Abstract
The bulbocavernosus (BC) is a sexually dimorphic muscle observed only in males. Androgen receptor knockout mouse studies show the loss of BC formation. This suggests that androgen signaling plays a vital role in its development. Androgen has been known to induce muscle hypertrophy through satellite cell activation and myonuclei accretion during muscle regeneration and growth. Whether the same mechanism is present during embryonic development is not yet elucidated. To identify the mechanism of sexual dimorphism during BC development, the timing of morphological differences was first established. It was revealed that the BC was morphologically different between male and female mice at embryonic day (E) 16.5. Differences in the myogenic process were detected at E15.5. The male BC possesses a higher number of proliferating undifferentiated myoblasts. To identify the role of androgen signaling in this process, muscle-specific androgen receptor (AR) mutation was introduced, which resulted in no observable phenotypes. Hence, the expression of AR in the BC was examined and found that the AR did not colocalize with any muscle markers such as Myogenic differentiation 1, Myogenin, and paired box transcription factor 7. It was revealed that the mesenchyme surrounding the BC expressed AR and the BC started to express AR at E15.5. AR mutation on the nonmyocytic cells using spalt-like transcription factor 1 (Sall1) Cre driver mouse was performed, which resulted in defective BC formation. It was revealed that the number of proliferating undifferentiated myoblasts was reduced in the Sall1 Cre:ARL−/Y mutant embryos, and the adult mutants were devoid of BC. The transition of myoblasts from proliferation to differentiation is mediated by cyclin-dependent kinase inhibitors. An increased expression of p21 was observed in the BC myoblast of the Sall1 Cre:ARL−/Y mutant and wild-type female. Altogether this study suggests that the nonmyocytic AR may paracrinely regulate the proliferation of myoblast possibly through inhibiting p21 expression in myoblasts of the BC.
The effect of androgen signaling on muscles is one of the most used experimental system for studying hormonal effects on tissues. In a physiological context, the use of steroids or androgen analogs has anabolic effects on muscles leading into increased mass, strength, and weight of muscles (1–3). Such effects are suggested to be due to the ability of androgen signaling to regulate satellite cell functions, induce stem cells into a myogenic differentiation, and control myogenic gene transcription (4–7). However, mouse studies on muscle-specific androgen receptor (AR) ablation reported contrasting results. Ablation of AR in postmitotic differentiating myoblasts affected muscle mass and caused altered fiber types (8). Another study shows no change in muscle mass but rather reduced contractile functions of myofibers (9). Effect of T treatment on the C2C12 muscle cell line also exhibits varying results on the proliferative potential of myoblasts (7, 10–12). This implies that the current understanding on the effect of androgen signaling on muscle development still requires further investigations.
Amid these discrepancies, skeletal muscle-specific AR ablation exhibits decreased size in one muscle complex, the bulbocavernosus (BC) and levator ani (LA) complex (8, 9). This indicates that the BC/LA complex is one of the most androgen-responsive skeletal muscles in the body. The BC is a striated muscle covering the bulb of the penis with its posterior extension, the LA (13). These muscles have been implicated with functions on erection and defecation (14–17). The BC/LA is characterized as well developed in the male but not in the female. A previous study (18) states that the BC/LA in male undergoes an androgen-dependent increase of muscle fibers during the first week after birth. More recently the BC/LA muscle with other perineum muscles were traced to originate from the ventral muscle mass of the hindlimb (19). In such a study, MyoD+ (early marker of muscle lineage) cells from the limb extend toward the base of the genital tubercle (GT) at embryonic day (E) 12.5 of mouse embryos (E12.5) and are separated into the different perineum muscles at E14.5. This might suggest that muscle precursor cells (MPCs) for the formation of the BC/LA are present in both male and female embryos. This leads to the question about the mechanism directing the differential development of embryonic BC in males and females. One study suggests that a higher number of apoptotic cells in female BC/LA at E18 and postnatal day (P) 1 may contribute to decreased muscle volume at postnatal stages (17). Knowing that MPCs from the hindlimb muscles reach the perineum at E14.5 and that the onset of GT masculinization upon androgen actions starts at E15.5 (20), it is possible that the BC muscle starts its dimorphic development at earlier embryonic stages.
The process of myogenesis is governed by a defined set of myogenic regulatory factors. MyoD is a transcription factor initiating cells into a myogenic pathway and acting upstream of Myogenin (Myog) (21). Myog mutant mice display normal initial myogenesis but display defective myocyte differentiation (22). Hence, MyoD+ cells could represent undifferentiated myoblasts, whereas Myog+ cells correspond to differentiated myoblasts. Myoblasts can proceed to differentiation after exiting proliferation regulated by different cyclin-dependent kinases (Cdk) and Cdk inhibitors (CKI) (23). Population of cells determined to become satellite cells express paired box transcription factor 7 (Pax7) (24–26). During muscle growth and regeneration, Pax7+ cells are activated, leading to the expression of MyoD followed by Myog during differentiation (4).
Global androgen receptor knockout (ARKO) (27) and muscle-specific ARKO mice (8, 9) exhibit defective BC growth. Testicular-feminization mutation (Tfm) rats, AR loss-of-function mutant, also exhibit feminized BC/LA. A gain-of-function experiment by introducing wild-type (WT)-AR transgene into Tfm rats failed to rescue the feminized phenotypes in the BC/LA complex (28). This questions the function of AR during BC/LA muscle development. Aside from myogenic cells, the muscles are composed of other cell types such as nonsomitic progenitors, fibroblasts, adipocytes, and endothelial and nerve cells (29–31). AR is known to be expressed in satellite cells, CD34+ mesenchymal stem cells, and myonuclei in the muscle (31). Overall, these previous studies could suggest that different cell types, other than myocytic cells, may respond to androgen signaling. Androgen signaling is also known to regulate prostate development through interaction of epithelial and mesenchymal cells (32, 33). The distinct role of AR in each different cell types within the BC muscle population is not yet reported.
In this study, we investigated the role of androgen in the embryonic sexually dimorphic BC development. First, we examined the primary developmental differences of male and female BC at postnatal and embryonic stages. We found that male BC possesses a higher number of proliferating myoblasts at E15.5, which may account for the size difference at later embryonic stages. Next, the involvement of AR was examined by analyzing AR expression, and it was found that AR was expressed in the nonmyocytic cells in the BC and its adjacent mesenchyme at E15.5. The nonmyocytic AR mutation was introduced by using spalt-like transcription factor 1 (Sall1)-Cre driver mouse strain, and such mutation resulted in a decreased number of proliferating myoblasts in the mutant BC. Reduced expression of p21 was also observed in the mutant. This suggests that nonmyocytic AR may possibly affect sexually dimorphic BC formation by regulating the number of proliferating myoblasts through modulating CKI expression such as p21. Furthermore, the Sall1-expressing cells derived from the perineum mesenchyme appear to contribute to the BC region starting at E15.5. These Sall1+ cells, which are also the AR+ cells, may represent a population of cells regulating the sexual dimorphic embryonic BC, possibly through paracrine signaling.
Materials and Methods
Mouse
The mutant mouse lines used were R26 LacZ (34), Sall1-CreER+2 (35), AR-null (36), and MCK-Cre;ARKO (37, 38). ICR mice were used for the analyses of normal histogenesis determining male and female differences. Embryos were collected from at least three pregnant mice. The pregnant mice were checked for vaginal plug at noon and embryos were designated as E0.5. All mice-handling procedures are in accordance with the guidelines of the Animal Care and Use Committee of Wakayama Medical University, Japan. SallAR male mutants were generated by crossing the AR-null (ARL−/L−) female mice with Sall1-CreER/+ male mice. Sall1-Cre lineage analysis was performed on embryos derived from ICR female mated with Sall1-CreER/+ Rosa26 LacZ male mice. SallAR embryos were compared with WT littermates in all analyses. The tamoxifen (TM)-inducible Cre recombinase system removes the floxed sequence of the target gene upon TM treatment (39–41). TM treatment was prepared by dissolving TM (Sigma) in sesame oil (Kanto Chemical) with a final concentration of 10 mg/mL. four milligrams of TM per 40 g body weight was injected ip to pregnant mice. No overt teratological effects were observed in embryos treated with such concentration (42).
Histology and immunohistochemistry
Embryos were dissected and fixed in 4% paraformaldehyde (PFA) overnight and dehydrated with methanol. The perineum regions were dissected, paraffinized, and embedded. hematoxylin and eosin and Masson trichrome staining were performed by standard procedures as previously described (43, 44). For immunohistochemistry, antigen retrieval was performed by incubating the tissue sections mounted on slides in 0.1 mM citrate buffer (pH 6.0) in an autoclave (121°C) for 1 minute. Endogenous peroxidase activity was inactivated by incubating tissues in PBS containing 3% H2O2. For cryosection samples, freshly dissected embryos were embedded and sectioned in 10 μm thickness. The primary antibodies, dilutions, and sources were as follows: anti-MyoD (1:100; BD Pharmingen), anti-Myog (1:100) and anti-Pax7 (1:200) from the Developmental Studies Hybridoma Bank; anti-green fluorescent protein (1:100; Abcam; and 1:100; Roche); anti-AR (1:200; Santa Cruz Biotechnology). Anti-p21 (1:100; Santa Cruz Biotechnology) was dissolved in PBS with either goat or rabbit serum. A M.O.M. (mouse on mouse) kit (Vector Labs) was used for cryosectioned tissues and double staining. Tissue sections were incubated with diluted primary antibodies overnight at 4°C. Antibody detection was carried out using immunofluorescence with Alexa fluor 488 antimouse-IgG (Life Technologies) and Alexa fluor 546 antirabbit-IgG (Life Technologies) as secondary antibodies. Tissue sections were counterstained with Hoechst 33342 (Sigma). Alternatively, some sections were visualized using the streptavidin-biotin system with diaminobenzidine as the final chromogen. The tissue sections were counterstained with hematoxylin for 3 minutes.
Cell proliferation assay
Cell proliferation was examined using the 5-ethynyl-2′-deoxyuridine (EdU) labeling kit (C10337; Invitrogen). All procedures were conducted according to the instructions of the manufacturer. At least three embryos per stage were used for this assay. Each embryo was collected from independent pregnant mice. Double staining was conducted in the following order: antigen retrieval followed by EDU staining and anti-MyoD staining overnight. Tissue sections were counterstained with Hoechst 33342 (Sigma).
Section LacZ staining
Freshly dissected embryos were fixed overnight in a solution composed of 2% PFA and PBS with 1 M MgCl2 at 4°C. The embryos were washed with PBS MgCl2 solution for 1 hour and dehydrated with sucrose solution overnight. The perineum region was embedded in optimal cutting temperature compound (Sakura Finetechnical Co) and was sectioned in 10 μm thickness. Color development was done for 4 hours to 2 days at 37°C. The developing solution was composed of X-gal (1 mg/mL), 5 mM K3Fe(CN)6, 5 mM K4FE(CN)6, and 0.2 mM MgCl2 dissolved in PBS. Color development was terminated by washing the embryos with PBS and refixation with paraformaldehyde.
Scanning electron microscope photomicrograph and three-dimensional (3D) reconstruction of BC/LA muscles
Mouse embryos were fixed in 4% PFA and were dehydrated and processed as described previously (45). For 3D reconstruction, 6-μm sections of the perineum region were stained with MyoD (diaminobenzidine), and approximately 70 photomicrographs from serial sections were obtained. The images were converted to gray scale and the MyoD-expressing region was outlined and stacked using ImageJ (Image Processing and Analysis in Java; National Institutes of Health) software. The resulting stacked file was processed using Amira software (FEI Visualization Sciences Group).
Statistical analyses
Data from counting EDU and immunostaining signals were analyzed using an unpaired Student's t test. Three independent trials were conducted. Data are expressed as mean with SE. Values were considered as statistically significant if P < .05.
Results
Timing of sexual dimorphism of the BC muscle during embryogenesis
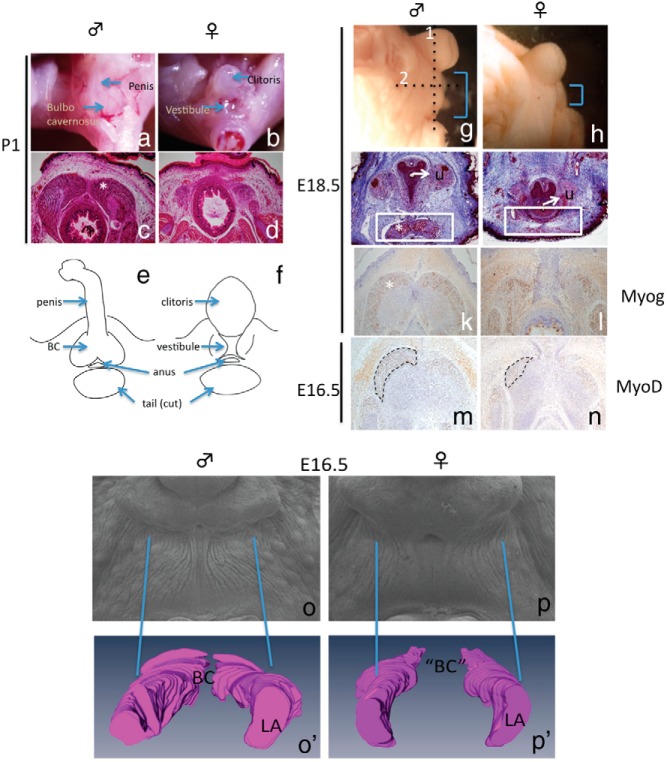
Anatomical studies of adult perineum acknowledge the presence of the BC in male but its postnatal characterization is scarce. We confirmed that the BC was present in the perineum of the male mouse located proximally from the penis at P1 (Figure 1A). The female was devoid of BC, and a vestibule or part of the vaginal opening was observed at the base of the clitoris (Figure 1B). Similarly, histological sections of the perineum at P1 showed the formation of prominent BC muscle in male and a remnant of BC in the female (Figure 1, C and D). Postnatal staged samples showed a highly dimorphic perineum region due to the presence of BC in the male but not in the female (Figure 1, E and F). We further investigated on embryonic stages to identify the onset of BC sexual dimorphism.
Figure 1.
Characterization of male and female BC muscles. Whole mount (A and B) and transverse sections of BC (C and D) from postnatal samples of the male and female are shown. E and F, A schematic representation of the structures in the perineum. G and H, Embryonic investigations of the perineum with AGD difference observed at E18.5. I and J, Transverse section (dotted line 1 in G) of E18.5 perineum stained with Masson trichrome showing the BC (*) located ventral to the urethra (white box). Horizontal sections (dotted line 2 in G) of E18.5 and E16.5 perineum of the BC region (* and traced line) were stained with anti-Myog (K and L) and MyoD (M and N), respectively. Scanning electron microscope photomicrograph of the perineum (O and P) and a 3D reconstruction (O′ and P′) of the BC or remnant of BC/LA complex based on MyoD-stained sections at E16.5. U, urethra.
To identify the morphological differences in embryonic male and female BC, histological analyses of embryonic BC with MyoD and Myog staining for early and late muscle development were performed, respectively. The perineum length or anogenital distance (AGD) was notably longer in the male than in the female at E18.5 (Figure 1, G and H). Transverse sections revealed that the BC was already formed in the male but not in the female (Figure 1, I and J). Horizontal sections stained with anti-Myog confirmed the presence of BC muscles in the male (Figure 1K) and some Myog positive cells in the female (Figure 1L). Similarly, a prominent BC was observed in the male at E16.5. However, MyoD staining in horizontal sections revealed the presence of a MyoD+ muscle structure in female that was relatively smaller to the male (Figure 1, M and N). A 3D model of the BC muscle was constructed using serial horizontal sections of the BC stained with anti-MyoD (Figure 1, O′ and P′ and Supplemental Figure 1A and B), which displayed a larger BC muscle region in the male. These observations suggest that the morphological difference in the male and the female BC was evident at E16.5. To refine the timing of sexual dimorphism of BC, further analysis on myogenesis was conducted.
Male BC muscles develop higher numbers of proliferating myoblasts after E14.5
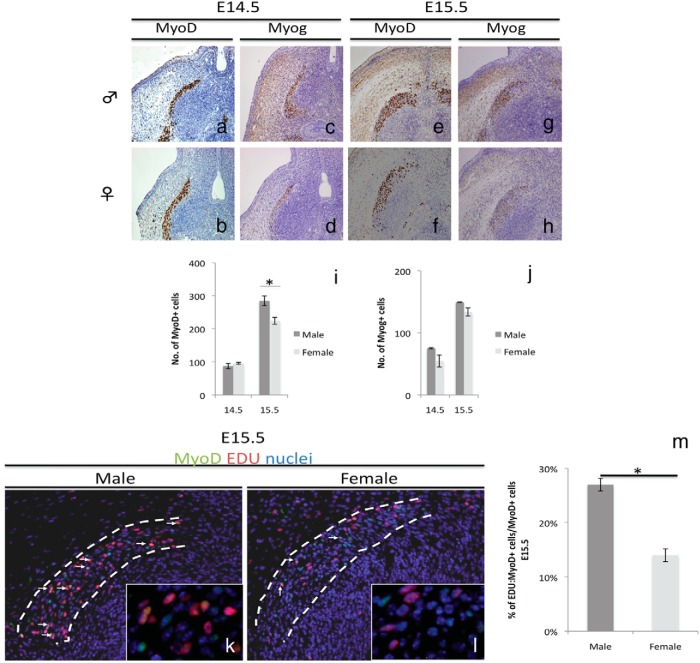
Elucidation of the myogenic events leading to well-developed male BC muscles was investigated. For this purpose, the numbers of MyoD- and Myog-expressing cells were examined at stages before E16.5, the stage of initial morphological difference in BC muscles. This analysis determines the differences of undifferentiated myoblasts and differentiated myoblasts between male and female BC. Both male and female possessed a MyoD+ and Myog+ region at the base of the GT (Figure 2, A–D). The number of MyoD+ and Myog+ cells did not show significant differences between the male and the female at E14.5, the stage of BC muscle establishment in the perineum (Figure 2, I and J). These observations confirm that both male and female embryos possessed precursor cells for BC formation. A wider MyoD-expressing region could be observed in male by analyzing transverse sections at E15.5 (Supplemental Figure 2A). Furthermore, the number of MyoD+ cells was significantly higher in the male (Figure 2I) but the number of Myog+ cells did not show a significant difference (Figure 2J) between the male and the female. In addition, the number of Pax7+ cells representing the satellite cell population was also examined. The number of satellite cells was also significantly higher in male at E15.5 (Supplemental Figure 2D). These data show that the male possesses a higher number of undifferentiated myoblasts and satellite cells during the onset of BC sexual dimorphism (E15.5).
Figure 2.
Differences in male and female BC myogenesis. A–H, Expression of muscle markers MyoD and Myog in E14.5 and E15.5 horizontal sections of the BC. Quantitative assessment of MyoD+ (I) and Myog+ (J) cells at E14.5 and E15.5 is shown. Immunostaining (K and L) and quantitative assessment (M) of MyoD:EDU double-positive cells at E15.5 are shown. Three embryonic specimens from different litters were analyzed by counting cells in the whole BC region (three to four sections per sample). *, P < .05. Error bars, means ± SE.
Cell proliferation occurs in undifferentiated myoblasts and ceases when the cells proceed to differentiation (23, 46). Hence, differences in cell proliferation were investigated through EDU staining. To examine the state of proliferating undifferentiated myoblasts in the muscle, EDU was costained with anti-MyoD. The total number MyoD+EDU+ was not significantly different between male and female at E14.5 (Supplemental Figure 2C). However, the total number of EDU+ cells, MyoD+EDU+ cells and percentage of MyoD:EDU double-positive cells over the total number of MyoD+ cells were significantly higher in the male than in the female at E15.5 (Supplemental Figure 2, B and C, and Figure 2, K–M). We thus demonstrate that the male exhibited increased numbers of proliferating myoblasts for BC muscle in comparison with females between E14.5 and E15.5.
AR is not expressed in the muscle precursors of embryonic BC
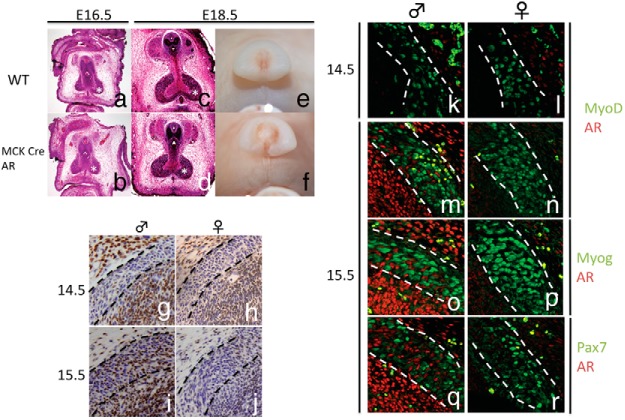
A previous study showed that muscle-specific deletion of AR resulted in decreased BC/LA mass of the AR mutants (9). In the current study, male BC showed the sexual dimorphism during myogenesis as early as E15.5, indicating that AR signaling may contribute to the formation of the BC at such stage. To identify the role of AR in the sexually dimorphic embryonic BC development, AR was ablated in the muscle using a muscle creatine kinase (MCK)-Cre driver mouse strain. MCK expression is observed in myocytic cells of the skeletal muscles starting at E13.0 (47). Adult phenotypes of MCK:AR mutants include decreased weight of the LA and BC (8, 9).
We examined this mutant mouse during embryogenesis, and we found no discernible differences in AGD and BC muscle formation between the WT male and MCK-Cre AR mutants at E16.5 (Figure 3, A and B) and E18.5 (Figure 3, C–F). This prompted us to examine whether the AR is expressed within the embryonic BC muscles. AR expression was not observed in either the male or female presumptive BC muscle region at E14.5 (Figure 3, G and H). AR expression starts sporadically in the male BC but not in the female at E15.5 (Figure 3, I and J). To identify the cellular subtype expressing the AR, fluorescent double staining of AR with muscle markers MyoD, Myog, and Pax7 were performed. There was no AR expression in the female, and there was no colocalization with any of the muscle markers in the male at E15.5 (Figure 3, K–R). These results show that although AR is present in the male BC region from E15.5, it is not expressed in the MPCs (cells expressing MyoD, Myog, or Pax7).
Figure 3.
Phenotypes of MCK-Cre AR mutants by histological (A–D) and whole-mount (E and F) representations at E16.5 and E18.5 are shown. Expression of AR in male and female BC (G–J, traced lines) are also shown. K–R, Coexpression analysis of AR and muscle markers MyoD, Myog, and Pax7.
Ablation of AR in nonmyocytic cells results in defective BC formation
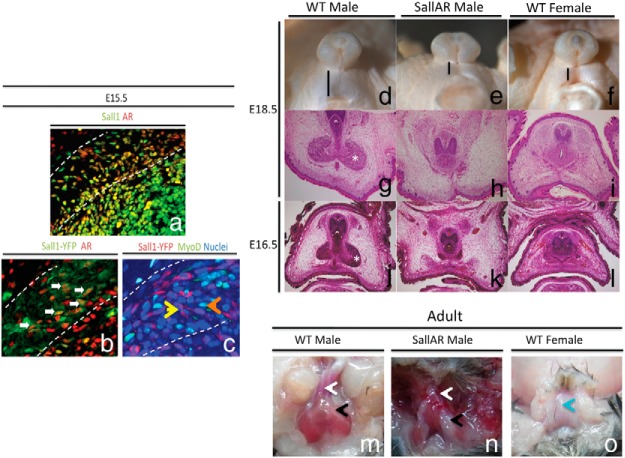
We identified that AR is not expressed in MPCs, and muscle-specific AR deletion did not result in defective embryonic BC formation. Hence, AR should be ablated in nonmyocytic cells. The Sall1 Cre driver mouse expresses Cre in the undifferentiated mesenchyme of some embryonic tissues (35). Sall1 is of particular interest because its expressing region in the GT shows high responsiveness to masculinizing factors (Suzuki K. unpublished observations). Mutation in Sall1 is also known to cause Townes-Brocks syndrome, which exhibits anorectal malformation, attesting to its expression in the perineum (48). Furthermore, immunostaining of Sall1 and AR showed colocalization in the BC and perineum mesenchyme (Figure 4A). The LacZ signal in the Sall1-CreER/+ Rosa26 reporter line was then examined. LacZ signals were not observed within the BC muscle region at E14.5 and start to be detected within the BC muscle region at E15.5 (Supplemental Figure 3). To identify whether Sall1Cre driver mouse line is suitable to ablate AR expression, the Sall1Cre driver mice were crossed with yellow fluorescent protein (YFP)+/+ mice, and the offspring were analyzed by costaining AR with YFP. AR and YFP expression exhibited colocalization in the surrounding mesenchyme and BC muscle at E15.5 (Figure 4B), whereas YFP and MyoD expression did not colocalize (Figure 4C).
Figure 4.
A, Coimmunostaining with antibodies for Sall1 and AR.YFP expression in Sall1 Cre YFP reporter mice was costained with anti-AR (B) and MyoD (C) at E15.5. White arrows mark colocalization of YFP and AR signals. Arrowheads mark separate staining from MyoD (orange) and AR (yellow). Morphological comparison of embryonic (D–L) and adult (M–O) BC between WT male and SallAR mutants is shown. *, embryonic BC; white arrow, base of the penis; black arrow, adult BC region; blue arrow, vagina.
To identify the role of the nonmyocytic AR in the sexually dimorphic embryonic BC development, Sall1-CreER/+ male mice were crossed with ARL−/L− female mice to generate Sall1-CreER/+ ARL−/Y (SallAR) mouse. After TM treatment at E11.5, the embryos were examined for phenotypes. During the sexually dimorphic stage of BC development (E16.5 and E18.5), SallAR mutant male embryos showed a decreased AGD length (Figure 4, D–F) and defective BC formation, resembling that of the WT female (Figure 4, G–J). BC muscle was completely absent in the adult SallAR mutant (Figure 4, M and N).
The Sall1+ AR+ cells represent a population of cells in the BC muscle originating from the mesenchyme of the perineum
The finding that nonmyocytic AR of Sall1-expressing cells contributes to the development of the sexually dimorphic BC development prompted us to further examine the morphogenetic significance of Sall1-expressing cells. We used embryos from female ICR mouse mated with Sall1-CreER/+ R26 LacZ male and treated with TM at E11.5 and E12.5, inducing Cre recombination of Sall1-expressing cells at approximately E12-E13 and E13-E14, respectively (at the stages of perineal muscles establishment). The BC muscle region showed no observable LacZ+ cells at E14.5 (Figure 5, A and B), whereas the surrounding mesenchyme of the BC was positive with LacZ signals. However, expression of LacZ was detected in the BC muscle region starting at E15.5 (Figure 5, C and D) and became more evident at later stages (E18.5; Figure 5E). Taken together, this suggests that Sall1-expressing cells from the surrounding mesenchyme of the BC may contribute to the muscle population starting at E15.5. The effect of AR ablation on Sall1+ cells was evident at E14.5, but no overt differences were observed between WT male and SallAR mutants (Supplemental Figure 4). Whether nonmyocytic AR on the surrounding mesenchyme or the nonmyocytic AR of the BC is mainly responsible for male specific BC formation was not clarified.
Figure 5.
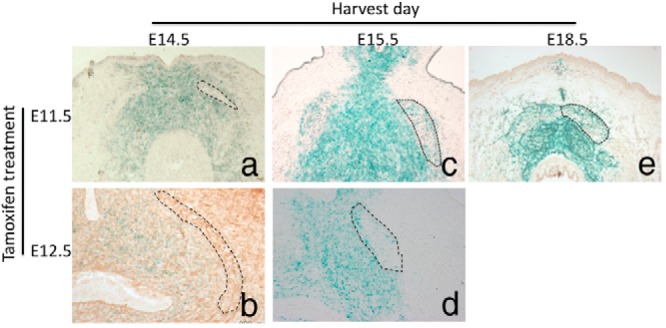
LacZ expression of Sall1Cre reporter mouse obtained at E14.5 (A and B), E15.5 (C and D), and E18.5 (E) after TM treatment at E11.5 (A, C, and E) and E12.5 (B and D) is shown. Such stages correspond to the timing of muscle precursor establishment in the perineum. Traced regions represent the BC region.
Loss of nonmyocytic AR results in decreased myoblast proliferation
To elucidate the effect of nonmyocytic AR on myogenesis, the expression of myogenic markers in SallAR mutants was examined during the onset of the sexual dimorphism in myogenesis (E15.5). The number of MyoD-expressing cells in the SallAR mutant was significantly reduced as compared with WT male (Figure 6, A–E). Such reduction of MyoD+ cells became prominent at later stages in the mutant (Supplemental Figure 5). However, the number of Myog-expressing cells showed no significant difference between the WT and SallAR mutant at E15.5 (Figure 6, C–E). This indicates that the primary effect on the SallAR mutant BC myogenesis is the decreased number of undifferentiated myoblasts.
Figure 6.
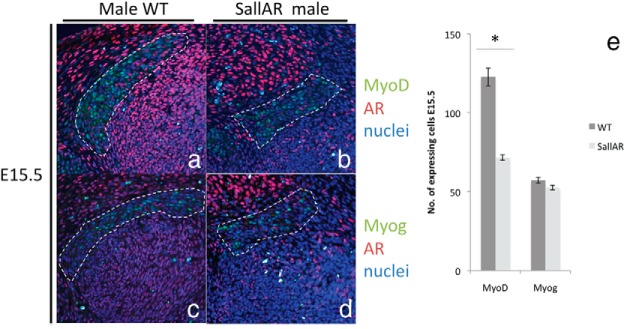
Immunostaining (A–D) and quantitative assessment (E) of the MyoD- and Myog-expressing region, costained with anti-AR, between male WT and SallAR mutants at E15.5 are shown. The BC is inside the traced region. Three embryonic specimens from different litters were analyzed by counting cells in the whole BC region (three to four sections per sample). *, P < .05. Error bars, means ± SE.
Next, to identify whether the decreased number of MyoD-positive cells is due to defective myoblast proliferation, the BC muscle was costained with anti-MyoD and EDU to examine the proliferating myoblasts. In the WT male, MyoD and EDU double-positive cells were observed in the BC of the E15.5 male (Figure 7A, blue arrows). However, a significant reduction in MyoD+EDU+ cells was evident in the SallAR mutant at E15.5 (Figure 7, A and B, blue arrows, Supplemental Figure 6). The percentage of MyoD:EDU double-positive cells over the total number of MyoD+ cells also showed a significant reduction in the SallAR mutants compared with WT males (Figure 7C). This indicates that AR ablation resulted in fewer proliferating myoblasts. Such a difference in myoblast proliferation was further evident at E16.5 (Figure 7, E and F). Furthermore, Pax7+ and EDU+ cells were also reduced in SallAR mutants at E15.5 (Supplemental Figure 7, A and B).
Figure 7.
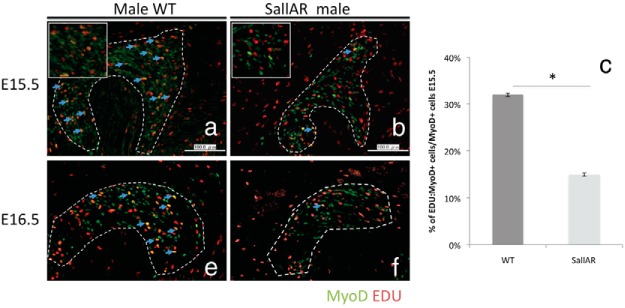
Immunostaining (A and B) and quantitative assessment (C) of the MyoD:EDU double-positive cells (blue arrows) between male WT and SallAR mutants at E15.5 and E16.5 (E and F) are shown. The BC is inside the traced region. Three embryonic specimens from different litters were analyzed by counting cells in the whole BC region (three to four sections per sample). *, P < .05. Error bars, means ± SE.
It is also notable that the loss of nonmyocytic AR influenced the development of non-AR-expressing MPCs. Examination of proliferative potential of the AR+ cells by costaining AR with EDU demonstrates that the AR+ cells are negative for EDU signals (Supplemental Figure 7, C and D), indicating that cell autonomous dysregulation of proliferation of the AR+ cells is not the cause of smaller embryonic BC in the mutant.
p21 expression is up-regulated in the MPCs of SallAR mutants
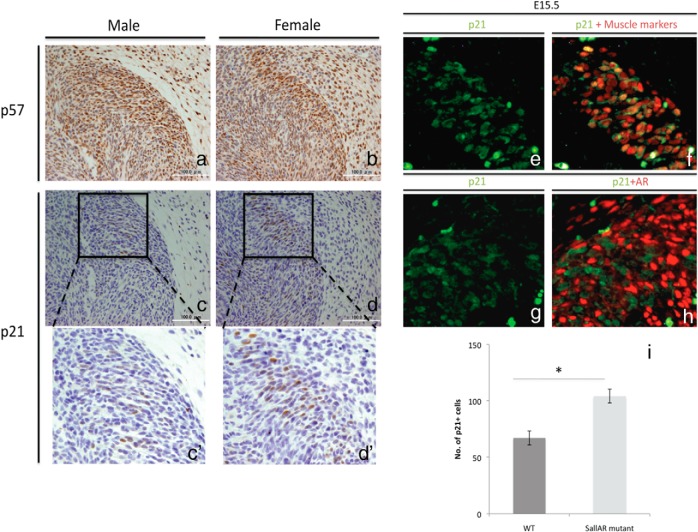
One mode of regulating cell proliferation is through the inhibition of Cdks. Previous studies have implicated the role of p21, p27, and p57 in regulating cell proliferation during muscle development (23, 49). The expressions of p21, p27, and p57 in male and female BC were examined at E15.5. p27 expression was not observed in the BC muscles, whereas p57 was expressed ubiquitously in the perineum, both in muscle and nonmuscle regions (Figure 8, A and B). In contrast, p21 expression was restricted in muscle regions including the BC. Hence, p21 expression was further examined (Figure 8, C and D). A noticeable difference in the expression of p21 was detected between male and female BC at E15.5, with a higher p21 expression in the female (Figure 8, C′ and D′).
Figure 8.
Expression of CKIs p57 (A and B) and p21 (C, D, C′, and D′) in males and females by horizontal section of the perineum at E15.5 is shown. Coexpression analyses of p21 and muscle markers (E and F) and p21 and AR (G and H) are also shown. J, Quantitative assessment of p21 expression in WT males and SallAR mutant males at E15.5. Three embryonic specimens from different litters were analyzed by counting cells in the whole BC region (three to four sections per sample). *, P < .05. Error bars, means ± SE.
To identify whether the p21 is expressed in myocytes or nonmyocytic AR-expressing cells, p21 was costained with muscle marker cocktail (MyoD, Myog, and Pax7) and anti-AR (Figure 8, E and F). p21 was coexpressed with muscle markers (Figure 8F) but not with AR (Figure 8H). We then examined whether the expression of p21 was altered by the loss of nonmyocytic AR. The SallAR mutant showed a higher expression of p21 as compared with WT male at E15.5 (Figure 8I). This may suggest that nonmyocytic AR may regulate myoblast proliferation possibly through repression of CKIs.
Discussion
Sexual dimorphism in embryonic myogenesis during BC/LA development
The BC/LA has been commonly recognized as a muscle complex developed only in males. However, the onset of its sexual dimorphism and characterization of events leading to its dimorphism are still unclear. Previous study has suggested that the LA attained its initial sexual dimorphic morphology starting at P1 (18). They reported a higher number of muscle fibers in male in the first postnatal week followed by formation of larger muscle cross-sectional area in the later postnatal stages in response to increased plasma testosterone (18). Although such study did not focus on the BC, it represents the anabolic effects of T in sexually dimorphic muscle. Another study suggested the possible role of differential embryonic cell death during BC formation, which is at its peak at E18 and P1 in the female (17). This implies that the increased apoptosis in female BC/LA could not account for the initial sexual dimorphism observed at E17, albeit explaining the decreased BC/LA growth at E18 until postnatal days.
In the current study, it was observed that the number of proliferating myoblasts was higher in the male than in the female, whereas the differentiated myoblasts was not significantly different at E15.5. The undifferentiated state of myoblast is necessary to expand the myoblast population (23, 50), which accounts for larger muscle size of the BC in the male at E16.5. Hence, the initial differences in male and female embryonic BC are likely caused by the difference of myoblast proliferation. Furthermore, the number of satellite cell precursor (Pax7+ cells) observed in the current study was lower in the female as compared with the male. This result corroborates with previous finding that the number of satellite cells is higher in the male at P2.5 (18, 51). The current study may suggest that such dimorphism in satellite cells number was also determined at embryonic stages.
Nonmyocytic AR affects muscle precursor cell proliferation
A previous study identified the initial expression of AR in the perineum region to occur at approximately E15 (52). However, the specific cell type expressing AR was not distinguished. In the current study, AR was not expressed in the BC muscle area at E14.5 but was highly expressed in the surrounding mesenchyme. AR was expressed at the BC region at E15.5. However, the AR did not colocalize with any of the muscle markers, namely MyoD, Pax7, and Myog. This suggests that the AR-expressing cells responsible for the sexually dimorphic development of the embryonic BC are nonmyocytic in origin. Furthermore, muscle-specific AR deletion through MCK-Cre exhibits no discernable phenotypes at embryonic stages although adult MCK:AR exhibits a reduced size of BC and LA (data not shown) (8). This suggests that muscle-specific AR is dispensable in attaining the sexual dimorphic embryonic BC development.
Deletion of nonmyocytic AR through Sall1 Cre resulted in a feminized development of the BC. Such a phenotype could not be attributed to defective testis development because Sall1 Cre did not show its activity in the testis (data not shown). The BC of the SallAR mutant displays a reduced number of proliferating myoblasts resulting in the formation of a smaller BC at embryonic stages. The effects of nonmuscle tissues on muscle development are well-recognized phenomena. The induction of myogenesis in the somite is regulated by signals derived from the neural tube and notochord (53). Additionally, factors from nonmyogenic cells such as scatter factor/hepatocyte growth factor and Tcf4 has been suggested to be essential during myogenesis and muscle patterning, respectively (54, 55). Another example of nonmyogenic cells regulating muscle development is observed in the cremaster muscles. Gubernaculum-specific AR deletion resulted in increased Pax7 expression and decreased expression of skeletal muscle actin, suggesting defective development of the cremaster muscle. However, cremaster muscle-specific AR deletion did not show any observable phenotypes. They suggested that the deficiency in AR results in the loss of AR-induced paracrine signals from the gubernacular cells to the myoblasts (56).
In the current study, the proliferation of myoblasts was reduced when nonmyocytic AR was ablated. Proliferation of myoblasts is suggested to be regulated by CKIs, the most notable of which is p21. Both embryonic female BC and SallAR mutant males exhibited increased expression of p21 at E15.5. Muscle regeneration in p21 null mutants shows enhanced myoblast proliferation (49). This may suggest that cell proliferation in the absence of androgen signaling was impeded due to the increase of p21 expression. Supplementation of T is reported to reverse the increased p21 expression in aged muscles (57). In addition, p21 overexpression in vascular smooth muscles results in growth inhibition through increased apoptosis. Such increased p21 was correlated with an increased expression of Bcl-2-associated X protein proapoptotic protein (50). Deletion of Bax and Bak genes are reported to induce masculinized development of BC/LA by reducing the incidence of apoptosis (17). Hence, the increased p21 expression in female detected at E15.5 in the current study may explain the increased apoptosis in female BC at later stages (E18 and P1) as previously reported (17).
Androgen signaling has been recognized to positively induce cell proliferation through activating satellite cells. Additionally, T administration affects smooth muscle patterning in prostate development (58) and increased myoblast proliferation in skeletal muscles. However, whether this occurs cell autonomously has not been elucidated because many cell types in the muscles express AR. It is noteworthy that the effects of AR ablation on nonmyocytic Sall1+ cells were directed toward the non-AR-expressing muscle precursor cells. AR did not colocalize with EDU and p21, suggesting that proliferation of AR-expressing cells was not affected. This may suggest that AR is affecting cell proliferation through paracrine events. A recent study has investigated paracrine control of myoblast proliferation. They identified that the muscle side population expresses bone morphogenetic protein-4 (Bmp4) ligand, which serves as a trophic factor to Bmpr1a+ muscle cells (59).
Other signaling pathways have been identified to regulate myoblast proliferation in paracrine manner such as Wnt and Notch signaling. Wnt signaling is an essential regulator of dimorphic gonadal development (60), and it is suggested to affect muscle formation in conjunction with Notch signaling (61). Nandrolone, an anabolic steroid, activates the androgen and Wnt signaling pathways, resulting in stabilization of the Numb protein (61). Numb reduced Notch signaling, which may lead to an increase in the muscle progenitor pool size (62). Notch is of particular interest in the current study because it is cited as a possible upstream regulator of p21 expression (63). The female BC region showed a higher expression of Jagged1 (data not shown), which corroborates with the observed higher p21 expression in the female and the SallAR mutant male. Further investigations are necessary to confirm the involvement of Notch in such putative juxtacrine signaling. Notch signaling has been implicated in the survival and differentiation of other androgen-dependent tissues (64, 65). It would be thus possible that AR regulates the expression of growth factors in the nonmyocytic cells, paracrinely affecting p21 expression in the neighboring myoblasts possibly leading to altered cell proliferation. Thus, such paracrine regulation by nonmyocytic AR may explain the previously reported inability of muscle specific WT-AR to rescue the feminized phenotype of Tfm rats.
Nonmyocytic Sall1+ cells possibly contribute to muscle development
The ventral limb muscle mass extends to the perineal region starting at E12.5 and is separated into distinct muscles at E14.5 (19). TM treatment of Sall1CreER/+ at stages of limb muscle extension did not show observable LacZ signals in the BC region at E14.5. However, examination of embryos at E15.5 displayed LacZ+ cells in the BC. The mesenchyme surrounding the BC was positive for LacZ, regardless of the TM treatment timing. This possibly suggests that the Sall1+ cells of the BC did not originate from the limb muscle but rather from surrounding mesenchyme. Furthermore, AR expressed in Sall1+ cells plays a vital role in determining the sexually dimorphic BC formation suggesting the role of the nonmyocytic cell populations in the muscle.
Side population, mesoangioblasts, and muscle-derived stem-cells constitute the nonmyocytic cell population, which plays a vital role for muscle growth and regeneration (66, 67). PW1+Pax7− interstitial cells (PICs) contribute to muscle regeneration, suggesting a satellite cell-like character, but their origin is not derived from Pax3+ cell lineage (68). Additionally, not all PICs adapt a myogenic fate. A recent study has suggested the separation of myogenic and nonmyogenic PICs based on the expression of platelet-derived growth factor receptor-α (PDGFRa) (69). The PDGFRa+ cells are the nonmyogenic population that secretes factors for myogenic differentiation (70). However, the origin of this cell population is currently unknown. The mesenchyme surrounding the BC and the nonmyocitic cells were the AR-expressing cells of the perineum. The coexpression of AR with PDGFRa in embryonic BC and its localization in the muscle insterstitium in postnatal BC (data not shown) suggest that these cells could be a part of the interstitial cell population. The interstitial cells are comprised of fibroblasts, mesenchymal progenitors, endothelial cells, and connective tissues (71). Coupled with the findings that not all satellite cells and SP are somitic in origin (72), it might be then possible that nonmyocytic Sall1+ cells in the BC could represent a population of cells in the muscles that are derived from nonmyogenic lineage of its surrounding mesenchyme.
In summary, this study documents a possible mode of mechanism for sexually dimorphic muscle development through androgen signaling via AR-expressing neighboring nonmuscle cell. Cell proliferation of myoblasts through p21 inhibition was the observed effect on myoblasts. Further analysis of mediating factors would be necessary to clarify the mechanism of paracrine control of proliferation by AR-expressing cells.
Acknowledgments
We express our deepest gratitude to Drs Anne Moon, Pierre Chambon, Hiroshi Asahara, and Takahiro Matsumoto for their continuous support. We also thank Dr Daisuke Matsumaru, Dr Mika Okazawa, Dr Hiroko Suzuki, Shoko Matsushita, Dr Mylah Villacorte, Dr Liu Liqing, and Iola Iba for their invaluable suggestions and assistance.
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas: Molecular mechanisms for establishment of sex differences (Grant 22132006) and the National Institutes of Health Grant R01ES016597. This work was also supported by the Monbukagusho scholarship from the Ministry of Education, Culture, Sports, and Science, Japan.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- AGD
- anogenital distance
- AR
- androgen receptor
- ARKO
- AR knockout
- BC
- bulbocavernosus
- Cdk
- cyclin-dependent kinase
- CKI
- Cdk inhibitor
- 3D
- three-dimensional
- E
- embryonic day
- EdU
- 5-ethynyl-2′-deoxyuridine
- GT
- genital tubercle
- LA
- levator ani
- MCK
- muscle creatine kinase
- MPC
- muscle precursor cell
- MyoD
- myogenic differentiation 1
- Myog
- myogenin
- Pax7
- paired box transcription factor 7
- PDGFRa
- platelet-derived growth factor receptor-α
- PFA
- paraformaldehyde
- PIC
- PW1+Pax7− interstitial cell
- Sall1
- spalt-like transcription factor 1
- Tfm
- testicular-feminization mutation
- TM
- tamoxifen
- P
- postnatal day
- WT
- wild type
- YFP
- yellow fluorescent protein.
References
- 1. Bhasin S, Woodhouse L, Casaburi R, et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281(6):E1172–E1181 [DOI] [PubMed] [Google Scholar]
- 2. Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90(2):678–688 [DOI] [PubMed] [Google Scholar]
- 3. Mauras N, Hayes V, Welch S, et al. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab. 1998;83(6):1886–1892 [DOI] [PubMed] [Google Scholar]
- 4. Chen Y, Zajac JD, MacLean HE. Androgen regulation of satellite cell function. J Endocrinol. 2005;186(1):21–31 [DOI] [PubMed] [Google Scholar]
- 5. Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144(11):5081–5088 [DOI] [PubMed] [Google Scholar]
- 6. Sheffield-Moore M. Androgens and the control of skeletal muscle protein synthesis. Ann Med. 2000;32(3):181–186 [DOI] [PubMed] [Google Scholar]
- 7. Powers ML, Florini JR. A direct effect of testosterone on muscle cells in tissue culture. Endocrinology. 1975;97(4):1043–1047 [DOI] [PubMed] [Google Scholar]
- 8. Ophoff J, Van Proeyen K, Callewaert F, et al. Androgen signaling in myocytes contributes to the maintenance of muscle mass and fiber type regulation but not to muscle strength or fatigue. Endocrinology. 2009;150(8):3558–3566 [DOI] [PubMed] [Google Scholar]
- 9. Chambon C, Duteil D, Vignaud A, et al. Myocytic androgen receptor controls the strength but not the mass of limb muscles. Proc Natl Acad Sci USA. 2010;107(32):14327–14332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diel P, Baadners D, Schlüpmann K, Velders M, Schwarz JP. C2C12 myoblastoma cell differentiation and proliferation is stimulated by androgens and associated with a modulation of myostatin and Pax7 expression. J Mol Endocrinol. 2008;40(5):231–241 [DOI] [PubMed] [Google Scholar]
- 11. Chen Y, Lee NK, Zajac JD, MacLean HE. Generation and analysis of an androgen-responsive myoblast cell line indicates that androgens regulate myotube protein accretion. J Endocrinol Invest. 2008;31(10):910–918 [DOI] [PubMed] [Google Scholar]
- 12. Desler MM, Jones SJ, Smith CW, Woods TL. Effects of dexamethasone and anabolic agents on proliferation and protein synthesis and degradation in C2C12 myogenic cells. J Anim Sci. 1996;74(6):1265–1273 [DOI] [PubMed] [Google Scholar]
- 13. Yiou R, Delmas V, Carmeliet P, et al. The pathophysiology of pelvic floor disorders: evidence from a histomorphologic study of the perineum and a mouse model of rectal prolapse. J Anat. 2001;199(Pt 5):599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sachs BD. Role of striated penile muscles in penile reflexes, copulation, and induction of pregnancy in the rat. J Reprod Fertil. 1982;66(2):433–443 [DOI] [PubMed] [Google Scholar]
- 15. Karacan I, Aslan C, Hirshkowitz M. Erectile mechanisms in man. Science. 1983;220(4601):1080–1082 [DOI] [PubMed] [Google Scholar]
- 16. Wallach SJ, Hart BL. The role of the striated penile muscles of the male rat in seminal plug dislodgement and deposition. Physiol Behav. 1983;31(6):815–821 [DOI] [PubMed] [Google Scholar]
- 17. Jacob DA, Ray T, Bengston CL, et al. The role of cell death in sexually dimorphic muscle development: male-specific muscles are retained in female bax/bak knockout mice. Dev Neurobiol. 2008;68(11):1303–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tobin C, Joubert Y. Testosterone-induced development of the rat levator ani muscle. Dev Biol. 1991;146(1):131–138 [DOI] [PubMed] [Google Scholar]
- 19. Valasek P, Evans DJ, Maina F, Grim M, Patel K. A dual fate of the hindlimb muscle mass: cloacal/perineal musculature develops from leg muscle cells. Development. 2005;132(3):447–458 [DOI] [PubMed] [Google Scholar]
- 20. Miyagawa S, Satoh Y, Haraguchi R, et al. Genetic interactions of the androgen and Wnt/β-catenin pathways for the masculinization of external genitalia. Mol Endocrinol. 2009;23(6):871–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tapscott SJ. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development. 2005;132(12):2685–2695 [DOI] [PubMed] [Google Scholar]
- 22. Hasty P, Bradley A, Morris JH, et al. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993;364(6437):501–506 [DOI] [PubMed] [Google Scholar]
- 23. Zhang P, Wong C, Liu D, Finegold M, Harper JW, Elledge SJ. p21(CIP1) and p57(KIP2) control muscle differentiation at the myogenin step. Genes Dev. 1999;13(2):213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102(6):777–786 [DOI] [PubMed] [Google Scholar]
- 25. Relaix F, Montarras D, Zaffran S, et al. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;172(1):91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J. 2004;23(16):3430–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. MacLean HE, Chiu WS, Notini AJ, et al. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. FASEB J. 2008;22(8):2676–2689 [DOI] [PubMed] [Google Scholar]
- 28. Niel L, Shah AH, Lewis GA, et al. Sexual differentiation of the spinal nucleus of the bulbocavernosus is not mediated solely by androgen receptors in muscle fibers. Endocrinology. 2009;150(7):3207–3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Monks DA, O'Bryant EL, Jordan CL. Androgen receptor immunoreactivity in skeletal muscle: enrichment at the neuromuscular junction. J Comp Neurol. 2004;473(1):59–72 [DOI] [PubMed] [Google Scholar]
- 30. Swift-Gallant A, Monks DA. Androgen receptor expression in satellite cells of the neonatal levator ani of the rat. Dev Neurobiol. 2013;73(6):448–454 [DOI] [PubMed] [Google Scholar]
- 31. Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, Zheng W, Bhasin S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab. 2004;89(10):5245–5255 [DOI] [PubMed] [Google Scholar]
- 32. Prins GS, Putz O. Molecular signaling pathways that regulate prostate gland development. Differentiation. 2008;76(6):641–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prins GS, Birch L. The developmental pattern of androgen receptor expression in rat prostate lobes is altered after neonatal exposure to estrogen. Endocrinology. 1995;136(3):1303–1314 [DOI] [PubMed] [Google Scholar]
- 34. Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21(1):70–71 [DOI] [PubMed] [Google Scholar]
- 35. Inoue S, Inoue M, Fujimura S, Nishinakamura R. A mouse line expressing Sall1-driven inducible Cre recombinase in the kidney mesenchyme. Genesis. 2010;48(3):207–212 [DOI] [PubMed] [Google Scholar]
- 36. Sato T, Matsumoto T, Kawano H, et al. Brain masculinization requires androgen receptor function. Proc Natl Acad Sci USA. 2004;101(6):1673–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. MacLean HE, Chiu WS, Ma C, et al. A floxed allele of the androgen receptor gene causes hyperandrogenization in male mice. Physiol Genomics. 2008;33(1):133–137 [DOI] [PubMed] [Google Scholar]
- 38. Brüning JC, Michael MD, Winnay JN, et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2(5):559–569 [DOI] [PubMed] [Google Scholar]
- 39. Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8(24):1323–1326 [DOI] [PubMed] [Google Scholar]
- 40. Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D, Chambon P. Ligand-activated site-specific recombination in mice. Proc Natl Acad Sci USA. 1996;93(20):10887–10890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237(3):752–757 [DOI] [PubMed] [Google Scholar]
- 42. Haraguchi R, Motoyama J, Sasaki H, et al. Molecular analysis of coordinated bladder and urogenital organ formation by Hedgehog signaling. Development. 2007;134(3):525–533 [DOI] [PubMed] [Google Scholar]
- 43. Haraguchi R, Suzuki K, Murakami R, et al. Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development. 2000;127(11):2471–2479 [DOI] [PubMed] [Google Scholar]
- 44. Ogi H, Suzuki K, Ogino Y, et al. Ventral abdominal wall dysmorphogenesis of Msx1/Msx2 double-mutant mice. Anat Rec A Discov Mol Cell Evol Biol. 2005;284(1):424–430 [DOI] [PubMed] [Google Scholar]
- 45. Suzuki K, Yamanishi K, Mori O, et al. Defective terminal differentiation and hypoplasia of the epidermis in mice lacking the Fgf10 gene. FEBS Lett. 2000;481(1):53–56 [DOI] [PubMed] [Google Scholar]
- 46. Olson EN. Interplay between proliferation and differentiation within the myogenic lineage. Dev Biol. 1992;154(2):261–272 [DOI] [PubMed] [Google Scholar]
- 47. Lyons GE, Mühlebach S, Moser A, et al. Developmental regulation of creatine kinase gene expression by myogenic factors in embryonic mouse and chick skeletal muscle. Development. 1991;113(3):1017–1029 [DOI] [PubMed] [Google Scholar]
- 48. Netzer C, Rieger L, Brero A, et al. SALL1, the gene mutated in Townes-Brocks syndrome, encodes a transcriptional repressor which interacts with TRF1/PIN2 and localizes to pericentromeric heterochromatin. Hum Mol Genet. 2001;10(26):3017–3024 [DOI] [PubMed] [Google Scholar]
- 49. Hawke TJ, Meeson AP, Jiang N, et al. p21 is essential for normal myogenic progenitor cell function in regenerating skeletal muscle. Am J Physiol Cell Physiol. 2003;285(5):C1019–C1027 [DOI] [PubMed] [Google Scholar]
- 50. Matsushita H, Morishita R, Kida I, et al. Inhibition of growth of human vascular smooth muscle cells by overexpression of p21 gene through induction of apoptosis. Hypertension. 1998;31(1 Pt 2):493–498 [DOI] [PubMed] [Google Scholar]
- 51. Niel L, Willemsen KR, Volante SN, Monks DA. Sexual dimorphism and androgen regulation of satellite cell population in differentiating rat levator ani muscle. Dev Neurobiol. 2008;68(1):115–122 [DOI] [PubMed] [Google Scholar]
- 52. Smith MR, Hamson DK, Poort JE, Jordan CL, Breedlove SM. Ontogeny of androgen receptor expression in spinal nucleus of the bulbocavernosus motoneurons and their target muscles in male mice. Neurosci Lett. 2012;513(2):119–123 [DOI] [PubMed] [Google Scholar]
- 53. Bryson-Richardson RJ, Currie PD. The genetics of vertebrate myogenesis. Nat Rev Genet. 2008;9(8):632–646 [DOI] [PubMed] [Google Scholar]
- 54. Kardon G, Harfe BD, Tabin CJ. A Tcf4-positive mesodermal population provides a prepattern for vertebrate limb muscle patterning. Dev Cell. 2003;5(6):937–944 [DOI] [PubMed] [Google Scholar]
- 55. Mathew SJ, Hansen JM, Merrell AJ, et al. Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development. 2011;138(2):371–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kaftanovskaya EM, Huang Z, Barbara AM, et al. Cryptorchidism in mice with an androgen receptor ablation in gubernaculum testis. Mol Endocrinol. 2012;26(4):598–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kovacheva EL, Hikim AP, Shen R, Sinha I, Sinha-Hikim I. Testosterone supplementation reverses sarcopenia in aging through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways. Endocrinology. 2010;151(2):628–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chrisman H, Thomson AA. Regulation of urogenital smooth muscle patterning by testosterone and estrogen during prostatic induction. Prostate. 2006;66(7):696–707 [DOI] [PubMed] [Google Scholar]
- 59. Frank NY, Kho AT, Schatton T, et al. Regulation of myogenic progenitor proliferation in human fetal skeletal muscle by BMP4 and its antagonist Gremlin. J Cell Biol. 2006;175(1):99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yu H, Pask AJ, Shaw G, Renfree MB. Differential expression of WNT4 in testicular and ovarian development in a marsupial. BMC Dev Biol. 2006;6:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liu XH, Wu Y, Yao S, et al. Androgens up-regulate transcription of the Notch inhibitor Numb in C2C12 myoblasts via Wnt/β-catenin signaling to T cell factor elements in the Numb promoter. J Biol Chem. 2013;288(25):17990–17998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jory A, Le Roux I, Gayraud-Morel B, et al. Numb promotes an increase in skeletal muscle progenitor cells in the embryonic somite. Stem Cells. 2009;27(11):2769–2780 [DOI] [PubMed] [Google Scholar]
- 63. Noseda M, Chang L, McLean G, et al. Notch activation induces endothelial cell cycle arrest and participates in contact inhibition: role of p21Cip1 repression. Mol Cell Biol. 2004;24(20):8813–8822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huang Z, Rivas B, Agoulnik AI. NOTCH1 gain of function in germ cells causes failure of spermatogenesis in male mice. PLoS One. 2013;8(7):e71213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Orr B, Grace OC, Vanpoucke G, Ashley GR, Thomson AA. A role for notch signaling in stromal survival and differentiation during prostate development. Endocrinology. 2009;150(1):463–472 [DOI] [PubMed] [Google Scholar]
- 66. Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159(1):123–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Péault B, Rudnicki M, Torrente Y, et al. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15(5):867–877 [DOI] [PubMed] [Google Scholar]
- 68. Mitchell KJ, Pannérec A, Cadot B, et al. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol. 2010;12(3):257–266 [DOI] [PubMed] [Google Scholar]
- 69. Pannérec A, Formicola L, Besson V, Marazzi G, Sassoon DA. Defining skeletal muscle resident progenitors and their cell fate potentials. Development. 2013;140(14):2879–2891 [DOI] [PubMed] [Google Scholar]
- 70. Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12(2):153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pannérec A, Marazzi G, Sassoon D. Stem cells in the hood: the skeletal muscle niche. Trends Mol Med. 2012;18(10):599–606 [DOI] [PubMed] [Google Scholar]
- 72. Schienda J, Engleka KA, Jun S, et al. Somitic origin of limb muscle satellite and side population cells. Proc Natl Acad Sci USA. 2006;103(4):945–950 [DOI] [PMC free article] [PubMed] [Google Scholar]