Abstract
Our aim was to evaluate lipid peroxidation, expressed as thiobarbituric acid-reactive substances (TBARS), nitric oxide metabolites (nitrite + nitrate) expressed as NOx, and TBARS/NOx ratio in a group of subjects with metabolic syndrome (MS). In this regard we enrolled 106 subjects with MS defined according to the IDF criteria, subsequently subdivided into diabetic (DMS) and nondiabetic (NDMS) and also into subjects with a low triglycerides/HDL-cholesterol (TG/HDL-C) index or with a high TG/HDL-C index. In the entire group and in the four subgroups of MS subjects we found an increase in TBARS and NOx levels and a decrease in TBARS/NOx ratio in comparison with normal controls. Regarding all these parameters no statistical difference between DMS and NDMS was evident, but a significant increase in NOx was present in subjects with a high TG/HDL-C index in comparison with those with a low index. In MS subjects we also found a negative correlation between TBARS/NOx ratio and TG/HDL-C index. Considering the hyperactivity of the inducible NO synthase in MS, these data confirm the altered redox and inflammatory status that characterizes the MS and suggest a link between lipid peroxidation, inflammation, and insulin resistance, evaluated as TG/HDL-C index.
1. Introduction
The metabolic syndrome (MS) is characterized by an elevated cardiovascular morbidity and mortality [1].
A strong association between MS and oxidative stress has been demonstrated [2, 3]. The end products of lipid peroxidation, which refers to the oxidative degradation of lipids resulting in cell membrane damage, are malondialdehyde (MDA), 4-hydroxynonenal (HNE), and 4-oxy-2-nonenal (ONE), obtained by the oxidation of polyunsaturated fatty acids. Other systemic markers of lipid peroxidation are oxidized low-density lipoprotein (oxLDL), isoprostanes, and thiobarbituric acid-reactive substances (TBARS), which are increased in MS subjects [4–10]. Plasma lipid peroxidation products levels are higher in subjects with 5 MS components compared with those with 2 or 3 MS components [11]. A multivariate analysis, performed by Leiva et al., showed that elevated levels of TBARS are associated with a 21-fold risk for the development of MS [12]. OxLDLs are increased in newly diagnosed MS subjects [13] and are also considered a predictive biomarker for MS [14]. OxLDLs are significantly correlated with coronary artery calcium measured using computerized tomography and with greater internal carotid intima-media thickness in MS subjects [15]. Recently, in diabetic subjects with coronary artery disease, elevated serum MDA-modified LDL (MDA-LDL) levels have been observed and, in subjects with more than 4 MS criteria, a higher MDA-LDL/LDL-cholesterol ratio has been found. MDA-LDLs are correlated with lipid profile, C-reactive protein (CRP), and adiponectin levels [16]. Also the urinary 8-epi-prostaglandin F2α (8-epi-PGF2α) is increased and significantly correlated with CRP in MS subjects, underlining the close link between oxidative stress and systemic inflammation [17].
Recently in a group of MS subjects we observed a marked increase of the nitric oxide metabolites (NOx), not associated with the presence of diabetes mellitus (DM) [18]. Our data were in agreement with other papers [19–21] that described a NOx increase in MS. The explanation of this finding is ascribed to the degree of inflammation accompanying MS that induces the expression of inducible nitric oxide synthase (iNOS) by macrophages. As it is known, in addition, the iNOS is activated by cytokines, such as TNF-α, IL-1β, and interferon [22–24], even if in literature there are contrasting data regarding the direct relationship between NOx and some of these cytokines [25–28].
In this reexamination of our group of MS subjects, we calculated also the TBARS/NOx ratio that, up to now, has been evaluated in preeclampsia [29], in juvenile essential hypertension [30], and in hypertensive adolescents with obesity or uraemia [31, 32]. In MS the TBARS/NOx ratio may be considered as an integrated marker of plasma lipid peroxidation and degree of inflammation.
In this study, we evaluated lipid peroxidation, expressed as TBARS, nitric oxide metabolites (NOx), and their ratio (TBARS/NOx) in a group of MS subjects subdivided according to the presence or not of diabetes mellitus and also according to the degree of insulin resistance, expressed as triglycerides/HDL-cholesterol (TG/HDL-C) index; this index, in fact, may be considered an indicator of insulin resistance [33–35] its logarithm being inversely correlated with insulin sensitivity measures, such as the homeostasis model assessment and the quantitative insulin sensitivity check index [36].
2. Subjects and Methods
We enrolled 106 subjects (45 women and 61 men, mean age: 53.5 ± 8.9 years) with MS defined according to the International Diabetes Federation criteria [37]. Subsequently, MS subjects were subdivided, respectively, into diabetics (DMS) (14 women and 29 men) and nondiabetics (NDMS) (31 women and 32 men) and into MS subjects with low (27 women and 26 men) or with high (18 women and 35 men) TG/HDL-C ratio. These two last subgroups of 53 subjects were selected according to the median value of the TG/HDL-C ratio. The control group consisted of 41 subjects (14 women and 27 men, mean age: 41.6 ± 7.9 years) selected from the hospital staff; control subjects were free of medical diseases as assessed by clinical history, physical examination, electrocardiography, and routine hematological and urine analysis. In all the participants cholesterol and triglycerides were measured by standard enzymatic procedures, high-density lipoprotein (HDL) cholesterol after phosphotungstic acid/magnesium chloride precipitation and enzymatic determination of cholesterol, and low-density lipoprotein (LDL) cholesterol was determined by the Friedewald formula. Mean and S.D. of age, anthropometric profile, glycometabolic pattern, lipid profile, and blood pressure values of the entire group and of the four subgroups of MS subjects (subdivided according to the presence of DM or to the TG/HDL-C ratio) are shown in Tables 1 and 2.
Table 1.
| Mean ± S.D. | Range | |
|---|---|---|
| Waist circumference (cm) | 106.7 ± 11.2 | 86–135 |
| Body mass index (Kg/m2) | 32.21 ± 4.53 | 22.5–45.2 |
| Systolic blood pressure (mmHg) | 132.1 ± 16.3 | 100–210 |
| Diastolic blood pressure (mmHg) | 81.2 ± 9.9 | 50–110 |
| Fasting glucose (mg/dL) | 114.3 ± 44.3 | 68–347 |
| Total cholesterol (mg/dL) | 213.9 ± 53.0 | 106–390 |
| HDL-cholesterol (mg/dL) | 40.4 ± 10.8 | 14–80 |
| LDL-cholesterol (mg/dL) | 133.2 ± 46.5 | 52.4–292.0 |
| Triglycerides (mg/dL) | 220.2 ± 147.8 | 52–759 |
| TG/HDL index | 6.399 ± 6.033 | 1.060–32.52 |
Means ± S.D. and range of the anthropometric profile, blood pressure values, and metabolic pattern in all MS subjects.
MS: metabolic syndrome.
Table 2.
| MS patients withoutDM | MS patients withDM | MS patients with low TG/HDL index | MS patients with high TG/HDL index | |
|---|---|---|---|---|
| Waist circumference (cm) | 102.8 ± 8.8 | 114.4 ± 11.7§ | 106.7 ± 11.16 | 106.8 ± 11.46 |
| Body mass index (Kg/m2) | 31.5 ± 4.2 | 33.2 ± 5.0* | 32.45 ± 4.55 | 31.94 ± 4.55 |
| Systolic blood pressure (mmHg) | 130.0 ± 13.3 | 136.0 ± 20.5 | 133.2 ± 18.8 | 131.1 ± 13.7 |
| Diastolic blood pressure (mmHg) | 82.2 ± 8.9 | 79.6 ± 11.4 | 80.5 ± 10.9 | 81.94 ± 8.9 |
| Fasting glucose (mg/dL) | 92.2 ± 10.3 | 147.5 ± 54.2§ | 114.5 ± 39.7 | 114.2 ± 48.7 |
| Total cholesterol (mg/dL) | 228.0 ± 48.3 | 193.1 ± 53.1§ | 201.2 ± 40.8 | 226.6 ± 60.6† |
| HDL-cholesterol (mg/dL) | 39.7 ± 9.3 | 41.4 ± 12.8 | 46.9 ± 9.3 | 33.9 ± 8.1‡ |
| LDL-cholesterol (mg/dL) | 147.9 ± 45.8 | 112.8 ± 39.7§ | 123.5 ± 37.5 | 143.0 ± 58.7 |
| Triglycerides (mg/dL) | 231.0 ± 145.9 | 204.2 ± 150.9 | 137.4 ± 42.6 | 302.9 ± 168.4‡ |
| TG/HDL index | 6.739 ± 6.190 | 5.900 ± 5.832 | 3.00 ± 0.95 | 9.80 ± 7.00‡ |
Means ± S.D. of the anthropometric profile, blood pressure values, and metabolic pattern in MS patients subdivided, respectively, into nondiabetics and diabetics and into patients with low and high TG/HDL index.
*P < 0.05; § P < 0.001 versus MS patients without DM (Student's t-test).
† P < 0.05; ‡ P < 0.001 versus MS patients with low TG/HDL index (Student's t-test).
MS: metabolic syndrome.
DM: diabetes mellitus.
At fasting blood samples were collected by venous puncture from the antecubital vein of each subject and immediately transferred to glass tubes anticoagulated with EDTA-K3; then we evaluated lipid peroxidation and NO metabolites.
2.1. Lipid Peroxidation
The oxidation of polyunsaturated fatty acids was determined in plasma by the detection of the thiobarbituric acid-reactive substances (TBARS) generated by peroxidative processes, which include lipid peroxides and MDA. The evaluation of TBARS was made by fluorimetry, using the 1,1,3,3-tetramethoxypropane as standard [38].
2.2. NO Metabolites
Considering that in vivo NO has a very short life (less than 0.1 sec) and it is converted into nitrite (NO2 −), which has a half-life of few minutes, and into the more stable nitrate (NO3 −), NOx represents almost only the nitrate concentration. In the laboratory method adopted by us at first nitrate was converted into nitrite by a nitrate reductase, and then nitrite was assessed by spectrophotometry after the addition of the Griess reagent [39].
2.3. Statistical Analysis
The values were expressed as means ± S.D. The difference between the control group and MS subjects was evaluated according to Student's t-test for unpaired data. The comparison between control group and MS subjects subdivided according to the presence or not of DM and according to the TG/HDL-C index was performed using the one-way analysis of variance (ANOVA), integrated with Bonferroni's multiple posttest. The values of TBARS and NOx and of TBARS/NOx ratio were correlated with the age, the anthropometric profile, the blood pressure values, and the glycometabolic and lipid pattern using the linear regression test. The null hypothesis was rejected for P values less than 0.5.
3. Results
Examining the age, the anthropometric profile, the blood pressure values, and the glycometabolic pattern of MS subjects subdivided according to the presence or not of DM, we observed (Table 2) that age, waist circumference, and blood glucose levels were higher in DMS, while total cholesterol and LDL-cholesterol were higher in NDMS.
Examining the same parameters in MS subjects subdivided according to the TG/HDL-C index (Table 2), we observed that total cholesterol and triglycerides were increased in the subgroup with high TG/HDL-C index and that the HDL-cholesterol was increased in the subgroup with low TG/HDL-C index.
In the entire group of MS subjects an increase in TBARS and in NOx levels and a marked decrease in TBARS/NOx ratio were evident in comparison with normal controls (Table 3). In the control group, as well as in the whole group of MS subjects, no statistical correlation between TBARS and NOx was observed (data not shown). Subdividing MS subjects into DMS and NDMS (Table 4) and also according to the low or high TG/HDL-C index (Table 5), we found an increase in TBARS and in NOx levels and a decrease in TBARS/NOx ratio in all the subgroups compared with normal controls. Employing the Bonferroni posttest, instead, we found no significant difference between DMS and NDMS regarding TBARS, NOx, and TBARS/NOx ratio (Table 4), while we noted a significant increase in NOx in subjects with a high TG/HDL-C index in comparison with those with a low index (Table 5).
Table 3.
| Control subjects | All MS patients | |
|---|---|---|
| TBARS (nmol/mL) | 5.902 ± 1.211 | 8.983 ± 0.722§ |
| NOx (nmol/mL) | 28.07 ± 18.83 | 79.82 ± 29.22§ |
| TBARS/NOx ratio | 0.363 ± 0.311 | 0.128 ± 0.049§ |
Means ± S.D. of TBARS, NOx, and TBARS/NOx ratio in control subjects and in all MS patients.
§ P < 0.001 versus control subjects (Student's t-test).
MS: metabolic syndrome.
TBARS: thiobarbituric acid-reactive substances.
NOx: nitric oxide metabolites (nitrite + nitrate).
Table 4.
| Control subjects | MS patients without DM | MS patients with DM | F | |
|---|---|---|---|---|
| TBARS (nmol/mL) | 5.902 ± 1.211 | 8.834 ± 0.628§ | 9.200 ± 0.801§ | 185.81 |
| NOx (nmol/mL) | 28.07 ± 18.83 | 80.99 ± 33.93§ | 78.10 ± 20.76§ | 55.11 |
| TBARS/NOx ratio | 0.363 ± 0.311 | 0.129 ± 0.057§ | 0.125 ± 0.033§ | 28.51 |
Means ± S.D. of TBARS, NOx, and TBARS/NOx ratio in control subjects and in MS patients subdivided into nondiabetics and diabetics.
1 P < 0.001 (ANOVA).
§ P < 0.001 versus control subjects (Bonferroni's test).
MS: metabolic syndrome.
DM: diabetes mellitus.
TBARS: thiobarbituric acid-reactive substances.
NOx: nitric oxide metabolites (nitrite + nitrate).
Table 5.
| Control subjects | MS patients with low TG/HDL index | MS patients with high TG/HDL index | F | |
|---|---|---|---|---|
| TBARS (nmol/mL) | 5.902 ± 1.211 | 8.902 ± 0.581§ | 9.063 ± 0.838§ | 179.61 |
| NOx (nmol/mL) | 28.07 ± 18.83 | 72.67 ± 27.34§ | 86.97 ± 29.54§# | 61.91 |
| TBARS/NOx ratio | 0.363 ± 0.311 | 0.140 ± 0.057§ | 0.115 ± 0.035§ | 28.91 |
Means ± S.D. of TBARS, NOx, and TBARS/NOx ratio in control subjects and in MS patients subdivided according to the TG/HDL index.
1 P < 0.001 (ANOVA).
§ P < 0.001 versus control subjects (Bonferroni's test).
# P < 0.05 versus MS patients with low TG/HDL index (Bonferroni's test).
MS: metabolic syndrome.
TBARS: thiobarbituric acid-reactive substances.
NOx: nitric oxide metabolites (nitrite + nitrate).
In the entire group of MS subjects we found a positive correlation between TBARS and TG/HDL-C index (r = 0.250; P < 0.01) and between NOx and TG/HDL-C index (r = 0.321; P < 0.001) and a negative correlation between TBARS/NOx ratio and TG/HDL-C index (r = −0.238; P < 0.05) (Figure 1).
Figure 1.
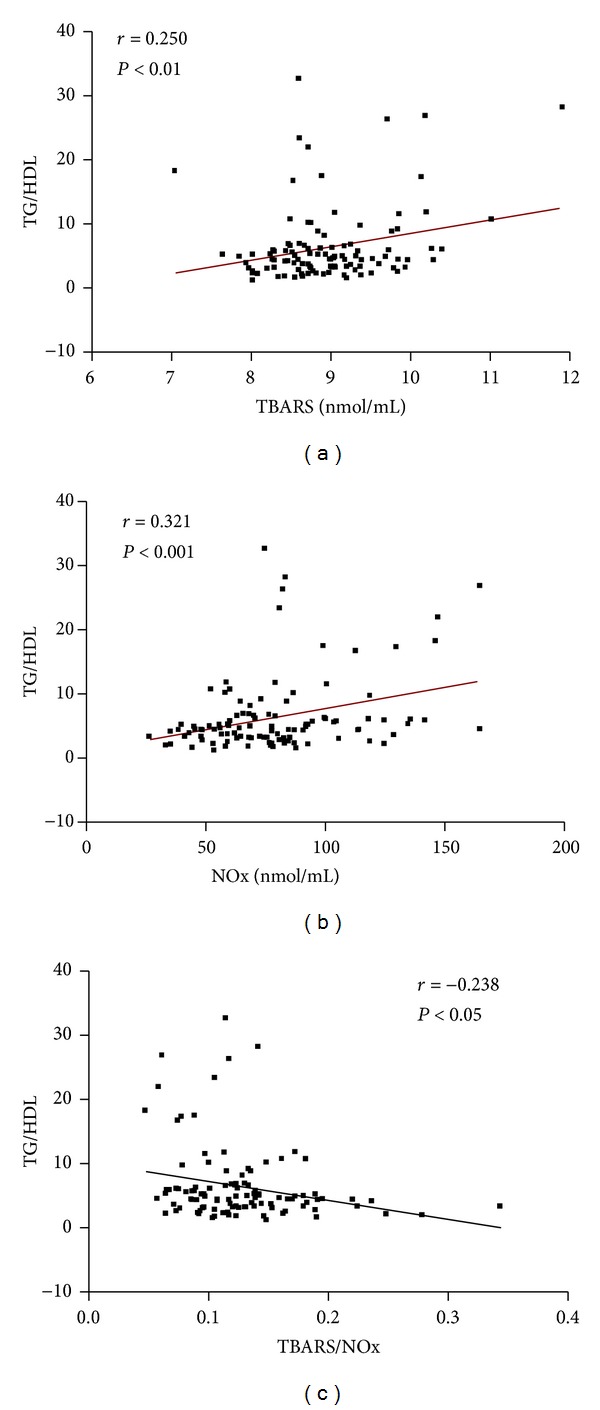
Correlations between TG/HDL index and, respectively, TBARS, NOx, and TBARS/NOx ratio in MS patients.
Examining the linear regression among TBARS, age, anthropometric profile, blood pressure values, and glycometabolic pattern, in the entire group of MS subjects we observed only a positive correlation between TBARS and fasting blood glucose level (r = 0.206; P < 0.02).
Examining the linear regression among NOx and all the parameters previously considered, in the entire group of MS subjects we found only a positive correlation between NOx and triglycerides (r = 0.344; P < 0.001).
Examining instead the linear regression among TBARS/NOx ratio and the above-mentioned characteristics of the MS subjects, we found in the whole group only a negative correlation between TBARS/NOx and triglycerides (r = −0.259; P < 0.007).
4. Discussion
The analysis of the data concerning the evaluation of lipid peroxidation, nitric oxide metabolites, and their ratio in MS shows some aspects that deserve to be underlined.
The first point concerns the asymmetrical increase in TBARS and NOx in the entire group of MS subjects; in fact, the TBARS increase is of 52.2% while the NOx increase is of 184.4%; therefore, the TBARS/NOx ratio is markedly decreased (−64.7%) in comparison with normal controls. This asymmetrical behavior might explain the lack of correlation between TBARS and NOx observed by us, differently from others, who found a positive correlation between hydroperoxide levels and NOx in MS [40]. In this regard, it must be considered that we evaluated a parameter of lipid peroxidation (TBARS) that includes not only hydroperoxides but also malondialdehyde.
The second point concerns the different behavior of TBARS, NOx, and TBARS/NOx ratio in MS subjects subdivided according to the presence or not of DM or according to the TG/HDL-C index. In fact, the subdivision of MS subjects into DMS and NDMS does not show any difference in these parameters, while the subdivision according to the TG/HDL-C index shows a difference in NOx between low TG/HDL-C index subjects and high TG/HDL-C index subjects. This behavior is imputable to the marked increase in NOx observed in the subgroup where a condition of greater insulin resistance is evident.
The third point that needs to be investigated is the bidirectional role played by the insulin resistance in lipid peroxidation and NOx. The pivotal role of the insulin resistance in MS is well known and the results observed in this research seem to confirm this assumption. In the entire group of MS subjects a positive correlation between TBARS and TG/HDL-C index and between NOx and TG/HDL-C index is evident, suggesting a close link between the degree of insulin resistance, the lipid peroxidation, and the synthesis of NOx. In other clinical conditions that are included among the principal criteria for MS, such as obesity [25] and arterial hypertension [41], a significant correlation between NOx and insulin levels was found. However, no correlation between NOx and insulin resistance (evaluated as steady state plasma glucose) has been observed in DM subjects [42]. In nondiabetic hypercholesterolemic subjects a positive correlation between the urinary 8-epi-PGF2α and the HOMA-IR was observed [43]. From our data we observed an interesting negative correlation between TG/HDL-C index and TBARS/NOx ratio, which underlines how the reduction of TBARS/NOx ratio is associated with the degree of insulin resistance.
All the aspects described in this third point call the attention to the link between lipid peroxidation, iNOS activity, and insulin resistance. In animal models [44, 45] the iNOS seems to play an indirect role in the pathogenesis of the insulin resistance in peripheral tissues, and in particular in the skeletal muscle. Similarly, recent research on experimental models has underlined how some bioproducts of lipid peroxidation, such as HNE and ONE, are able to induce structural and functional changes in human insulin [46] and how HNE causes insulin resistance [47]. The role played by lipid peroxidation in the insulin resistance development has been suggested also in sedentary adults [48], in which lipid peroxides seem to act on the skeletal muscle. Recently [49] other authors have hypothesized that HNE and oxysterols may be the link between the adipose tissue dysfunction and the abnormality of glucose homeostasis.
In agreement with our data, some authors observed in DM subjects a correlation between TBARS and fasting glucose level [50]. Differently from others, who found a positive correlation between oxLDL, age, systolic blood pressure, and body mass index (BMI) [51], a positive correlation between MDA and BMI [52], and a positive correlation between lipoperoxides and systolic blood pressure [53], we did not note any correlation between TBARS, age, anthropometric parameters, and blood pressure values. In this research no correlation between TBARS, total cholesterol, LDL-cholesterol, and HDL-cholesterol was noted, while we observed a negative correlation between TBARS and triglycerides, considered by us occasional and physiologically inexplicable. Other authors in hypercholesterolemic subjects found a positive correlation between MDA, total cholesterol, and LDL-cholesterol and a negative correlation between MDA and HDL-C [54]. Regarding the NOx we observed neither correlation between NOx and age nor correlation between NOx, anthropometric profile, and blood pressure values, differently from other authors [55–57]. In addition, we did not find any correlation between NOx and lipid profile, although some significant correlations between NOx and lipid parameters have been observed in adolescent subjects [58]. The positive correlation between NOx and triglycerides found by us in these subjects agrees with that reported by other authors in postmenopausal women with MS [21] and in healthy population [56], but it has not been observed in normal-weight obese syndrome [28].
From the analysis of the data reported in this study, the data reported in this study clearly confirmed the abnormality of the oxidative status accompanying the MS, as previously described by us in the same group of MS subjects, in which we have examined the behavior of NOx [18], and also the protein oxidation [59] and the total antioxidant status (in press).
5. Conclusions
In conclusion, the simultaneous examination of TBARS, NOx, and TBARS/NOx ratio in MS subjects, subdivided, respectively, according to the presence of DM or to the TG/HDL-C index, shows a different trend of these parameters in relation to the subdivision criteria and, in particular, a significant positive association between NOx and the degree of insulin resistance. This datum, like other aspects discussed in the text, might suggest considering the possible use of the antioxidant treatment in MS subjects in order to attenuate the insulin resistance that seems to affect the peripheral tissues, and in particular the skeletal muscle. In this regard, up to now, several molecules, such as vitamins C and E and flavonoids [49], α-lipoic acid, α-tocopherol, glutathione, N-acetylcysteine, coenzyme Q10, and taurine [60], have been employed to prevent or to treat MS and associated diseases.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Simons LA, Simons J, Friedlander Y, McCallum J. Is prediction of cardiovascular disease and al-cause mortality genuinely driven by the metabolic syndrome, and independently from its component variables? The Dubbo Study. Heart Lung and Circulation. 2011;20(4):214–219. doi: 10.1016/j.hlc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Hopps E, Noto D, Caimi G, Averna MR. A novel component of the metabolic syndrome: the oxidative stress. Nutrition, Metabolism and Cardiovascular Diseases. 2010;20(1):72–77. doi: 10.1016/j.numecd.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Demircan N, Gürel A, Armutcu F, Ünalacak M, Aktunç E, Atmaca H. The evaluation of serum cystatin C, malondialdehyde, and total antioxidant status in patients with metabolic syndrome. Medical Science Monitor. 2008;14(2):CR97–CR101. [PubMed] [Google Scholar]
- 4.Barona J, Jonesa JJ, Kopecc RE, et al. A Mediterranean-style low-glycemic-load diet increases plasma carotenoids and decreases LDL oxidation in women with metabolic syndrome. Journal of Nutritional Biochemistry. 2012;23:609–615. doi: 10.1016/j.jnutbio.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones JL, Comperatore M, Barona J, et al. A Mediterranean-style, low-glycemic-load diet decreases atherogenic lipoproteins and reduces lipoprotein (a) and oxidized low-density lipoprotein in women with metabolic syndrome. Metabolism: Clinical and Experimental. 2012;61(3):366–372. doi: 10.1016/j.metabol.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Kim M, Paik JK, Kang R, Kim SY, Lee SH, Lee JH. Increased oxidative stress in normal-weight postmenopausal women with metabolic syndrome compared with metabolically healthy overweight/obese individuals. Metabolism: Clinical and Experimental. 2013;62(4):554–560. doi: 10.1016/j.metabol.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Kosola J, Vaara JP, Ahotupa M, et al. Elevated concentration of oxidized LDL together with poor cardiorespiratory and abdominal muscle fitness predicts metabolic syndrome in young men. Metabolism: Clinical and Experimental. 2013;62(7):992–999. doi: 10.1016/j.metabol.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Mitjavila MT, Fandos M, Salas-Salvadó J, et al. The Mediterranean diet improves the systemic lipid and DNA oxidative damage in metabolic syndrome individuals. A randomized, controlled, trial. Clinical Nutrition. 2013;32(2):172–178. doi: 10.1016/j.clnu.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Park SH, Kim JY, Lee JH, Park HY. Elevated oxidized low-density lipoprotein concentrations in postmenopausal women with the metabolic syndrome. Clinica Chimica Acta. 2011;412(5-6):435–440. doi: 10.1016/j.cca.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Yokota T, Kinugawa S, Yamato M, et al. Systemic oxidative stress is associated with lower aerobic capacity and impaired skeletal muscle energy metabolism in patients withmetabolic syndrome. Diabetes Care. 2013;36(5):1341–1346. doi: 10.2337/dc12-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yubero-Serrano EM, Delgado-Lista J, Peña-Orihuela P, et al. Oxidative stress is associated with the number of components of metabolic syndrome: LIPGENE study. Experimental and Molecular Medicine. 2013;45(6, article e28) doi: 10.1038/emm.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leiva E, Mujica V, Sepúlveda P, et al. High levels of iron status and oxidative stress in patients with metabolic syndrome. Biological Trace Element Research. 2013;151(1):1–8. doi: 10.1007/s12011-012-9525-3. [DOI] [PubMed] [Google Scholar]
- 13.Jialal I, Devaraj S, Adams-Huet B, Chen X, Kaur H. Increased cellular and circulating biomarkers of oxidative stress in nascent metabolic syndrome. Journal of Clinical Endocrinology and Metabolism. 2012;97(10):E1844–E1850. doi: 10.1210/jc.2012-2498. [DOI] [PubMed] [Google Scholar]
- 14.Rao VS, Nagaraj RK, Hebbagodi S, Kadarinarasimhiah NB, Kakkar VV. Association of inflammatory and oxidative stress markers with metabolic syndrome in Asian Indians in India. Cardiology Research and Practice. 2011;2011:8 pages. doi: 10.4061/2011/295976.295976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaidya D, Szklo M, Cushman M, et al. Association of endothelial and oxidative stress with metabolic syndrome and subclinical atherosclerosis: multi-ethnic study of atherosclerosis. European Journal of Clinical Nutrition. 2011;65(7):818–825. doi: 10.1038/ejcn.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuda M, Tamura R, Kanno K, et al. Impact of dyslipidemic components of metabolic syndrome, adiponectin levels, and anti-diabetes medications on malondialdehyde-modified low-density lipoprotein levels in statin-treated diabetes patients with coronary artery disease. Diabetology & Metabolic Syndrome. 2013;5(article 77) doi: 10.1186/1758-5996-5-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park S, Kim M, Paik JK, Jang YJ, Lee SH, Lee JH. Oxidative stress is associated with C-reactive protein in nondiabetic postmenopausal women, independent of obesity and insulin resistance. Clinical Endocrinology. 2013;79(1):65–70. doi: 10.1111/j.1365-2265.2012.04512.x. [DOI] [PubMed] [Google Scholar]
- 18.Caimi G, Hopps E, Montana M, et al. Evaluation of nitric oxide metabolites in a group of subjects with metabolic syndrome. Diabetes and Metabolic Syndrome: Clinical Research and Reviews. 2012;6(3):132–135. doi: 10.1016/j.dsx.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Zahedi Asl S, Ghasemi A, Azizi F. Serum nitric oxide metabolites in subjects with metabolic syndrome. Clinical Biochemistry. 2008;41(16-17):1342–1347. doi: 10.1016/j.clinbiochem.2008.08.076. [DOI] [PubMed] [Google Scholar]
- 20.Stühlinger MC, Abbasi F, Chu JW, et al. Relationship between insulin resistance and an endogenous nitric oxide synthase inhibitor. Journal of the American Medical Association. 2002;287(11):1420–1426. doi: 10.1001/jama.287.11.1420. [DOI] [PubMed] [Google Scholar]
- 21.Chedraui P, Escobar GS, Ramírez C, et al. Nitric oxide and pro-inflammatory cytokine serum levels in postmenopausal women with the metabolic syndrome. Gynecological Endocrinology. 2012;28(10):787–791. doi: 10.3109/09513590.2012.671395. [DOI] [PubMed] [Google Scholar]
- 22.Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacological Reviews. 1991;43(2):109–142. [PubMed] [Google Scholar]
- 23.Förstermann U, Kleinert H, Gath I, Schwarz P, Closs EI, Dun NJ. Expression and expressional control of nitric oxide synthases in various cell types. Advances in Pharmacology. 1995;34:171–186. doi: 10.1016/s1054-3589(08)61085-6. [DOI] [PubMed] [Google Scholar]
- 24.Weinberg JB. Nitric oxide production and nitric oxide synthase type 2 expression by human mononuclear phagocytes: a review. Molecular Medicine. 1998;4(9):557–591. doi: 10.1007/BF03401758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olszanecka-Glinianowicz M, Zahorska-Markiewicz B, Janowska J, Zurakowski A. Serum concentrations of nitric oxide, tumor necrosis factor (TNF)-α and TNF soluble receptors in women with overweight and obesity. Metabolism: Clinical and Experimental. 2004;53(10):1268–1273. doi: 10.1016/j.metabol.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Pereira FO, Frode TS, Medeiros YS. Evaluation of tumour necrosis factor alpha, interleukin-2 soluble receptor, nitric oxide metabolites, and lipids as inflammatory markers in type 2 diabetes mellitus. Mediators of Inflammation. 2006;2006:7 pages. doi: 10.1155/MI/2006/39062.39062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouvia SM, La Greca RD, Zanaro NL, Palmer L, Sassetti B. Endothelial dysfunction, nitric oxide and platelet activation in hypertensive and diabetic type II patients. Thrombosis Research. 2001;102(2):107–114. doi: 10.1016/s0049-3848(01)00237-7. [DOI] [PubMed] [Google Scholar]
- 28.di Renzo L, Galvano F, Orlandi C, et al. Oxidative stress in normal-weight obese syndrome. Obesity. 2010;18(11):2125–2130. doi: 10.1038/oby.2010.50. [DOI] [PubMed] [Google Scholar]
- 29.Kumar CA, Das UN. Lipid peroxides, anti-oxidants and nitric oxide in patients with pre-eclampsia and essential hypertension. Medical Science Monitor. 2000;6(5):901–907. [PubMed] [Google Scholar]
- 30.Túri S, Friedman A, Bereczki C, et al. Oxidative stress in juvenile essential hypertension. Journal of Hypertension. 2003;21(1):145–152. doi: 10.1097/00004872-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 31.Baráth Á, Németh I, Karg E, et al. Roles of paraoxonase and oxidative stress in adolescents with uraemic, essential or obesity-induced hypertension. Kidney and Blood Pressure Research. 2006;29(3):144–151. doi: 10.1159/000095124. [DOI] [PubMed] [Google Scholar]
- 32.Baráth Á, Túri S, Németh I, et al. Different pathomechanisms of essential and obesity-associated hypertension in adolescents. Pediatric Nephrology. 2006;21(10):1419–1425. doi: 10.1007/s00467-006-0215-2. [DOI] [PubMed] [Google Scholar]
- 33.Díaz González L, Suárez García S, López Fernández V, Álvarez Cosmea A, Arias García MT, Álvarez Menéndez F. Identification of individuals with insulin resistance by means of clinical measurements and routine biochemical markers. Construction of an individual risk index. Revista Clinica Espanola. 2007;207(6):271–277. doi: 10.1157/13106848. [DOI] [PubMed] [Google Scholar]
- 34.González-Chávez A, Simental-Mendía LE, Elizondo-Argueta S. Elevated triglycerides/HDL-cholesterol ratio associated with insulin resistance. Cirugia y Cirujanos. 2011;79(2):126–131. [PubMed] [Google Scholar]
- 35.Soutelo J, Graffigna M, Honfi M, et al. Triglicéridos/HDL-cholesterol ratio: in adolescents without cardiovascular risk factors. Archivos Latinoamericanos de Nutricion. 2012;62(2):167–171. [PubMed] [Google Scholar]
- 36.Tan MH, Johns D, Glazer NB. Pioglitazone reduces atherogenic index of plasma in patients with type 2 diabetes. Clinical Chemistry. 2004;50(7):1184–1188. doi: 10.1373/clinchem.2004.031757. [DOI] [PubMed] [Google Scholar]
- 37.Alberti KGMM, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. The Lancet. 2005;366(9491):1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 38.di Massimo C, Scarpelli P, Lorenzo ND, Caimi G, Orio FD, Ciancarelli MGT. Impaired plasma nitric oxide availability and extracellular superoxide dismutase activity in healthy humans with advancing age. Life Sciences. 2006;78(11):1163–1167. doi: 10.1016/j.lfs.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 39.Nims RW, Darbyshire JF, Saavedra JE, et al. Colorimetric methods for the determination of nitric oxide concentration in neutral aqueous solutions. Methods: A Companion to Methods in Enzymology. 1995;7(1):48–54. [Google Scholar]
- 40.Simão ANC, Lozovoy MAB, Simão TNC, et al. Immunological and biochemical parameters of patients with metabolic syndrome and the participation of oxidative and nitroactive stress. Brazilian Journal of Medical and Biological Research. 2011;44(7):707–712. doi: 10.1590/s0100-879x2011007500069. [DOI] [PubMed] [Google Scholar]
- 41.Zavaroni I, Ardigo D, Rossi PC, et al. Relationship between plasma nitric oxide concentration and insulin resistance in essential hypertension. American Journal of Hypertension. 2004;17(7):549–552. doi: 10.1016/j.amjhyper.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Chien WY, Yang KD, Eng HL, et al. Increased plasma concentration of nitric oxide in type 2 diabetes but not in nondiabetic individuals with insulin resistance. Diabetes and Metabolism. 2005;31(1):63–68. doi: 10.1016/s1262-3636(07)70168-4. [DOI] [PubMed] [Google Scholar]
- 43.Shin MJ, Lee JH, Jang Y, et al. Insulin resistance, adipokines, and oxidative stress in nondiabetic, hypercholesterolemic patients: leptin as an 8-epi-prostaglandin F2α determinant. Metabolism: Clinical and Experimental. 2006;55(7):918–922. doi: 10.1016/j.metabol.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 44.Cha HN, Kim YW, Kim JY, et al. Lack of inducible nitric oxide synthase does not prevent aging-associated insulin resistance. Experimental Gerontology. 2010;45(9):711–718. doi: 10.1016/j.exger.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Cha HN, Song SE, Kim YW, Kim JY, Won KC, Park SY. Lack of inducible nitric oxide synthase prevents lipid-induced skeletal muscle insulin resistance without attenuating cytokine level. Journal of Pharmacological Sciences. 2011;117(2):77–86. doi: 10.1254/jphs.11093fp. [DOI] [PubMed] [Google Scholar]
- 46.Pillon NJ, Vella RE, Soulère L, Becchi M, Lagarde M, Soulage CO. Structural and functional changes in human insulin induced by the lipid peroxidation byproducts 4-hydroxy-2-nonenal and 4-hydroxy-2-hexenal. Chemical Research in Toxicology. 2011;24(5):752–762. doi: 10.1021/tx200084d. [DOI] [PubMed] [Google Scholar]
- 47.Pillon NJ, Croze ML, Vella RE, Soulère L, Lagarde M, Soulage CO. The lipid peroxidation by-product 4-hydroxy-2-nonenal (4-HNE) induces insulin resistance in skeletal muscle through both carbonyl and oxidative stress. Endocrinology. 2012;153(5):2099–2111. doi: 10.1210/en.2011-1957. [DOI] [PubMed] [Google Scholar]
- 48.Ingram KH, Hill H, Moellering DR, et al. Skeletal muscle lipid peroxidation and insulin resistance in humans. Journal of Clinical Endocrinology and Metabolism. 2012;97(7):E1182–E1186. doi: 10.1210/jc.2011-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murdolo G, Piroddi M, Luchetti F, et al. Oxidative stress and lipid peroxidation by-products at the crossroad between adipose organ dysregulation and obesity-linked insulin resistance. Biochimie. 2013;95(3):585–594. doi: 10.1016/j.biochi.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 50.Komosińska-Vassev K, Olczyk K, Olczyk P, Winsz-Szczotka K. Effects of metabolic control and vascular complications on indices of oxidative stress in type 2 diabetic patients. Diabetes Research and Clinical Practice. 2005;68(3):207–216. doi: 10.1016/j.diabres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Chrysohoou C, Panagiotakos DB, Pitsavos C, et al. The association between pre-hypertension status and oxidative stress markers related to atherosclerotic disease: the ATTICA study. Atherosclerosis. 2007;192(1):169–176. doi: 10.1016/j.atherosclerosis.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 52.Abdilla N, Tormo MC, Fabia MJ, Chaves FJ, Saez G, Redon J. Impact of the components of metabolic syndrome on oxidative stress and enzymatic antioxidant activity in essential hypertension. Journal of Human Hypertension. 2007;21(1):68–75. doi: 10.1038/sj.jhh.1002105. [DOI] [PubMed] [Google Scholar]
- 53.Sánchez-Rodríguez MA, Martínez-Cruz M, Correa-Muñoz E, Mendoza-Núñez VM. Relationship between metabolic syndrome components and oxidative stress in elderly community-dwelling Mexicans. Annals of Nutrition & Metabolism. 2010;56(4):302–307. doi: 10.1159/000309601. [DOI] [PubMed] [Google Scholar]
- 54.Pirinccioglu AG, Gökalp D, Pirinccioglu M, Kizil G, Kizil M. Malondialdehyde (MDA) and protein carbonyl (PCO) levels as biomarkers of oxidative stress in subjects with familial hypercholesterolemia. Clinical Biochemistry. 2010;43(15):1220–1224. doi: 10.1016/j.clinbiochem.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 55.Toprakci M, Ozmen D, Mutaf I, et al. Age-associated changes in nitric oxide metabolites nitrite and nitrate. International Journal of Clinical and Laboratory Research. 2000;30(2):83–85. doi: 10.1007/BF02874163. [DOI] [PubMed] [Google Scholar]
- 56.Ghasemi A, Zahedi Asl S, Mehrabi Y, Saadat N, Azizi F. Serum nitric oxide metabolite levels in a general healthy population: relation to sex and age. Life Sciences. 2008;83(9-10):326–331. doi: 10.1016/j.lfs.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 57.Li R, Lyn D, Lapu-Bula R, et al. Relation of endothelial nitric oxide synthase gene to plasma nitric oxide level, endothelial function, and blood pressure in African Americans. American Journal of Hypertension. 2004;17(7):560–567. doi: 10.1016/j.amjhyper.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 58.Choi JW. Enhanced nitric oxide production is closely associated with serum lipid concentrations in adolescents. Clinica Chimica Acta. 2004;347(1-2):151–156. doi: 10.1016/j.cccn.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 59.Caimi G, Hopps E, Noto D, et al. Protein oxidation in a group of subjects with metabolic syndrome. Diabetes and Metabolic Syndrome: Clinical Research and Reviews. 2013;7(1):38–41. doi: 10.1016/j.dsx.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 60.Mattson MP. Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Experimental Gerontology. 2009;44(10):625–633. doi: 10.1016/j.exger.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


