nM). After 48 hrs of incubation, the relative cell proliferation was determined using a Cell Counting kit-8. (C) Plot of effects of CTM on dendrite extension of SK-MEL-2 cells. (D) Photographs of effects of CTM on dendrite extension of SK-MEL-2 cells. In (C) and (D), SK-MEL-2 human melanoma cells were cultured in DMEM (2% FBS) media. The cells were seeded in a 6-well plate with or without CTM and α-MSH. After 48h of incubation, the cells with and without “dendricity” (i.e. the appearance of cell extensions as a function of time) were counted and photographed under Zeiss microscopy (20x magnification) using a Real-time image system. In (C), the average dendrite lengths (n=20) in the melanoma cells were measured using the Image tool program. *p<0.05.
CTM suppressed α-MSH-induced expressions of melanogenic proteins and Tgase-2
We examined the effect of CTM on the expressions of SK-MEL-2 melanogenic proteins including TRP-1, TRP-2 and MITF by Western blotting. Treatment of the SK-MEL-2 cells with α-MSH resulted in increased expressions of TRP-1, TRP-2 and MITF (Fig. 2). However, pretreatment with CTM decreased their expressions. Next, because Tgase-2 expression is up-regulated during advanced stages of malignant melanomas (Fok et al., 2006), we investigated whether CTM suppression of melanogenic proteins is correlated with Tgase-2 expression. Interestingly, the α-MSH-treated cells showed elevated Tgase-2 expression, which was inhibited by CTM in accordance with the melanogenic proteins’ expression changes (Fig. 2).
Fig. 2.
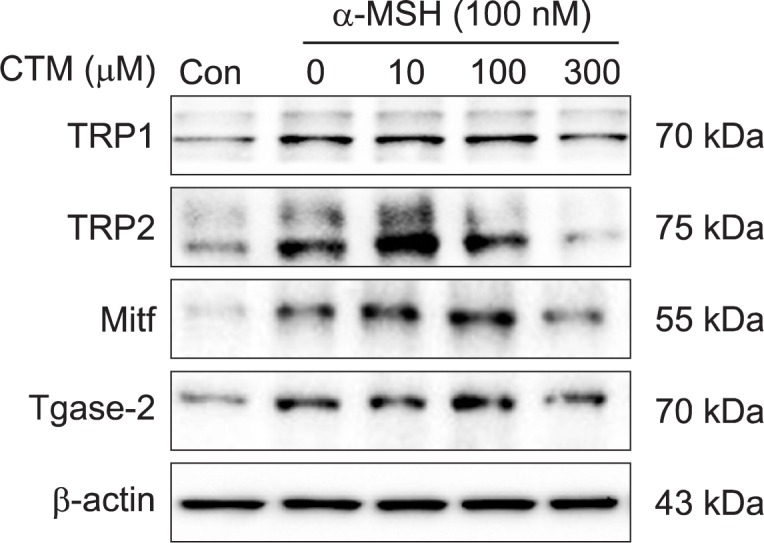
Effects of CTM on MITF, TRP1, and TRP2 expressions of SK-MEL-2 cells. SK-MEL-2 cells were treated with control media or CTM (10, 100, 300 μM) for the indicated time. Whole-cell lysates (30 μg) were prepared, the protein level was subjected to 10% SDS-PAGE, and the expressions of several proteins were determined by Western blotting.
CTM reduced α-MSH-induced migration of SK-MEL-2 cells
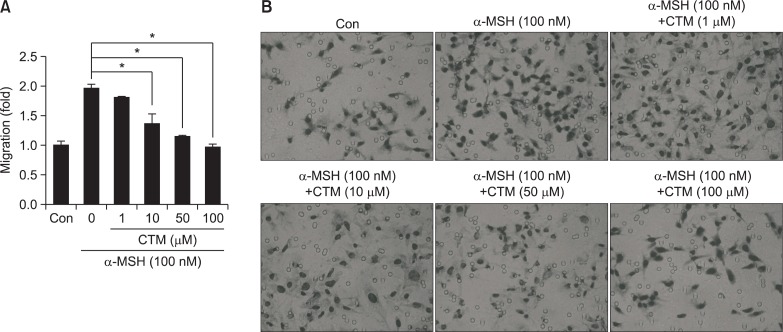
As α-MSH is well-known to increase melanocyte migration (Brzoska et al., 2008), we utilized a chemotactic migration assay to determine whether CTM, as a Tgase inhibitor, can inhibit α-MSH-induced migration of SK-MEL-2 cells. We found that α-MSH induced SK-MEL-2 cell migration and that CTM dose-dependently suppressed it (Fig. 3). These results indicated that CTM is an effective anti-migratory compound against melanoma cells.
Fig. 3.
Effect of CTM on chemotactic migration in SK-MEL-2 cells. (A) Plot of effect of CTM on chemotactic migration in SK-MEL-2 cells. (B) Photograph of effect of CTM on chemotactic migration in SK-MEL-2 cells. In (A) and (B), for the migration assay, the lower chamber transwells were coated with fibronectin (10 μg/ml). SK-MEL-2 cells (5×104/well) were treated with vehicle or increasing concentrations (1,10, 50, 100 μM) of cyst amine for 4 hrs. After incubation, the cells on the bottom side of the filter were fixed, stained by Diff-quick, and then subsequently counted under four randomly chosen high-power fields (400×). In (B), SK-MEL-2 cells that had migrated through the membrane after 5 hrs of incubation were stained and photographed. *p<0.05.
Tgase-2 is involved in α-MSH-induced melanogenesis of SK-MEL-2 cells
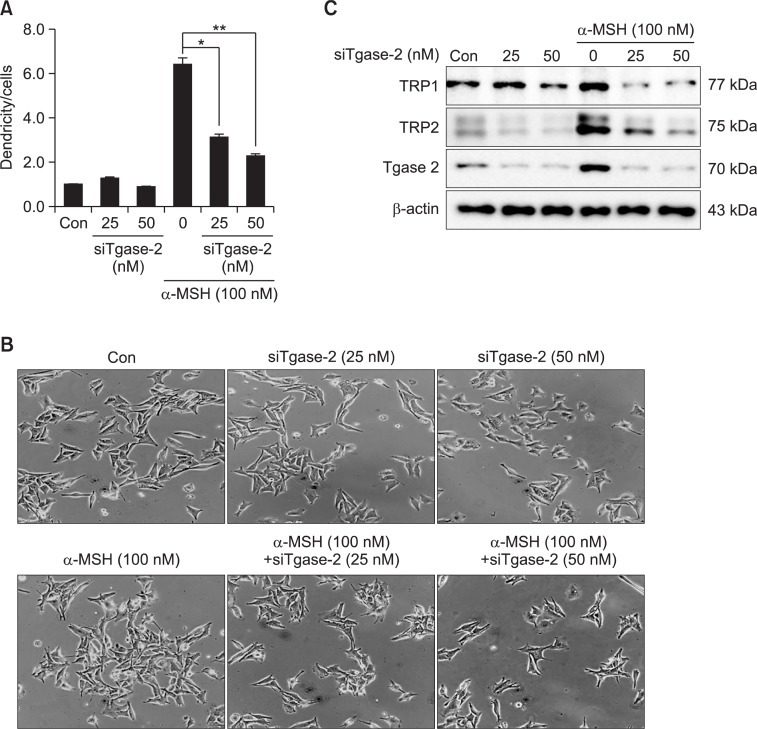
To confirm the involvement of Tgase-2 in α-MSH-induced melanogenesis of SK-MEL-2 cells, we also examined the effects of Tgase-2 gene silencing on dendrite extension and expressions of melanogenic proteins. As shown in Fig. 4A, gene silencing of Tgase-2 effectively suppressed the SK-MEL-2 cells’ α-MSH-induced dendrite extension (Fig. 4A, B). Gene silencing of Tgase-2, moreover, significantly inhibited α-MSH-induced expressions of TRP-1 and TRP-2 (Fig. 4C). These results indicate Tgase-2’s probable involvement in α-MSH-induced melanogenesis of SK-MEL-2 cells.
Fig. 4.
Effects of Tgase2 gene silencing on dendricity and MITF, TRP1, and TRP2 expressions of SK-MEL-2 cells. (A) Effect of Tgase2 gene silencing on dendrite extension of SK-MEL-2 cells. (B) Photograph of effect of Tgase2 gene silencing on dendrite extension of SK-MEL-2 cells. (C) Effect of Tgase-2 gene silencing on MITF, TRP1, and TRP2 expressions of SK-MEL-2 cells. In (A), and (B), the SK-MEL-2 cells were transfected with siRNA of Tgase-2 for 48 hrs. After 48 hrs of incubation, the cells were treated with α-MSH. Then, the cells with and without “dendricity” (i.e. the appearance of cell extensions as a function of time) were counted and photographed under Zeiss microscopy (20× magnification) using a Real-time image system. In (A), the average dendrite lengths (n=20) in the melanoma cells were measured using the Image tool program. In (C), whole-cell lysates (30 μg) were prepared, the protein level was subjected to 4–12% SDS-PAGE, and the expressions of several proteins were determined by Western blotting. *p<0.05, **p<0.01.
DISCUSSION
Skin pigmentation disorders including vitiligo and melasma can result from problems that arise in melanocyte proliferation, differentiation, melanogenesis, migration or dendricity (Lee et al., 2012b). Pigmentation modulation is important to aesthetics and quality of life. Regardless, the involved melanogenetic mechanism is still not fully understood.
Many intrinsic biological factors, including α-MSH and endothelin-1, are implicated in the pigmentation and tanning response to UV irradiation (Son et al., 2014). α-MSH binding to MC1R induced the up-regulation of TYR, TRP-1, and TRP-2 via the mediation of MITF (Lee and Noh, 2013).
In the present study, we showed that α-MSH increases the production of melanin in SK-MEL-2 cells and that CTM inhibits such α-MSH-induced melanin production (Fig. 1A). CTM’s anti-melanogenic activity also has been reported in other melanoma cell lines, namely MM96L and MM418c5 (Bolognia et al., 1995; Qiu et al., 2000). CTM is believed to be reduced to cysteamine via sequestering quinines (Qiu et al., 2000). Whereas such studies usually have focused on CTM’s involvement via the inhibition of TYR activity, we also examined the effects of CTM on expressions of melanogenesis-implicated proteins such as TRP-1, TRP-2, MITF and Tgase-2, as well as the dendricity and chemotactic migration of SK-MEL-2 cells (Fig. 2, 3). In the results, CTM at high dose did not show cytotoxic effects on SK-MEL-18 melanoma cells (Fig. 1B) (Bolognia et al., 1995). α-MSH induced dendrite formation in SK-MEL-2 cells, and CTM dose-dependently inhibited it (Fig. 1C). CTM suppressed a-MSH-induced induction of TRP-1, TRP-2, MITF and Tgase-2 (Fig. 2). However, we could not observe significant effects of CTM on TYR expression (data not shown). CTM inhibited a-MSH-induced migration of SKMEL-2 cells (Fig. 3).
CTM also is regarded as a Tgase inhibitor, though its specificity to Tgase-2 is low. Because Tgase-2 usually is involved in dendrite formation in neuronal cells (Tucholski et al., 2001), and given α-MSH’s induction of Tgase-2 expression (Fig. 2), we speculated that CTM’s effects on α-MSH-induced melanogenesis might be attributable to Tgase-2 inhibition. Consistent with our expectation, gene silencing of Tgase-2 suppressed α-MSH-induced dendrite formation (Fig. 4A, B). Tgase-2 gene silencing, furthermore, blocked α-MSH-induced TRP-1, TRP-2, MITF, and Tgase-2 expression (Fig. 4C). These results all pointed to Tgase-2’s involvement in SK-MEL-2 cell melanogenesis, though the specific mechanism remains unclear. However, its known involvement in the differentiation of several cell types, including chondorocytes and oligodendrocytes suggests that its role in melanogenesis might be via the differentiation of melanocytes (Van Strien et al., 2011; Niger et al., 2013). Recently, autophagy is involved in regulation of melanogenesis and Tgase-2 is involved in regulation of autophagy (Ozpolat et al., 2007; Ho and Ganesan, 2011). So Tgase-2-involvement in autophagy might be one of plausible explanations for its involvement in α-MSH-induced melanogenesis. However this speculation requires further studies. In summation, we showed both that Tgase-2 is involved in α-MSH-induced melanogenesis and that it is a promising target for its control.
Acknowledgments
This study was supported by grants from the GRRC program of Gyeonggi Province (GRRC Dongguk2013-B01).
REFERENCES
- Bolognia JL, Sodi SA, Osber MP, Pawelek JM. Enhancement of the depigmenting effect of hydroquinone by cystamine and buthionine sulfoximine. Br J Dermatol. 1995;133:349–357. doi: 10.1111/j.1365-2133.1995.tb02660.x. [DOI] [PubMed] [Google Scholar]
- Borrell-Pages M, Canals JM, Cordelieres FP, Parker JA, Pineda JR, Grange G, Bryson EA, Guillermier M, Hirsch E, Hantraye P, Cheetham ME, Neri C, Alberch J, Brouillet E, Saudou F, Humbert S. Cystamine and cysteamine increase brain levels of BDNF in Huntington disease via HSJ1b and transglutaminase. J Clin Invest. 2006;116:1410–1424. doi: 10.1172/JCI27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzoska T, Luger TA, Maaser C, Abels C, Bohm M. Alpha-melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocr Rev. 2008;29:581–602. doi: 10.1210/er.2007-0027. [DOI] [PubMed] [Google Scholar]
- Chang TS, Chen CT. Inhibitory effect of homochlorcyclizine on melanogenesis in alpha-melanocyte stimulating hormone-stimulated mouse B16 melanoma cells. Arch Pharm Res. 2012;35:119–127. doi: 10.1007/s12272-012-0113-z. [DOI] [PubMed] [Google Scholar]
- Fok JY, Ekmekcioglu S, Mehta K. Implications of tissue transglutaminase expression in malignant melanoma. Mol Cancer Ther. 2006;5:1493–1503. doi: 10.1158/1535-7163.MCT-06-0083. [DOI] [PubMed] [Google Scholar]
- Ho H, Ganesan AK. The pleiotropic roles of autophagy regulators in melanogenesis. Pigment Cell Melanoma Res. 2011;24:595–604. doi: 10.1111/j.1755-148X.2011.00889.x. [DOI] [PubMed] [Google Scholar]
- Kim NH, Lee CH, Lee AY. H19 RNA downregulation stimulated melanogenesis in melasma. Pigment Cell Melanoma Res. 2010;23:84–92. doi: 10.1111/j.1755-148X.2009.00659.x. [DOI] [PubMed] [Google Scholar]
- Lee AY, Noh M. The regulation of epidermal melano-genesis via cAMP and/or PKC signaling pathways: insights for the development of hypopigmenting agents. Arch Pharm Res. 2013;36:792–801. doi: 10.1007/s12272-013-0130-6. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Park MK, Bae HC, Yoon HJ, Kim SY, Lee CH. Transglutaminase-2 is involved in all-trans retinoic acid-induced invasion and matrix metalloproteinases expression of SH-SY5Y neuroblastoma cells via NF-kappaB pathway. Biomol Ther. 2012a;20:286–292. doi: 10.4062/biomolther.2012.20.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Park MK, Kim SY, Park Choo HY, Lee AY, Lee CH. Serotonin induces melanogenesis via serotonin receptor 2A. Br J Dermatol. 2011;165:1344–1348. doi: 10.1111/j.1365-2133.2011.10490.x. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Park MK, Lee EJ, Kim YL, Kim HJ, Kang JH, Kim HM, Lee AY, Lee CH. Histamine receptor 2-mediated growth-differentiation factor-15 expression is involved in histamine-induced melanogenesis. Int J Biochem Cell Biol. 2012b;44:2124–2128. doi: 10.1016/j.biocel.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Lim YJ, Lee EH, Kang TH, Ha SK, Oh MS, Kim SM, Yoon TJ, Kang C, Park JH, Kim SY. Inhibitory effects of arbutin on melanin biosynthesis of alpha-melanocyte stimulating hormone-induced hyperpigmentation in cultured brownish guinea pig skin tissues. Arch Pharm Res. 2009;32:367–373. doi: 10.1007/s12272-009-1309-8. [DOI] [PubMed] [Google Scholar]
- Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- Mhaouty-Kodja S. Ghalpha/tissue transglutaminase 2: an emerging G protein in signal transduction. Biol. Cell. 2004;96:363–367. doi: 10.1016/j.biolcel.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Niger C, Beazley KE, Nurminskaya M. Induction of chondrogenic differentiation in mesenchymal stem cells by TGF-beta cross-linked to collagen-PLLA [poly(L-lactic acid)] scaffold by transglutaminase 2. Biotechnol Lett. 2013;35:2193–2199. doi: 10.1007/s10529-013-1301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozpolat B, Akar U, Mehta K, Lopez-Berestein G. PKC delta and tissue transglutaminase are novel inhibitors of autophagy in pancreatic cancer cells. Autophagy. 2007;3:480–483. doi: 10.4161/auto.4349. [DOI] [PubMed] [Google Scholar]
- Park HY, Kosmadaki M, Yaar M, Gilchrest BA. Cellular mechanisms regulating human melanogenesis. Cell Mol Life Sci. 2009;66:1493–1506. doi: 10.1007/s00018-009-8703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plensdorf S, Martinez J. Common pigmentation disorders. Am. Fam. Physician. 2009;79:109–116. [PubMed] [Google Scholar]
- Qiu L, Zhang M, Sturm RA, Gardiner B, Tonks I, Kay G, Parsons PG. Inhibition of melanin synthesis by cystamine in human melanoma cells. J Invest Dermatol. 2000;114:21–27. doi: 10.1046/j.1523-1747.2000.00826.x. [DOI] [PubMed] [Google Scholar]
- Son J, Kim M, Jou I, Park KC, Kang HY. IFN-gamma Inhibits Basal and alpha-MSH-Induced Melanogenesis. Pigment Cell Melanoma Res. 2014;27:201–208. doi: 10.1111/pcmr.12190. [DOI] [PubMed] [Google Scholar]
- Tucholski J, Lesort M, Johnson GV. Tissue transglutaminase is essential for neurite outgrowth in human neuroblastoma SH-SY5Y cells. Neuroscience. 2001;102:481–491. doi: 10.1016/s0306-4522(00)00482-6. [DOI] [PubMed] [Google Scholar]
- Van Strien ME, Baron W, Bakker EN, Bauer J, Bol JG, Breve JJ, Binnekade R, Van Der Laarse WJ, Drukarch B, Van Dam AM. Tissue transglutaminase activity is involved in the differentiation of oligodendrocyte precursor cells into myelin-forming oligodendrocytes during CNS remyelination. Glia. 2011;59:1622–1634. doi: 10.1002/glia.21204. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Brenner M, Hearing VJ. The regulation of skin pigmentation. J Biol Chem. 2007;282:27557–27561. doi: 10.1074/jbc.R700026200. [DOI] [PubMed] [Google Scholar]
- Yang Y, Dickinson C, Haskell-Luevano C, Gantz I. Molecular basis for the interaction of [Nle4,D-Phe7]melanocyte stimulating hormone with the human melanocortin-1 receptor. J Biol Chem. 1997;272:23000–23010. doi: 10.1074/jbc.272.37.23000. [DOI] [PubMed] [Google Scholar]