Abstract
Aldosterone synthesis is initiated upon the transport of cholesterol from the outer to the inner mitochondrial membrane, where the cholesterol is hydrolyzed to pregnenolone. This process is the rate-limiting step in acute aldosterone production and is mediated by the steroidogenic acute regulatory (StAR) protein. We have previously shown that angiotensin II (AngII) activation of the serine/threonine protein kinase D (PKD) promotes acute aldosterone production in bovine adrenal glomerulosa cells, but the mechanism remains unclear. Thus, the purpose of this study was to determine the downstream signaling effectors of AngII-stimulated PKD activity. Our results demonstrate that overexpression of the constitutively active serine-to-glutamate PKD mutant enhances, whereas the dominant-negative serine-to-alanine PKD mutant inhibits, AngII-induced StAR mRNA expression relative to the vector control. PKD has been shown to phosphorylate members of the activating transcription factor (ATF)/cAMP response element binding protein (CREB) family of leucine zipper transcription factors, which have been shown previously to bind the StAR proximal promoter and induce StAR mRNA expression. In primary glomerulosa cells, AngII induces ATF-2 and CREB phosphorylation in a time-dependent manner. Furthermore, overexpression of the constitutively active PKD mutant enhances the AngII-elicited phosphorylation of ATF-2 and CREB, and the dominant-negative mutant inhibits this response. Furthermore, the constitutively active PKD mutant increases the binding of phosphorylated CREB to the StAR promoter. Thus, these data provide insight into the previously reported role of PKD in AngII-induced acute aldosterone production, providing a mechanism by which PKD may be mediating steroidogenesis in primary bovine adrenal glomerulosa cells.
The mineralocorticoid aldosterone is responsible for sodium reabsorption in the kidney, thereby regulating blood volume and pressure. Angiotensin II (AngII) and elevated potassium levels are the primary secretagogues of aldosterone production from adrenal zona glomerulosa cells (1, 2). Aberrant aldosterone synthesis has been implicated in various cardiovascular disease processes, including hypertension (3), as well as a number of pathological consequences such as cardiac fibrosis (4), kidney disease (5), and congestive heart failure (CHF) (6).
Studies have suggested the significance of aldosterone in CHF patients and in individuals after myocardial infarction. Thus, mineralocorticoid receptor antagonists, added to standard therapies, improved the morbidity and mortality associated with these conditions, as demonstrated in the Randomized Aldactone Evaluation Study (RALES) (7) and the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) (8). This result suggests the importance of inappropriate mineralocorticoid receptor activation to cardiovascular disease, and it seems likely that the direct effects of aldosterone on cardiac fibrosis (reviewed in Refs. 9 and 10) may exacerbate these pathologies.
We and others have shown that protein kinase D (PKD) is integral to AngII signaling (11, 12). Aldosterone production in response to AngII occurs as a result of AngII receptor type 1 (AT1R) binding and, through a heterotrimeric G protein, activation of a phosphoinositide-specific phospholipase C (13). Upon phospholipase C activation, 2 second messengers, inositol 1,4,5-trisphosphate and diacylglycerol (DAG) are generated and stimulate Ca2+ release from intracellular stores (14) and protein kinase C (PKC) activity, respectively. DAG can also be generated through phospholipase D-mediated hydrolysis of phosphatidylcholine induced through AT1R activation (15, 16). AngII has also been shown to activate several tyrosine kinases via the AT1R, including Src family kinases (17).
PKD is a serine/threonine kinase with a high homology to conventional PKC isoforms in the regulatory domain and is activated by phorbol esters, DAG, growth factors, and hormones (18). Novel PKCs activate PKD by phosphorylating serines 744 and 748 in the activation loop (serines 738 and 742 in human) (19), with inhibition of PKC activity abrogating PKD transphosphorylation and thus its activity in many cell types (20). We have recently shown that PKD mediates AngII-induced aldosterone synthesis acutely (12), and there is also a report implicating PKD in chronic production of aldosterone (11). Thus, Gomez-Sanchez's group showed that PKD is able to modulate the expression of 11β-hydroxylase and aldosterone synthase steroidogenic enzymes (11). However, the mechanisms by which PKD activation results in acute aldosterone secretion remain unclear.
Steroidogenic acute regulatory (StAR) protein is a transport protein that requires phosphorylation for activation (21) and traffics cholesterol from the outer mitochondrial membrane (OMM) to the inner mitochondrial membrane (IMM) (22), where the lipid is cleaved to produce pregnenolone. The delivery of cholesterol to the IMM is the rate-limiting step in the acute production of steroid hormones (23). Mutations in the StAR gene have been implicated in the development of congenital lipoid adrenal hyperplasia, a disease characterized by the inability to synthesize steroids due to the failure of affected individuals to shuttle cholesterol to the IMM (24, 25). Thus, because previous studies showed that PKD up-regulated aldosterone production in primary bovine adrenal glomerulosa cells, it was of interest to determine the role of this enzyme in modulating StAR expression. In addition, there are data to support the involvement of the activating transcription factor (ATF)/cAMP response element binding protein (CREB) family of transcription factors in AngII-mediated steroidogenesis (26), and ATF/CREB family of transcription factors have been demonstrated to bind to the promoter and modulate the transcription of StAR (27, 28). More interestingly, in addition to several known downstream targets of PKD (29, 30), CREB has been shown to be phosphorylated and activated by PKD at Ser133 in cardiac myocytes (31). Thus, in this study, we investigated the hypothesis that PKD phosphorylates and activates the ATF/CREB family of transcription factors, in particular ATF-2 and CREB, to induce transcription of StAR and underlie AngII-stimulated acute aldosterone secretion.
Materials and Methods
Adrenal glomerulosa cell culture
Glomerulosa cell slices were prepared from near-term fetal bovine adrenal glands obtained from a local meat-packing plant, and the cells were dispersed from collagenase-digested slices by mechanical agitation. Freshly isolated cells were cultured overnight in Falcon Primaria dishes in a DMEM-Ham's F-12 medium (1:1) containing 10% horse serum (vol/vol), 2% fetal bovine serum (vol/vol), ascorbate (100μM), α-tocopherol (1.2μM), Na2SeO3 (0.05μM), butylated hydroxyanisole (50μM), metyrapone (5μM), penicillin (100 U/mL), streptomycin (100 μg/mL), and amphotericin-B (0.25 μg/mL) with or without 0.1% gentamycin. After replacement of the serum-containing medium with serum-free medium plus 0.2% BSA, the cells were incubated for an additional 20–24 hours before treatment. Before stimulation, the medium was removed, and the cells were washed 3 times with Krebs-Ringer bicarbonate-buffered (KRB+) solution containing sodium acetate (KRB+ containing 120mM NaCl, 24.9mM NaHCO3, 3.5mM KCl, 1.2mM MgSO4, 1.2mM NaH2PO4, 1.25mM CaCl2, 20.1% dextrose, 0.2% BSA, and 2.5mM NaAcetate) and incubated at 37°C in KRB+ equilibrated with 5% CO2 for 30–60 minutes (eqKRB+).
Measurement of aldosterone production
Cultured adrenal glomerulosa cells were incubated with eqKRB+ containing the appropriate agents for the indicated times, and the supernatants were collected and stored frozen until aldosterone was assayed using a solid-phase RIA kit (Siemens).
Plasmid constructs
Recombinant DNA techniques were performed as described in Ref. 32. Human PKD constructs, with serines 738/742 mutated to alanines to produce a dominant-negative mutant or to glutamate to generate a constitutively active mutant, were obtained from Dr Alex Toker (Harvard Medical School) and have been previously described (33). The recombinant adenovirus constructs carrying these genes were generated by homologous recombination between the pAdTrack-CMV shuttle plasmid and an adenoviral backbone vector, pAdEasy-1, in electrocompetent BJ5183 cells. Recombinant plasmids were then transfected into Ad-293 cells to generate the replication-deficient adenoviruses. Large-scale virus production was achieved by amplification in Ad-293 cells and purification using cesium chloride gradient banding followed by dialysis with storage buffer (10% glycerol, 10mM Tris base, and 0.9% NaCl [pH 8.1]) for 1 hour, with multiple changes of buffer. Titers were determined by measuring OD260. Primary cultures were infected with the adenoviral constructs at a multiplicity of infection of 25 in serum-free medium. After 4 hours of incubation at 37°C, green fluorescent protein expression was observed using confocal microscopy to confirm infection, and media were replaced with serum-free media for 20 hours.
RNA extraction, cDNA synthesis, and real-time RT-PCR
Total RNA was extracted from cells using the RNeasy kit (QIAGEN) according to protocols of the manufacturer. Purity and integrity of the RNA was checked spectroscopically using a Nanodrop instrument (NanoDrop Technologies). Total RNA was reverse transcribed using iScript Reverse Transcription Supermix for quantitative RT-PCR (qRT-PCR) (Life Sciences Research) following the manufacturer's protocol. Primers for the amplification of target sequences were purchased from Applied Biosystems, and PCR amplifications were performed using the ABI StepOne Real-Time PCR System following the reaction parameters recommended by the manufacturer (20 μL of total volumes consisting of Fast Reagent Master Mix, primers/probes mix with bovine StAR [catalog number Bt03213118_g1], or bovine glyceraldehyde 3-phosphate dehydrogenase [GAPDH] [catalog number Bt03210909_g1], with 5 μL of cDNA added to the mix). GAPDH was used as an endogenous control gene. Negative controls contained water instead of cDNA. In all experiments, the relative gene expression was calculated by the ΔΔCt method. The resultant mRNA was normalized to a calibrator; in each case, the calibrator chosen is the basal (untreated) sample. Final results were expressed as n-fold difference in gene expression relative to calibrator.
Western blot analysis
Cultured adrenal glomerulosa cells were stimulated with eqKRB+ containing the appropriate agents for the indicated times. Cells were solubilized with warm lysis buffer (Tris-HCl [0.175M; pH 8.5], sodium dodecyl sulfate (SDS) [3%, wt/vol], and EGTA [1.5mM]) and scraped, and buffer containing 30% (vol/vol) glycerol, 15% 2-mercaptoethanol (vol/vol), and 0.1% bromophenol blue (wt/vol) was added to constitute loading buffer. Because measured protein concentrations were within 5% in each experiment, equal volumes of sample were subjected to SDS-PAGE on an 8% gel and transferred to Immobilon-P membrane, which was then incubated with blocking buffer and appropriate primary antibodies. Antibodies used for experiments included anti-PKD, anti-ATF-2, and anti-CREB, and antiphospho-Thr69/71 ATF-2 and antiphospho-Ser133 CREB from Cell Signaling and antiactin antibody from Sigma. Infrared imaging was performed on an Odyssey imaging system, LI-COR Biosciences.
Chromatin immunoprecipitation (ChIP)
This assay was performed using a ChIP assay kit from Millipore-Upstate Technologies according to the manufacturer's instructions. Briefly, on day 1, primary cell cultures were infected for 4 hours with empty vector and the serine-to-glutamate PKD mutant adenovirus, and media were replaced with serum-free media for an additional 16–20 hours. On the second day, cell cultures were preincubated in eqKRB+ for 30 minutes before treatment with 10nM AngII for 1 hour. After treatment, DNA and protein complexes were cross-linked with 1% formaldehyde at 37°C for 10 minutes. Next, cells were collected and resuspended in 500 μL of SDS lysis buffer and left on ice for 10 minutes before sonication; 10 μL of the supernatant were retained as input (starting material, to normalize results), whereas 100 μL were diluted 1:10 in 900 μL of ChIP dilution buffer and cleared with 80 μL of sonicated salmon sperm DNA protein A agarose for 6 hours at 4°C. Immunocomplexes were formed using 2-μg antiphospho-Ser133 CREB antibody overnight at 4°C (d 2). Immunoprecipitation with salmon sperm DNA and protein A agarose was performed at 4°C for 2 hours. DNA and protein complexes were reverse cross-linked overnight at 65°C by the addition of 5 μL of 5M NaCl per sample (d 3). DNA was extracted using a QIAGEN DNeasy kit and resuspended in 20 μL of Tris-EDTA buffer (d 4).
Primer sequences were designed according to the sequence of the putative CREB binding site on the proximal portion of the bovine StAR promoter (forward, 5′-TGCAGAGCCACACTCTAAACAGCA-3′ and reverse, 5′-CTCACCAGGAGTCTTTGGCCATTT-3′). A 5-μL volume of each sample and input was used for quantitative PCR according to the IQ SYBR Green Supermix protocol (Life Sciences Research). Negative controls contained water instead of DNA. Final results were calculated using the ΔΔCt method as described in the real-time PCR section above, using the input Ct value to normalize the data and with the vector-infected, untreated sample used as the calibrator. A melt curve was performed to determine the efficiency of the StAR primers used for the CREB ChIP experiments. Gel electrophoresis was also performed; no contaminating products were present in the reaction.
Statistical analysis
All experiments were repeated a minimum of 3 times, and within each experiment, variables were performed at least in duplicate. Statistically significant differences for the time-course experiments and ChIP analysis were determined by ANOVA with a Newman-Keuls post hoc test using the computer program Prism (GraphPad Software). Alternatively, for the Western blotting experiments, the values were log transformed before analysis in order to stabilize the variance in relation to the mean, and ANOVA was then used to compare the different treatment groups. Finally, a Dunnett's test was used to compare all other groups with the control group and a contrast was used to compare AngII vs treatment + AngII. For the qRT-PCR experiments, the ΔCt (target gene − control gene) values were the data used in the analyses. ANOVA was then used to compare the different groups, and the ΔΔ Ct values were calculated using contrasts that subtracted control from all other groups. SAS 9.3 (SAS Institute, Inc) was used for the analyses, and statistical significance was determined at P < .05. For ease of understanding, the graphed values are presented as either fold over control or percent of the maximal response.
Results
AngII induced a time-dependent increase in StAR mRNA expression
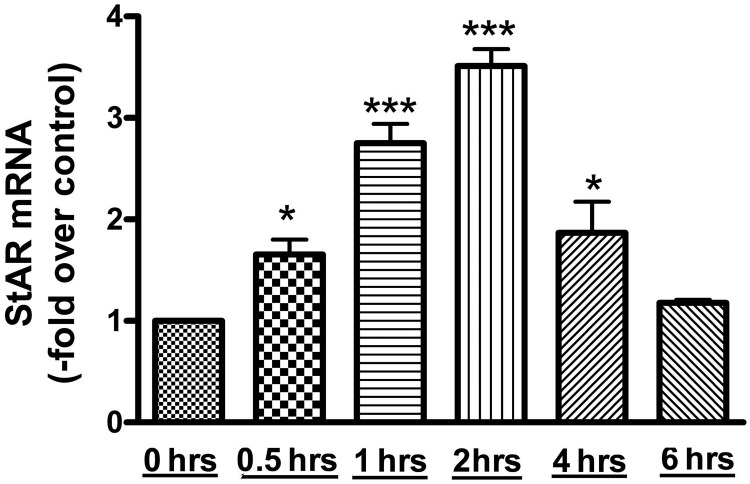
AngII is an important regulator of aldosterone production, and StAR protein is required for cholesterol transport to the IMM; this is the rate-limiting step in aldosterone synthesis. By microarray analysis, we have previously observed that StAR expression is increased after a 1-hour treatment with AngII (34). Thus, to determine the time point at which AngII induces maximal StAR mRNA expression, time-course experiments were performed. StAR mRNA expression was measured at time points of 0, 0.5, 1, 2, 4, and 6 hours. StAR expression was greatest at the 2-hour time point, with a mean value of 3.51 ± 0.17-fold over the control, and returned to almost basal levels by 6 hours (Figure 1).
Figure 1.
AngII induced StAR mRNA expression in primary bovine adrenal glomerulosa cells in a time-dependent manner. After a 30-minute preincubation with eqKRB+, primary cultures of bovine adrenal glomerulosa cells were treated for 30 minutes or 1, 2, 4, or 6 hours with 10nM AngII. Controls without AngII treatment were performed at times 0 and 6 hours. RNA was isolated and used for qRT-PCR with data normalized to GAPDH and shown as the fold change compared with basal (control 0 min). Values represent means ± SEM from 4 experiments performed in duplicate; *, P < .05 vs control; ***, P < .001 vs control.
Overexpression of the constitutively active Ser738/742-to-glutamate PKD mutant increased, and the dominant-negative Ser738/742-to-alanine PKD mutant inhibited, AngII-induced StAR mRNA expression
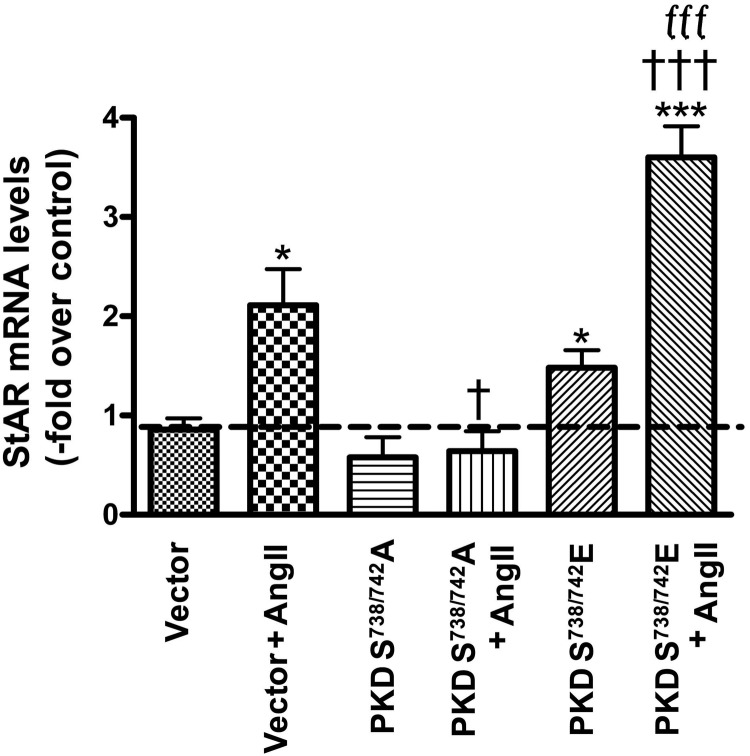
Next, we sought to determine the mechanism by which AngII-induced PKD activation mediates aldosterone production. Manna et al (35) have previously demonstrated in Leydig tumor cells that phorbol 12-myristate 13-acetate (PMA)-induced PKD activation increases steroidogenesis, whereas small interfering RNA knockdown of PKD activity suppresses both PMA- and dibutyryl-cAMP-induced StAR expression and progesterone synthesis (35). This result, together with the findings that PKD mediates both acute and chronic aldosterone production (11, 12) and cortisol secretion (11), suggests that PKD affects a step common to all steroidogenesis, such as StAR. Given these results, we hypothesized that PKD might increase StAR expression to regulate aldosterone secretion acutely in primary bovine adrenal glomerulosa cells. To determine whether PKD promotes StAR mRNA expression, primary bovine adrenal glomerulosa cells were infected with either vector, the dominant-negative Ser738/742-to-alanine PKD mutant or the constitutively active Ser738/742-to-glutamate PKD mutant, and the infected cells were then incubated with 10nM AngII for 2 hours, after which time RNA was isolated for qRT-PCR analysis. Overexpression of the constitutively active PKD mutant promoted StAR mRNA expression compared with vector-infected cells, and this effect was even more pronounced upon AngII stimulation. On the other hand, the dominant-negative PKD mutant inhibited AngII-induced StAR gene expression in comparison with vector-infected cells (Figure 2). This result is consistent with our previous report that the PKD mutants had little or no effect on basal aldosterone secretion but enhanced (constitutively active) or inhibited (dominant-negative) AngII-induced steroidogenesis (12).
Figure 2.
The constitutively active Ser738/742-to-glutamate PKD mutant promoted, and the dominant-negative Ser738/742-to-alanine PKD mutant inhibited, StAR mRNA expression. Cultured primary bovine glomerulosa cells were incubated for 4 hours with adenovirus expressing pAdtrackCMV (empty vector), or the dominant-negative Ser738/742-to-alanine (PKDS738/742A) or constitutively active Ser738/742-to-glutamate (PKDS738/742E) PKD mutants. On the second day of culture, cells were treated with or without 10nM AngII for 2 hours, and RNA was isolated and used for qRT-PCR with data normalized to GAPDH. The ΔΔCt method was used to analyze the data, and values were expressed as the fold change compared with basal (vector without AngII stimulation) as described in Materials and Methods. Values represent means ± SEM from 4 experiments performed in duplicate; *, P < .05; ***, P < .001 vs control (vector); †, P < .05; †††, P < .001 vs vector + AngII; fff, P < .001 vs PKDS738/742A + AngII.
AngII induced a time-dependent increase in CREB and ATF-2 phosphorylation
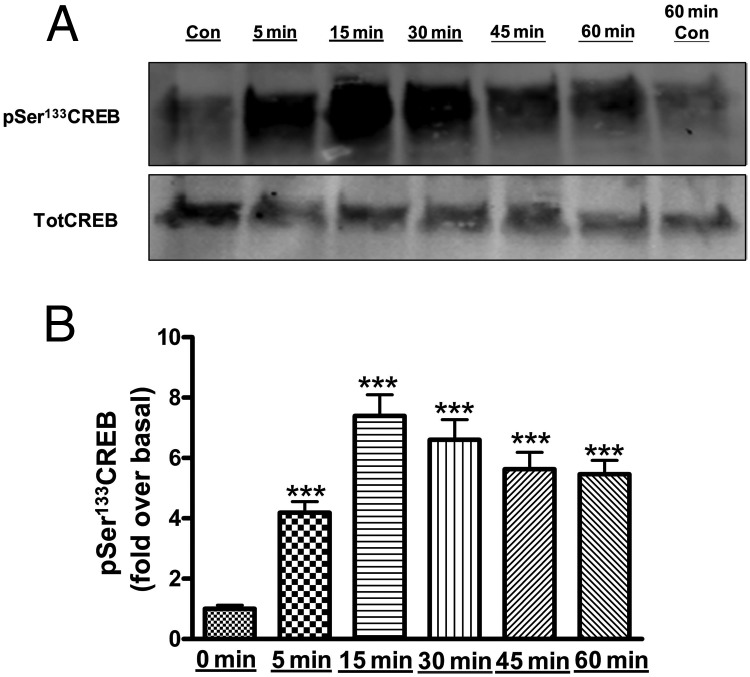
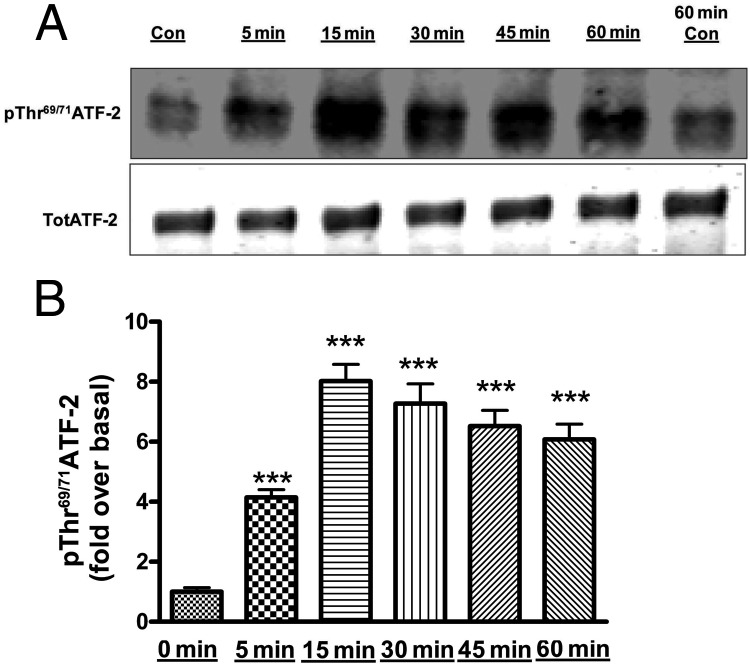
Members of the ATF/CREB family of transcription factors have been shown to interact with the StAR promoter to enhance steroidogenesis in a Leydig cell model in response to stimulation with a cAMP analog (27). To determine whether ATF/CREB family members are also phosphorylated/activated by AngII in primary bovine adrenal glomerulosa cells, immunoblot analysis was performed using phospho-specific antibodies to monitor AngII-induced CREB and ATF-2 phosphorylation at different time points (0, 5, 15, 30, 45, and 60 min). The phosphorylation of both CREB (Figure 3, A and B) and ATF-2 (Figure 4, A and B) was increased at 5 minutes, reaching a maximum at 15 minutes, followed by a slow decline in the remaining 45 minutes of exposure. Similarly, we observed an increase in aldosterone production by 5 minutes, with maximal secretion at 15 minutes compared with control (468.3 ± 58.1 vs 12 ± 0.7 pg/mL).
Figure 3.
AngII induced phosphorylation (activation) of CREB in primary bovine adrenal glomerulosa cells in a time-dependent manner. After a 30-minute preincubation with eqKRB+, primary cultures of bovine adrenal glomerulosa cells were treated for 5, 15, 30, 45, or 60 minutes with 10nM AngII. Controls (Con; no AngII treatment) were performed at times 0 and 60 minutes. Samples were analyzed by Western blotting to determine phospho-Ser133 CREB levels. A, Representative blot. B, Band intensities from multiple experiments were quantified and normalized to total CREB (TotCREB). Values represent means ± SEM of 6 samples from 3 separate experiments and are expressed as fold over control (the average of control times 0 and 60 min); ***, P < .001 vs control.
Figure 4.
AngII induced phosphorylation (activation) of ATF-2 in primary bovine adrenal glomerulosa cells in a time-dependent manner. After a 30-minute preincubation with eqKRB+, primary cultures of bovine adrenal glomerulosa cells were treated for 5, 15, 30, 45, and 60 minutes with 10nM AngII. Controls (Con; no AngII treatment) were performed at times 0 and 60 minutes. Samples were analyzed by Western blotting to determine phospho-Thr69/71 ATF-2 levels. A, Representative blot. B, Band intensities from multiple experiments were quantified and normalized to total ATF-2 (TotATF-2). Values represent means ± SEM of 6 samples from 3 separate experiments and are expressed as fold over control (the average of control times 0 and 60 min); ***, P < .001 vs control.
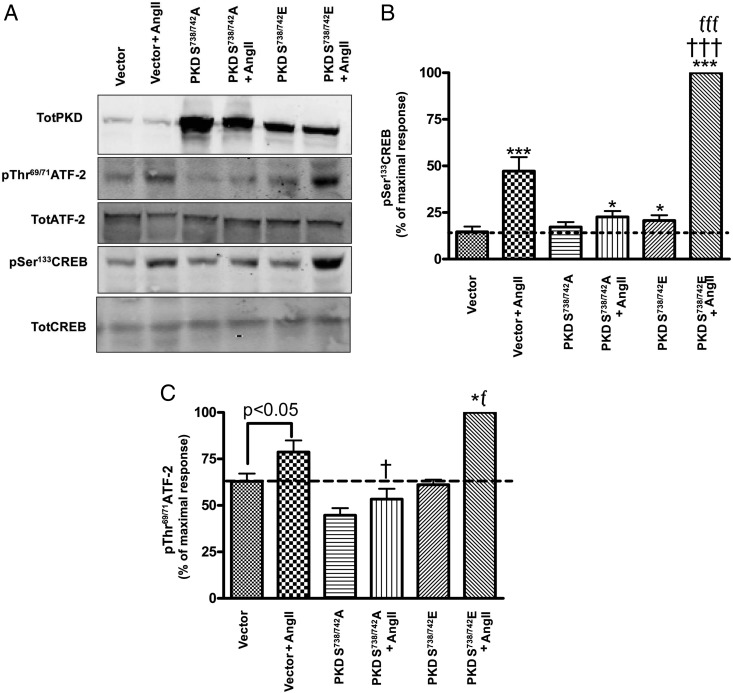
Overexpression of the constitutively active Ser738/742-to-glutamate PKD mutant increased, and the dominant-negative Ser738/742-to-alanine PKD mutant inhibited, AngII-induced CREB and ATF-2 phosphorylation
Studies by Johannessen et al (31) demonstrated the involvement of PKD in phosphorylating and activating the CREB family of transcription factors, and increasing transcription of target genes. Transcription of the StAR gene is influenced by multiple DNA regulatory factors, including the ATF/CREB family of transcription factors and activator protein-1. Furthermore, it has also been previously demonstrated that members of the ATF/CREB family of transcription factors bind the StAR promoter (27). Because PKD phosphorylates CREB at Ser133 and thereby stimulates its transcriptional activity (31), we sought to determine whether AngII-induced PKD activation increased the phosphorylation of CREB at Ser133. In addition, we examined the effect of PKD on the phosphorylation of ATF-2, which is also phosphorylated in response to AngII in the primary bovine adrenal glomerulosa cells. Primary bovine adrenal glomerulosa cells were infected with either empty vector (control) or dominant-negative serine-to-alanine or constitutively active serine-to-glutamate PKD mutants for 4 hours, after which time the media were replaced with serum-free media for 20 hours. Cells were then treated with or without 10nM AngII for 1 hour and collected for Western blot analysis. The constitutively active PKD mutant increased CREB phosphorylation, and this effect was greatly enhanced upon AngII stimulation. On the other hand, the dominant-negative PKD severely blunted the AngII-induced increase in CREB phosphorylation (Figure 5, A and B). Although the effect of AngII on the phosphorylation of ATF2 was less than that of CREB, similar results were observed with ATF-2 phosphorylation (Figure 5, A and C). Thus, constitutively active PKD increased ATF-2 phosphorylation, and this effect was augmented upon AngII stimulation; the dominant-negative mutant inhibited the AngII response. We also studied the phosphorylation of CREM in response to AngII, because in the adrenocortical cells NCI-H295R, AngII- and cAMP-induced cAMP response element modulator (CREM; a member of the ATF/CREB family of transcription factors) activation are important for StAR expression (36, 37). However, we observed no induction of CREM phosphorylation by AngII (data not shown). Additionally, we performed PKD overexpression experiments to determine whether AngII-mediated PKD activation induces ATF-1 phosphorylation, another member of the ATF/CREB family of transcription factors, which has been shown to regulate aldosterone synthase expression in NCI-H295R cells (38). However, AngII did not increase ATF-1 phosphorylation in vector-infected cells (data not shown).
Figure 5.
The constitutively active Ser738/742-to-glutamate PKD mutant promoted, and the dominant-negative Ser738/742-to-alanine PKD mutant inhibited, AngII-induced CREB and ATF-2 phosphorylation. Cultured primary bovine glomerulosa cells were incubated for 4 hours with adenovirus expressing pAdtrackCMV (empty vector), or the Ser738/742-to-alanine (PKDS738/742A) or Ser738/742-to-glutamate (PKDS738/742E) PKD mutants. On the second day of culture, media were replaced with serum-free media for an additional 16–20 hours before treatment with or without AngII (10nM) for 1 hour. A, Representative blot. B and C, Band intensities from multiple experiments were quantified and normalized to their respective total proteins (TotCREB or TotATF-2), CREB (B) and ATF-2 (C). Analysis was performed on transformed data, and values were expressed relative to the maximal response (PKDS738/742E + AngII) as described in Materials and Methods. Values represent the means ± SEM from 4 experiments performed in duplicate; *, P < .05 vs control (vector); ***, P < .001 vs control (vector); †, P < .05 vs vector + AngII; †††, P < .001 vs vector + AngII; f, P < .05 vs PKDS738/742A + AngII; fff, P < .001 vs PKDS738/742A + AngII.
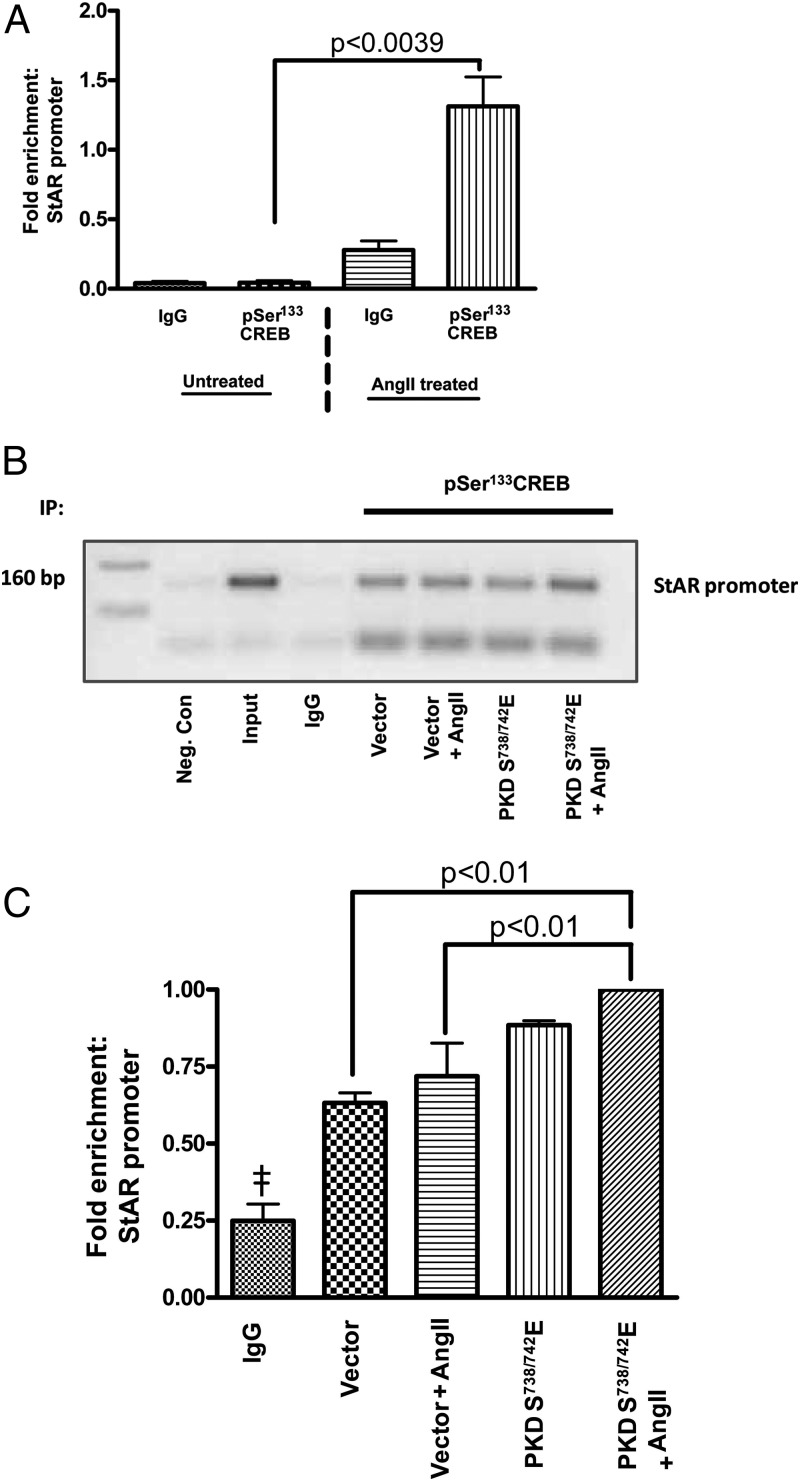
Overexpression of the constitutively active Ser738/742-to-glutamate PKD mutant increased phospho-CREB binding to the StAR promoter
The results shown above indicated that PKD phosphorylates members of the ATF/CREB family of transcription factor at sites that have been shown to activate their transcriptional effects (31, 39). PKD also mediates an increase in StAR expression (Figure 2), and CREB has been found to bind to the StAR promoter (35). To further define the molecular mechanism of PKD-mediated aldosterone production, we determined whether PKD increases StAR expression by phosphorylating CREB and activating CREB-mediated StAR expression by ChIP analysis. Primary bovine glomerulosa cells were infected with either vector or the constitutively active serine-to-glutamate PKD mutant, and ChIP was performed using an antiphospho-CREB antibody to precipitate DNA. This ChIP analysis demonstrated that in intact cells, AngII treatment of the cells expressing the constitutively active PKD mutant increased phospho-CREB binding to the proximal StAR promoter when compared with vector-infected samples (Figure 6). These results support the studies performed by Stocco and coworkers (35), who demonstrated that PKD knockdown decreases the association of CREB, c-Jun, and c-Fos with the StAR promoter and offer more conclusive evidence for a mechanism by which PKD mediates aldosterone production. Taken together, our results demonstrate that the activation of PKD signaling by AngII promotes ATF/CREB phosphorylation, which activates these factors to trigger StAR mRNA expression and therefore aldosterone production in primary bovine adrenal glomerulosa cells.
Figure 6.
Constitutively active Ser738/742-to-glutamate PKD increased phospho-CREB association with the StAR promoter. Cultured primary bovine glomerulosa cells were treated with or without AngII on day 2 before analysis by ChIP. Alternatively, the cells were incubated for 4 hours with adenovirus expressing pAdtrackCMV (empty vector) or the Ser738/742-to-glutamate PKD mutant (PKDS738/742E). On the second day of culture, media were replaced with serum-free media for an additional 16–20 hours before treatment with or without AngII (10nM) for 1 hour. The ChIP assay was performed as described in Materials and Methods. Cross-linked sheared chromatin was immunoprecipitated with either IgG or antiphospho-CREB antibody. We performed PCR analysis on the recovered chromatin using the −160-bp region of the bovine proximal StAR promoter. A, qRT-PCR analysis from 3 experiments showing the fold enrichment upon AngII treatment relative to the control cells (no AngII treatment) and the IgG immunoprecipitation (IP) control. B, Representative gel using semiquantitative RT-PCR to show the association of phospho-CREB with the proximal StAR promoter. A negative control (Neg. Con) containing water instead of DNA is also shown. C, qRT-PCR analysis from 3 experiments demonstrates the fold enrichment of the StAR promoter relative to the IgG control. Values represent the means ± SEM; ‡, P < .05 vs (vector, vector + AngII, PKDS738/742E, PKDS738/742E + AngII).
Discussion
As discussed previously, the rate-limiting step in acute aldosterone production is the transfer of cholesterol from the OMM to the IMM, a process mediated by the StAR protein (23). The regulation of StAR protein is mediated by multiple signaling events, including the cAMP-dependent protein kinase or protein kinase A (PKA) and PKC/PKD pathways, and involves both transcriptional, translational, and posttranslational regulation (reviewed in Refs. 40, 41). However, the mechanisms by which the PKC/PKD pathway regulates StAR activity are yet to be fully understood. PKA regulates StAR activity both by stimulating StAR transcription and StAR protein phosphorylation (42). Nevertheless, studies in MA-10 mouse Leydig tumor cells have demonstrated not only that silencing PKD inhibits PMA-induced StAR mRNA expression and progesterone synthesis (35) but also that stimulation with cAMP could not rescue the steroidogenic capacity of the Leydig tumor cells with knocked down PKD. Transcription factor binding within the proximal region of the mouse StAR promoter has been shown to regulate StAR gene expression (43). These authors also demonstrated that CREB-binding protein and its functional homolog, p300, are transcriptional coactivators that interact with a variety of transcription factors, including ATF/CREB, to play critical roles in steroidogenic gene regulation (43). These results suggest the important role that PKD plays in integrating signaling by both PKC and PKA to control steroidogenesis through regulating StAR.
Our results are consistent with previously reported findings in mouse Leydig tumor cells and suggest a possible mechanism by which the PKC/PKD signaling might promote steroidogenesis. These data indicate for the first time that PKD mediates StAR expression in response to AngII in primary cultures of bovine glomerulosa cells, because overexpression of the constitutively active serine-to-glutamate PKD mutant enhanced AngII-induced StAR gene expression (Figure 2). Using H295R human adrenocortical carcinoma cells, Rainey's group has previously demonstrated that CREM, as well as ATF-1 and ATF-2, is activated by AngII and that the activation of these transcription factors stimulates chronic aldosterone production by increasing CYP11B2, the gene encoding aldosterone synthase, mRNA expression (44). We are the first to demonstrate that AngII-induced PKD activation increases the phosphorylation and activation of ATF-2 and CREB, members of the ATF/CREB family of transcription factors, and that this may be the mechanism through which PKD mediates StAR mRNA expression and acute aldosterone production in bovine glomerulosa cells. Our results demonstrating a lack of effect of AngII treatment on CREM and ATF1 phosphorylation were unexpected given a previous report in the literature for the involvement of these family members in steroidogenesis (27). However, this previous study, performed in mouse Leydig tumor cells, demonstrated that CREM does not bind the StAR proximal promoter. In contrast, NCI-H295R cells do not even express CREB (45), and in these cells, CREM regulates StAR expression (36). Thus, the ATF/CREB family member involved in regulating StAR expression might be specific to the cell type and/or species, and our results indicate that bovine adrenal glomerulosa cells primarily use CREB (and perhaps ATF-2) to influence aldosterone synthesis. In addition, PKD has previously been shown to increase chronic aldosterone production by up-regulating aldosterone synthase (CYP11B2) expression (11). These authors demonstrated an up-regulation of CYP11B2 and 11β-hydroxylase expression in NCI-H295R cells infected with constitutively active PKD mutant constructs (a pleckstrin homology-deletion mutant and the serine-to-glutamate PKD mutant used in this study). These studies indicate a role for PKD also in chronic aldosterone production and aldosterone synthetic capacity, because CYP11B2 represents the rate-limiting late regulatory step in aldosterone production.
In many cell types, the serine-to-glutamate PKD mutant exhibits constitutive activity (46, 47). However, previous results from our laboratory (12) indicated that the serine-to-glutamate PKD mutant alone did not enhance aldosterone secretion, and hence, an increase in steroidogenesis with overexpression of the mutant (relative to vector-infected cells) was only observed when AngII was added. Romero et al (11) obtained similar results, showing that the serine-to-glutamate PKD mutant did not enhance aldosterone secretion in the absence of AngII stimulation. Nonetheless, the current results suggest that the serine-to-glutamate PKD mutant exhibits some constitutive, although perhaps not maximal, activity, because cells overexpressing this mutant displayed a slight but significant increase in StAR mRNA expression, as well as in CREB phosphorylation, in the absence of AngII. Indeed, Wood et al (48) demonstrated that in lymphoma cells, the serine-to-glutamate PKD mutant requires an additional signal to be fully active. These authors observed that the purportedly constitutively active serine-to-glutamate PKD mutant was only minimally activated/phosphorylated at Ser916 basally. However, stimulation with the phorbol ester, phorbol 12,13-dibutyrate (a pharmacological mimic of DAG) or activation of the antigen receptor (which results in DAG production) induced a robust PKD activation as measured by autophosphorylation at Ser916 (48). These results suggest that the serine-to-glutamate PKD mutant no longer requires PKC transphosphorylation at serines 738 and 742 for its activation but nevertheless requires a DAG signal for full activity. Similarly, we have found that in bovine adrenal glomerulosa cells, the activation of the serine-to-glutamate PKD mutant, as monitored by Ser916 phosphorylation, is enhanced by AngII treatment (Supplemental Figure 1), suggesting that as in lymphoma cells (48), the serine-to-glutamate PKD mutant is not fully active in the absence of a second signal. Our previous results indicate that the AngII-stimulated activity of phospholipase D, which underlies in part increased DAG levels in response to the hormone (49), also contributes to AngII-induced PKD activation (50). Taken together, these results suggest that the “constitutively active” serine-to-glutamate PKD mutant requires a second signal, likely DAG, for full activation.
On the other hand, it is also known that aldosterone secretion requires 2 signals, a DAG/PKC/PKD signal and a calcium signal (reviewed in Refs. 51, 52). This requirement may result, in part, from the fact that StAR activity is regulated not only at the transcriptional level but also translationally and posttranslationally, eg, by phosphorylation (reviewed in Ref. 53); further, calcium is likely required for StAR activation. Indeed, Cherradi et al (54) have shown that changes in mitochondrial calcium concentration are required for AngII-induced aldosterone production and cholesterol translocation from the OMM to the IMM. Thus, even in the presence of a second DAG signal, the constitutively active serine-to-glutamate PKD may require an additional calcium signal to induce aldosterone secretion. However, whether or not PKD itself can phosphorylate StAR and stimulate its activity requires further research. In addition, DAG/PKC/PKD is not the only mechanism underlying aldosterone production in response to AngII, which activates a number of other signaling pathways, including phosphoinositide hydrolysis, increases in cytosolic calcium levels and calcium/calmodulin-dependent protein kinase activity, production of lipoxygenase metabolites, such as 12-hydroxyeicosatetraenoic acid and tyrosine kinase activation (reviewed in Ref. 53). The relationship between these other signaling pathways and PKD is unclear and warrants additional investigation.
Taken together, our results provide insight into the previously reported role of PKD in AngII-induced acute aldosterone production and provide a mechanism by which PKD may be mediating the increased acute steroidogenic response in primary adrenal glomerulosa cells. Thus, PKD may be a potential target for therapeutic intervention in the development of better strategies to alleviate hypertension and to treat CHF.
Acknowledgments
We thank Dr Alex Toker for generously providing the various PKD constructs as well as for his useful discussions, Dr Bert Vogelstein for his kind gift of the AdEasy adenovirus system, and the excellent technical assistance of Mr Peter Parker for preparation of the bovine glomerulosa cells. This work was submitted in partial fulfillment of the requirements for a doctoral degree from Georgia Regents University (L.O.O.). The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government.
This work was supported by in part by the National Institutes of Health/National Heart, Lung and Blood Institute award HL70046 and the VA Merit award I01BX001344. W.B.B. is supported by a VA Research Career Scientist award.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AngII
- angiotensin II
- ATF
- activating transcription factor
- AT1R
- angiotensin II receptor type 1
- CHF
- congestive heart failure
- ChIP
- chromatin immunoprecipitation
- CREB
- cAMP response element binding protein
- CREM
- cAMP response element modulator
- Ct
- cycle threshold
- CYP11B2
- the gene encoding aldosterone synthase
- DAG
- diacylglycerol
- eqKRB+
- KRB+ equilibrated with 5% CO2 for 30–60 minutes
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- IMM
- inner mitochondrial membrane
- KRB+
- Krebs-Ringer bicarbonate buffered
- OMM
- outer mitochondrial membrane
- PKA
- protein kinase A
- PKC
- protein kinase C
- PKD
- protein kinase D
- PMA
- phorbol 12-myristate 13-acetate
- qRT-PCR
- quantitative RT-PCR
- SDS
- sodium dodecyl sulfate
- StAR
- steroidogenic acute regulatory.
References
- 1. Müller J. Aldosterone: the minority hormone of the adrenal cortex. Steroids. 1995;60(1):2–9 [DOI] [PubMed] [Google Scholar]
- 2. Quinn SJ, Williams GH. Regulation of aldosterone secretion. Annu Rev Physiol. 1988;50:409–426 [DOI] [PubMed] [Google Scholar]
- 3. Calhoun DA. Aldosterone and cardiovascular disease: smoke and fire. Circulation. 2006;114:2572–2574 [DOI] [PubMed] [Google Scholar]
- 4. Delcayre C, Swynghedauw B. Molecular mechanisms of myocardial remodeling. The role of aldosterone. J Mol Cell Cardiol. 2002;34(12):1577–1584 [DOI] [PubMed] [Google Scholar]
- 5. Rocha R, Stier CT, Jr, Kifor I, et al. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141(10):3871–3878 [DOI] [PubMed] [Google Scholar]
- 6. Weber KT, Sun Y, Guarda E. Structural remodeling in hypertensive heart disease and the role of hormones. Hypertension. 1994;23:869–877 [DOI] [PubMed] [Google Scholar]
- 7. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–717 [DOI] [PubMed] [Google Scholar]
- 8. Pitt B, Williams G, Remme W, et al. The EPHESUS trial: eplerenone in patients with heart failure due to systolic dysfunction complicating acute myocardial infarction. Eplerenone Post-AMI Heart Failure Efficacy and Survival Study. Cardiovasc Drugs Ther. 2001;15(1):79–87 [DOI] [PubMed] [Google Scholar]
- 9. Brilla CG, Maisch B, Zhou G, Weber KT. Hormonal regulation of cardiac fibroblast function. Eur Heart J. 1995;16(suppl C):45–50 [DOI] [PubMed] [Google Scholar]
- 10. DiBianco R. The changing syndrome of heart failure: an annotated review as we approach the 21st century. J Hypertens Suppl. 1994;12(4):S73–S87 [PubMed] [Google Scholar]
- 11. Romero DG, Welsh BL, Gomez-Sanchez EP, Yanes LL, Rilli S, Gomez-Sanchez CE. Angiotensin II-mediated protein kinase D activation stimulates aldosterone and cortisol secretion in H295R human adrenocortical cells. Endocrinology. 2006;147(12):6046–6055 [DOI] [PubMed] [Google Scholar]
- 12. Shapiro BA, Olala L, Arun SN, Parker PM, George MV, Bollag WB. Angiotensin II-activated protein kinase D mediates acute aldosterone secretion. Mol Cell Endocrinol. 2010;317(1–2):99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kojima I, Shibata H, Ogata E. Phorbol ester inhibits angiotensin-induced activation of phospholipase C in adrenal glomerulosa cells. Its implication in the sustained action of angiotensin. Biochem J. 1986;237:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spät A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev. 2004;84:489–539 [DOI] [PubMed] [Google Scholar]
- 15. Zheng X, Bollag WB. AngII induces transient phospholipase D activity in the H295R glomerulosa cell model. Mol Cell Endocrinol. 2003;206(1–2):113–122 [DOI] [PubMed] [Google Scholar]
- 16. Bollag WB, Jung E, Calle RA. Mechanism of angiotensin II-induced phospholipase D activation in bovine adrenal glomerulosa cells. Mol Cell Endocrinol. 2002;192:7–16 [DOI] [PubMed] [Google Scholar]
- 17. Sirianni R, Sirianni R, Carr BR, Pezzi V, Rainey WE. A role for src tyrosine kinase in regulating adrenal aldosterone production. J Mol Endocrinol. 2001;26(3):207–215 [DOI] [PubMed] [Google Scholar]
- 18. Zugaza JL, Waldron RT, Sinnett-Smith J, Rozengurt E. Bombesin, vasopressin, endothelin, bradykinin, and platelet-derived growth factor rapidly activate protein kinase D through a protein kinase C-dependent signal transduction pathway. J Biol Chem. 1997;272(38):23952–23960 [DOI] [PubMed] [Google Scholar]
- 19. Waldron RT, Rozengurt E. Protein kinase C phosphorylates protein kinase D activation loop Ser744 and Ser748 and releases autoinhibition by the pleckstrin homology domain. J Biol Chem. 2003;278(1):154–163 [DOI] [PubMed] [Google Scholar]
- 20. Waldron RT, Iglesias T, Rozengurt E. Phosphorylation-dependent protein kinase D activation. Electrophoresis. 1999;20(2):382–390 [DOI] [PubMed] [Google Scholar]
- 21. Arakane F, King SR, Du Y, et al. Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J Biol Chem. 1997;272(51):32656–32662 [DOI] [PubMed] [Google Scholar]
- 22. Arakane F, Kallen CB, Watari H, et al. The mechanism of action of steroidogenic acute regulatory protein (StAR). StAR acts on the outside of mitochondria to stimulate steroidogenesis. J Biol Chem. 1998;273:16339–16345 [DOI] [PubMed] [Google Scholar]
- 23. Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem. 1994;269(45):28314–28322 [PubMed] [Google Scholar]
- 24. Bose HS, Sugawara T, Strauss JF, 3rd, Miller WL. The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. N Engl J Med. 1996;335(25):1870–1878 [DOI] [PubMed] [Google Scholar]
- 25. Caron KM, Soo SC, Wetsel WC, Stocco DM, Clark BJ, Parker KL. Targeted disruption of the mouse gene encoding steroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. Proc Natl Acad Sci USA. 1997;94(21):11540–11545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nogueira EF, Xing Y, Morris CA, Rainey WE. Role of angiotensin II-induced rapid response genes in the regulation of enzymes needed for aldosterone synthesis. J Mol Endocrinol. 2009;42:319–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clem BF, Hudson EA, Clark BJ. Cyclic adenosine 3′,5′-monophosphate (cAMP) enhances cAMP-responsive element binding (CREB) protein phosphorylation and phospho-CREB interaction with the mouse steroidogenic acute regulatory protein gene promoter. Endocrinology. 2005;146(3):1348–1356 [DOI] [PubMed] [Google Scholar]
- 28. Jo Y, King SR, Khan SA, Stocco DM. Involvement of protein kinase C and cyclic adenosine 3′,5′-monophosphate-dependent kinase in steroidogenic acute regulatory protein expression and steroid biosynthesis in Leydig cells. Biol Reprod. 2005;73(2):244–255 [DOI] [PubMed] [Google Scholar]
- 29. Lint JV, Rykx A, Vantus T, Vandenheede JR. Getting to know protein kinase D. Int J Biochem Cell Biol. 2002;34(6):577–581 [DOI] [PubMed] [Google Scholar]
- 30. Bollag WB, Dodd ME, Shapiro BA. Protein kinase D and keratinocyte proliferation. Drug News Perspect. 2004;17(2):117–126 [DOI] [PubMed] [Google Scholar]
- 31. Johannessen M, Delghandi MP, Rykx A, et al. Protein kinase D induces transcription through direct phosphorylation of the cAMP-response element-binding protein. J Biol Chem. 2007;282(20):14777–14787 [DOI] [PubMed] [Google Scholar]
- 32. Luo J, Deng ZL, Luo X, et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc. 2007;2(5):1236–1247 [DOI] [PubMed] [Google Scholar]
- 33. Storz P, Döppler H, Toker A. Protein kinase Cδ selectively regulates protein kinase D-dependent activation of NF-κB in oxidative stress signaling. Mol Cell Biol. 2004;24:2614–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nogueira EF, Vargas CA, Otis M, Gallo-Payet N, Bollag WB, Rainey WE. Angiotensin-II acute regulation of rapid response genes in human, bovine, and rat adrenocortical cells. J Mol Endocrinol. 2007;39:365–374 [DOI] [PubMed] [Google Scholar]
- 35. Manna PR, Soh JW, Stocco DM. The involvement of specific PKC isoenzymes in phorbol ester-mediated regulation of steroidogenic acute regulatory protein expression and steroid synthesis in mouse Leydig cells. Endocrinology. 2011;152(1):313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meier RK, Clark BJ. Angiotensin II-dependent transcriptional activation of human steroidogenic acute regulatory protein gene by a 25-kDa cAMP-responsive element modulator protein isoform and Yin Yang 1. Endocrinology. 2012;153(3):1256–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sugawara T, Sakuragi N, Minakami H. CREM confers cAMP responsiveness in human steroidogenic acute regulatory protein expression in NCI-H295R cells rather than SF-1/Ad4BP. J Endocrinol. 2006;191(1):327–337 [DOI] [PubMed] [Google Scholar]
- 38. Nogueira EF, Rainey WE. Regulation of aldosterone synthase by activator transcription factor/cAMP response element-binding protein family members. Endocrinology. 2010;151:1060–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59(4):675–680 [DOI] [PubMed] [Google Scholar]
- 40. Kallen CB, Arakane F, Christenson LK, Watari H, Devoto L, Strauss JF., 3rd Unveiling the mechanism of action and regulation of the steroidogenic acute regulatory protein. Mol Cell Endocrinol. 1998;145(1–2):39–45 [DOI] [PubMed] [Google Scholar]
- 41. Christenson LK, Strauss JF., 3rd Steroidogenic acute regulatory protein: an update on its regulation and mechanism of action. Arch Med Res. 2001;32(6):576–586 [DOI] [PubMed] [Google Scholar]
- 42. Jefcoate CR, Lee J, Cherradi N, Takemori H, Duan H. cAMP stimulation of StAR expression and cholesterol metabolism is modulated by co-expression of labile suppressors of transcription and mRNA turnover. Mol Cell Endocrinol. 2011;336(1–2):53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Manna PR, Dyson MT, Stocco DM. Role of basic leucine zipper proteins in transcriptional regulation of the steroidogenic acute regulatory protein gene. Mol Cell Endocrinol. 2009;302(1):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nogueira EF, Rainey WE. Regulation of aldosterone synthase by activator transcription factor/cAMP response element-binding protein family members. Endocrinology. 2010;151(3):1060–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Groussin L, Massias JF, Bertagna X, Bertherat J. Loss of expression of the ubiquitous transcription factor cAMP response element-binding protein (CREB) and compensatory overexpression of the activator CREMtau in the human adrenocortical cancer cell line H295R. J Clin Endocrinol Metab. 2000;85(1):345–354 [DOI] [PubMed] [Google Scholar]
- 46. Rozengurt E, Rey O, Waldron RT. Protein kinase D signaling. J Biol Chem. 2005;280(14):13205–13208 [DOI] [PubMed] [Google Scholar]
- 47. Rykx A, De Kimpe L, Mikhalap S, et al. Protein kinase D: a family affair. FEBS Lett. 2003;546:81–86 [DOI] [PubMed] [Google Scholar]
- 48. Wood CD, Marklund U, Cantrell DA. Dual phospholipase C/diacylglycerol requirement for protein kinase D1 activation in lymphocytes. J Biol Chem. 2005;280(7):6245–6251 [DOI] [PubMed] [Google Scholar]
- 49. Bollag WB, Jung E, Calle RA. Mechanism of angiotensin II-induced phospholipase D activation in bovine adrenal glomerulosa cells. Mol Cell Endocrinol. 2002;192(1–2):7–16 [DOI] [PubMed] [Google Scholar]
- 50. Olala LO, Seremwe M, Tsai YY, Bollag WB. A role for phospholipase D in angiotensin II-induced protein kinase D activation in adrenal glomerulosa cell models. Mol Cell Endocrinol. 2013;66(1):31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rasmussen H, Isales CM, Calle R, et al. Diacylglycerol production, Ca2+ influx, and protein kinase C activation in sustained cellular responses. Endocr Rev. 1995;16:649–681 [DOI] [PubMed] [Google Scholar]
- 52. Bollag WB, Shapiro BA. Angiotensin II signaling in the adrenal cortex: a role for protein kinases C and D in acute aldosterone secretion? In: Miura H, Sasaki Y, eds. Angiotensin Research Progress Hauppauge, NY: Nova Science Publishers, Inc; 2008:1–33 [Google Scholar]
- 53. Hattangady NG, Olala LO, Bollag WB, Rainey WE. Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol. 2012;350(2):151–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cherradi N, Brandenburger Y, Capponi AM. Mitochondrial regulation of mineralocorticoid biosynthesis by calcium and the StAR protein. Eur J Endocrinol. 1998;139(3):249–256 [DOI] [PubMed] [Google Scholar]