Abstract
This study evaluated the pharmacokinetic profile and therapeutic efficacy of piroxicam (PX), a long acting non-steroidal anti-inflammatory drug for the treatment of arthritis, following intra-articular (IA) injection in comparison to the pharmacokinetic profile and therapeutic efficacy of PX after intramuscular (IM) injection. In the pharmacokinetic study in rats, systemic exposure and pharmacokinetic parameters of PX after a single IA dose were compared with systemic exposure and pharmacokinetic parameters of PX after administration of the same dose IM (0.6 mg/kg). The anti-inflammatory and analgesic effects of IA PX were evaluated simultaneously in a monoiodoacetate-induced osteoarthritis rat model. The plasma PX concentration rapidly rose following IA injection, and it was comparable to the plasma PX concentration following IM injection, suggesting the rapid efflux of the drug molecule from the joint cavity. However, in the efficacy study, the IA PX administration significantly reduced the knee swelling by reducing the level of prostaglandin E2 in the joint, compared to that following administration of IA vehicle and after administration of the IM PX dose. In addition, we found that the anti-inflammatory and anti-nociceptive efficacies of IA PX were synergistically increased upon co-treatment with hyaluronic acid (HA), a potent agent for the treatment of osteoarthritis, at the weight ratio of 1:1 or 1:2, and these effects were more pronounced than those following administration of HA or PX alone. In conclusion, this study demonstrated the efficacy of the IA use of PX alone and/or in combination with HA in osteoarthritis.
Keywords: Piroxicam, Intra-articular injection, Pharmacokinetics, Anti-inflammation, Hyaluronic acid, Osteoarthritis
INTRODUCTION
Osteoarthritis (OA) is the most common form of arthritis with an average incidence after the age of 65 years in about 60% of men and 70% of women (Sarzi-puttini et al., 2005; Blagojevic et al., 2010). This musculo-skeletal disease is characterized pathologically by deterioration and loss of the articular cartilage, subchondral sclerosis and osteophyte formation, and it is often accompanied by inflammation of the synovium and deterioration of the supporting structures of the joint (Haywood et al., 2003; Buckland-Wright, 2004). The currently available therapy is symptomatic treatment directed towards relieving the pain and regaining the physical function, including oral analgesics and non-steroidal anti-inflammatory drugs (NSAIDs) and intra-articular (IA) corticosteroid or hyaluronic acid (HA) injection (Pendleton et al., 2000; Moreland, 2003).
Among them, oral NSAIDs are considered as the standard treatment for OA. NSAIDs inhibit the production of arachidonic acid metabolites such as prostaglandins and thromboxanes, which mediate the inflammatory process. Piroxicam (PX; 4-hydroxy-2-methyl-N-2-pyridinyl-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide) is a member of the oxicam group of NSAIDs. Its long plasma half-life of approximately 2 days allows once-a-day dosing, and thus may lead to improved compliance, particularly in the elderly (Richardson et al., 1985). However, many patients, especially elderly patients, either cannot tolerate daily oral doses of NSAIDs such as PX, and/or suffer from NSAID-induced systemic side effects including gastroduodenal ulceration, bleeding and renal disorder (Hart and Huskisson, 1984; Laake et al., 1984; Deeks et al., 2002). HA, a high molecular weight polymer of glucosamine and glucuronic acid residues, is a principle constituent of normal synovial fluid and contributes significantly to its rheological properties and joint homeostasis. Intra-articular viscosupplementation with HA can improve activity and can reduce pain in patients with arthritis of the knee (Peyron, 1993; Altman and Moskowitz, 1998). But, HA is a slow-acting symptom-modifying agent, requiring a series of weekly injections and commonly prescribed with oral NSAIDs (most protocols require three or more injections) (Altman and Moskowitz, 1998).
Several clinical studies have demonstrated that IA administration of NSAIDs provides advantageous results with respect to the analgesic efficacy, by delivering higher drug concentration in local tissue (Unlu et al., 2006; Oztuna et al., 2007; Lee et al., 2011). The weekly IA tenoxicam-treated group showed more rapid pain relief than the daily oral treatment group of OA patients (Oztuna et al., 2007). Lee et al. (2011) also reported that a significant improvement, as demonstrated by pain assessment tools such as visual analog scale, pain rating scale, and Rubin’s scale, was observed in the ketorolac and HA combination-treated group as compared with the HA alone-treated group at the 5-week follow-up after IA injection.
However, a consensus on whether local infiltration with NSAIDs including PX, offers relevant pain and/or inflammation relief is still lacking in the treatment of OA. Some investigators have reported no analgesic effect or any benefit in terms of pain scores after the use of IA NSAIDs over placebo after knee surgery (Cook et al., 1997; Gupta et al., 1999). Therefore, this paper reports the results of a study undertaken to characterize the pharmacokinetic profile and therapeutic efficacy of PX after IA injection in comparison to the those of PX after administration of the same dose intramuscularly (IM), for the treatment of OA. The plasma concentration profile of PX was evaluated in normal rats, using a validated LC-MS/MS assay. Second, we evaluated whether treatment with IA PX was better than IM PX in terms of anti-inflammatory and anti-nociceptive effects, using a monoiodoacetate-induced OA rat model. Third, we further evaluated the anti-inflammatory and analgesic efficacies of IA PX combined with HA, an effective agent for the treatment of OA, versus each single drug, in a rat model of OA.
MATERIALS AND METHODS
Materials
Commercial PX injection (Rheoma® injection, Samsung Pharmaceuticals, 20 mg/ml as PX) for IA and IM use was obtained from the local hospital pharmacy. Sodium hyaluronate of microbial origin (molecular weight: 1500–2500 kDa) was purchased from Humedix Co., Ltd (Sungnam, Korea). Mo nosodium iodoacetate and isoxicam were purchased from Sigma-Aldrich (St Louis, MO, USA). Acetonitrile, methanol, formic acid, and deionized water were obtained from J. T. Baker (Phillipsburg, NJ, USA). All other chemicals used were of the highest commercial grade available.
Animals
Eight-week-old male Sprague-Dawley rats were obtained from Samtako (Kyungki-do, Korea). Rats were housed under conditions that included a controlled light cycle (light/dark: 12 h each) and controlled temperature (23 ± 1°C). Tap water and standard laboratory chow were available ad libitum. Rats were allowed to habituate themselves to the housing facilities for at least 3 days before agent treatments. All animal experiments were performed in accordance with the “Principles of Laboratory Animal Care”(NIH publication No.85-23, revised 1996), and were approved by the Committee for Animal Experiments of Dong-A Pharmaceutical (Seoul, Korea).
Pharmacokinetic study in rats
Experimental design: Rats were assigned to three groups by a stratified randomization scheme designed to achieve similar group mean body weights. One group received 0.6 mg/kg of PX intra-articularly by an insulin syringe (31 G) into the right knee. Rats in the second group were given the same dose by IM injection in the hind leg. Third group received PX/HA combination intra-articularly at a dose of 0.6 mg/kg each. After single administration of each drug, blood samples were collected up to 24 h post-dose for the pharmacokinetic evaluation of PX in plasma, using a fully validated method. In brief, blood samples were drawn from the jugular vein and collected in heparinized tubes. Plasma samples were then obtained by centrifuging the blood samples at 3,000 g for 10 min at 4°C in a microcentrifuge (Microfuge 22R Beckman Coulter, Fullerton, CA, USA). Plasma samples were analyzed for PX as described below.
Quantification of PX in rat plasma: An LC-MS/MS assay was developed to determine the concentrations of PX in rat plasma. A 50 μl aliquot of plasma was transferred into a glass tube, followed by the addition of 10 μl of isoxicam as an internal standard (100 μg/ml), and 200 μl of methanol containing 0.1% formic acid for protein precipitation. The mixture was vortexed for 30 s and then centrifuged at 3000 g for 5 min. The supernatant was subsequently injected into the LC-MS/MS system. An API 2000 mass spectrometer (Applied Biosystems, USA) with electrospray ionization (ESI) in positive ion mode for ion production was used for PX detection. Chromatography was performed on an XBridge C18 column (2.1 mm×100 mm, 5 μm, Waters, USA). The mobile phase consisted of 0.1% formic acid in water and 0.1% formic acid in acetonitrile (20:80, v/v) at a flow rate of 0.2 ml/min. The ion-spray potential was set at 5.5 kV, and the source temperature was 550°C. Multiple reaction monitoring (MRM) was performed using nitrogen as the collision gas. The analytes were detected by monitoring the transitions m/z 332.1→121.2 and 336.0→210.0, with collision energies of 30 and 30 eV for PX and isoxicam, respectively. The calibration equation was determined by least-squares linear regression (weighted 1/x) over the range 0.05010 μg/ml in plasma.
Calculation of pharmacokinetic parameters: The reported pharmacokinetic parameters (Tmax, Cmax and area under the curve (AUC)) were obtained using WinNonlin pharmacokinetic software (Version 6.1) (Pharsight, Inc., Mountain View, CA, USA), through a non-compartmental analysis.
Efficacy study in rats with experimentally induced OA
Induction of OA in rats: Monoiodoacetate (MIA)-induced arthritis model in rats was used (Fernihough et al., 2004). Under anesthesia with 10% chloral hydrate (4 ml/kg body weight), male SD rats were injected with 3 mg of MIA (30 μl in saline) into the right knee, and saline was injected in the sham group of rats. One day after the MIA injection, substantial inflammation of the synovial joints was observed in the model.
Drug treatment: Treatment was applied one day after the MIA injection and the time of treatment was defined as 24 h. At first, the therapeutic efficacy of PX after IA injection was compared to that of PX after IM dose. There were mainly four animal groups (n=8 per group) for drug treatments: (i) sham group injected with normal saline; (ii) MIA-treated group injected with IA vehicle; (iii) MIA-treated group injected with IA PX; and (iv) MIA-treated group injected with IM PX solution. PX solution was prepared by diluting the commercial product with distilled water at a concentration of 10 mg/ml. Drugs were administered using insulin syringe (31 G) at a dose of 0.6 mg/kg. All injection volumes were about 20 μl.
In order to assess the effect of PX and HA ratio on their anti-inflammatory and analgesic activities, individual agents or combinations of agents at a different weight ratios (PX:HA, 4:1, 2:1, 1:1, 1:2, and 1:4) were used. All MIA-induced OA rats were divided into 12 groups (n=8 per group), and they received vehicle solution; HA 0.3 mg/kg; PX 0.075 mg/kg, 0.15 mg/kg, 0.3 mg/kg, 0.6 mg/kg, 1.2 mg/kg, respectively; or PX/HA combination 0.075/0.3 mg/kg, 0.15/0.3 mg/kg, 0.3/0.3 mg/kg, 0.6/0.3 mg/kg, and 1.2/0.3 mg/kg, respectively, prior to being subjected to the test mentioned above.
Joint swelling measurement: Each formulation was administered 1 day after MIA treatment and its anti-inflammatory effect, as demonstrated by the reduction of knee swelling, was measured 2 days later with digital electronic calipers (Mitutoyo, UK), representing asymmetry of knee diameters (millimeters) between the ipsilateral and contralateral knee joints (Ashraf et al., 2011).
Prostaglandin E2 metabolite measurement: The level of prostaglandin E2 metabolite (PGEM) in the joint was measured by the modified method as described previously (Magari et al., 2003). In brief, the right knee of each rat was amputated using a bone cutter and stored at −70°C until use. The tissues were immersed in 5 ml of normal saline and then homogenized on ice by using a high speed homogenizer (T 25 Ultra turrax, Ika, Germany) and incubated on ice for 3 days. Supernatants were collected by centrifugation at 13,000 rpm for 10 min. The concentration of PGE2 was determined by EIA following the manual of the EIA kit (Cayman Chemical Company, 514531).
Pain assessment: OA was characterized by changes in weight distribution of each hind paw (Yoshimi et al., 2010). Thus, a hind limb weight-bearing apparatus (incapacitance tester; Linton Instrumentation, Norfolk, UK) was used to assess the difference in the distribution of weight between the right (osteoarthritic) and the left (contralateral control) hind limbs at 72 h (Yoshimi et al., 2010). Animals were placed into a Plexiglas chamber with each hind paw on the separate force plate, and were allowed to become accustomed to the apparatus. When stationary, the force exerted on the plate by each hind paw was recorded over a period of 5 s and expressed in grams. A total of four readings were taken for each rat at each time point, and the mean was used for calculation. The study was performed in an unblinded fashion, and % weight distribution of the right limb was calculated by the following formula: % weight of the right leg=100×[Right limb weight÷(left limb weight+right limb weight)].
Statistical analysis
Statistical significance was determined using Student’s t-test and was considered to be significant at p<0.05, unless otherwise indicated.
RESULTS
Bioanalytical method validation
The LC-MS/MS method for the quantitation of the NSAID in rat plasma met all of the validation criteria per the FDA Guidance. Retention times for PX and isoxicam were 1.74 min and 1.81 min, respectively. The mean recovery of PX and IS from rat plasma was 99.6% and 96.5%, respectively. The assay was linear over the range 50.0–10,000 ng/ml for the NSAID and the calibration curve could be described by the equation: y=1.01χ+0.0175 (r2=0.9996). The lower limit of quantification was 50 ng/ml with a relative standard deviation of 5.3%. The intra-assay precision ranged between 3.7% and 7.1% coefficient of variation (CV) with an accuracy of −1.8–9.5% relative error (RE), over the range 100–8,000 ng/ml (Table 1). The inter-assay precision was below 10.1% CV and the accuracy was below 10.3% RE. Organic extracts were stable at room temperature for at least 48 h. Plasma samples were stable for at least 6 months at −80°C and also after three freeze-thaw cycles. The results indicated that the analyte was stable under any of the storage conditions described above and that no stability-related problems would be expected during the routine analysis of samples for the pharmacokinetic studies.
Table 1.
Accuracy and precision of the LC-MS/MS analysis for PX in rat plasma
| Conc. (μg/ml) | Accuracy (%)a
|
Precision (%)b
|
||
|---|---|---|---|---|
| Intra-day | Inter-day | Intra-day | Inter-day | |
| 0.05 | 90.5 | 103.4 | 5.3 | 10.1 |
| 0.1 | 92.7 | 95.8 | 7.1 | 5.8 |
| 0.8 | 93.7 | 89.7 | 3.9 | 5.2 |
| 8.0 | 101.8 | 98.9 | 3.7 | 3.5 |
Expressed as mean measured concentrations /nominal concentrations×100.
Expressed as the relative standard deviation.
Pharmacokinetic evaluation in rats
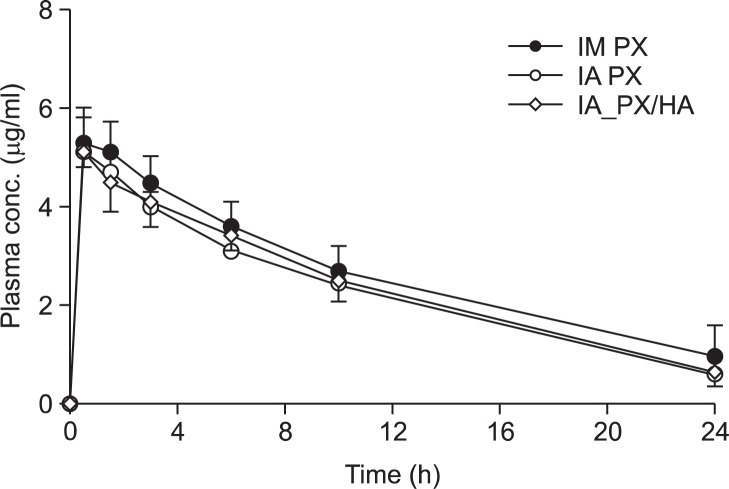
Fig. 1 shows the plasma concentration of PX versus time profiles after IM and IA administration in rats. The corresponding exposure data in terms of Cmax, Tmax, and AUC of PX are given in Table 2. The plasma PX concentration rapidly rose and peaked at 0.5 h following IA and IM injection, regardless of the route of injection, and it was eliminated from the circulation with the t1/2 values between 6 and 9. Accordingly, there were no significant differences in the Tmax, Cmax, and AUC values between the IA PX and IM PX, suggesting the rapid efflux of the drug molecule from the joint cavity after IA injection. The relative bioavailability of PX after IA injection, calculated as percentage of the AUC(0–24h) to IM PX, was about 85%, with no significance (p>0.05).
Fig. 1.
Mean (± S.D.) plasma concentration-time profile of PX after IM injection of PX and IA injection of either PX or PX/HA combination.
Table 2.
Comparison between the mean pharmacokinetic parameters of PX after IM or IA injection of PX alone or after IA injection of PX/HA combination in rats
| IM PX | IA PX | IA PX/HA | |
|---|---|---|---|
| AUC(0–24h) (μg•h/ml)a | 69.5 ± 22.4 | 59.0 ± 12.1 | 62.5 ± 11.5 |
| AUC(0-∞) (μg•h/ml)a | 70.8 ±22.7 | 60.1 ± 13.1 | 64.4 ± 12.6 |
| Cmax (μg/ml)a | 5.3 ± 0.7 | 5.1 ± 0.7 | 5.1 ± 0.3 |
| Tmax (h)a | 0.80 ± 0.48 | 0.90 ± 0.52 | 1.10 ± 0.52 |
| t1/2 (h)a | 7.3 ± 1.4 | 8.1 ± 0.9 | 9.0 ± 0.8 |
| Relative BAb | - | 84.9 | 89.9 |
Data are expressed as mean ± S.D.
Calculated as percentage of the mean AUC(0–24h) of each group to that of IM PX-treated group.
Note: There were no statistically significant differences (p>0.05) between the groups in all parameters.
The time course versus plasma PX concentrations after IA administration of either single agent or in combination with HA were further evaluated (Fig. 1). After single administration of PX in the presence/absence of HA, the plasma concentrations of PX, when compared at each time point, were statistically identical (Fig. 1). PX was rapidly absorbed from the joint, yielding a Cmax of 5.1 μg/ml at approximately 0.5 h (Tmax) after dosing. The clearance of the therapeutic agent from the blood in rats was not altered by the natural polymer. This finding is supported by the observation that the calculated AUC value for PX after administration of PX/HA combination was identical to that after administration of PX alone (Table 2).
Efficacy study in rats with experimentally induced OA
Therapeutic efficacy of PX after IA and IM administration: The anti-inflammatory effect of PX on the joint swelling is shown in Fig. 2A. IA administration of MIA (3 mg/30 μl) in the right knee produced a significant knee joint swelling 1 day later, with an approximately 2 mm increase in the right knee joint diameter compared with that in the sham group (p<0.05). The diameter of the knee joint was only minimally decreased (about 15%) for a further 72 h in the vehicle-treated group. In contrast, both IA and IM injections of PX solution resulted in marked improvements, causing about 63% and 46% decrease in knee joint swelling, respectively, compared to that in the vehicle-treated group (Fig. 2A). In particular, the IA PX-treated group exhibited the greatest reduction in knee joint swelling, more than 63% after 2 days of dosing, showing a significant difference from the IM PX-treated group (p<0.05).
Fig. 2.
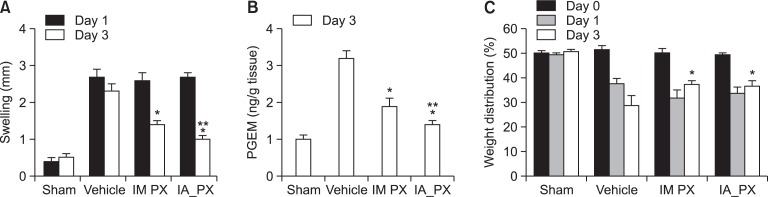
Changes in knee swelling (A), the level of PGEM in the joint tissue (B), and weight distribution of each hind paw (C) in MIA-induced OA rats after administration of PX via different routes: sham group injected with normal saline (sham); MIA-treated group injected with vehicle intraarticularly (vehicle); MIA-treated group injected with PX intramuscularly (IM PX); and MIA-treated group injected with PX intraarticularly (IA PX). A, the anti-inflammatory effect, as demonstrated by reduction of the knee swelling, was determined with calipers at 2 days after dosing and calculated based on the difference in diameter between the right and left knee. B, PGEM concentration in the joint was measured using EIA kit. C, the analgesic effects were investigated by measuring weight imbalance. Weight distribution (%) of the right limb was calculated by the following formula: % weight of the right leg=100×[Right limb weight÷(left limb weight+right limb weight)]. Bar represents S.E. (n=8), and statistical analysis was performed using the Student’s t-test; *p<0.05 versus vehicle-treated group; **p<0.05 versus IM PX-treated group).
The level of PGEM in the joint tissue was determined as the anti-nociceptive and anti-inflammatory effects of NSAIDs are understood to be caused by the decrease in PGE2 in the inflamed tissue. Because PGE2 produced in the synovial fluid is rapidly converted in vivo to its more stable metabolites, an estimate of the amount released was quantified (Cialdai et al., 2009). Single IA or IM administration of PX (0.6 mg/kg) strongly reduced the level of PGEM in the joint by 39% and 56%, respectively compared to that in the vehicle-treated group (Fig. 2B). The IA PX-treated group showed a remarkably greater reduction in the enzyme level compared to the IM PX-treated group (p<0.05).
We further assessed the weight distribution of the injured hind paw and normal paw as a parameter for estimating the analgesic effect of PX. The change in % weight distribution of the right hind paw during the administration period is shown in Fig. 2C. Weight-bearing asymmetry in the experimental rat OA model was thought to be due to the pain induced by destruction of cartilage (Mihara et al., 2007). The rats in the MIA-injected groups showed weight-bearing asymmetry and the values were between 32-37%, whereas the rats in the sham group with no MIA injection showed weight-bearing symmetry, with a value of about 50%. In the vehicle group, there was gradual decrease in weight distribution of the right hind paw over the entire period, reaching 28% at 72 h after MIA injection. Compared to the vehicle group, both IM and IA PX-treated groups showed greater values at 48 h after drug injection (about 37%) (p<0.05), suggesting an analgesic effect. There were no significant differences in the analgesic effect between the two groups.
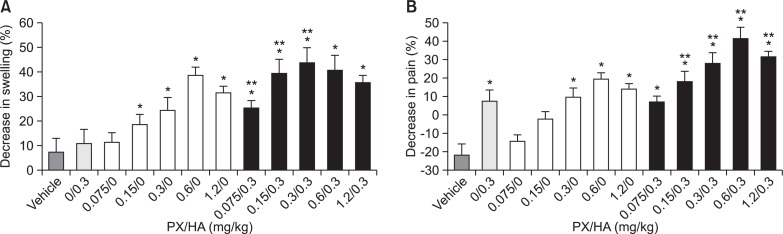
Therapeutic efficacy of PX combined with HA after IA administration: The anti-inflammatory and analgesic efficacies of combined IA PX and HA versus each single drug were evaluated at the PX to HA ratio ranging from 4:1 to 1:4 (Fig. 3). Synergistic anti-inflammatory activity was observed, as indicated by the joint swelling, at HA:PX weight ratios of 4:1, 2:1 and 1:1; while synergistic anti-nociceptive effect was observed at molar ratios of 2:1, 1:1, 1:2, and 1:4. Especially, combination of HA with PX at the ratio of 1:1 and 2:1 resulted in a clear amelioration in both inflammation and pain related parameters, and this effect was more pronounced than that after the administration of HA or PX alone.
Fig. 3.
Decrease (%) in knee swelling (A), and pain (%) in each hind paw (B) in MIA-induced OA rats after IA administration of PX/HA at different weight ratios. A, % decrease in knee swelling at 2 days after dosing versus prior to drug injection. B, % suppression of weight distribution imbalance at 2 days after dosing. Bar represents S.E. (n=8), and statistical analysis was performed using the Student’s t-test (*p<0.05 versus vehicle-treated group; **p<0.05 versus greater than the sum of the values produced by each drug at the same dose).
DISCUSSION
It has been reported that delivering an NSAID at the site of injury might provide more profound pain relief compared with that after less targeted systemic administration. Several studies comparing IA NSAIDs with systemic administration reported better pain relief after IA administration, indicating a peripheral analgesic effect in OA and/or in postoperative pain in the knee (Elhakim et al., 1996; Colbert et al., 1999; Unlu et al., 2006). When administered locally, high concentrations of NSAIDs can be achieved at the site of cell injury, and local administration may lead to clinical benefits such as the use of lower doses, lower subsequent systemic exposure, and a reduced frequency of adverse events. In contrast, Caruso et al. (1964) reported that after IA injection of indomethacin, a member of NSAIDs family, in patients with rheumatoid arthritis, the local pain was only slightly alleviated and the functional capacity was modestly improved, due to rapid disappearance of the drug from the joint cavity. In order to provide a sound basis for preclinical efficacy of the IA use of PX alone and/or in combination with HA, preclinical pharmacokinetic and efficacy studies were conducted.
It is now obvious that the rate and extent of systemic absorption of PX following IA injection was rapid and substantial. The finding of rapid efflux of the drug from the joint is consistent with that in an earlier report, which documented that the NSAIDs paracetamol, salicylate, and diclofenac had mean terminal half-lives of 1.1, 2.4, and 5.2 h, respectively in the synovial fluid from patients with knee rheumatoid arthritis (Owen et al., 1994). These short half-lives of IA administered drugs including PX in the joint tissue can be explained by the loose structure of the synovium, which offers little barrier to the diffusion of molecules in and out of the joint. Knight and Levick (1984) demonstrated that the synovial surface consists of a discontinuous layer of synoviocytes with extensive intercellular gaps ranging from 0.1 to 5.5 μm. As a consequence of the synovial miscellaneous arrangement, there is free transsynovial flux of water, solutes, and even small molecules (MW <10,000 Da) such as NSAIDs (Okuyama and Aihara, 1984; Gerwin et al., 2006). However, in spite of the rapid disappearance of PX from the joint after IA injection, it is worth noting that the systemic bioavailability of PX after IA administration was relatively lower, at an average of 85%, compared to that after IM injection, indicating that the IA route provided a higher PX concentration in the local tissue.
In our study, an MIA model was used to evaluate the pre-clinical efficacy of IA PX formulation in treating OA pain and swelling in comparison with each single drug. The injection of iodoacetate induces the loss of cartilage proteoglycans, followed by severe thinning of the cartilage and the development of lesions in the region of the subchondral bone and calcified cartilage consisting of fibrous tissue, infiltrating mononuclear cells and blood vessels (Williams and Thonar, 1989; Janusz et al., 2001). Morphological characteristics and response to conventional analgesics suggest similarities between the MIA model and OA patients.
In the MIA-induced OA model, administration of IA PX caused a substantial amelioration in inflammation symptoms and relevant prostaglandin level in the joint, which was even more pronounced compared to that after administration of IM PX injection. An IA PX administration significantly relieved the knee swelling and/or pain, by reducing the level of PGE2 by approximately 39% compared to that after vehicle treatment. It is assumed that following local administration of the NSAID, high concentrations of the drug were achieved in the inflamed synovium as proven by the pharmacokinetic study, with potential for a more effective reduction of inflammation and/or pain. This finding is supported by the earlier report, which suggests that NSAIDs delivered via IA injection effectively inhibit the activation of peripheral nociceptors, by reducing the levels of arachidonic acid metabolites such as prostaglandins and thromboxanes in the joint tissue (Izdes et al., 2003). NSAIDs injected intraarticularly may also reduce the pain by modifying the local inflammatory process (Unlu et al., 2006). Actually, Oztuna et al. (2007) demonstrated that the weekly IA administration of tenoxicam provided more rapid and profound pain relief compared to the daily oral administration in OA patients. Further investigations on the elimination half-life of an NSAID in the inflammatory exudate and the correlation between exposure pattern and enzyme inhibition are needed for gaining a more comprehensive understanding.
The relationship between the PX:HA ratio and the anti-inflammatory and analgesic effects was further assessed in rats with experimentally induced OA. The pharmacological mechanisms of HA are not clear, but IA HA supplementation was reported to prevent IL-1-induced proteoglycan release from cultured chondrocytes and cartilage explants, stimulate proteoglycan synthesis in chondrocytes, enhance chondrocyte proliferation, and enhance collagen synthesis in animal models of experimentally induced OA (Larsen et al., 1992; Morris et al., 1992; Shimazu et al., 1993; Sonoda et al., 1997; Frean et al., 1999; Kang et al., 1999; Kawasaki et al., 1999). Our preclinical study clearly demonstrated that both the long-acting NSAID and HA are effective in treating OA via two distinctive mechanisms that involve a synergistic effect of intraarticular co-administration. Especially, the PX:HA ratio of 1:1 or 1:2 showed outstanding synergistic anti-inflammatory and anti-nociceptive effects. The finding of the synergistic activity of two agents is supported by an earlier clinical report, which demonstrated that a significant improvement in pain, as indicated by the visual analog scale and Rubin’s scale, was achieved in the IA NSAID/HA-treated group as compared with the HA alone-treated group (Lee et al., 2011). Furthermore, Hashizume and Mihara (2009) found that HA inhibited NSAID-accelerated matrix metalloproteinase production, which was followed by inflammatory cytokine production from cytokine-activated chondrocytes. From these findings, we concluded that the use of NSAIDs in combination with HA, which have complementary mechanisms of action, could potentially provide synergistic efficacy in the treatment of OA.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2013R1A1A1058935).
REFERENCES
- Altman RD, Moskowitz R. Intra-articular sodium hyaluronate (Hyalgan) in the treatment of patients with osteoarthritis of the knee: a randomized clinical trial. J Rheumatol. 1998;25:2203–2212. [PubMed] [Google Scholar]
- Ashraf S, Mapp PI, Walsh DA. Contributions of angio-genesis to inflammation, joint damage, and pain in a rat model of osteoarthritis. Arthritis Rheum. 2011;63:2700–2710. doi: 10.1002/art.30422. [DOI] [PubMed] [Google Scholar]
- Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2010;18:24–33. doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Buckland-Wright C. Subchondral bone changes in hand and knee osteoarthritis detected by radiography. Osteoarthritis Cartilage. 2004;18(Suppla A):S10–S19. doi: 10.1016/j.joca.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Caruso I, Forcella E, Marcazzan E. Behavior of the plasma level of indomethacin. Reumatismo. 1964;16:393–403. [PubMed] [Google Scholar]
- Cialdai C, Giuliani S, Valenti C, Tramontana M, Maggi CA. Effect of Intra-articular 4-(S)-amino-5-(4-{4-[2,4-dichloro-3-(2,4-dimethyl-8-quinolyloxymethyl)phenylsulfonamido]-tetrahydro-2H-4-pyranylcarbonyl} piperazino)-5-oxopentyl](trimethyl)ammonium chloride hydrochloride (MEN16132), a kinin B2 receptor antagonist, on nociceptive response in monosodium iodoacetate-induced experimental osteoarthritis in rats. J Pharmacol Exp Ther. 2009;331:1025–1032. doi: 10.1124/jpet.109.159657. [DOI] [PubMed] [Google Scholar]
- Colbert ST, Curran E, O'Hanlon DM, Moran R, McCarroll M. Intra-articular tenoxicam improves postoperative analgesia in knee arthroscopy. Can J Anaesth. 1999;46:653–657. doi: 10.1007/BF03013953. [DOI] [PubMed] [Google Scholar]
- Cook TM, Tucker JP, Nolan JP. Analgesia after day-case knee arthroscopy: double-blind study of intra-articular tenoxicam, intra-articular bupivacaine and placebo. Br J Anaesth. 1997;78:163–168. doi: 10.1093/bja/78.2.163. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Smith LA, Bradley MD. Efficacy, tolerability, and upper gastrointestinal safety of celecoxib for treatment of osteoarthritis and rheumatoid arthritis: systematic review of randomised controlled trials. BMJ. 2002;325:619. doi: 10.1136/bmj.325.7365.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhakim M, Fathy A, Elkott M, Said MM. Intra-articular tenoxicam relieves post-arthroscopy pain. Acta Anaesthesiol Scand. 1996;40:1223–1226. doi: 10.1111/j.1399-6576.1996.tb05554.x. [DOI] [PubMed] [Google Scholar]
- Fernihough J, Gentry C, Malcangio M, Fox A, Rediske J, Pellas T, Kidd B, Bevan S, Winter J. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain. 2004;112:83–93. doi: 10.1016/j.pain.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Frean SP, Abraham LA, Lees P. In vitro stimulation of equine articular cartilage proteoglycan synthesis by hyaluronan and carprofen. Res Vet Sci. 1999;67:183–190. doi: 10.1053/rvsc.1999.0328. [DOI] [PubMed] [Google Scholar]
- Gerwin N, Hops C, Lucke A. Intraarticular drug delivery in osteoarthritis. Adv Drug Deliv Rev. 2006;58:226–242. doi: 10.1016/j.addr.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Gupta A, Axelsson K, Allvin R, Liszka-Hackzell J, Rawal N, Althoff B, Augustini BG. Postoperative pain following knee arthroscopy:the effects of intra-articular ketorolac and/or morphine. Reg Anesth Pain Med. 1999;24:225–230. doi: 10.1016/s1098-7339(99)90132-3. [DOI] [PubMed] [Google Scholar]
- Hart FD, Huskisson EC. Non-steroidal anti-inflammatory drugs: Current status and rational therapeutic use. Drugs. 1984;27:232–255. doi: 10.2165/00003495-198427030-00004. [DOI] [PubMed] [Google Scholar]
- Hashizume M, Mihara M. Desirable effect of combination therapy with high molecular weight hyaluronate and NSAIDs on MMP production. Osteoarthritis Cartilage. 2009;17:1513–1518. doi: 10.1016/j.joca.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Haywood L, McWilliams DF, Pearson CI, Gill SE, Ganesan A, Wilson D, Walsh DA. Inflammation and angiogenesis in osteoarthritis. Arthritis Rheum. 2003;48:2173–2177. doi: 10.1002/art.11094. [DOI] [PubMed] [Google Scholar]
- Izdes S, Orhun S, Turanli S, Erkilic E, Kanbak O. The effects of preoperative inflammation on the analgesic efficacy of intraarticular piroxicam for outpatient knee arthroscopy. Anesth Analg. 2003;97:1016–1019. doi: 10.1213/01.ANE.0000081725.81280.17. [DOI] [PubMed] [Google Scholar]
- Janusz MJ, Hookfin EB, Heitmeyer SA, Woessner JF, Freemont AJ, Hoyland JA, Brown KK, Hsieh LC, Almstead NG, De B, Natchus MG, Pikul S, Taiwo YO. Moderation of iodoacetate-induced experimental osteoarthritis in rats by matrix metalloproteinase inhibitors. Osteoarthritis Cartilage. 2001;9:751–760. doi: 10.1053/joca.2001.0472. [DOI] [PubMed] [Google Scholar]
- Kang Y, Eger W, Koepp H, Williams JM, Kuettner KE, Homandberg GA. Hyaluronan suppresses fibronectin fragment-mediated damage to human cartilage explants cultures by enhancing proteoglycan synthesis. J. Orthop. Res. 1999;17:858–869. doi: 10.1002/jor.1100170611. [DOI] [PubMed] [Google Scholar]
- Kawasaki K, Ochi M, Uchio Y, Adachi N, Matsusaki M. Hyaluronic acid enhances proliferation and chondroitin sulfate synthesis in cultured chondrocytes embedded in collagen gels. J Cell Physiol. 1999;179:142–148. doi: 10.1002/(SICI)1097-4652(199905)179:2<142::AID-JCP4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Knight AD, Levick JR. Morphometry of the ultrastructure of the blood-joint barrier in the rabbit knee. Q J Exp Physiol. 1984;69:271–288. doi: 10.1113/expphysiol.1984.sp002805. [DOI] [PubMed] [Google Scholar]
- Laake K, Kjeldaas L, Borchgrevink CF. Side-effects of piroxicam (Feldenee). A one-year material of 103 reports from Norway. Acta Med Scand. 1984;215:81–83. [PubMed] [Google Scholar]
- Larsen NE, Lombard KM, Parent EG, Balazs EA. Effect of hylan on cartilage and chondrocyte cultures. J Orthop Res. 1992;10:23–32. doi: 10.1002/jor.1100100104. [DOI] [PubMed] [Google Scholar]
- Lee SC, Rha DW, Chang WH. Rapid analgesic onset of intra-articular hyaluronic acid with ketorolac in osteoarthritis of the knee. J Back Musculoskelet Rehabil. 2011;24:31–38. doi: 10.3233/BMR-2011-0272. [DOI] [PubMed] [Google Scholar]
- Magari K, Miyata S, Ohkubo Y, Mutoh S, Goto T. Calcineurin inhibitors exert rapid reduction of inflammatory pain in rat adjuvant-induced arthritis. Br J Pharmacol. 2003;139:927–934. doi: 10.1038/sj.bjp.0705310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara M, Higo S, Uchiyama Y, Tanabe K, Saito K. Different effects of high molecular weight sodium hyaluronate and NSAID on the progression of the cartilage degeneration in rabbit OA model. Osteoarthritis Cartilage. 2007;15:543–549. doi: 10.1016/j.joca.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther. 2003;5:54–67. doi: 10.1186/ar623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris EA, Wilcon S, Treadwell BV. Inhibition of interleukin 1-mediated proteoglycan degradation in bovine articular cartilage explants by addition of sodium hyaluronate. Am J Vet Res. 1992;53:1977–1982. [PubMed] [Google Scholar]
- Okuyama S, Aihara H. The mode of action of analgesic drugs in adjuvant arthritic rats as an experimental model of chronic inflammatory pain: possible central analgesic action of acidic nonsteroidal antiinflammatory drugs. Jpn J Pharmacol. 1984;35:95–103. doi: 10.1254/jjp.35.95. [DOI] [PubMed] [Google Scholar]
- Owen SG, Francis HW, Roberts MS. Disappearance kinetics of solutes from synovial fluid after intra-articular injection. Br J Clin Pharmacol. 1994;38:349–355. doi: 10.1111/j.1365-2125.1994.tb04365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztuna V, Eskandari M, Bugdayci R, Kuyurtar F. Intraarticular injection of tenoxicam in osteoarthritic knee joints with effusion. Orthopedics. 2007;30:1039–1042. doi: 10.3928/01477447-20071201-07. [DOI] [PubMed] [Google Scholar]
- Pendleton A, Arden N, Dougados M, Doherty M, Bannwarth B, Bijlsma JWJ, Cluzeau F, Cooper C, Dieppe PA, GHYPERL KP, Hauselmann HJ, Herrero-Beaumont G, Kaklamanis PM, Leeb B, Lequesne M, Lohmander S, Mazieres B, Mola EM, Pavelka K, Serni U, Swoboda B, Verbruggen AA, Weseloh G, Zimmermann-Gorska I. EULAR recommendations for the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT) Ann Rheum Dis. 2000;59:936–944. doi: 10.1136/ard.59.12.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron JG. Intrarticular hyaluronan injections in the treatment of osteoarthritis: state of the art review. J Rheumatol Suppl. 1993;39:10–15. [PubMed] [Google Scholar]
- Richardson CJ, Blocka KL, Ross SG, Verbeeck RK. Effects of age and sex on piroxicam disposition. Clin Pharmacol Ther. 1985;37:13–18. doi: 10.1038/clpt.1985.4. [DOI] [PubMed] [Google Scholar]
- Sarzi-puttini P, Cimmino MA, Scarpa R, Caporali R, Parazzin F, Zaninelli A, Atzeni F, Canesi B. Osteoarthritis: an overview of the disease and its treatment strategies. Semin Arthritis Rheum. 2005;35:1–10. doi: 10.1016/j.semarthrit.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Shimazu A, Jikko A, Iwamoto M, Koike T, Yan W, Okada Y, Shinmei M, Nakamura S, Kato Y. Effects of hyaluronic acid on the release of proteoglycan from the cell matrix in rabbit chondrocyte cultures in the presence and absence of cytokines. Arthritis Rheum. 1993;36:247–253. doi: 10.1002/art.1780360217. [DOI] [PubMed] [Google Scholar]
- Sonoda M, Harwood FL, Wada Y, Moriya H, Amiel D. The effects of hyaluronan on the meniscus and on the articular cartilage after partial meniscectomy. Am J Sports Med. 1997;25:755–762. doi: 10.1177/036354659702500606. [DOI] [PubMed] [Google Scholar]
- Unlu Z, Ay K, Tuzun C. Comparison of intra-articular tenoxicam and oral tenoxicam for pain and physical functioning in osteoarthritis of the knee. Clin Rheumatol. 2006;25:54–61. doi: 10.1007/s10067-005-1136-3. [DOI] [PubMed] [Google Scholar]
- Williams JA, Thonar EJM. Early osteophyte formation after chemically induced articular cartilage injury. Am J Sports Med. 1989;17:7–15. doi: 10.1177/036354658901700102. [DOI] [PubMed] [Google Scholar]
- Yoshimi E, Kumakura F, Hatori C, Hamachi E, Iwashita A, Ishii N, Terasawa T, Shimizu Y, Takeshita N. Antinociceptive effects of AS1892802, a novel rho kinase inhibitor, in rat models of inflammatory and noninflammatory arthritis. J Pharmacol Exp Ther. 2010;334:955–963. doi: 10.1124/jpet.110.167924. [DOI] [PubMed] [Google Scholar]