Abstract
Background
IDH1 and IDH2 gene mutations are novel, recurring molecular aberrations among patients with normal karyotype acute myeloid leukemia (AML).
Methods
Among 358 patients with AML treated on 4 protocols using high dose ara-C plus idarubicin induction, pre-treatment samples were available for 170 [median age 53 years, (range, 17 - 73); 96% ≤ 65] and were evaluated for IDH1R132, IDH2R172 and IDH2R140 mutations or the codon 105 SNP in IDH1.
Results
IDH1 and IDH2 mutations were present in 12 (7%) and 24 (14%) patients, and IDH1 G105 SNP in 24 (14%). Overall, 52 (30%) patients had IDH gene alterations. There was no association with complete response (CR), remission duration, overall and event-free survival and any of the IDH alterations and no association with a higher CR rate or survival with the 4 regimens for the 52 patients with aberrant IDH. Among the patients with diploid karyotype and NPM1mutFLT3WTgenotype, those with IDH1 or IDH2 mutations had an inferior outcome.
Conclusions
IDH aberrations and IDH1 codon 105 SNP occur in about 30% of younger patients with AML, mostly with diploid karyotype. Using high-dose ara-C based induction regimens, we did not detect an association with outcome for any of the aberrations.
Keywords: AML, IDH, Mutations, SNP, outcome
INTRODUCTION
Isocitrate dehydrogenase (IDH) is a member of the β-decarboxylating dehydrogenase family of enzymes and catalyzes the oxidative decarboxylation of isocitrate into α-ketoglutarate (α-KG) in the tricarboxylic acid (TCA) cycle, an important biochemical pathway in the synthesis of nucleotides, lipids, and amino acids.1 Three isoforms of the IDH enzymes with different subcellular localization have been described in mammalian cells: IDH1 is localized in the cytoplasm and the peroxisomes, whereas IDH2 and IDH3 are mitochondrial enzymes.2 These enzymes use nicotanomide adenine dinucleotide (NAD) or NAD phosphate as cofactors to catalyze the conversion of isocitrate to α-KG yielding NADH or NADPH as a result.
Recent interest in this family of enzymes in relation to carcinogenesis is based on the initial observations in colorectal cancer and gliomas with several reports of recurring mutations in the IDH1 gene on chromosome band 2q33.3 and the IDH2 gene on chromosome band 15q26.1 in patients with grade II and III gliomas and, in particular, secondary glioblastoma multiforme (GBM) developing from lower grade gliomas.3-7 Overall, these mutations have been associated with a longer survival in patients with GBM.
The IDH mutations identified in brain tumors (and in patients with AML) alter the amino acid residues R132 in IDH1 and R172 in IDH2. The mutated enzyme protein products have been associated with a loss of function in the forward reaction that decorboxylates isocitrate as well as a gain of function in the reverse reaction that reduces α-KG to 2-hydroxyglutarate (2HG).8 Both these loss and gain of function reactions have significant implications for cellular metabolism with 2HG potentially deregulating several α-KG-dependent enzymes and transcription factors, such as HIF-1α, implicated in carcinogenesis.9,10
Initial studies failed to detect mutations of the IDH genes in patients with leukemia.6,11,12 Recently, Mardis and colleagues examined the entire genome sequence of leukemia cells from a single patient with acute myeloid leukemia (AML) with normal cytogenetics (CN-AML) comparing it with the sequence from normal skin cells from the same patient.13 Among 750 possible mutations, several (including mutations in NPM1 and NRAS, as well as IDH1) were recurrent in other AML genomes suggesting their potential role in leukemogenesis.13 This had led to several groups investigating the incidence and prognostic implications of mutations in IDH1 and IDH2 in patients with AML, (particularly those with normal karyotype) in an effort to further improve stratification of these patients and identify potential targets for therapeutic intervention. These studies have reported conflicting associations between the presence of these mutations and clinical outcomes.
In a study by the Cancer and Leukemia Group B (CALGB), leukemia cells from a third of 358 patients examined harbored the IDH1 or IDH2 mutation.14 Among the younger patients (< 60 years) with molecular low-risk group [mutated NPM1 but without fms-like tyrosine kinase-3- internal tandem duplication (FLT3-ITD)], patients with mutated IDH1 (IDH1 R132 had a significantly worse disease-free survival (DFS) with a trend for worse overall survival (OS).14 Patients with R140 IDH2 had no differences in disease outcomes compared to those with wild-type (WT) IDH1/IDH2, whereas older patients with R172 IDH2 had a lower complete remission (CR) rate than those with IDH1/IDH2 wild-type (WT) gene.14
In another study conducted by the German-Austrian AML study group (AMLSG), 129 (16%) of 805 adults (age range, 16-60 years) with AML had one of the IDH mutations.15 Among patients with CN-AML, mutated NPM1, and FLT3WT AML, IDH mutations adversely affected relapse-free survival (RFS) and OS whereas the outcome was not affected in patients lacking this genotype.15 In a study by the Acute Leukemia French Association (ALFA), IDH1 and IDH2 mutations were identified in 9.6% and 3% of adults with AML with an association with a worse outcome for IDH2 among the patients with CN-AML and for IDH2 for those patients with CN-AML and a favorable genotype (NPM1 mutated, FLT3 WT).16 Other groups have also reported on an adverse prognostic effect for IDH mutations as well as the single nucleotide polymorphism (SNP) rs1554137 located in codon 105 in the same exon as the IDH1 R132 mutations in patients with CN-AML, particularly those with NPM1 mutated, FLT3 wild type genotype.17-19 Although this SNP does not result in a change in the amino acid sequence, it has been reported to be associated with a higher mRNA expression compared to patients with two WT alleles.
Here we examined the incidence and prognostic impact of IDH mutations as well as the G105 polymorphism among 358 younger patients with AML treated with an induction regimen containing idarubicin, high dose cytarabine with or without tipifarnib, sorafenib, or vorinostat; we also sought to determine whether any of the 4 regimens had an impact on the outcome of the IDH mutated patients. Although IDH1 G105 polymorphism is not a true aberration and has not been to date shown to be associated with generation of 2HG, we decided to include these patients in the analysis due to recent reports suggesting their negative impact.
MATERIALS AND METHODS
Patients and treatment
From October 2004 to February 2010, 358 patients with newly diagnosed AML were treated on 4 consecutive protocols using high dose ara-C plus idarubicin induction therapy (IA alone, IA plus tipifarnib [IAT], IA plus sorafenib [IAS], and IA plus vorinostat [IAV]). The details of the 4 regimens have been published previously.20-22 All patients reviewed and signed a consent, approved by the M. D. Anderson Cancer Center institutional review board to participate in the clinical trials and to provide bone marrow samples for analysis. The induction regimen in all patients included ara-C 1.5 g/m2 given by 24 hour continuous infusion daily for 4 days (3 days in patients 60 years or older) and idarubicin 12 mg/m2 intravenously daily for 3 days. Patients received tipifarnib 300 mg twice daily for 21 days, sorafenib 400 mg twice daily for 7 days, or vorinostat 500 mg 3 times daily for 3 days, with IAT, IAS, or IAV regimens, respectively. Patients could receive up to 2 induction courses and if in CR or CR without platelet recovery (CRp) would proceed to receive consolidation which consisted of up to 5 courses of idarubicin 8 mg/m2 per day on days 1-2, ara-C 0.75 g/m2 per day on days 1-3, and tipifarnib, sorafenib or vorinostat as appropriate to the protocol. Maintenance with tipifarnib, sorafenib or vorinostat was given for up to 12 months. Patients treated with the IA regimen would receive no further therapy after the completion of the 5 courses of consolidation chemotherapy with no maintenance (Table 1). It should be further emphasized that cytarabine was administered by continuous infusion during induction in all regimens.
Table 1.
Details of the treatment regimens
| IA (From 2006-2009) (n*=78) |
IAT21 (From 2004-2006) (n*=33) |
IAS20 (From 2007-2010) (n*=38) |
IAV22 (From 2008-2010)) (n*=21) |
|
|---|---|---|---|---|
| Induction | Ara-C 1.5 g/m2 by CI daily × 4* |
Ara-C 1.5 g/m2 by CI daily × 4* |
Ara-C 1.5 g/m2 by CI daily × 4* |
Ara-C 1.5 g/m2 by CI daily × 4* |
| Idarubicin 12 mg/m2 IV daily × 3 |
Idarubicin 12 mg/m2 IV daily × 3 |
Idarubicin 12 mg/m2 IV daily × 3 |
Idarubicin 12 mg/m2 IV daily × 3 |
|
| Tipifarnib 300 mg po bid × 21 days |
Sorafenib 400 mg po bid × 7 days |
Vorinostat 500 mg po tid × 3 days |
||
| Consolidation | Up to 5 courses Ara-C 0.75 g/m2 by CI × 3 days |
Up to 5 courses Ara-C 0.75 g/m2 by CI × 3 days |
Up to 5 courses Ara-C 0.75 g/m2 by CI × 3 days |
Up to 5 courses Ara-C 0.75 g/m2 by CI × 3 days |
| Idarubicin 8 mg/m2 daily × 2 days |
Idarubicin 8 mg/m2 daily × 2 days |
Idarubicin 8 mg/m2 daily × 2 days |
Idarubicin 8 mg/m2 daily × 2 days |
|
| Tipifarnib 300 mg po bid × 14 days |
Sorafenib 400 mg po bid for up to 28 days per cycle |
Vorinostat 500 mg po tid × 3 days per cycle |
||
| Maintenance | None | Tipifarnib 300 mg po bid × 21 days every 4-6 weeks, up to 6 months |
Sorafenib 400 mg po bid, up to 1 year |
Vorinostat 200 mg po tid × 14 every 28 days for 12 cycles |
IA: idarubicin plus ara-C; T: tipifarnib; S: sorafenib; V: vorinostat; CI: continuous infusion;
number of patients with pre-treatment samples included in this study
Pre-treatment samples for testing for IDH1R132, IDH2R172 and IDH2R140 mutations were available for 170 patients and these are the subjects of this report. Their median age was 53 years (range, 17 - 73) and the majority of the patients (96%) were 65 years or younger. Other pre-treatment characteristics of the patients are shown in Table 2.
Table 2.
Patient characteristics
| Characteristic | Number (%) |
|---|---|
| Patients | 170 |
| Age in years, median [range] | 53 (17-73) |
| De novo AML | 150 (88) |
| Secondary AML | 20 (12) |
| Plt × 109/L, median [range] | 53 (5-306) |
| WBC × 109/L, median [range] | 4.9 (0.3–161.5) |
| % bone marrow blasts, median [range] | 52 (20-98) |
| Cytogenetics | |
| Diploid | 104 (61) |
| Chromosome 5/7deletion/complex | 32 (18) |
| Other | 34 (20) |
| NPM1 mutated | 46 (27) |
| NPM1 wild-type | 92 (54) |
| NPM1 not done | 32 (19) |
| FLT3-ITD Positive | 36 (21) |
| FLT3-ITD Negative | 132 (78) |
| FLT3-ITD not done | 2 (1) |
| NPM1 mutated/ FLT3-ITD negative | 28 (16) |
Molecular analysis for IDH1 and IDH2 mutations and SNP
For IDH1, a 214-bp partial exon 4 sequence containing both G105 and R132 was amplified using a forward primer within exon 4 and a reverse primer within intron 4. For IDH2, a 288-bp sequence with the entire exon 4 containing R140 and R172 was amplified using forward primer within intron 3 and a reverse primer within intron 4. All primers were tagged with M13 sequence to allow Sanger sequencing using M13 primers. This design allowed interrogation of all three active site arginine residues for both IDH1 (R100, R109, R132) and IDH2 (R140, R149, R172). The primer sequences were as follows: M13-IDH1 forward, TGTAAAACGACGGCCAGTTGAGAAGAGGGTTGAGGAGTT; M13-IDH1 reverse, CAGGAAACAGCTATGACCAACATGCAAAATCACATTATTGCC; M13-IDH2 forward, TGTAAAACGACGGCCAGTGGGTTCAAATTCTGGTTGAA; M13-IDH2 reverse; CAGGAAACAGCTATGACCTAGGCGAGGAGCTCCAGT; M13 forward, TGTAAAACGACGGCCAGT; M13 reverse, CAGGAAACAGCTATGACC. All Sanger assays were performed bi-directionally using M13 primers. Bidirectional Sanger sequencing is an accepted and more stringent way of confirming the presence of a mutation compared to simply repeating a unidirectional sequencing. For Sanger sequencing performed directly from the DNA aliquot, reaction mixtures of 50 μL contained 1 mM dNTPs, 2 mM MgCl2, 200 nM primers, 1.5 U Taq Polymerase (Applied Biosystems, Carlsbad, CA). For each reaction, 200 ng of genomic DNA was amplified with the following PCR conditions: an initial 10-minute denaturation at 95°C followed by 40 cycles of 30 seconds at 95°C; 30 seconds at 60°C; 30 seconds at 72°C, and a final extension of 7 minutes at 72°C.
PCR products were purified using Agencourt Ampure magnetic beads kit (Beckman Coulter, Brea, CA) prior to quality and quantity assessment by gel electrophoresis. Sequencing analysis was performed using a multi-capillary sequencer (ABI 3130 Genetic Analyzer; Applied Biosystems) following cycle sequencing using dye-terminator chemistry according to manufacturer’s protocol (BigDye Terminator v1.1 Ready Reaction Cycle Sequencing Kit; Applied Biosystems). Briefly, 4 L of PCR product in a total volume 20 L containing, 3.2 pmol of either the forward or reverse M13 primer and 6 L of the sequencing mixture were placed in a DNA thermal cycler and amplified at 96°C for 1 minute, 25 cycles at 96°C for 10 seconds, 58°C for 5 seconds, 60°C for 4 minutes, and final hold at 4°C. Sequencing reactions were purified using the Qiagen DyeEx™ purification kit (Qiagen, Valencia, CA) as per manufacturer’s protocol. The resulting data was analyzed by Seqscape software (Applied Biosystems).
Sequencing reactions were purified using the Qiagen DyeEx™ purification kit (Qiagen) as per manufacturer’s protocol. The resulting data was analyzed by Seqscape software (Applied Biosystems).
Molecular analysis for NPM1 and FLT3 mutations
Mutations in coding regions of exon 12 of NPM1 were detected using PCR amplification of a 168-base-pair segment followed by capillary gel electrophoresis. PCR primers included forward, 5′-FAM-GATGTCTATGAAGTGTTGTGGT-TCC-3′ and reverse, 5′- GGACAGCCAGATCAACTG-3′. PCR was performed in a 50- L reaction volume that contained 2 L of patient DNA (100 ng/ L), 5 L of 10× ThermoPol Buffer (New England BioLabs, Ipswich, MA) with magnesium sulfate, 5 L of 10 mmol/L dNTP, 1 L of NPM1 forward primer (10 mol/L), 1 L of NPM1 reverse primer (10 mol/L), 35.2 L of water, and 0.75 L of Vent DNA polymerase (New England Biolabs) (2 U/ L). PCR conditions included initial denaturing at 95°C for 10 minutes, 40 cycles of 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds, and final extension at 72°C for 7 minutes. PCR products were analyzed by capillary electrophoresis on a 3100 or 3130 genetic analyzer (Applied Biosystems).
A multiplex fluorescent-based PCR method was used to detect ITD and D835 point mutations in FLT3. DNA was isolated from bone marrow aspirate samples by using a standard phenol-chloroform extraction method. The presence of ITD was assessed by amplification of the juxtamembrane domain using primers from exons 11 and 12 as described by Kiyoi and Naoe.23 The presence of D835 point mutations was assessed by a restriction fragment length polymorphism–mediated assay using primers flanking the mutation site.15 To facilitate the detection of PCR products by capillary electrophoresis on a 3100 genetic analyzer (Applied Biosystems, Foster City, CA), forward primers for ITD and D835 were labeled with a fluorescent dye, 6-carboxyfluorescein (FAM).16 The presence of any PCR fragment larger than the wild-type allele was considered positive for ITD. D835 PCR products were digested with the EcoRV restriction enzyme before capillary electrophoresis. The wild-type allele cut by this enzyme resulted in 2 fragments of 64 and 48 base pairs. In contrast, mutations at D835 alter the EcoRV recognition site and result in one 112-base-pair fragment. The sensitivity of these assays is approximately 2%, ie, 1 mutated cell in 50 total cells, as established by dilutional studies.
Statistical methods
Patient characteristics are summarized using median (range) for continuous variables and frequency (percentage) for categorical variables. OS is defined as the time interval between the date of initiation of treatment and the date of death due to any cause; patients alive at last follow-up are censored at the last follow-up date. Event-free survival (EFS) is defined as the time interval between the date of initiation of treatment and date of treatment failure, relapse, or death, whichever occurred first; patients were otherwise censored at the last follow-up date. The probabilities of OS and EFS were estimated using the Kaplan-Meier method and were compared among subgroups of patients using the log-rank test. The predictive effects of IDH mutations and other patient characteristics on OS and EFS were examined using univariate and multivariate Cox proportional hazards models. Univariate and multivariate logistic regression models were also used to assess the association between patient covariates and the probability of response. All statistical analyses were conducted in SAS 9.0.
RESULTS
Incidence of IDH mutations and the G105 SNP
Mutations of IDH1 and IDH2 were present in 12 (7%) and 24 (14%) patients, respectively, and IDH1G105 SNP was noted in 24 (14%). Overall, 52 (30%) patients had IDH gene mutations or the previously described SNP rs 11554137 in codon 105 of IDH1.17 In concordance with previous reports IDH1 and IDH2 mutations did not commonly occur in the same patient with the exception of a single patient who had IDH1R132 mutation, IDH1G105 SNP, and IDH2R140 mutation. 14-16 Two patients had concomitant IDH1R132 mutation and IDH1G105 SNP, 3 patients had IDH2R140 mutation and IDH1G105 SNP, 1 patient IDH2R172 mutation and IDH1G105 SNP. IDH1 mutations were most commonly R132H or R132C with the exception of one which was R132G. Twenty four patients had IDH2 mutations, either IDH2 R172 or R140. All IDH2 R172 mutations led to a R172K substitution.
Association of clinical characteristics with IDH mutations and SNP
There was a strong association with normal karyotype with 11 of 12 (92%) of IDH1 mutated, 18 of 24 (75%) of IDH2 mutated, and 17 of 24 (71%) of IDH1 SNP being diploid (Table 3). There was no association between any of the IDH alterations and patient age, sex, therapy-related vs. de novo AML, presenting WBC, peripheral blood blasts, or FAB subtype.
Table 3.
Patient characteristics and their association with IDH1 and IDH2 mutations and IDH1 SNP
| Character | IDH1 R 132 | P value |
IDH1 G105 | P value |
IDH2 R172 & R140 | P value |
|||
|---|---|---|---|---|---|---|---|---|---|
| + | − | + | − | + | − | ||||
| Number | 12 (7) | 158 (93) | 24 (14) | 146 (86) | 24 (15) | 141 (85) | |||
|
Age, median
(range) |
53 (36-73) |
53 (17-72) |
NS | 53 (18-72) |
53 (17-73) |
NS | 56 (19-68) |
53 (17-73) |
NS |
| Sex, Male | 5 (42) | 85 (54) | NS | 10 (42) | 80 (55) | NS | 10 (42) | 78 (55) | NS |
|
De novo
Secondary |
11 (92) 1 (8) |
139 (89) 19 (12) |
NS | 22 (92) 2 (8) |
128 (88) 18 (12) |
NS | 23 (96) 1 (4) |
124 (88) 17 (12) |
NS |
|
Plt × 109/L,
median (range) |
99 (7-214) |
50 (5-306) |
0.047 | 68 (24-242) |
46 (5-306) |
0.04 | 67 (15- 238) |
49 (5- 306) |
NS |
|
WBC × 109/L,
median, (range) |
8.8 (0.6- 50.7) |
4.9 (0.3- 161.5) |
NS | 4.8 (1.4-84) |
5.0 (0.3- 161.5) |
NS | 4.5 (0.5-50.7) |
5.1 (0.3- 161.5) |
NS |
|
% BM blasts,
median (range) |
73 (24-92) |
52 (10-98) |
NS | 39 (18-90) |
54 (10-98) |
0.03 | 61 (10-94) |
52 (14-98) |
NS |
|
% PB blasts,
median (range) |
65 (0-91) |
16 (0-98) |
NS | 15 (0-95) |
17 (0-98) |
NS | 13 (0-93) |
18 (0-98) |
NS |
| Cytogenetics | |||||||||
|
Diploid
5/7/comples Other |
11 (92) 1 (8) |
93 (59) 31 (20) 34 (22) |
0.07 | 17 (71) 1 (4) 6 (25) |
87 (60) 31 (21) 28 (19) |
NS | 18 (75) 1 (4) 5 (21) |
83 (59) 29 (21) 29 (21) |
NS |
| NPM1 | |||||||||
|
Pos
Neg |
8 (67) 3 (25) |
38 (24) 89 (56) |
0.004 | 7 (29) 9 (38) |
39 (27) 83 (57) |
NS | 10 (42) 8 (33) |
36 (26) 82 958) |
0.04 |
| FLT3-ITD | |||||||||
|
Pos
Neg |
4 (33) 8 (67) |
32 (20) 124 (78) |
NS | 4 (17) 20 (83) |
32 (22) 112 (77) |
NS | 5 (21) 19 (79) |
31 (22) 109 (77) |
NS |
| NPM1 Pos/ | |||||||||
| FLT3-Neg Otherwise |
4 (33) 8 (67) |
24 (15) 134 (85) |
NS | 3 (13) 21 (88) |
25 917) 121 (83) |
NS | 6 (25) 18 (75) |
22 (16) 119 (84) |
NS |
|
Induction
regimen |
|||||||||
|
IA
IAT IAS IAV |
3 (25) 6 (50) 1 (8) 2 (17) |
75 (47) 27 (17) 37 (23) 19 (12) |
0.032 | 10 (42) 6 (25) 6 (25) 2 (8) |
68 (47) 27 (18) 32 (22) 19 (103) |
NS | 10 (42) 6 (25) 5 (21) 3 (13) |
65 (46) 27 (19) 32 (23) 17 (12) |
NS |
Among the 52 patients with IDH mutation or SNP, 12 had FLT3-ITD, 5 FLT3-TKD (including 2 patients with both ITD, and TKD), and 21 had NPM1 mutation. There was a strong association between IDH1 and IDH2 mutations, and NPM1 mutations (P values 0.0004 and 0.04, respectively). However, 12 of 52 patients with IDH alterations also had FLT3-ITD compared to 24 of 116 patients with IDHWT (p=NS). The favorable genotype, diploid, NPM1mut and FLT3 WT occurred in 9 patients.
Association of IDH mutations with outcome of therapy
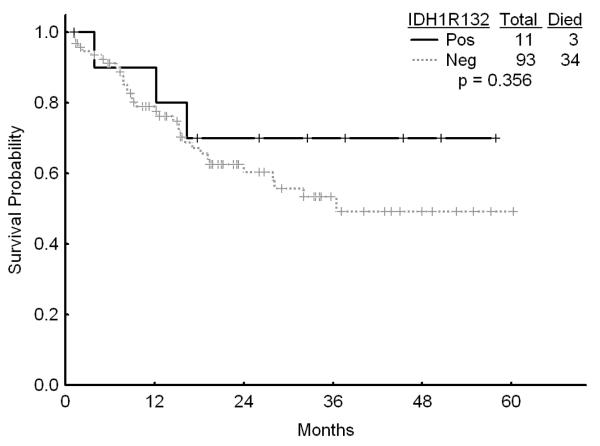
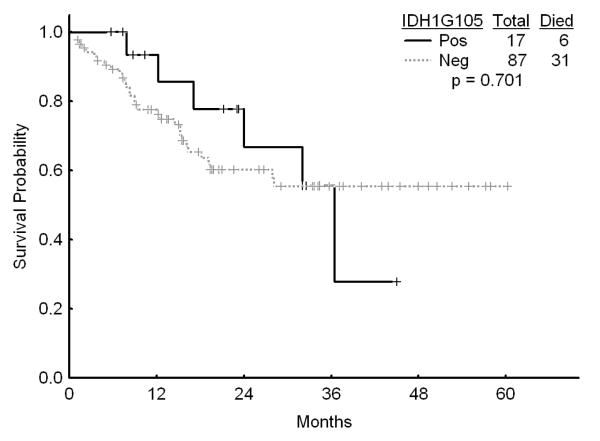
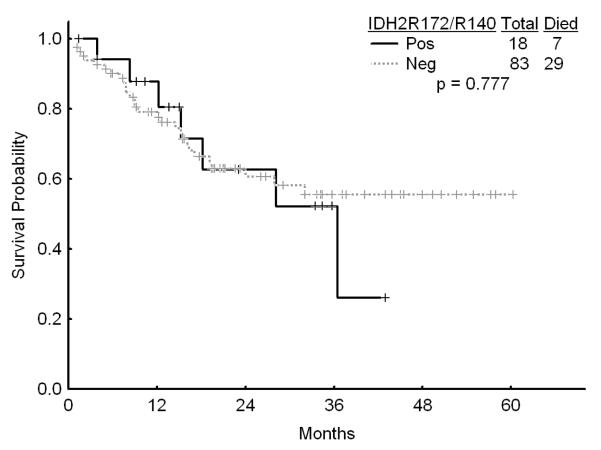
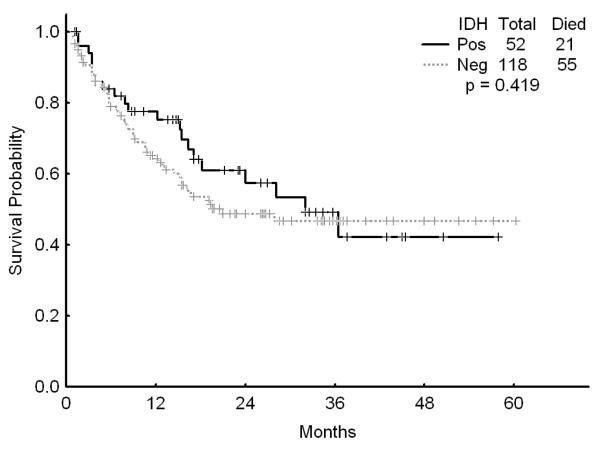
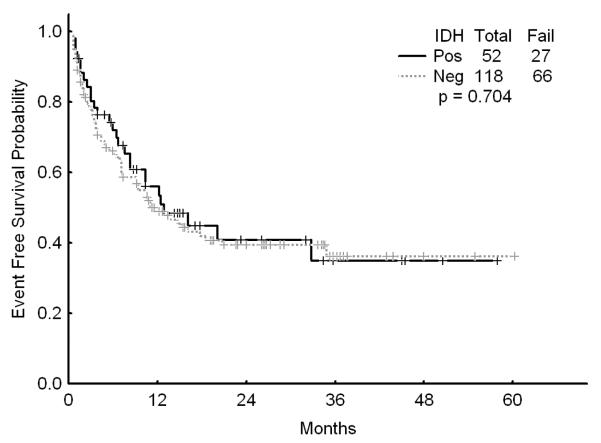
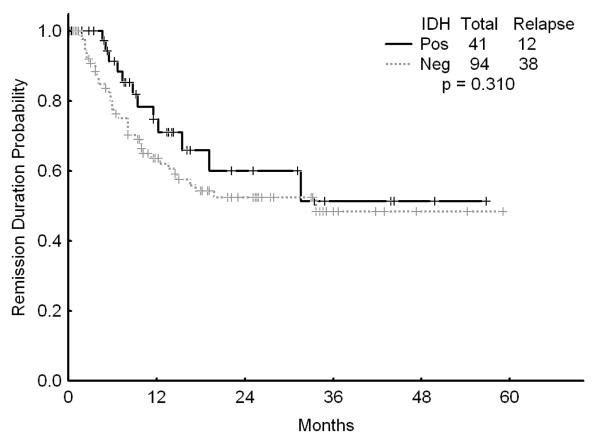
There was no association with achievement of complete response (CR), remission duration, overall and event-free survival and either of the IDH mutations or IDH1 SNP (Figures 1a, b, and c). Similarly, combining all patients with IDH mutations or SNP G105, we were not able show any difference in survival, EFS or CR duration (Figures 2a, b, and c). Furthermore, there was no association with a higher CR rate or survival among any of the 4 different regimens for the 52 patients with IDH gene mutations or IDH1 SNP. On univariate analysis for response, EFS, and OS having IDH1, or IDH2 mutations or any IDH aberration was not significantly associated with the outcome and predictors known to be important such as cytogenetics, NPM1mut/FLT3wt genotype, and de novo vs, secondary AML were significant on the multivariate model (data not shown).
Figure 1.
a Survival among patients with diploid karyotype by the presence of IDH1 mutation
b Survival among patients with diploid karyotype by the presence of IDH1 G105 SNP
c Survival among patients with diploid karyotype by the presence of IDH2 mutations
Figure 2.
a Survival for patients with IDH mutations or G105 SNP (dubbed “IDH positive) versus other
b Event-free survival for patients with IDH mutations or G105 SNP (dubbed “IDH positive) versus other
c Complete remission duration for patients with IDH mutations or G105 SNP (dubbed “IDH positive) versus other
IDH mutations and outcome in molecular subsets
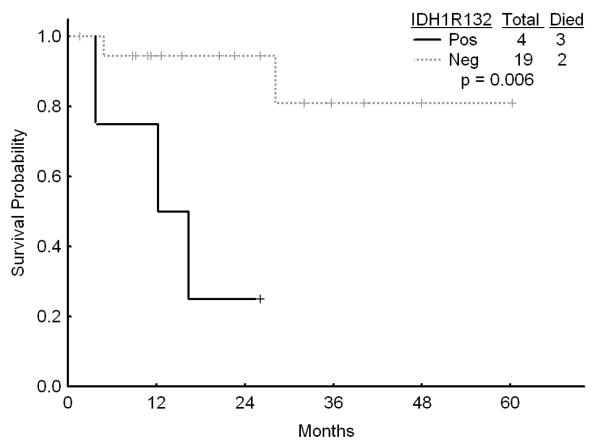
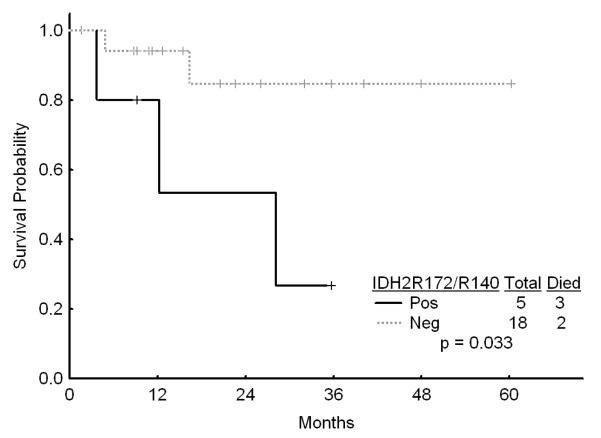
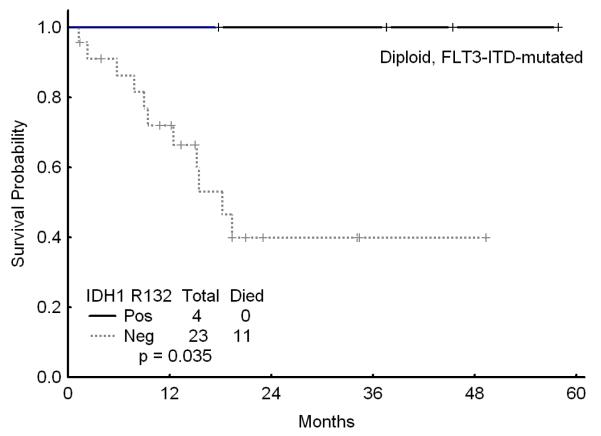
Among the 104 patients with diploid cytogenetics, IDH1 mutations occurred in 11 (11%), IDH2 mutations in 18 (17%), and G105 SNP in 17 (16%) of patients. The presence of IDH1 or IDH2 mutations or the IDH1 SNP did not affect the outcome and was not significant on univariate analysis (data not shown). However, among the 23 patients with diploid karyotype and the favorable genotype of NPM1mutFLT3WT, those with IDH1R132, IDH2R140, or IDH2R172 had a significantly worse survival as compared to those without these mutations (Figures 3a and b). However, the outcome of the patients with the diploid cytogenetics and the favorable genotype NPM1mutFLTWT was not affected by the presence or absence of the G105 SNP. Furthermore, among the patientas with diploid cytogenetics who were FLT3 mutated, presence of IDH1 mutation (but not IDH1 SNP G105 or IDH2 mutations) was associated with an improved outcome although this was based on a small number of patients (Figure 3c).
Figure 3.
a Survival in patients with diploid karyotype and NPM1mut/FLT3WT genotype by the presence of absence of IDH1 R132
b Survival in patients with diploid karyotype and NPM1mut/FLT3WT genotype by the presence of absence of IDH2 mutations
c Survival of patients with diploid karyotype and FLT3-ITD by the presence or absence of IDH1 R132
Association of outcome of therapy with the 4 regimens used in the molecular subsets
We also examined whether any of the 4 regimens used to treat the patients (IA, IAT, IAS, or IAV) had an impact on the response rate or survival of patients with IDH1, IDH2 mutations or G105 SNP and did not find any correlation with a better or worse outcome among the 4 regimens.
DISCUSSION
Identification of IDH1 mutations by sequencing the whole leukemia genome of a patient with diploid AML has generated significant interest in the IDH genes and their potential role in leukemogenesis.10,13,24 IDH mutations were previously described in patients with glioma where they are associated with a more favorable outcome.4,25 A number of studies have suggested that these mutations may be an early event in the oncogenesis of secondary GBM.24 Similarly, a number of reports have shown a low incidence of these mutations in myelodysplastic syndromes (MDS) and myeloproliferative disorders but have suggested a possible role for them in transformation into acute leukemia.26-28
In AML, a number of groups have examined the incidence and clinico-pathological features of these mutations in patients, particularly those with diploid cytogenetics.13-19,29,30 These studies have suggested that IDH1 and IDH2 mutations each occur in 5% to 15% of patients with a strong association with the diploid karyotype. The IDH1 SNP in codon G105 occurs in about 12% of cytogenetically normal patients and healthy volunteers and is associated with an inferior outcome with the impact most pronounced among those with the high risk genotype (NPMWT or FLT3-ITD positive).17
In the current study, we detected IDH1 and IDH2 mutations in 12 (7%) and 24 (14%) of patients (overall 21%) and IDH1 G105 in another 24 (14%) of patients. Among the patients with diploid cytogenetics, IDH1 mutations occurred in 11 (11%), IDH2 mutations in 18 (17%), and G105 SNP in 17 (16%) of patients. Overall, the presence of the mutations and/or the G105 SNP was not associated with any impact on the achievement of CR, EFS, and overall survival. Similarly, among the patients with diploid cytogenetics (n=104), the presence of these IDH alterations did not affect any of the outcome measures adversely. However, when we examined the group of patients with diploid karyotype and the favorable genotype of NPM1mut/FLT3WT (n=23), patients with either IDH1 or IDH2 mutations had a significantly worse survival than those without the mutations but the presence of G105 SNP did not affect the outcome, although it should be stressed that these findings are in small numbers of patients.
Our results are consistent with those from other investigators reporting the incidence of these mutations as about 5% to 15% (overall up to 30%) of patients with AML with a strong association with those with cytogenetically normal disease. 14-16,19,29,30 The variations amongst the various reports is likely due to different patient populations including differences in the age of the patients evaluated in the studies. We also detected a similar incidence of the G105 SNP among the patients with AML as was previously reported by Wagner et al but failed to show an adverse prognostic impact on survival as suggested by them.17 Overall, alterations in the IDH genes account for a sizable proportion of patients, particularly those with normal cytogenetics and appear to have an impact on survival among those with a favorable genotype of NPM1mut/FLT3WT. This is considerable importance in further refining the prognosis of this group of patients and the potential for different forms of post-remission therapy. Importantly, we also were not able to show any impact of the selection of the 4 different treatment regimens on the outcome of patients with IDH alterations.
Further studies on the role of IDH enzymes in cellular metabolism is likely to provide further insight into the association of the IDH gene alterations with diploid karyotype AML and their impact on the outcome of the subset with NPM1 but without FLT3 ITD mutations. In gliomas, one possible theory of the improved outcome of the patients with IDH1 mutation is sensitization of the glioma cells to chemotherapy and radiation through the reduction of intracellular pools of NADPH.31 If this were to be a true mechanism in gliomas, the paradox in AML where, among the patients with favorable genotype, those with IDH mutations appear to have a worse outcome has to be reconciled. Other important effects of the deregulated IDH function such as reduction of decorboxylation of isocitrate to α-KG as well as increased conversion of α-KG to 2-hydroxyglutarate (2HG) may be potentially responsible for their prognostic influence in specific subsets; recent studies have demonstrated the accumulation of 2HG in AML cells.32,33 A potential hypothesis is that, through its structural similarities to α-KG, 2HG competitively inhibits several α-KG-dependent enzymes important in leukemogenesis. A recent report suggested that IDH mutated AML cells display global DNA hypermethylation with a specific signature, and expression of 2HG producing IDH alleles in cells induced global hypermethylation. 34 The authors further demonstrated that loss-of-function mutations in TET2, an α-KG dependent enzyme, also produced similar methylation defects as IDH mutants and expression of mutatnt IDH1/2 or depletion of TET2 resulted in impaired hematopoietic differentiation.34
ACKNOWLEDGMENTS
None
Footnotes
CONFLICT OF INTEREST The authors declare no relevant conflict of interest
AUTHOR CONTRIBUTIONS Farhad Ravandi, Keyur Patel, and Rajyalakshmi Luthra designed the study, provided material, analyzed the data, and wrote the manuscript. Stefan Faderl, Marina Konopleva, Tapan Kadia, Steven Kornblau, Michael Andreeff, Guillermo Garcia-Manero, Jorge Cortes, and Hagop Kantarjian provided patients and material, critically reviewed the manuscript and provided final approval.
Mark Brandt, Sherry Pierce, and Xuemei Wang collected and analyzed the data
REFERENCES
- 1.Haselbeck RJ, McAlister-Henn L. Function and expression of yeast mitochondrial NAD- and NADP-specific isocitrate dehydrogenases. J Biol Chem. 1993;268:12116–12122. [PubMed] [Google Scholar]
- 2.Plaut GW, Cook M, Aogaichi T. The subcellular location of isozymes of NADP-isocitrate dehydrogenase in tissues from pig, ox and rat. Biochim Biophys Acta. 1983;760:300–308. doi: 10.1016/0304-4165(83)90177-0. [DOI] [PubMed] [Google Scholar]
- 3.Sjoblom T, Jones S, Wood LD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 4.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bleeker FE, Lamba S, Leenstra S, et al. IDH1 mutations at residue p.R132 (IDH1(R132)) occur frequently in high-grade gliomas but not in other solid tumors. Hum Mutat. 2009;30:7–11. doi: 10.1002/humu.20937. [DOI] [PubMed] [Google Scholar]
- 6.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118:469–474. doi: 10.1007/s00401-009-0561-9. [DOI] [PubMed] [Google Scholar]
- 8.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao S, Lin Y, Xu W, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009;324:261–265. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reitman ZJ, Yan H. Isocitrate dehydrogenase 1 and 2 mutations in cancer: alterations at a crossroads of cellular metabolism. J Natl Cancer Inst. 102:932–941. doi: 10.1093/jnci/djq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang MR, Kim MS, Oh JE, et al. Mutational analysis of IDH1 codon 132 in glioblastomas and other common cancers. Int J Cancer. 2009;125:353–355. doi: 10.1002/ijc.24379. [DOI] [PubMed] [Google Scholar]
- 12.Park SW, Chung NG, Han JY, et al. Absence of IDH2 codon 172 mutation in common human cancers. Int J Cancer. 2009;125:2485–2486. doi: 10.1002/ijc.24647. [DOI] [PubMed] [Google Scholar]
- 13.Mardis ER, Ding L, Dooling DJ, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcucci G, Maharry K, Wu YZ, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol. 28:2348–2355. doi: 10.1200/JCO.2009.27.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paschka P, Schlenk RF, Gaidzik VI, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol. 28:3636–3643. doi: 10.1200/JCO.2010.28.3762. [DOI] [PubMed] [Google Scholar]
- 16.Boissel N, Nibourel O, Renneville A, et al. Prognostic impact of isocitrate dehydrogenase enzyme isoforms 1 and 2 mutations in acute myeloid leukemia: a study by the Acute Leukemia French Association group. J Clin Oncol. 28:3717–3723. doi: 10.1200/JCO.2010.28.2285. [DOI] [PubMed] [Google Scholar]
- 17.Wagner K, Damm F, Gohring G, et al. Impact of IDH1 R132 mutations and an IDH1 single nucleotide polymorphism in cytogenetically normal acute myeloid leukemia: SNP rs11554137 is an adverse prognostic factor. J Clin Oncol. 28:2356–2364. doi: 10.1200/JCO.2009.27.6899. [DOI] [PubMed] [Google Scholar]
- 18.Green CL, Evans CM, Hills RK, Burnett AK, Linch DC, Gale RE. The prognostic significance of IDH1 mutations in younger adult patients with acute myeloid leukemia is dependent on FLT3/ITD status. Blood. 116:2779–2782. doi: 10.1182/blood-2010-02-270926. [DOI] [PubMed] [Google Scholar]
- 19.Abbas S, Lugthart S, Kavelaars FG, et al. Acquired mutations in the genes encoding IDH1 and IDH2 both are recurrent aberrations in acute myeloid leukemia: prevalence and prognostic value. Blood. 116:2122–2126. doi: 10.1182/blood-2009-11-250878. [DOI] [PubMed] [Google Scholar]
- 20.Ravandi F, Cortes JE, Jones D, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol. 28:1856–1862. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jabbour E, Kantarjian H, Ravandi F, et al. A phase 1-2 study of a farnesyltransferase inhibitor, tipifarnib, combined with idarubicin and cytarabine for patients with newly diagnosed acute myeloid leukemia and high-risk myelodysplastic syndrome. Cancer. doi: 10.1002/cncr.25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Manero G, Tambaro FP, Bekele N, et al. Final report of a phase II trial of vorinostat, idarubicin and cytarabine in previously untreated acute myelogenous leukemia (AML) or high risk myelodysplastic syndrome (MDS) Blood. 2010;116 [Google Scholar]
- 23.Kiyoi H, Naoe T. FLT3 in human hematologic malignancies. Leuk Lymphoma. 2002;43:1541–1547. doi: 10.1080/1042819021000002866. [DOI] [PubMed] [Google Scholar]
- 24.Dang L, Jin S, Su SM. IDH mutations in glioma and acute myeloid leukemia. Trends Mol Med. 16:387–397. doi: 10.1016/j.molmed.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Weller M, Felsberg J, Hartmann C, et al. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol. 2009;27:5743–5750. doi: 10.1200/JCO.2009.23.0805. [DOI] [PubMed] [Google Scholar]
- 26.Pardanani A, Patnaik MM, Lasho TL, et al. Recurrent IDH mutations in high-risk myelodysplastic syndrome or acute myeloid leukemia with isolated del(5q) Leukemia. 24:1370–1372. doi: 10.1038/leu.2010.98. [DOI] [PubMed] [Google Scholar]
- 27.Thol F, Weissinger EM, Krauter J, et al. IDH1 mutations in patients with myelodysplastic syndromes are associated with an unfavorable prognosis. Haematologica. 95:1668–1674. doi: 10.3324/haematol.2010.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green A, Beer P. Somatic mutations of IDH1 and IDH2 in the leukemic transformation of myeloproliferative neoplasms. N Engl J Med. 362:369–370. doi: 10.1056/NEJMc0910063. [DOI] [PubMed] [Google Scholar]
- 29.Chou WC, Hou HA, Chen CY, et al. Distinct clinical and biologic characteristics in adult acute myeloid leukemia bearing the isocitrate dehydrogenase 1 mutation. Blood. 115:2749–2754. doi: 10.1182/blood-2009-11-253070. [DOI] [PubMed] [Google Scholar]
- 30.Thol F, Damm F, Wagner K, et al. Prognostic impact of IDH2 mutations in cytogenetically normal acute myeloid leukemia. Blood. 116:614–616. doi: 10.1182/blood-2010-03-272146. [DOI] [PubMed] [Google Scholar]
- 31.Bleeker FE, Atai NA, Lamba S, et al. The prognostic IDH1( R132 ) mutation is associated with reduced NADP+-dependent IDH activity in glioblastoma. Acta Neuropathol. 119:487–494. doi: 10.1007/s00401-010-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gross S, Cairns RA, Minden MD, et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med. 207:339–344. doi: 10.1084/jem.20092506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward PS, Patel J, Wise DR, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]