Abstract
Patients with cutis laxa (CL) have wrinkled, sagging skin with decreased elasticity. Skin symptoms are associated with variable systemic involvement. The most common, genetically highly heterogeneous form of autosomal recessive CL, ARCL2, is frequently associated with variable metabolic and neurological symptoms. Progeroid symptoms, dysmorphic features, hypotonia and psychomotor retardation are highly overlapping in the early phase of these disorders. This makes the genetic diagnosis often challenging. In search for discriminatory symptoms, we prospectively evaluated clinical, neurologic, metabolic and genetic features in our patient cohort referred for suspected ARCL. From a cohort of 26 children, we confirmed mutations in genes associated with ARCL in 16 children (14 probands), including 12 novel mutations. Abnormal glycosylation and gyration abnormalities were mostly, but not always associated with ATP6V0A2 mutations. Epilepsy was most common in ATP6V0A2 defects. Corpus callosum dysgenesis was associated with PYCR1 and ALDH18A1 mutations. Dystonic posturing was discriminatory for PYCR1 and ALDH18A1 defects. Metabolic markers of mitochondrial dysfunction were found in one patient with PYCR1 mutations. So far unreported white matter abnormalities were found associated with GORAB and RIN2 mutations. We describe a large cohort of CL patients with neurologic involvement. Migration defects and corpus callosum hypoplasia were not always diagnostic for a specific genetic defect in CL. All patients with ATP6V0A2 defects had abnormal glycosylation. To conclude, central nervous system and metabolic abnormalities were discriminatory in this genetically heterogeneous group, although not always diagnostic for a certain genetic defect in CL.
Keywords: cutis laxa, metabolic, inborn errors of glycosylation, mitochondrial function, neurologic, mutation
INTRODUCTION
In inherited cutis laxa (CL), patients have progeroid, inelastic, sagging skin, associated with abnormal structure or function of elastic fibers. These abnormalities are either because of direct structural defects, or abnormal maturation and secretion of the involved proteins. The skin symptoms are associated with variable systemic involvement. The underlying pathomechanism of this multisystem involvement is not fully understood.
CL syndromes show two major phenotypes: syndromes with severe multiorgan involvement and syndromes with central nervous system (CNS) involvement and growth delay.1, 2 CL has an estimated incidence of 1:2–400 000, and shows X-linked, autosomal dominant and autosomal recessive inheritance.2 Only around 100 cases have been described with a known mutation, and in most patients the underlying genetic defect has not been discovered yet. Diagnosis based on clinical features is highly challenging because of significant phenotypic overlap.
X-linked CL (XLCL or occipital horn syndrome, OHS, MIM 304150) is clinically distinct from the other CL syndromes because of early presentation, occipital horns and variable CNS involvement. XLCL is caused by mutations in ATP7A, a copper transporter gene.3, 4 Diagnosis can be made based on clinical features, low serum levels of copper and ceruloplasmin and mutation analysis.3, 4
In autosomal dominant CL (MIM 123700), the family history, the appearance of skin signs and organ involvement is variable. Mental development is normal. Some patients carry mutations in the elastin (ELN) or fibulin 5 (FBLN5) gene.5, 6 Autosomal recessive CL (ARCL) is the most prevalent and variable form of inherited neonatal CL. ARCL is divided in three major groups: type 1, 2 and 3 (ARCL1, MIM 219100; ARCL2A, MIM 219200; ARCL2B, MIM 612940; WSS, MIM 278250; ARCL3A, MIM 219150; ARCL 3B, MIM 614438; geroderma osteodysplasticum (GO), MIM 231070; De Barsy Syndrome (DBS), MIM 219150; macrocephaly, alopecia, CL, scoliosis syndrome (MACS), MIM 613075). ARCL1 is a life-threatening disease with generalized neonatal CL and severe systemic, mainly cardiopulmonary involvement because of mutations in FBLN4 (EFEMP2), FBLN5 or LTBP4.7, 8, 9, 10, 11
ARCL2 is a group of syndromes due to variable gene defects, resulting in overlapping phenotypes without major cardiopulmonary symptoms, but with frequent CNS involvement. ARCL2A (including ATP6V0A2-CDG, Debré-type CL and wrinkly skin syndrome) is caused by mutations in the ATP6V0A2 gene.2, 12, 13 Generalized CL is already present at birth and intriguingly improves over time. Patients have suggestive facial features with hanging eyelids, down-slanting palpebral fissures, muscle hypotonia, epilepsy and mild-to-moderate developmental delay.2, 12, 14 The combination of the biochemical marker of abnormal protein glycosylation (congenital disorder of glycosylation (CDGII)) and cobblestone-like brain dysgenesis in ARCL2 is pathognomonic for ARCL2A.2, 15, 16 The recently defined ARCL2B is caused by mutations in the PYCR1 gene, involved in de novo mitochondrial proline synthesis, leading to a progeroid appearance, parchment-like skin, triangular face, joint luxations, muscle hypotonia, dystonic posturing and developmental delay.17, 18 Some patients have normal intelligence.19, 20 Corpus callosum dysgenesis is common in ARCL2B.18 The similar and metabolically related P5CS deficiency (P5CSD) is caused by mutations in ALDH18A1.21, 22
Patients with DBS, or ARCL3, have CL, growth retardation, corneal clouding, athetoid movements and developmental delay.23 Some children with a clinical diagnosis of DBS also carry mutations in the PYCR1 or ALDH18A1 genes.18, 24
GO, caused by GORAB mutations is another ARCL involving the Golgi apparatus function. Frequent bone fractures and joint luxations are characteristic, however, intellectual disability is unusual.25 MACS syndrome, caused by mutations in the RIN2 gene is a disorder with very characteristic, discriminatory facial features with sagging chin and severe developmental delay.26, 27, 28
Several other syndromes are described in which CL is an associated feature. Intriguing examples are the syndromes caused by genetic alterations in the RAS-MAPK pathway. It is well accepted that Costello syndrome (CS; MIM 218040) can present with CL, however, skin manifestations in other RAS-MAPK-associated syndromes are more variable. CL has been recently described in patients with both BRAF and PNTP11 mutations (causing cardiofaciocutaneous syndrome (CFC; MIM 115150) and Noonan syndrome (NS; MIM 163950), respectively, Figure 1a. The typical features diagnostic for the syndromes were not apparent at birth29 and patients could be therefore initially evaluated for a possible ARCL. Especially, progeroid features in patients with SHOC2 mutations overlap with those with PYCR1.
Figure 1.
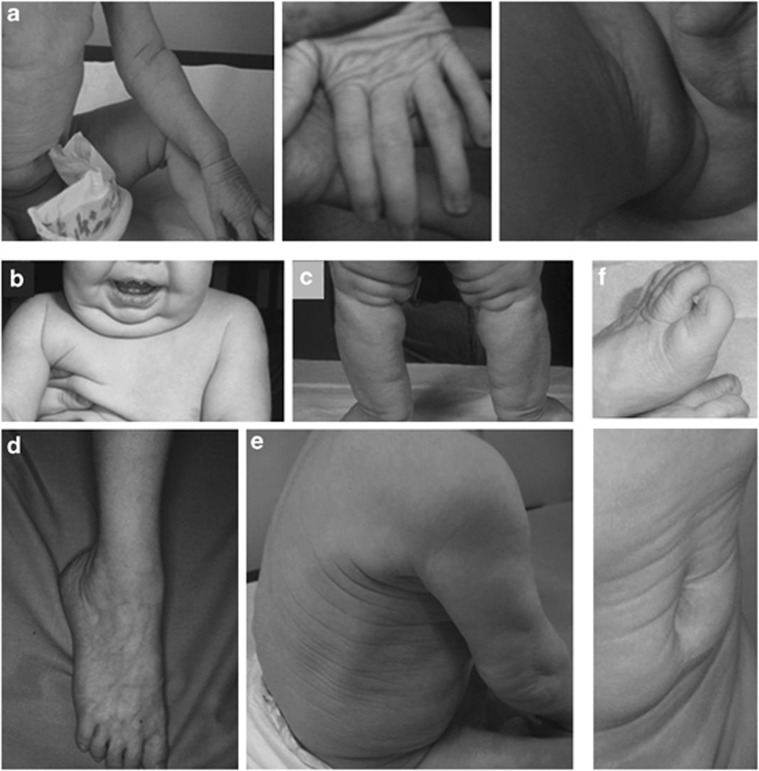
CL in patients with different genetic defects. (a) Patients with RAS-MAPK pathway-related defects (BRAF, SHOC2 and PTPN11 mutations, respectively). (b) Patient with RIN2 mutation, (c) patient with ATP6V0A2 mutation, (d) patient with ALDH18A1 mutation, (e) patient with GORAB mutation and (f) patient with PYCR1 mutation.
Other syndromes with neurological symptoms include, for example, Keutel syndrome, a disorder with hearing loss, cartilaginous ossification and intellectual disability and mild CL (MIM 245150); Sotos syndrome, an overgrowth syndrome with spasticity and developmental delay (MIM 117550); and Cantu syndrome, a syndrome of hypertrichosis, skeletal dysplasia, cardiomyopathy and intellectual disability (MIM 114620).30
In this article, we evaluate a large cohort of patients suspected with ARCL based on the clinical phenotype and the presence of neurological features. In search for discriminatory symptoms to find the underlying molecular defect, we propose a diagnostic approach in this genetically heterogeneous neurological disease.
PATIENTS AND METHODS
We prospectively evaluated the clinical, metabolic, neurological and genetic features of a cohort of patients referred between 2008 and 2011 for consult to our clinical center at the Institute of Genetic and Metabolic Disease (IGMD) with a suspected ARCL syndrome. In this period, 36 patients were referred from 12 countries. None of the patients included in this cohort had previous genetic diagnostic tests or metabolic analysis for glycosylation disorders or evaluation of mitochondrial function. However, screening for abnormal copper or ceruloplasmin levels to rule out OHS had been performed.
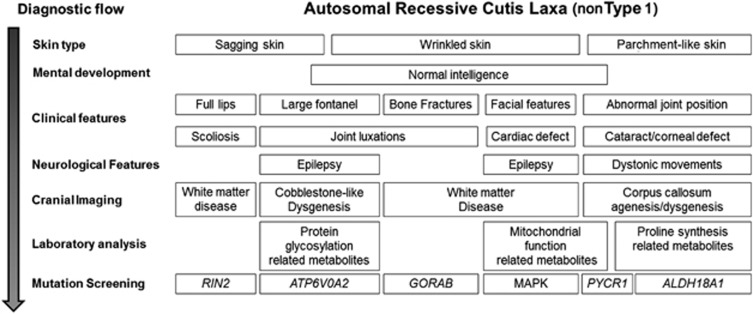
To exclude other inheritance patterns and ARCL type 1, we used the following diagnostic criteria for suspected ARCL (non-type 1) in our cohort: (1) neonatal CL, (2) no affected parents and (3) absence of severe cardiopulmonary or urogenital involvement. In order to evaluate the clinical association between CL and neurologic involvement and to select a coherent group, we used the additional selection term (4) central nervous involvement (see Supplementary Figure 1). Applying these criteria we excluded 10 patients. Based on clinical features and history, three patients were clinically diagnosed with either acquired CL (post infection; n=1) or autosomal dominant CL (n=2). One patient was clinically diagnosed with ARCL type 1 (this patient had lethal cardiopulmonary involvement and an affected brother, but the patient remained genetically unconfirmed).31 One patient, with a severe multiple malformation syndrome, suspected with a chromosomal abnormality had a de novo paternal inverted duplication of the entire short arm of chromosome 11 including the HRAS locus, described recently.32 In five children with progeroid skin anomalies and/or syndromal presentation associated with wrinkled skin, a MAPK pathway-related defect has been considered and confirmed. In one patient, a HRAS mutation was found, in two patients BRAF missense mutations were found, already described in association with CL29 and in two patients the common missense mutation in the SHOC2 gene33 was confirmed. This led us to a cohort of 26 patients meeting the criteria for suspected autosomal recessive inheritance non-type 1 (see Supplementary Figure 1). The same geneticist at IGMD evaluated all patients regarding clinical features, disease history and family history following our diagnostic flowchart protocol (Figure 2).
Figure 2.
Diagnostic flowchart for evaluation of a new CL patient with suspected autosomal recessive inheritance (non-type 1). The facial features of PYCR1, ALDH18A1 and GORAB-related syndromes are similar to each other and different from the ATP6V0A2-related ARCL2A. Mild mental retardation and frequent bone fractures suggest GORAB gene mutations and severe mental retardation and CCA on MRI is indicative of PYCR1 gene mutations. If no mutation is found in these genes, ALDH18A1 should be screened. N.B. RAS-MAPK-related defects are not part of the ARCL syndromes, however, because of the possible absence of the typical features diagnostic for these syndromes in early life some patients, later diagnosed with an MAPK-related defect, could be initially evaluated for a possible ARCL.
Patients
All patients were initially evaluated after birth because of CL and clinically followed for a minimum of 2 years (2–18 years). Patients 1, 2, 6–8, 14 and 15 were offspring of consanguineous parents. Patients 9 and 13 and patients 14 and 15 are siblings. All children, except for patients 2, 12, 13 and 15 underwent skin biopsy (for histology and methods see Supplementary Data). Clinical and neurological evaluation, ophthalmologic investigation, skeletal survey, echocardiogram and ECG, standard developmental assessment and IQ measurement and a standard cranial MRI were performed in all cases, except in patients 8 and 11. For clinical details see Table 1.
Table 1. Clinical and neurological features of 16 patients with autosomal recessive cutis laxa type II.
| Patients | Hypotonia | Developmental delay | Dystonia | Spasticity | Epilepsy | Visual loss | Abnormal brain MRI | Dysmorphic features | Metabolic abnormalities | Mutation (gene) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | +/− | + | − | − | + | − | Cystic white matter lesion | Sagging chin Long face | ApoC-III | RIN2 |
| 2 | + | + | + | + | + | Corneal clouding | Cerebral atrophy | Progeria, beaked nose | − | PYCR1 |
| 3 | + | + | + | − | + | Corneal clouding | Corpus callosum dysgenesis | Triangular face large ears | − | PYCR1 |
| 4 | + | + | − | − | + | Cataract Esotropia | Corpus callosum dysgenesis | Triangular face, long ears | − | PYCR1 |
| 5 | + | + | + | + | + | Cataract | Corpus callosum dysgenesis, CA | Long face large ears | − | ALDH18A1 |
| 6 | + | + | + | + | + | Corneal lesions | CCA, poli-microgyria | Triangular face, long ears | − | PYCR1 |
| 7 | + | + | − | − | + | Cataract esotropia | Cerebral atrophy | Progeria, beaked nose | Lactate, EMA | PYCR1 |
| 8 | + | − | + | − | − | Corneal clouding | NA | Down-slanting droopy eyes | TIEF/ApoC-III | ATP6V0A2 |
| 9a | + | + | − | − | − | Esotropia | Cobblestone-like dysgenesis | Down-slanting droopy eyes | TIEF/ApoC-III | ATP6V0A2 |
| 10 | + | + | − | − | + | Esotropia | Cobblestone-like dysgenesis | Down-slanting droopy eyes | TIEF/ApoC-III | ATP6V0A2 |
| 11 | + | + | − | − | + | − | NA | Down-slanting droopy eyes | TIEF/ApoC-III | ATP6V0A2 |
| 12 | + | + | − | − | − | Esotropia | Cobblestone-like dysgenesis | Down-slanting droopy eyes | TIEF/ApoC-III | ATP6V0A2 |
| 13a | + | + | − | +/− | − | − | Cobblestone-like dysgenesis | Down-slanting droopy eyes | NA | ATP6V0A2 |
| 14a | + | + | − | + | + | +/− | White matter lesions | Long face, large ears | − | GORAB |
| 15a | + | + | − | + | − | − | White matter lesions | Long face, large ears | − | GORAB |
| 16 | + | + | − | − | + | − | − | Extreme face laxity | − | GORAB |
Abbreviations: ApoC-III, apolipoprotein CIII isoelectrophoresis; CA, cerebellar atrophy; EMA, ethylmalonic acid; NA, not available; TIEF, transferrin isoelectric focussing.
Patients 9 and 13 and patients 14 and 15 are siblings.
Metabolic investigations
Blood lactate, blood gas, glucose, ammonia, liver function tests, creatine kinase, triglycerides, cholesterol, serum amino acids, serum acyl-carnitines, urine organic acids, amino acids and purines/pyrimidines were assessed by standard methods in all patients. Ceruloplasmin levels were previously screened. Transferrin isoelectric focussing (TIEF) and apolipoprotein-C isoelectrophoresis (ApoC-III IEF) were carried out as screening for glycosylation disorders by standard methods (see Supplementary Data).34, 35
Molecular genetic studies
Genomic DNA was extracted from peripheral blood lymphocytes using standard salting out procedures.36 Genotyping was performed by using the Affymetrix GeneChip Mapping 10K 2.0 array or the Affymetrix NspI 250K SNP array. All SNP array experiments were performed and analyzed according to the manufacturer's protocols (Affymetrix, Santa Clara, CA, USA). Homozygosity mapping was performed using pLINK v1.06 (Shaun Purcell, http://pngu.mgh.harvard.edu/purcell/plink/ Boston, MA, USA) using a homozygous window of 50 SNPs tolerating two heterozygous SNPs and 10 missing SNPs per window.
Mutation analysis of ATP6V0A2, GORAB, ALDH18A1, PYCR1, FBLN5, PTPN11, BRAF, HRAS, SHOC2, NSD1 and RIN2 was performed by standard methods and as described previously.4, 12, 13, 17, 18, 24, 25, 33 PCR products were sequenced using the ABI PRISM BigDye Terminator Cycle Sequencing V2.0 Ready Reaction Kit and analyzed with the ABI PRISM 3730 DNA analyzer (Applied Biosystems, Foster City, CA, USA).
RESULTS
Patients
Patients were initially suspected with a clinical diagnosis based on characteristic features, like characteristic face and alopecia (RIN2 mutations; MACS syndrome: patient 1), arthrogryposis or dystonia (suspected PYCR1/ALDH18A1; ARCL2B), or typical facial features suggesting ATP6V0A2 mutations (ARCL2A), or bone fractures suggesting GORAB mutations (GO). Several patients had no discriminatory features at initial clinical evaluation (further unspecified ARCL2-like syndrome).
All patients underwent extensive neurologic evaluation. For neurologic data see Table 1.
Mutation analysis
From the 26 patients, we confirmed a mutation in 16 cases (Table 2). In patient 2, homozygosity mapping detected a homozygous region of 3.1 Mb on chromosome 17q25.3 delimited by SNP_A-1939818 and SNP_A-2039852 with PYCR1 in the region. Direct DNA sequencing of PYCR1 revealed a homozygous splicing mutation. Homozygosity mapping in the family of patients 14 and 15 showed that the largest homozygous region of 24.8 Mb was defined by 102 homozygous SNPs on 1q23.3–1q31.1 and was delimited by SNP_A-1511542 and SNP_A-1509763. The region contained GORAB. By direct DNA sequencing of GORAB in both patients, we found a novel homozygous missense mutation. Direct sequencing of the candidate gene identified mutations in patients 1 (RIN2), 3, 4, 6, 7 (PYCR1), 5 (ALDH18A1), 8–13 (ATP6V0A2) and 16 (GORAB). For more details and prediction of pathogeneicity of novel mutations, see Table 2 and Supplementary Data, respectively.
Table 2. Confirmed mutations in 16 patients with cutis laxa.
| Patient | Gene | cDNA | Consequence | Mutation previously reported in other patients |
|---|---|---|---|---|
| 1 | RIN2 | c.1731delC | p.Ile578Serfs*4 | Albrecht et al27; Basel-Vanagaite et al26 |
| c.1731delC | p.Ile578Serfs*4 | |||
| 2 | PYCR1 | c.540+1G>A | splicing | Reversade et al18; Yildirim et al20 |
| c.540+1G>A | ||||
| 3 | PYCR1 | c.355C>T | p.Arg119Cys | — |
| c.356G>A | p.Arg119His | Reversade et al18 | ||
| 4 | PYCR1 | c.355C>T | p.Arg119Cys | — |
| c.355C>T | p.Arg119Cys | — | ||
| 5 | ALDH18A1 | c.1273C>T | p.Arg425Cys | Zampatti et al38 |
| c.2225G>T | p.Ser742Ile | |||
| 6 | PYCR1 | c.772G>A | p.Val258Met | — |
| c.772G>A | p.Val258Met | — | ||
| 7 | PYCR1 | c.535G>A | p.Ala179Thr | Reversade et al18 |
| c.535G>A | p.Ala179Thr | |||
| 8 | ATP6V0A2 | c.754delT | p.Tyr252Ilefs*15 | — |
| c.754delT | p.Tyr252Ilefs*15 | |||
| 9a | ATP6V0A2 | c.600delC | p.Ile201Serfs*20 | Fischer et al44 |
| 110-kb deletion | p. deletion | |||
| 10 | ATP6V0A2 | c.1101delC | pThr368Leufs*43 | — |
| c.1562_1563delins9 | p.Ile521Metfs*16 | |||
| 11 | ATP6V0A2 | c.1326+1G>A; | splicing/ | — |
| c.2287C>T | p.His763Tyr | |||
| 12 | ATP6V0A2 | c.1605+1G>A | splicing | — |
| c.1605+1G>A | ||||
| 13a | ATP6V0A2 | c.600delC | p.Ile201Serfs*20 | — |
| 110-kb deletion | p. deletion | |||
| 14a | GORAB | c.524C>T | p.Ser175Phe | — |
| c.524C>T | p.Ser175Phe | |||
| 15a | GORAB | c.524C>T | p.Ser175Phe | — |
| c.524C>T | p.Ser175Phe | |||
| 16 | GORAB | c.3676G>T | p.Glu123* | — |
| c.3676G>T | p.Glu123* |
Patients 9 and 13 and patients 14 and 15 are siblings.
Laboratory results
Lactate levels and blood alanine levels were increased in patient 7 once. No significant abnormalities were detected in the urine organic acids, except for mild ethylmalonic aciduria in patient 7 (22 and 27 μmol/mmol creatinine; normal <20 μmol/mmol creatinine), suspected with ARCL2B. Blood ammonia, triglycerides, cholesterol, serum acyl-carnitines, urine amino acids and purines/pyrimidines were normal in all patients.
TIEF results were abnormal in patients 8–12 showing a CDG type II pattern and abnormal ApoC-III isoforms (ApoC-III1: 72–76% control range 33–67%) and ApoC-III2: 18–21% (control range 27–60%). In patient 13, no TIEF was performed. In patient 1, the IEF was slightly abnormal.28
Ophthalmologic evaluation
Patients 2–8 had corneal clouding and/or cataract; patients 4, 7, 9, 10, 12, 13, 14 and 15 had strabismus.
Cranial imaging
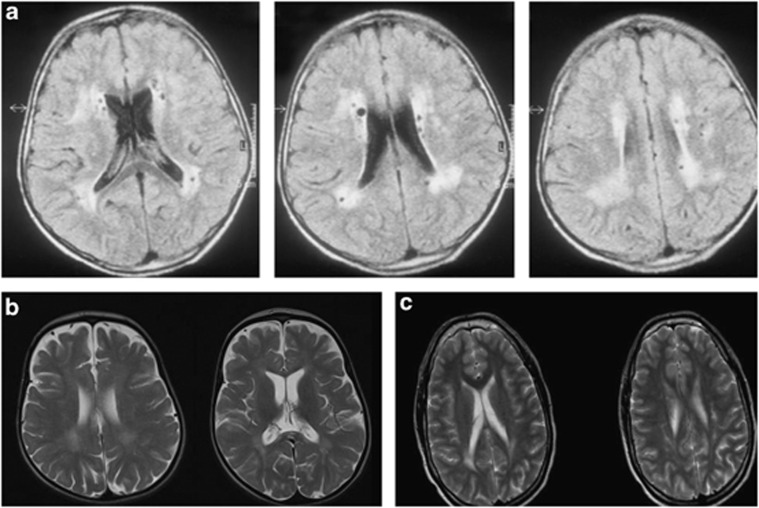
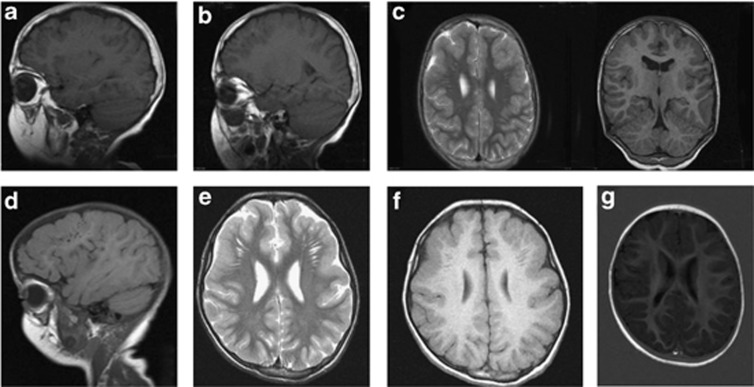
Cranial MRI in patient 1 at the age of 18 years showed cerebral atrophy. The gyration was normal. There were bilateral, cystic-gliotic, parietal periventricular white matter abnormalities observed (Figure 3a). The pontocerebellar structures and the corpus callosum were normal, and no abnormalities of the basal ganglia were present. Patients 3, 4 and 6 had partial dysgenesis of the corpus callosum. MRI in patient 5 revealed cerebellar vermis hypoplasia and a thin corpus callosum. In patient 6, cranial CT imaging showed severe cerebral atrophy, abnormal frontal gyration (polimicrogyria) and agenesis of the corpus callosum. Patients 9, 10, 12 and 13 had a frontotemporal cobblestone-like dysgenesis (Figure 4). MRI in patient 14 revealed mild periventricular white matter changes, besides cortical atrophy from the age of 10 years (Figure 3b). Patient 15 had only minimal, nonspecific periventricular white matter changes at the age of 16 years (Figure 3c). The cranial MRI of patient 16 at the age of 6 years was normal. Patients 2 and 7 had no specific MRI abnormalities, only variable degrees of cortical atrophy. A summary of the MRI findings is given in Table 1.
Figure 3.
White matter anomalies in patients diagnosed with RIN2 and GORAB mutations. (a) Axial FLAIR images of patient 1 diagnosed with MACS syndrome at the age of 18, showing periventricular white matter disease with cystic lesions in the white matter. The U fibers are spared. (b, c) Mild white matter changes in the periventricular white matter and in the corpus callosum on axial T2 images in patients 14 and 15 at age of 10 and 16 years, diagnosed with GO syndrome, intellectual disability and spasticity.
Figure 4.
MRI images of patients with ATP6V0A2 mutations. (a, b) Demonstrate frontal and frontotemporal cobblestone-like brain dysgenesis on sagittal T1 weighed images in patient 9 at the age of 9 years. Note the enlarged Virchow space under the abnormally developed brain region. (c, d) Show a T2 weighed (axial) and a T1 weighed (coronal) image both showing frontotemporal cobblestone-like brain dysgenesis. (e, f) Show axial images of frontotemporal cobblestone-like brain dysgenesis respectively on T2- and T1-weighted images in patient 10 at the age of 2. (g) Shows an axial image showing mild, frontal cobblestone-like brain dysgenesis.
Histology
From all children, except for patients 2, 8, 9 and 11, a skin biopsy was taken from either the lower forearm, the upper thigh or the lower abdominal region (not necessarily the location with the most severe CL). Light microscopy showed severe elastin abnormalities in all patients. In all samples, a decreased amount of irregular, short, thick and often fragmented elastic fibers was seen in addition to a more general disorganized extracellular matrix. Electron microscopy was performed in patients 1, 3, 7, 9, 16 and was characteristic for the disease localization in patients 1 and 9 and patients 3 and 7 (Golgi and mitochondrial involvement, respectively, for more details see Supplementary Data).
DISCUSSION
Since CL or wrinkled skin has been described in >50 syndromes (Oxford Medical Database), most of which are associated with neurological involvement, it is not easy to suggest a diagnostic strategy. Making the correct diagnosis is complicated because of phenotypic diversity in patients with the same genetic defect, but on the other hand there is a significant phenotypic overlap between the different syndromes.
There are only a few discriminatory or even pathognomonic symptoms for each type of disease. In addition, although not always, discriminatory metabolic alterations might give some clues, as they are surprisingly common.
Our study confirms that the combining of biochemical analyses, neurological findings and neuroimaging in CL patients will facilitate a guided mutational analysis and a quick correct diagnosis (see suggested diagnostic chart Figure 2).
Neurological features and the use of neuroimaging in ARCL2
Several CL syndromes are presenting with muscle hypotonia (ATP6V0A2 and PYCR1 defects), spasticity (PYCR1 and ALDH18A1 defects), dystonic, athetoid movements (ALDH18A1 and PYCR1 defect) or epilepsy (ALDH18A1 and ATP6V0A2 defects). Developmental delay is frequent in patients with ATP6V0A2 and PYCR1 mutations.19, 25 Up until now, no neurological involvement has been described in GO patients carrying GORAB mutations and most of the patients had a normal mental development.25 The finding of spasticity, intellectual disability and white matter involvement in our patients with GORAB mutations is therefore unique, and could be either because of a broad spectrum of phenotypic variability, or a consequence of a possible second disease based on the high consanguinity in this family.
Interestingly, several forms of CL syndromes occur in association with congenital brain developmental defects. As our first description of a novel CL syndrome with variable cortical anomalies and a genetic defect in ATP6V0A2, several new patients have been reported.12, 16 In children diagnosed with ATP6V0A2 mutations, the presence of cobblestone-like brain dysgenesis in combination with glycosylation abnormalities is pathognomonic; the cerebral abnormalities are dominated by bilateral cortical malformation of the posterior parts of the frontal lobes or the temporal lobes and enlarged perivascular spaces. Surprisingly, one of the patients (patient 6) with a confirmed PYCR1 mutation also had gyration anomalies: polymicrogyria, a recognizably different type than the cobblestone brain-like abnormalities and obviously, without glycosylation abnormalities.
An important observation is the common presence of corpus callosum dysgenesis or corpus callosum agenesis (CCA) in patients with PYCR1 mutations. Although the presentation of CCA is nonspecific, the combination of a CL syndrome with progeroid features and corpus callosum dysgenesis is highly suggestive.12, 17, 20
So far, no brain anomalies have been reported in the patients diagnosed with MACS syndrome. The finding of periventricular cystic lesions in our patient (patient 1) diagnosed with a RIN2 mutation could be a coincidence, however, no suggestive perinatal history or vascular aberrations could be confirmed as an underlying basis of this feature. Similar, periventricular, cystic abnormalities have been previously observed in other metabolic multiple malformation syndromes as well; for example, in patients with Lowe syndrome (MIM 309000), which is also due to defects in an endosomal protein.37
Metabolic abnormalities
All patients with ATP6V0A2 mutations had a CDG type II pattern and abnormal ApoC-III screening results. We also found metabolic abnormalities suggestive for mitochondrial dysfunction (lactate, alanine and Krebs cycle intermediates elevation) in one of the five patients diagnosed with PYCR1 mutations (patient 7). This is in line with the findings of Kornak et al, demonstrating mitochondrial dysfunction, altered membrane potential and apoptosis rate, abnormal mitochondrial network on oxidative stress and ultrastructural changes by electomicroscopy.18 In the medical literature, no metabolic abnormalities have been described in patients carrying PYCR1 mutations, however, out of the almost 30 reported patients17, 18, 19, 20, 38, 39, 40, 41 metabolic studies have been only performed in four cases.
Until now, ten patients have been described with mutations in ALDH18A1.21, 22, 38, 42, 43 The two patients, presented by Baumgartner et al,22 showed low ornithine, arginine, citrulline and proline. Except for low ornithine levels in two of the patients described by Bicknell et al,21 no amino-acid profile abnormalities have been described in the other patients. Ammonia was increased in three of the ten cases. Although not present in all patients, the finding of metabolic abnormalities is indicative for these two diseases and therefore we advise to evaluate lactate, ammonia and the serum amino acids in unsolved CL patients.
Clinical flowchart
Using this protocol combining clinical/neurological features, biochemical abnormalities and neuroimaging, we genetically solved 16 (14 probands) out of 26 patients with suspected ARCL2 (or 24 out of 36 unselected CL patients) from our patient cohort. Hereby, we suggest to follow this diagnostic flowchart (Figure 2). Although the facial features related to PYCR1, ALDH18A1 and GORAB mutations overlap, in case of unique clinical features like scoliosis with alopecia (RIN2) or frequent bone fractures (GORAB), seizures associated with improving CL pattern with aging (ATP6V0A2) or dystonia associated with peripheral distribution of CL (PYCR1) direct mutation analysis can be performed.
In patients with a CL syndrome, especially those with neurologic involvement, but without the obvious presence of clinical discriminatory features, we suggest to perform glycosylation studies, measure blood lactate, ammonia, serum amino acids, alanine and proline-related metabolites and perform urine organic acid analysis. MRI brain imaging is suggested in all patients because of the high prevalence of characteristic findings in different CL types.
CONCLUSION
Here we describe a large cohort of patients presenting with CL and variable neurological involvement. As a novel finding, we detected white matter anomalies in patients diagnosed with GORAB or RIN2 mutation. In nine patients (eight probands), the type of neurological involvement (eg, dystonia or cobblestone-like brain dysgenesis) served as a hallmark for the clinical and molecular diagnosis, while in seven cases combining the clinical information with metabolic data (eg, TIEF/ApoC-III, lactate) led to the correct genetic diagnosis, see also Table 1. We suggest to perform careful neurologic evaluation and neuroimaging in CL patients.
Acknowledgments
We are thankful for the technical support of Rolf Pfund and Karin Huyben. The study was supported by the Institute of Genetic and Metabolic Disease.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Van Maldergem L, Loeys B.FBLN5-related cutis laxain: Pagon RA, Bird TD, Dolan CR, Stephens K, Adam MP (eds): GeneReviews Seattle, WA, USA: University of Washington, Seattle; 1993 [Google Scholar]
- Morava E, Guillard M, Lefeber DJ, Wevers RA. Autosomal recessive cutis laxa syndrome revisited. Eur J Hum Genet. 2009;17:1099–1110. doi: 10.1038/ejhg.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler SG. ATP7A-related copper transport diseases-emerging concepts and future trends. Nat Rev Neurol. 2011;7:15–29. doi: 10.1038/nrneurol.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler SG, Gallo LK, Proud VK, et al. Occipital horn syndrome and a mild Menkes phenotype associated with splice site mutations at the MNK locus. Nat Genet. 1994;8:195–202. doi: 10.1038/ng1094-195. [DOI] [PubMed] [Google Scholar]
- Zhang MC, He L, Giro M, Yong SL, Tiller GE, Davidson JM. Cutis laxa arising from frameshift mutations in exon 30 of the elastin gene (ELN) J Biol Chem. 1999;274:981–986. doi: 10.1074/jbc.274.2.981. [DOI] [PubMed] [Google Scholar]
- Markova D, Zou Y, Ringpfeil F, et al. Genetic heterogeneity of cutis laxa: a heterozygous tandem duplication within the fibulin-5 (FBLN5) gene. Am J Hum Genet. 2003;72:998–1004. doi: 10.1086/373940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeys B, Van Maldergem L, Mortier G, et al. Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Hum Mol Genet. 2002;11:2113–2118. doi: 10.1093/hmg/11.18.2113. [DOI] [PubMed] [Google Scholar]
- Megarbane H, Florence J, Sass JO, et al. An autosomal-recessive form of cutis laxa is due to homozygous elastin mutations, and the phenotype may be modified by a heterozygous fibulin 5 polymorphism. J Invest Dermatol. 2009;129:1650–1655. doi: 10.1038/jid.2008.450. [DOI] [PubMed] [Google Scholar]
- Renard M, Holm T, Veith R, et al. Altered TGFbeta signaling and cardiovascular manifestations in patients with autosomal recessive cutis laxa type I caused by fibulin-4 deficiency. Eur J Hum Genet. 2010;18:895–901. doi: 10.1038/ejhg.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban Z, Hucthagowder V, Schurmann N, et al. Mutations in LTBP4 cause a syndrome of impaired pulmonary, gastrointestinal, genitourinary, musculoskeletal, and dermal development. Am J Hum Genet. 2009;85:593–605. doi: 10.1016/j.ajhg.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert B, Su CT, Van Damme T, et al. Comprehensive clinical and molecular analysis of 12 families with type 1 recessive cutis laxa. Hum Mutat. 2013;34:111–121. doi: 10.1002/humu.22165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucthagowder V, Morava E, Kornak U, et al. Loss-of-function mutations in ATP6V0A2 impair vesicular trafficking, tropoelastin secretion and cell survival. Hum Mol Genet. 2009;18:2149–2165. doi: 10.1093/hmg/ddp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornak U, Reynders E, Dimopoulou A, et al. Impaired glycosylation and cutis laxa caused by mutations in the vesicular H+-ATPase subunit ATP6V0A2. Nat Genet. 2008;40:32–34. doi: 10.1038/ng.2007.45. [DOI] [PubMed] [Google Scholar]
- Morava E, Lefeber DJ, Urban Z, et al. Defining the phenotype in an autosomal recessive cutis laxa syndrome with a combined congenital defect of glycosylation. Eur J Hum Genet. 2008;16:28–35. doi: 10.1038/sj.ejhg.5201947. [DOI] [PubMed] [Google Scholar]
- Mohamed M, Kouwenberg D, Gardeitchik T, Kornak U, Wevers RA, Morava E. Metabolic cutis laxa syndromes. J Inherit Metab Dis. 2011;34:907–916. doi: 10.1007/s10545-011-9305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Maldergem L, Yuksel-Apak M, Kayserili H, et al. Cobblestone-like brain dysgenesis and altered glycosylation in congenital cutis laxa, Debre type. Neurology. 2008;71:1602–1608. doi: 10.1212/01.wnl.0000327822.52212.c7. [DOI] [PubMed] [Google Scholar]
- Guernsey DL, Jiang H, Evans SC, et al. Mutation in pyrroline-5-carboxylate reductase 1 gene in families with cutis laxa type 2. Am J Hum Genet. 2009;85:120–129. doi: 10.1016/j.ajhg.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reversade B, Escande-Beillard N, Dimopoulou A, et al. Mutations in PYCR1 cause cutis laxa with progeroid features. Nat Genet. 2009;41:1016–1021. doi: 10.1038/ng.413. [DOI] [PubMed] [Google Scholar]
- Kretz R, Bozorgmehr B, Kariminejad MH, et al. Defect in proline synthesis: pyrroline-5-carboxylate reductase 1 deficiency leads to a complex clinical phenotype with collagen and elastin abnormalities. J Inherit Metab Dis. 2011;34:731–739. doi: 10.1007/s10545-011-9319-3. [DOI] [PubMed] [Google Scholar]
- Yildirim Y, Tolun A, Tuysuz B. The phenotype caused by PYCR1 mutations corresponds to geroderma osteodysplasticum rather than autosomal recessive cutis laxa type 2. Am J Med Genet Pt A. 2011;155A:134–140. doi: 10.1002/ajmg.a.33747. [DOI] [PubMed] [Google Scholar]
- Bicknell LS, Pitt J, Aftimos S, Ramadas R, Maw MA, Robertson SP. A missense mutation in ALDH18A1, encoding Delta1-pyrroline-5-carboxylate synthase (P5CS), causes an autosomal recessive neurocutaneous syndrome. Eur J Hum Genet. 2008;16:1176–1186. doi: 10.1038/ejhg.2008.91. [DOI] [PubMed] [Google Scholar]
- Baumgartner MR, Hu CA, Almashanu S, et al. Hyperammonemia with reduced ornithine, citrulline, arginine and proline: a new inborn error caused by a mutation in the gene encoding delta(1)-pyrroline-5-carboxylate synthase. Hum Mol Genet. 2000;9:2853–2858. doi: 10.1093/hmg/9.19.2853. [DOI] [PubMed] [Google Scholar]
- de Barsy AM, Moens E, Dierckx L. Dwarfism, oligophrenia and degeneration of the elastic tissue in skin and cornea. A new syndrome. Helvetica Paediatrica Acta. 1968;23:305–313. [PubMed] [Google Scholar]
- Kivuva EC, Parker MJ, Cohen MC, Wagner BE, Sobey G. De Barsy syndrome: a review of the phenotype. Clin Dysmorphol. 2008;17:99–107. doi: 10.1097/MCD.0b013e3282f4a964. [DOI] [PubMed] [Google Scholar]
- Hennies HC, Kornak U, Zhang H, et al. Gerodermia osteodysplastica is caused by mutations in SCYL1BP1, a Rab-6 interacting golgin. Nat Genet. 2008;40:1410–1412. doi: 10.1038/ng.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basel-Vanagaite L, Sarig O, Hershkovitz D, et al. RIN2 deficiency results in macrocephaly, alopecia, cutis laxa, and scoliosis: MACS syndrome. Am J Hum Genet. 2009;85:254–263. doi: 10.1016/j.ajhg.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht B, de Brouwer AP, Lefeber DJ, et al. MACS syndrome: a combined collagen and elastin disorder due to abnormal Golgi trafficking. Am J Med Genet Pt A. 2010;152A:2916–2918. doi: 10.1002/ajmg.a.33712. [DOI] [PubMed] [Google Scholar]
- Syx D, Malfait F, Van Laer L, et al. The RIN2 syndrome: a new autosomal recessive connective tissue disorder caused by deficiency of Ras and Rab interactor 2 (RIN2) Hum Genet. 2010;128:79–88. doi: 10.1007/s00439-010-0829-0. [DOI] [PubMed] [Google Scholar]
- Kleefstra T, Wortmann SB, Rodenburg RJ, et al. Mitochondrial dysfunction and organic aciduria in five patients carrying mutations in the Ras-MAPK pathway. Eur J Hum Genet. 2011;19:138–144. doi: 10.1038/ejhg.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk DR, Bentley DD, Bayliss SJ, Lind A, Urban Z. Cutis laxa: a review. J Am Acad Dermatol. 2012;66:842 e841–817. doi: 10.1016/j.jaad.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Piccolo P, Morava E, et al. Cutis laxa and fatal pulmonary hypertension: a newly recognized syndrome. Clin Dysmorphol. 2011;20:77–81. doi: 10.1097/MCD.0b013e3283439676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardeitchik T, de Leeuw N, Nijtmans L, et al. Infant with MCA and severe cutis laxa due to a de novo duplication 11p of paternal origin. Am J Med Genet Pt A. 2012;158A:469–472. doi: 10.1002/ajmg.a.34410. [DOI] [PubMed] [Google Scholar]
- Cordeddu V, Di Schiavi E, Pennacchio LA, et al. Mutation of SHOC2 promotes aberrant protein N-myristoylation and causes Noonan-like syndrome with loose anagen hair. Nat Genet. 2009;41:1022–1026. doi: 10.1038/ng.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eijk HG, van Noort WL. The analysis of human serum transferrins with the PhastSystem: quantitation of microheterogeneity. Electrophoresis. 1992;13:354–358. doi: 10.1002/elps.1150130173. [DOI] [PubMed] [Google Scholar]
- Wopereis S, Grunewald S, Morava E, et al. Apolipoprotein C-III isofocusing in the diagnosis of genetic defects in O-glycan biosynthesis. Clin Chem. 2003;49:1839–1845. doi: 10.1373/clinchem.2003.022541. [DOI] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvola N, Diao A, McKenzie E, Skippen A, Cockcroft S, Lowe M. Membrane targeting and activation of the Lowe syndrome protein OCRL1 by rab GTPases. EMBO J. 2006;25:3750–3761. doi: 10.1038/sj.emboj.7601274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampatti S, Castori M, Fischer B, et al. De Barsy syndrome: a genetically heterogeneous autosomal recessive cutis laxa syndrome related to P5CS and PYCR1 dysfunction. Am J Med Genet Pt A. 2012;158A:927–931. doi: 10.1002/ajmg.a.35231. [DOI] [PubMed] [Google Scholar]
- Kouwenberg D, Gardeitchik T, Wevers RA, Häberle J, Morava E. Recognizable phenotype with common occurrence of microcephaly, psychomotor retardation, but no spontaneous bone fractures in autosomal recessive cutis laxa type IIB due to PYCR1 mutations. Am J Med Genet Pt A. 2011;155A:2331–2332. doi: 10.1002/ajmg.a.34154. [DOI] [PubMed] [Google Scholar]
- Lin DS, Chang JH, Liu HL, et al. Compound heterozygous mutations in PYCR1 further expand the phenotypic spectrum of De Barsy syndrome. Am J Med Genet Pt A. 2011;155A:3095–3099. doi: 10.1002/ajmg.a.34326. [DOI] [PubMed] [Google Scholar]
- Lin DS, Chang JH, Liu HL, et al. A novel mutation in PYCR1 causes an autosomal recessive cutislaxa with premature aging features in a family. Am J Med Genet Pt A. 2011;155A:1285–1289. doi: 10.1002/ajmg.a.33963. [DOI] [PubMed] [Google Scholar]
- Skidmore DL, Chitayat D, Morgan T, et al. Further expansion of the phenotypic spectrum associated with mutations in ALDH18A1, encoding Δ1-pyrroline-5-carboxylate synthase (P5CS) Am J Med Genet Pt A. 2011;155A:1848–1856. doi: 10.1002/ajmg.a.34057. [DOI] [PubMed] [Google Scholar]
- Martinelli D, et al. Understanding pyrroline-5-carboxylate synthetase deficiency: clinical, molecular, functional, and expression studies, structure-based analysis, and novel therapy with arginine. J Inherit Metabol Dis. 2012;35:761–776. doi: 10.1007/s10545-011-9411-8. [DOI] [PubMed] [Google Scholar]
- Fischer B, Dimopoulou A, Egerer J, et al. Further characterization of ATP6V0A2-related autosomal recessive cutis laxa. Hum Genet. 2012;131:1761–1773. doi: 10.1007/s00439-012-1197-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.