Abstract
Neurons in the arcuate nucleus that coexpress kisspeptin, neurokinin B (NKB), and dynorphin (KNDy neurons) play an important role in the modulation of reproduction by estrogens. Here, we study the anatomical and electrophysiological properties of arcuate NKB neurons in heterozygous female transgenic mice with enhanced green fluorescent protein (EGFP) under the control of the Tac2 (NKB) promoter (Tac2-EGFP mice). The onset of puberty, estrous cyclicity, and serum LH were comparable between Tac2-EGFP and wild-type mice. The location of EGFP-immunoreactive neurons was consistent with previous descriptions of Tac2 mRNA-expressing neurons in the rodent. In the arcuate nucleus, nearly 80% of EGFP neurons expressed pro-NKB-immunoreactivity. Moreover, EGFP fluorescent intensity in arcuate neurons was increased by ovariectomy and reduced by 17β-estradiol (E2) treatment. Electrophysiology of single cells in tissue slices was used to examine the effects of chronic E2 treatment on Tac2-EGFP neurons in the arcuate nucleus of ovariectomized mice. Whole-cell recordings revealed arcuate NKB neurons to be either spontaneously active or silent in both groups. E2 had no significant effect on the basic electrophysiological properties or spontaneous firing frequencies. Arcuate NKB neurons exhibited either tonic or phasic firing patterns in response to a series of square-pulse current injections. Notably, E2 reduced the number of action potentials evoked by depolarizing current injections. This study demonstrates the utility of the Tac2-EGFP mouse for electrophysiological and morphological studies of KNDy neurons in tissue slices. In parallel to E2 negative feedback on LH secretion, E2 decreased the intensity of the EGFP signal and reduced the excitability of NKB neurons in the arcuate nucleus of ovariectomized Tac2-EGFP mice.
Loss-of-function mutations in the genes encoding neurokinin B (NKB) or the neurokinin 3 receptor (NK3R) lead to a failure of pubertal development, low levels of serum gonadotropins, and infertility in both women and men (1–3). These findings have stimulated intense interest in determining precisely how NKB signaling influences the reproductive axis. Although NKB neurons are distributed in multiple brain regions (4), studies of the hypothalamus of premenopausal and postmenopausal women have implicated the infundibular (arcuate) nucleus as a key site for the modulation of NKB gene expression by estrogens (5). NKB neurons in this site express estrogen receptor α and and are termed KNDy neurons based on the coexpression of kisspeptin, NKB and dynorphin (5–8).
KNDy neurons have been identified in the arcuate nucleus of the monkey, goat, sheep, rat, and mouse, enabling numerous studies of their structure and function (8–16). NKB and kisspeptin gene expression in the arcuate nucleus is increased by ovariectomy and reduced by estrogens, in parallel with the negative feedback effects of estrogens on LH secretion (6, 9, 10, 17–20). Selective ablation of KNDy neurons in the rat blocks the rise in serum LH after ovariectomy and lowers tonic levels of LH, documenting an essential requirement of KNDy neurons in reproductive hormone secretion (21). KNDy neurons may also play an important role in 17β-estradiol (E2) modulation of body weight (21) and body temperature (22, 23) and the metabolic control of reproduction (24).
The development of genetically altered mice with reporter genes linked to the Kiss1 promoter has greatly facilitated electrophysiological studies of kisspeptin neurons, including those in the arcuate nucleus (25–32). There have been limited reports, however, on transgenic mice with reporter genes linked to Tac2, the NKB gene in the mouse (33, 34). Here, we examine a mouse that contains a bacterial artificial chromosome (BAC) expressing enhanced green fluorescent protein (EGFP) under the control of the Tac2 promoter (35).
Our first goal was to evaluate the utility of heterozygous Tac2-EGFP mice for studies of arcuate NKB neurons. To ensure that the reporter BACs in KNDy neurons did not interfere with normal reproductive function, we evaluated whether the onset of puberty, estrous cyclicity, and estrogen negative feedback was comparable between Tac2-EGFP and wild-type littermate controls. In addition, to evaluate for ectopic expression, the distribution of EGFP-expressing neurons was carefully mapped using immunohistochemistry and computer microscopy, and these data were compared with previous descriptions of Tac2 mRNA. We also confirmed that NKB and EGFP were coexpressed in arcuate neurons and determined whether E2 altered EGFP expression in ovariectomized (OVX) mice in a similar manner to its effects on Tac2 mRNA. Once the utility of this model was verified, we used tissue slices from Tac2-EGFP mice to conduct whole-cell patch-clamp recordings of arcuate NKB neurons. Because E2 dramatically reduces Tac2 gene expression, we hypothesized that treatment of OVX mice with chronic physiological levels of E2 would alter the biophysical properties, spontaneous activity, or evoked activity of arcuate NKB neurons.
Materials and Methods
Animals
Animal protocols were approved by the University of Arizona Animal Care and Use Committee and conformed to National Institutes of Health guidelines. Animals were housed in the Animal Care Facility at the University of Arizona on a 12-hour light, 12-hour dark cycle (lights on at 7 am) with water and food ad libitum. Mice were fed a diet that was low in phytoestrogens (Teklad Global 2019; Harlan Laboratories).
The Tac2-EGFP mouse was developed by the Gene Expression Nervous System Atlas Project at The Rockefeller University. An EGFP DNA sequence was inserted directly downstream of the Tac2 promoter into a BAC vector using homologous recombination (35, 36). The modified BACs (4–8 copies) were then injected into fertilized ova. Because BACs are inserted into random sites in the DNA, they are not expected to alter the native copy of the Tac2 gene (35). Cryopreserved embryos were purchased from the Mutant Mouse Regional Resource Center (015495-UCD; STOCK Tg [Tac2-EGFP] 381Gsat/Mmcd; University of California, Davis) and implanted into 2 pseudopregnant, wild-type Swiss-Webster females at the Genetically Engineered Mouse Model Core Facility at the University of Arizona. This resulted in the delivery of 20 mice, which were genotyped by amplifying tail DNA by PCR (primers: Tac2 5′-CGCATCCATCCAACGCTCTTG-3′ and EGFP, 5′-GGTCGGGGTAGCGGCTGAA-3′).
All experimental animals were generated by breeding heterozygous Tac2-EGFP males to wild-type Swiss-Webster females. Except for the evaluation of puberty and estrous cyclicity, mice were studied at approximately 3 months of age. Groups consisted of either intact diestrous females or mice OVX at 2 months of age and implanted immediately with sc SILASTIC capsules (20-mm effective length, 1.57 mm in inner diameter, 3.18 mm in outer diameter; Dow Corning) containing either 180-μg/mL E2 dissolved in sesame oil (OVX + E2) or vehicle (OVX). All capsules were replaced 2 weeks after implantation, and the mice were studied 1 month after ovariectomy.
Experiment 1. Evaluation of puberty, estrous cycles, and LH secretion in Tac2-EGFP and wild-type mice
Daily examination for vaginal opening was started on the morning of postnatal day 22 in 5 Tac2-EGFP mice (from 2 litters) and 7 wild-type, littermate mice. After vaginal opening, estrous cycles were monitored by vaginal smears (37). Terminal blood samples were collected at 3 months of age in diestrous, OVX, and OVX + E2 rats between 9 and 11 am. Serum LH and estradiol were analyzed at the Ligand Assay and Analysis Core Laboratory at the University of Virginia. The mouse LH Sandwich Assay had an intra-assay variability of 3.1% and a sensitivity of 0.07 ng/mL. Estradiol was assayed using a mouse Calbiotech ELISA assay with an intra-assay variability of 3.1% and a sensitivity of 0.3 pg/mL. Serum hormones were compared by ANOVA and Tukey's post hoc test with α = 0.05.
Experiment 2. Computer-assisted mapping of EGFP-immunoreactive (-ir) neurons in female Tac2-EGFP mice
Four diestrous females were deeply anesthetized with an overdose of sodium pentobarbital and perfused with heparinized 0.1M phosphate buffered saline (PBS, 7.4) followed by 4% paraformaldehyde in phosphate buffer (pH 7.4). Brains were extracted and fixed in 4% paraformaldehyde for 4 hours (4°C) and cryoprotected in ascending sucrose solutions in PBS (10%, 20%, and 30%) over a 3-day interval. Tissue blocks were prepared with a mouse brain matrix (Braintree Scientific, Inc), frozen on dry ice, and serially sectioned on a sliding microtome (40 μm, coronal axis). The sections were stored at −20°C in cryoprotectant solution (38). Every tenth section was Nissl stained.
The polyclonal GFP antibody (GFP-1020, lot number 1229FP08; Aves Labs) was raised in chicken eggs against recombinant GFP emulsified in Freund's adjuvant. Western blots of whole mouse embryo homogenates identified a single band around 24 kDa (Aves Labs). Titration of the chicken GFP antibody revealed optimum staining at very low concentrations (1:100 000) for 3,3′-diaminobenzidine (DAB) immunohistochemistry. There was no specific DAB staining of Tac2-EGFP mice when primary or secondary antibodies were omitted. In parallel immunohistochemical procedures, the GFP antibody did not label neurons in wild-type mouse tissue.
Every fifth section throughout the hypothalamus was rinsed in 0.1M PBS (pH 7.4) to remove cryoprotectant solution. Unless stated otherwise, multiple rinses of 0.1M PBS for 8–10 minutes were performed between each step. Sections were placed in 0.3% H2O2 in PBS for 30 minutes, blocked for 60 minutes (3% normal goat serum and 0.4% Triton X-100 in PBS), and directly incubated for 48 hours at 4°C with the chicken anti-GFP antibody (1:100 000) in blocking solution. Sections were then incubated for 2 hours in biotinylated goat antichicken IgG (lot number W0408; Vector Laboratories, Inc) diluted 1:600 in blocking solution and followed by Vectastain Elite ABC solution (Vector Laboratories, Inc) for 60 minutes. A nickel-intensified DAB reaction was performed by prerinses in 0.175M sodium acetate (3 × 5 min), a 15-minute incubation in freshly prepared, filtered Ni-DAB solution (1.25 g of nickel sulfate, 10 mg of DAB in 50 mL of sodium acetate solution, and 41.5 μL of 30% H2O2 added after filtration), and rinsing with sodium acetate solution (3 × 5 min). Sections were rinsed in PBS, mounted on gelatinized slides, dried, dehydrated with ascending concentrations of ethanol, cleared in xylene, and coverslipped.
An image-combining computer microscope outfitted with a motorized stage (LUDL Electronic Products), a Lucivid miniature CRT and Neurolucida software (MicroBrightfield) was used to map the location of EGFP-ir neurons. Tissue boundaries and region outlines were digitized with a ×4 objective. The sections were systematically scanned using a ×10 objective, and labeled neurons were manually marked. For the illustrations, slides were scanned using a DMetrix DX-20 virtual slide scanner (DMetrix). Low-magnification digital images were obtained from the virtual slides, and mirror images were paired with the computer microscope maps.
Experiment 3. Colocalization of pro-NKB-immunoreactivity with EGFP in the arcuate nucleus of OVX Tac2-EGFP mice
To determine whether the EGFP signal would serve as a marker for arcuate NKB neurons in tissue slices, pro-NKB immunohistochemistry was performed on arcuate sections from 4 OVX Tac2-EGFP mice. We studied OVX mice because the NKB-ir cell bodies were obscured by immunoreactive fibers in intact and OVX + E2 mice. Mice were deeply anesthetized, perfused with fixatives, and the brains processed as described above. The polyclonal rabbit pro-NKB antibody (NB300-201, lot A2; Novus Biologicals) was raised against residues 50–79 of the mouse pro-NKB peptide. This antibody has been extensively used in our previous studies (11, 21, 39). The distribution of pro-NKB-ir neurons was identical to that seen with other pro-NKB antibodies (40, 41). Preadsorption with the synthetic peptide used for immunization (24-h incubation with 10μM synthetic peptide; Novus Biologicals) blocked specific labeling. The omission of either primary or secondary pro-NKB antibody resulted in the absence of specific signal. pro-NKB staining was abolished in the arcuate nucleus by ablating KNDy neurons (21).
Sections were matched to plate 45 and 49 of the mouse brain atlas (42). Unless stated otherwise, sections were rinsed in PBS between steps. Sections were rinsed, incubated in 0.3% H2O2 in PBS for 30 minutes, and blocked for 60 minutes (3% normal goat serum and 0.4% Triton X-100 in PBS). The sections were then directly incubated for 48 hours at 4°C with the pro-NKB antibody (1:5000) in blocking solution, followed by an overnight incubation at 4°C with biotinylated goat antirabbit IgG (lot number W0117; Vector Laboratories, Inc) diluted 1:5000 in blocking solution. The sections were placed in the Vectastain Elite ABC solution for 30 minutes, incubated for 20 minutes with Biotinyl Tyramide (1:200, lot number 560549; PerkinElmer) and 0.005% H2O2 in PBS, then incubated with SA-Alexa Fluor 568 (1:200, lot number 3508208; Invitrogen) for 3 hours in a 37°C water bath. Sections were mounted on gelatinized slides and coverslipped with the ProLong Antifade reagent (Invitrogen).
Fluorescent microscopy was performed using a Nikon E1000 microscope with an epifluorescent attachment, a motorized stage (LUDL Electronic Products), a Uniblitz model VMM-D1 shutter driver (Vincent Associates), and a Photometrics Coolsnap FX camera (Roper Scientific). EGFP and NKB-immunofluorescence were photographed with a ×20 objective and digital montages throughout the arcuate nucleus were created with the aid of MetaMorph imaging software (Molecular Devices). A ×40 objective was used to count the numbers of single- and double-labeled neurons per unilateral arcuate section. The percent colocalization for middle and posterior levels of the arcuate nucleus was calculated for each animal, and these values were used to calculate group means. For the illustrations, images were imported into Adobe Photoshop to adjust for brightness and contrast.
Experiment 4. Semiquantitative analysis of EGFP fluorescent intensity in the arcuate nucleus of diestrous, OVX, and OVX + E2 mice
We observed that the EGFP signal was brighter in the OVX mice relative to intact and OVX + E2 mice. To verify this observation, we used image analysis to evaluate EGFP fluorescent intensity in diestrous, OVX, and OVX + E2 Tac2-EGFP mice (n = 6 mice/group). The mice were euthanized by an overdose of phenobarbital, perfused with fixatives, and the brains processed and sectioned as described above. Sections through the middle arcuate nucleus were rinsed in PBS (pH 7.4, 6 × 10 min), mounted, and coverslipped with ProLong Gold antifade media (Invitrogen). Within 24 hours, imaging was performed with the fluorescent microscope system described above. Sections were evenly distributed so that sections from each group were processed simultaneously and analyzed during each microscopy session. Identical microscope settings and exposure times were used for each session. After coding the sections to prevent experimenter bias, photomicrographs were taken and saved as 12-bit images. Digital montages of the arcuate nucleus were created using MetaMorph Imaging software (Molecular Devices). To count the number of neurons, the labeled neurons were marked on the montages using Corel Draw software (Corel, Inc). For image analysis, thresholds were set to exclude background, and size limitations were applied to select individual neurons. The average fluorescent intensity for each cell (average white value of all pixels for a cell) was measured using MetaMorph tools. This program reports fluorescent units on a 12-bit scale, in which a saturated pixel registers at 4095 U. For statistical analysis, the fluorescent intensity of each cell of the arcuate nucleus was averaged for each animal, and these averages were used to compute group means. Groups were compared using one-way ANOVA with Tukey's post hoc test with α = 0.05. For illustrations of relative fluorescent intensity, no adjustments were made for brightness or contrast.
Experiment 5. Effects of chronic E2 on the biophysical properties, spontaneous activity, and evoked activity of arcuate NKB neurons in tissue slices of OVX Tac2-EGFP mice
Tac2-EGFP mice (OVX, n = 12; OVX + E2, n = 11) were decapitated under isoflurane anesthesia, and brains were quickly extracted. The brains were placed in cold (1°C–2°C), artificial cerebrospinal fluid (aCSF) solution containing: 125mM NaCl, 24mM NaHCO3, 1mM MgCl2, 2.5mM KCl, 1mM CaCl2, and 10mM D-glucose (pH 7.3–7.4). Coronal brain slices (250 μm) were prepared with a vibratome (VT1000P; Leica) in cold, oxygenated (O2, 95%; CO2, 5%) aCSF solution (pH 7.3–7.4). Slices were incubated in oxygenated aCSF in a slice chamber (Scientific Systems Design, Inc) at 32°C for approximately 2 hours before recording. Chemicals were purchased from (Sigma).
Recording pipettes (3–6 MΩ) were pulled from boroscillate glass (OD, 1.5 mm and ID, 0.75 mm; Sutter Instrument) and filled with intracellular solution containing: 10mM HEPES, 0.2mM EGTA, 6mM NaCl, 2mM MgCl2, 130mM K-gluconate, 4mM NaATP, 0.4mM NaGTP, 5mM glutathione, 0.05mM spermine, and 1% biocytin, with osmolarity adjusted to 270–280 mOsm. Tissue slices were transferred to a recording chamber mounted on the stage of an Olympus BX-50WI fixed-stage microscope (×40, 0.75 N.A. water-immersion objective) equipped with differential interference contrast optics, an infrared video camera (C25400-07; Hamamatsu), and appropriate filters. The slices were continuously perfused with oxygenated aCSF at a rate of 1.5 mL/min at approximately 31.5°C. Arcuate NKB neurons were initially identified by brief fluorescent illumination using epifluorescent excitation at 470 nm and approached with the aid of differential interference contrast optics. Whole-cell recordings in current- and voltage-clamp were acquired using an amplifier (Multiclamp 700B; Molecular Devices) connected to a digitizer (Digidata 1440A; Molecular Devices). Liquid junction potentials were corrected before recording. The recorded signal was low-pass filtered at 2 kHz, digitized at 20 kHz, and stored on a computer hard drive.
The experimental protocol consisted of the following sequence: after obtaining a tight seal (>1 GΩ), whole-cell recording mode was achieved by rupturing the membrane under the seal by quick and gentle suction. The spontaneous activity was recorded in voltage-clamp with the membrane potential held at −70 mV for 15 minutes. The number of excitatory postsynaptic current (EPSC) events per minute and their amplitude were determined while holding the cell at −70mV in voltage-clamp mode.
After switching to current-clamp, the spontaneous action potential activity at rest was recorded with no bias current for 5 minutes. In a few neurons, another 5-minute recording was performed with a small bias current to standardize the membrane potential at −60 mV. The latter protocol was not used, because there was no significant difference in resting membrane potential among neurons (Table 1). To measure excitability, current injection steps were delivered from resting membrane potential (no bias). These consisted of 800-millisecond rectangular current pulses, beginning with a single hyperpolarizing step (−150 pA), followed by 10 depolarizing steps (30 pA). The threshold current (defined as the minimum current needed to elicit a single action potential) was determined by injecting brief, small (30 pA) current steps.
Table 1.
Electrophysiological Characteristics of Arcuate Tac2-EGFP Neurons
| Treatment Group | Resting Potential (mV) | Input Resistance (MΩ) | Spike Amplitude (mV) | Mean Frequency (Hz) | EPSC Events/min | EPSC Amplitude (pA) | Time Constant | Threshold Current (pA) |
|---|---|---|---|---|---|---|---|---|
| OVX | −56.0 ± 1.3 | 988.7 ± 0.8 | 55.7 ± 2.8 | 1.3 ± 0.4 | 89.5 ± 17.5 | 48.4 ± 5.4 | 0.02 ± 0.004 | 24.4 ± 5.6 |
| n = 22 | n = 16 | n = 17 | n = 22 | n = 22 | n = 22 | n = 16 | n = 16 | |
| OVX + E2 | −57.4 ± 2.2 | 987.6 ± 0.7 | 48.9 ± 4.8 | 1.55 ± 0.67 | 101.1 ± 26.8 | 60.5 ± 7.8 | 0.01 ± 0.001 | 30.0 ± 7.2 |
| n = 15 | n = 14 | n = 15 | n = 15 | n = 15 | n = 15 | n = 15 | n = 15 |
Values represent mean ± SEM of n neurons from 12 OVX and 11 OVX + E2 mice. No significant differences were identified between groups (Student's t tests).
Neurons with a resting membrane potential higher than −50 mV were excluded from analysis. The resting membrane potential, input resistance, and threshold current were analyzed using pCLAMP 10 software (Molecular Devices). Spike amplitude, mean firing frequency, and EPSC events were analyzed with a custom script written with Spike2 software (Cambridge Electronic Design). Statistical comparisons were made either by Student's t test or ANOVA with Tukey's post hoc tests (α = 0.05), where appropriate. Fisher's exact test was used to compare nonparametric data.
Results
The timing of puberty, estrous cyclicity, and serum LH in response to OVX and E2 treatment was similar in Tac2-EGFP and wild-type mice
The day of vaginal opening was not significantly different between Tac2-EGFP females (postnatal d 29.0 ± 1.05, mean ± SEM, n = 5) and littermate controls (postnatal d 28.8 ± 0.80, n = 7). Most of the Tac2-EGFP mice (4 out of 5) and littermate controls (6 out of 7) exhibited 4- to 5-day estrous cycles, whereas 1 in each group had cycles longer than 6 days. Serum LH was also not significantly different between Tac2-EGFP and wild-type controls (Figure 1). In both groups, LH was significantly increased 1 month after ovariectomy and reduced by E2 treatment (Figure 1A). The levels of serum E2 achieved by the SILASTIC capsules were nearly identical to diestrous values (Figure 1B).
Figure 1.
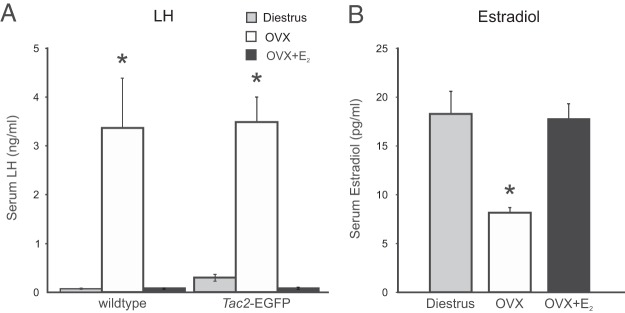
Serum LH and estradiol in diestrous, OVX, and OVX + E2-treated mice. Mice were OVX at 2 months of age and treated with sc capsules containing either E2 or vehicle. Blood samples were taken at 3 months of age. A, Serum LH was significantly increased in OVX mice, compared with diestrous and E2-treated OVX mice. Similar patterns of LH secretion were observed in wild-type and Tac2-EGFG mice. B, Treatment of OVX Tac2-EGFP mice with E2 capsules resulted in diestrous levels of serum E2. n = 5–10 mice/group. *, significantly different from diestrous and OVX + E2 mice (within wild-type and Tac2-EGFP groups, ANOVA with Tukey's post hoc test).
Distribution of EGFP-ir neurons in Tac2-EGFP mice
Using nickel-intensified DAB immunohistochemistry and low concentrations of a chicken GFP antibody (1:100 000), the ratio of signal to background was exceptional in Tac2-EGFP mice. Figure 2 shows a photomicrograph of a virtual slide (right) and computer-assisted map (left) of a section of through the tuberal hypothalamus. At this level, labeled neurons were identified in the arcuate nucleus, lateral hypothalamus, hippocampus, basolateral amygdala, claustrum, zona inserta, and cerebral cortex. Detailed descriptions, computer-assisted maps, and photomicrographs of EGFP-ir neurons at multiple levels of the hypothalamus are included in Supplemental Figures 1 and 2.
Figure 2.
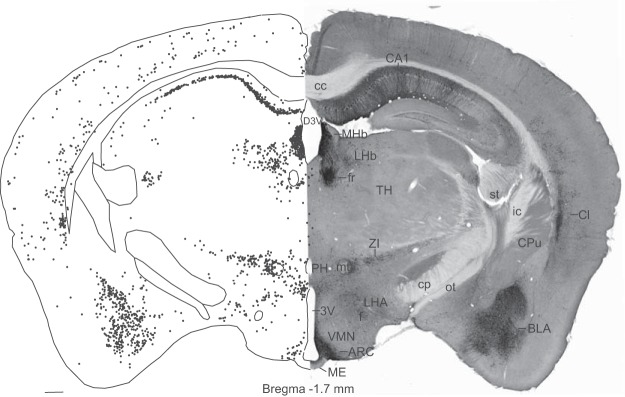
Photomicrograph (right) and computer-assisted map (left) of EGFP immunocytochemistry in a representative brain section from a Tac2-EFGP female mouse. Sections were visualized with nickel-intensified DAB. Each black dot on the left represents a single immunoreactive soma mapped with the Neurolucida image-combining computer microscope. See Supplemental Figures 1 and 2 for additional maps at multiple levels of the hypothalamus. 3V, third ventricle; ARC, arcuate nucleus; BLA, basolateral amygdaloid nucleus; CA1, field CA1 hippocampus; cc, corpus callosum; Cl, claustrum; cp, cerebral peduncle; CPu, caudate putamen (striatum); f, fornix; fr, fasciculus retroflexus; ic, internal capsule; LHA, lateral hypothalamic area; LHb, lateral habenular nucleus; ME, median eminence; MHb, med habenular nucleus; mt, mammilothalamic tract; ot, optic tract; PH, posterior hypothalamus; st, stria terminalis; TH, thalamus; VMN, ventromedial nucleus; ZI, zona incerta. Scale bar, 250 μm.
In the arcuate nucleus, there were heavily labeled EGFP-ir cell bodies that were obscured by a dense network of beaded immunoreactive fibers (Figure 3). Fiber labeling extended from the arcuate nucleus to the median eminence, periventricular zone, ependymal layer of the third ventricle, and ventrally to the pial surface. In the median eminence, the fibers were predominantly in the internal zone, with scattered fibers extending to the external zone (Figure 3).
Figure 3.
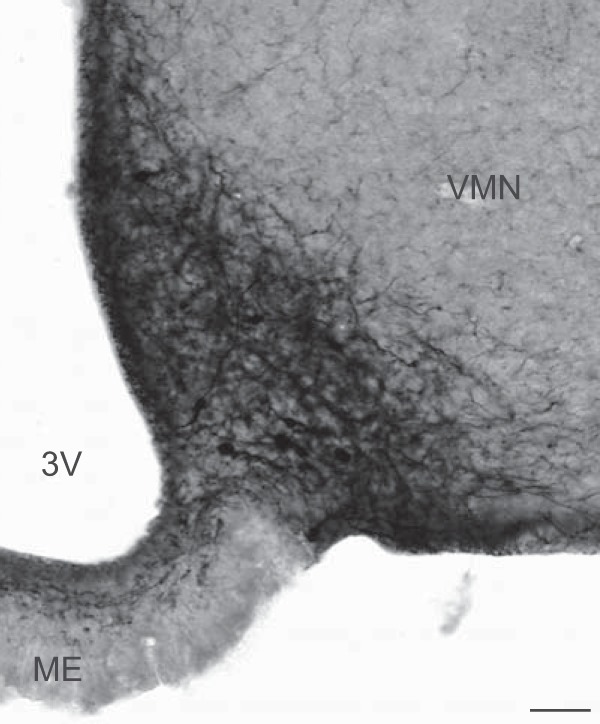
Photomicrograph of EGFP immunocytochemistry in the arcuate nucleus of a Tac2-EGFP female mouse. Sections were visualized with nickel-intensified DAB. Intensely labeled cell bodies were obscured by numerous beaded axons and dendrites. Axons extended to the median eminence, periventricular zone, ependymal layer, and the pial surface. The adjacent ventromedial nucleus was devoid of EGFP-ir somata. See Supplemental Figures 1 and 2 for photomicrographs of EGFP immunohistochemistry of other brain regions. 3V, third ventricle; ME, median eminence; VMN, ventromedial nucleus. Scale bar, 50 μm.
Most EGFP-positive neurons in the arcuate nucleus of Tac2-EGFP mice were labeled with a pro-NKB antibody
Colocalization of pro-NKB-immunofluorescence with EGFP was assessed in 4 OVX mice. The pro-NKB antibody labeled both cell bodies and fibers, whereas the native (unamplified) EGFP signal highlighted predominantly cell bodies (Figure 4). At the midlevel of the arcuate nucleus, 76.8% of EGFP neurons were labeled by the pro-NKB antibody. At posterior levels, 80.0% of EGFP neurons were pro-NKB-ir. Only 56% of the pro-NKB-ir neurons coexpressed EGFP, but because these sections were not processed for EGFP immunocytochemistry, this value likely underestimates the percentage of pro-NKB-ir neurons that coexpress EGFP.
Figure 4.
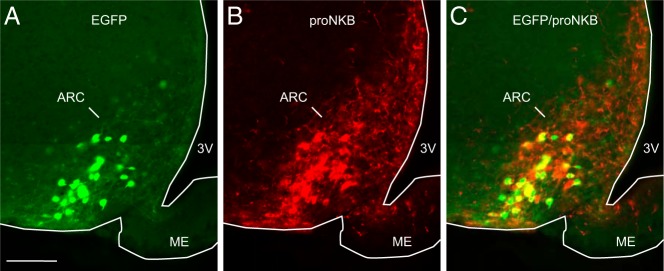
Representative photomicrographs of EGFP-fluorescence (A, green) and pro-NKB-immunofluorescence (B, red) in the arcuate nucleus of an OVX, Tac2-EGFP mouse. Nearly 80% of EGFP neurons in the arcuate nucleus were labeled by the pro-NKB antibody (C, yellow). ARC, arcuate nucleus; ME, median eminence; 3V, third ventricle. Scale bar in A, 100 μm (applies to A–C).
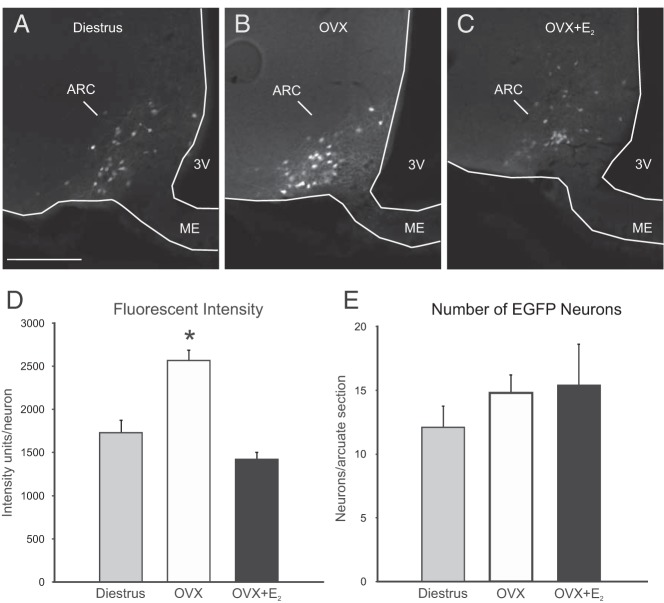
Fluorescent intensity of Tac2-EGFP neurons was increased in the arcuate nucleus of OVX mice and reduced by E2 treatment, with no change in cell number
A qualitatively brighter EGFP signal was observed in the arcuate nucleus of OVX mice (Figure 5, A–C). To verify this effect, semiquantitative analysis of native (unamplified) fluorescent signal was performed in diestrous, OVX, and OVX + E2 mice. Consistent with our observations, the average fluorescent intensity was significantly elevated in the OVX group (Figure 5D), compared with diestrous and OVX + E2 mice. No difference in fluorescent intensity was detected between OVX + E2 and diestrous mice (Figure 5D). Despite this difference in intensity, the number of EGFP neurons counted in each arcuate nucleus was not significantly different between groups (Figure 5E).
Figure 5.
EGFP fluorescence in the arcuate nucleus of Tac2-EGFP mice. Fluorescent arcuate neurons in the OVX mice (B) appeared qualitatively brighter than neurons in diestrous (A) and OVX + E2 (C) mice. D, Semiquantitative image analysis revealed a significant increase in the average fluorescent intensity units per neuron in the arcuate nucleus of OVX mice. E, The mean number of fluorescent neurons counted in a unilateral arcuate nucleus section was not significantly different between groups. 3V, third ventricle; ARC, arcuate nucleus, ME, median eminence. Values are mean ± SEM, n = 5–6 mice/group. *, significantly different from diestrous and OVX + E2 mice (one-way ANOVA with Tukey's post hoc test).
Chronic E2 treatment of OVX mice does not alter the basic electrophysiological properties or the spontaneous firing frequency of arcuate Tac2-EGFP neurons
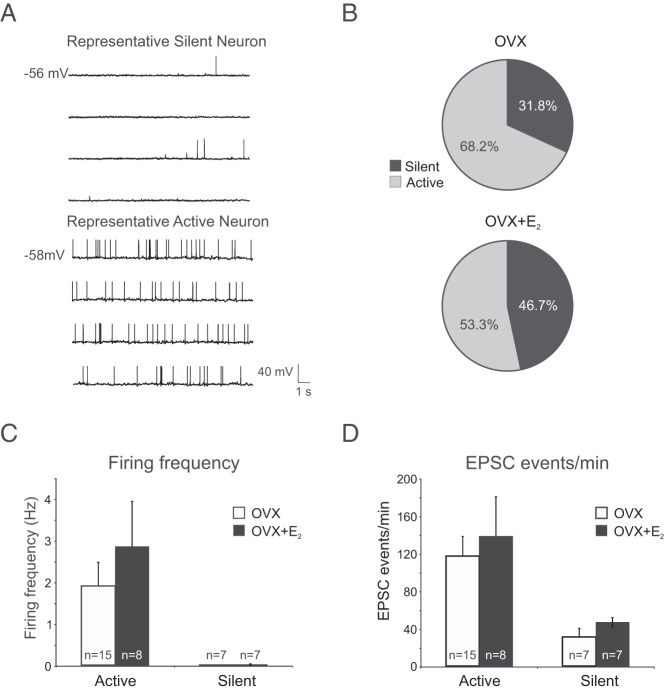
The electrophysiological properties and spontaneous firing frequency of Tac2-EGFP neurons in the arcuate nucleus were determined in 12 OVX mice (n = 22 neurons) and 11 OVX + E2 mice (n = 15 neurons). The basic properties of arcuate NKB neurons are summarized in Table 1. The resting membrane potential, input resistance, spike amplitude, mean firing frequency, EPSC events per minute, EPSC amplitude, and spike threshold were not statistically different between OVX and OVX + E2 groups. The neurons in both OVX and OVX + E2 groups exhibited spontaneous action potentials (defined as action potentials that occurred with no current injected through the recording electrode) with an irregular firing pattern. In current-clamp mode, 2 types of neurons could be identified based on their spontaneous firing at rest: active (mean firing frequency > 0.1 Hz) and silent (mean firing frequency 0–0.1 Hz, representative traces in Figure 6A). 68% of the neurons were classified active in OVX group and 53% in the OVX + E2 group (Figure 6B). In active neurons of both groups, the mean firing frequency at rest was above 1 Hz but not statistically different between treatment groups (Figure 6C). The average EPSC events per minute in voltage-clamp mode were positively correlated with the level of spontaneous action potentials in current-clamp (Figure 6D).
Figure 6.
Spontaneous activity of arcuate Tac2-EGFP neurons in OVX and OVX + E2 mice. A, Representative recordings of neurons classified as either silent (mean firing frequency <0.1 Hz) or active. B, Proportions of silent or active or silent neurons in OVX mice (n = 22 neurons from 12 mice) and OVX + E2 mice (n = 15 neurons from 11 mice). More active neurons were identified in OVX mice, but this difference was not statistically significant (Fisher's exact test). C, There was no significant difference between OVX and OVX + E2 mice in the mean spontaneous firing frequency of neurons classified as either spontaneously active or silent. D, Similarly, no difference was detected in the EPSC events per minute between treatment groups; mean ± SEM; n, number of Tac2-EGFP neurons.
Chronic E2 treatment reduces the excitability of arcuate NKB neurons in Tac2-EGFP mice
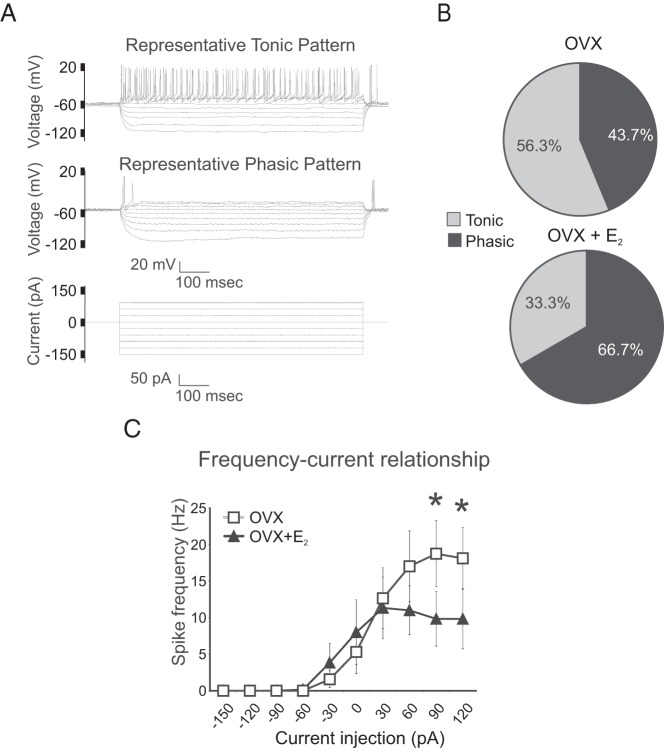
The response to rectangular-pulse current injections was studied in 9 OVX mice (n = 16 neurons) and 11 OVX + E2 mice (n = 15 neurons). When a series of rectangular-pulse current injections were applied, 2 evoked firing patterns were identified: tonic and phasic (Figure 7A). A tonic pattern was identified as persistent firing and little frequency adaptation during the first 400 milliseconds of threshold current injection and consequent depolarizing steps. The phasic pattern was characterized as a single spike or a few spikes followed by rapid adaptation at the threshold current injection and subsequent depolarizing steps. Tonic or phasic evoked firing categories were identified in both OVX and OVX + E2 mice. The majority (56.3%) of neurons in the OVX group were classified as tonic, compared with 33.3% in the OVX+E2 group (Figure 7B), but these differences were not statistically significant (Fisher's exact test); 13% of neurons in the OVX group were classified as silent with a phasic firing pattern, whereas 40% of neurons in the OVX + E2 group were silent with a phasic firing pattern (silent at rest and less responsive to depolarizing current injection). Neurons from the OVX group fired at a higher frequency at more depolarizing current injections, with significant differences between OVX and OVX +E2 mice at current injections of 90 and 120 pA (Figure 7C).
Figure 7.
Evoked activity of arcuate Tac2-EGFP neurons in OVX and OVX + E2 mice. A, Representative patterns of activity evoked by a series of rectangular-pulse current injections. A tonic pattern was identified with little frequency adaptation (top), and a phasic pattern was characterized by a few spikes and rapid adaptation (middle). B, Proportions of neurons identified as tonic or phasic in OVX and OVX + E2 mice. A greater percentage of tonic neurons was detected in the OVX mice, but these changes were not statistically significant (Fisher's exact test). C, In response to depolarizing current injections, OVX + E2 mice exhibited reduced spike frequencies, compared with OVX mice (mean ± SEM; OVX, n = 16 neurons from 9 mice; OVX + E2, n = 15 neurons from 11 mice). *, significantly different, OVX vs OVX + E2 (two-way ANOVA with Tukey's post hoc tests).
Discussion
In the present study, we show the Tac2-EGFP mouse line to be a useful model for anatomical and electrophysiological studies of arcuate KNDy neurons. Nearly 80% of EGFP-positive neurons in the arcuate nucleus of the Tac2-EGFP mouse were labeled with the pro-NKB antibody, confirming the utility of this mouse line for studies of arcuate NKB neurons. Moreover, in a previous study, Ruka et al (34) showed that 94%–100% of fluorescent neurons from the arcuate nucleus of Tac2-EFGP male mice express kisspeptin or dynorphin mRNA. Thus, the fluorescently labeled neurons in the arcuate nucleus of Tac2-EGFP mice exhibit the phenotype of KNDy neurons.
Because KNDy neurons are essential for a functional reproductive axis (21), we examined several parameters to evaluate the status of this physiological system. Importantly, there was no difference in timing of puberty or length of estrous cycles between transgenic Tac2-EGFP female mice and wild-type controls. Moreover, in both Tac2-EGFP and wild-type mice, serum LH was increased after ovariectomy and reduced to intact levels by E2 treatment. Thus, estrogen negative feedback on LH secretion, a major function attributed to KNDy neurons (43), was preserved in Tac2-EGFP mice.
Visualization of EGFP-ir with DAB and nickel intensification allowed robust labeling of EGFP-ir neurons in multiple regions of the central nervous system (see Supplemental Figures 1 and 2). The distribution was consistent with published descriptions of Tac2 mRNA in the mouse (44, 45), with the exception that we did not identify EGFP-ir neurons in the supraoptic nucleus, in agreement with studies in the rat (46). A Tac2-Cre/Tomato mouse line also had similar findings to our study (33), except the Tac2-Cre/Tomato mouse had intense labeling in the hippocampal dentate gyrus that was not seen here (33). Overall, comparisons with previous anatomic data suggest that Tac2-EFGP mouse will be useful for studies of NKB neurons in many brain regions. Additional studies (including dual-labeling procedures) will be necessary to determine whether the modulation by E2 extends to NKB neurons in other regions of the central nervous system.
Ovariectomy has been previously shown to increase Tac2 mRNA in the arcuate nucleus of mice and rats, and this change is reversed by treatment with estrogens (9, 12, 17). In parallel with these findings, we observed a brighter EGFP signal in the arcuate nucleus of OVX mice, compared with diestrous or intact mice. This observation was confirmed by semiquantitative measurements of the fluorescent intensity of arcuate neurons. These data show that EGFP expression in the arcuate nucleus of the Tac2-EGFP mouse is modulated by E2 in the same direction as E2 modulates the endogenous Tac2 gene. Although the signal was less bright in the OVX + E2 group, we counted nearly identical numbers of fluorescent neurons in the OVX and OVX + E2-treated mice. These data indicate that the same population of neurons could be studied, regardless of E2 status.
Based on the reduction of NKB gene expression by E2, we have long postulated that estrogen negative feedback decreases serum LH by reducing the activity of arcuate KNDy neurons. To test this hypothesis, we studied the effects of chronic, physiological levels of E2 on the electrical activity of arcuate Tac2-EGFP neurons in tissue slices. Overall, the basic electrophysiological properties of Tac2-EGFP neurons were not different between OVX and OVX + E2 mice. Although a higher percentage of neurons in the OVX group displayed spontaneous action potential activity, the difference was not significant. We also found no significant effect of E2 on spontaneous firing frequency or EPSC events per minute in the arcuate nucleus of OVX Tac2-EGFP mice. Thus, we were unable to demonstrate that chronic E2 treatment reduces the spontaneous activity of arcuate Tac2-EGFP neurons in tissue slices.
Consistent with our findings, E2 treatment did not significantly decrease the spontaneous activity of KNDy neurons in OVX kisspeptin/Cre/GFP mice (30). In other transgenic models, the percentage of spontaneously active KNDy neurons in OVX mice has ranged from 40% to 52% (26, 30). In contrast, no spontaneous activity was recorded in KNDy neurons in OVX Kiss1-IRES-GFP mice using cell-attached recordings (28). Despite these disparate data, there is general agreement that spontaneous KNDy neuron activity is not lower in intact or gonadal steroid-treated mice, compared with gonadectomized mice (28, 30, 31, 34). An important consideration is that brain sectioning to create tissue slices severs the connections from other brain regions and from KNDy neurons in adjacent brain sections. As a result, in vitro tissue slice studies may not provide an accurate representation of the spontaneous activity occurring in vivo (47), and there could be changes in excitability that are not reflected by changes in spontaneous activity. Another limitation of the whole-cell recording used here is that dialysis of the cytoplasm may remove critical intracellular signaling molecules.
In addition to characterizing biophysical properties and spontaneous activity, we provide the first information on the response of KNDy neurons to injections of depolarizing currents in OVX and OVX + E2 mice. Two response patterns were identified; persistent firing (tonic) and those that responded with rapid adaptation (phasic). Both firing patterns were identified in OVX and OVX + E2 mice, with a nonsignificant trend for more tonically firing neurons in the OVX group. Although E2 did not change the firing threshold, it significantly reduced the firing frequency in response to depolarizing current injections. Thus, E2 treatment decreased the excitability of Tac2-EGFP neurons in the arcuate nucleus of OVX mice.
Further studies are necessary to understand how E2 decreases the excitability of KNDy neurons. For example, these changes could be due to alterations in the expression of potassium channels, which are essential determinants of neuronal excitability (48, 49). Although estradiol alters neurons excitability via voltage-gated potassium channels in hypothalamic neurons (50–53), the effects of chronic E2 replacement on these channels in KNDy neurons are not known. Alternatively, because synaptic or peptidergic input was not blocked in our experiments, the reduced excitability in KNDy neurons by E2 may be mediated indirectly. For example, changes in peptidergic transmission from KNDy neuron collaterals within the tissue slices could have altered the response to depolarizing current. Notably, a recent study of arcuate Tac2-EGFP neurons showed a reduced excitatory response to senktide (an NK3R agonist) in intact male mice, compared with orchidectomized mice (34). Inhibition of KNDy neuron activity by dynorphin was also more pronounced in intact vs orchidectomized males (34). Thus, the reduced response to depolarization in E2-treated mice in the present study could be secondary to reduced excitatory NK3R signaling and/or increased inhibitory dynorphin signaling within the KNDy neuron network.
There is increasing evidence that KNDy neurons are a component of the GnRH pulse generator (12, 15, 54, 55). The GnRH pulse generator consists of bursts of multiunit activity in the arcuate nucleus that are timed with pulses of serum LH, and this phenomenon has been demonstrated in the monkey (56), goat (13, 55), and rat (57, 58). Morphological studies have shown that KNDy neurons are bilaterally interconnected within the arcuate nucleus (via NK3R) and project to GnRH terminals in the median eminence (11, 15, 39, 59–61). Thus, there is an anatomic substrate for KNDy neurons to coordinate their activity to control pulsatile GnRH secretion. Interestingly, electrophysiological studies have shown that arcuate kisspeptin neurons express the ion channels necessary for burst firing (32), but the effects of estrogens on these channels or burst activity in KNDy neurons is not known. In the present study, we demonstrate that chronic E2 treatment reduces the excitability of arcuate KNDy neurons. This phenomenon could play a role in the inhibitory effects of estrogens on the GnRH pulse generator and pulsatile LH secretion (13, 62).
In summary, our studies show that the Tac2-EGFP mouse is a valuable transgenic line for the study of KNDy neurons in the arcuate nucleus. The utility of this transgenic mouse was verified by their preserved reproductive function, the coexpression of EGFP and NKB-ir in arcuate neurons, and the similar distribution of EGFP to previous descriptions of Tac2 mRNA. In OVX Tac2-EGFP mice, E2 treatment reduced the EGFP signal intensity in the arcuate nucleus, similar to the well-described E2 inhibition of Tac2 gene expression. Chronic, physiological levels of E2 did not alter the basic electrophysiological properties or the spontaneous firing frequency of arcuate Tac2-EGFP neurons in tissue slices from OVX mice. However, E2 treatment decreased the excitability of KNDy neurons to pulses of depolarizing current. This decrease in electrical excitability may play a role in the E2 suppression of pulsatile LH secretion that is hypothesized to be mediated via KNDy neurons.
Acknowledgments
We thank Dr Jason Q. Pilarski for expert electrophysiology advice and Dr Ralph F. Fregosi for the use of his electrophysiology laboratory. We also thank Dr Kelly J. Suter, who graciously trained M.C. in hypothalamic slice preparation.
This work was supported by the National Institutes of Health, National Institute of Aging Grant R01 AG-032315. M.C. was supported by Science Foundation Arizona, Achievement Rewards for College Scientists Foundation, and The Evelyn F. McKnight Brain Institute. The Tac2-EGFP mouse was developed by the Gene Expression Nervous System Atlas project at The Rockefeller University (National Institute of Neurological Disorders and Stroke N01NS02331 and HHSN271200723701C) (New York, NY). Hormone assays were conducted at the Ligand Assay and Analysis Core Laboratory at the University of Virginia Center for Research in Reproduction (National Institute of Child Health and Human Development U54-HD28934) (Charlottesville, VA).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aCSF
- artificial cerebrospinal fluid
- BAC
- bacterial artificial chromosome
- DAB
- 3,3′-diaminobenzidine
- E2
- 17β-estradiol
- EGFP
- enhanced green fluorescent protein
- EPSC
- excitatory postsynaptic current
- ir
- immunoreactive
- KNDy neurons
- arcuate neurons coexpressing kisspeptin, NKB, and dynorphin
- NKB
- neurokinin B
- NK3R
- neurokinin 3 receptor
- OVX
- ovariectomized.
References
- 1. Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gianetti E, Tusset C, Noel SD, et al. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab. 2010;95:2857–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Young J, Bouligand J, Francou B, et al. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab. 2010;95:2287–2295 [DOI] [PubMed] [Google Scholar]
- 4. Chawla MK, Gutierrez GM, Young WS, 3rd, McMullen NT, Rance NE. Localization of neurons expressing substance P and neurokinin B gene transcripts in the human hypothalamus and basal forebrain. J Comp Neurol. 1997;384:429–442 [DOI] [PubMed] [Google Scholar]
- 5. Rance NE, Young WS., 3rd Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128:2239–2247 [DOI] [PubMed] [Google Scholar]
- 6. Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92:2744–2750 [DOI] [PubMed] [Google Scholar]
- 7. Hrabovszky E, Ciofi P, Vida B, et al. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci. 2010;31:1984–1998 [DOI] [PubMed] [Google Scholar]
- 8. Goodman RL, Lehman MN, Smith JT, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760 [DOI] [PubMed] [Google Scholar]
- 9. Rance NE, Bruce TR. Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology. 1994;60:337–345 [DOI] [PubMed] [Google Scholar]
- 10. Sandoval-Guzmán T, Stalcup ST, Krajewski SJ, Voytko ML, Rance NE. Effects of ovariectomy on the neuroendocrine axes regulating reproduction and energy balance in young cynomolgus macaques. J Neuroendocrinol. 2004;16:146–153 [DOI] [PubMed] [Google Scholar]
- 11. Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498:712–726 [DOI] [PubMed] [Google Scholar]
- 12. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wakabayashi Y, Nakada T, Murata K, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30:3124–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151:4494–4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J Neuroendocrinol. 2011;23:52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dellovade TL, Merchenthaler I. Estrogen regulation of neurokinin B gene expression in the mouse arcuate nucleus is mediated by estrogen receptor α. Endocrinology. 2004;145:736–742 [DOI] [PubMed] [Google Scholar]
- 18. Abel TW, Voytko ML, Rance NE. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J Clin Endocrinol Metab. 1999;84:2111–2118 [DOI] [PubMed] [Google Scholar]
- 19. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692 [DOI] [PubMed] [Google Scholar]
- 20. Alçin E, Sahu A, Ramaswamy S, et al. Ovarian regulation of kisspeptin neurones in the arcuate nucleus of the rhesus monkey (Macaca mulatta). J Neuroendocrinol. 2013;25:488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, et al. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153:2800–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, McMullen NT, Rance NE. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci USA. 2012;109:19846–19851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34:211–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sanchez-Garrido MA, Tena-Sempere M. Metabolic control of puberty: roles of leptin and kisspeptins. Horm Behav. 2013;64:187–194 [DOI] [PubMed] [Google Scholar]
- 25. Cravo RM, Margatho LO, Osborne-Lawrence S, et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gottsch ML, Popa SM, Lawhorn JK, et al. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology. 2011;152:4298–4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Navarro VM, Gottsch ML, Wu M, et al. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152:4265–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Croft S, Piet R, Mayer C, Mai O, Boehm U, Herbison AE. Spontaneous kisspeptin neuron firing in the adult mouse reveals marked sex and brain region differences but no support for a direct role in negative feedback. Endocrinology. 2012;153:5384–5393 [DOI] [PubMed] [Google Scholar]
- 29. de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154:2750–2760 [DOI] [PubMed] [Google Scholar]
- 30. Frazão R, Cravo RM, Donato J, Jr, et al. Shift in Kiss1 cell activity requires estrogen receptor α. J Neurosci. 2013;33:2807–2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alreja M. Electrophysiology of kisspeptin neurons. Adv Exp Med Biol. 2013;784:349–362 [DOI] [PubMed] [Google Scholar]
- 32. Kelly MJ, Zhang C, Qiu J, Rønnekleiv OK. Pacemaking kisspeptin neurons. Exp Physiol. 2013;98:1535–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mar L, Yang FC, Ma Q. Genetic marking and characterization of Tac2-expressing neurons in the central and peripheral nervous system. Mol Brain. 2012;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and κ-opioid receptors in adult male mice. Endocrinology. 2013;154:2761–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gong S, Zheng C, Doughty ML, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925 [DOI] [PubMed] [Google Scholar]
- 36. Heintz N. BAC to the future: the use of bac transgenic mice for neuroscience research. Nat Rev Neurosci. 2001;2:861–870 [DOI] [PubMed] [Google Scholar]
- 37. Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009;Appendix 4:Appendix 4I [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159 [DOI] [PubMed] [Google Scholar]
- 39. Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489:372–386 [DOI] [PubMed] [Google Scholar]
- 40. Lucas LR, Hurley DL, Krause JE, Harlan RE. Localization of the tachykinin neurokinin B precursor peptide in rat brain by immunocytochemistry and in situ hybridization. Neuroscience. 1992;51:317–345 [DOI] [PubMed] [Google Scholar]
- 41. Ciofi P, Krause JE, Prins GS, Mazzuca M. Presence of nuclear androgen receptor-like immunoreactivity in neurokinin B-containing neurons of the hypothalamic arcuate nucleus of the adult male rat. Neurosci Lett. 1994;182:193–196 [DOI] [PubMed] [Google Scholar]
- 42. Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. 3rd ed New York, NY: Elsevier; 2007 [Google Scholar]
- 43. Rance NE. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 2009;30:111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Duarte CR, Schütz B, Zimmer A. Incongruent pattern of neurokinin B expression in rat and mouse brains. Cell Tissue Res. 2006;323:43–51 [DOI] [PubMed] [Google Scholar]
- 45. Navarro VM, Castellano JM, McConkey SM, et al. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2011;300:E202–E210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Warden MK, Young WS., 3rd Distribution of cells containing mRNAs encoding substance P and neurokinin B in the rat central nervous system. J Comp Neurol. 1988;272:90–113 [DOI] [PubMed] [Google Scholar]
- 47. Constantin S, Iremonger KJ, Herbison AE. In vivo recordings of GnRH neuron firing reveal heterogeneity and dependence upon GABAA receptor signaling. J Neurosci. 2013;33:9394–9401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol. 2009;156:1185–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lv¨scher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nature Rev Neurosci. 2010;11:301–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. DeFazio RA, Moenter SM. Estradiol feedback alters potassium currents and firing properties of gonadotropin-releasing hormone neurons. Mol Endocrinol. 2002;16:2255–2265 [DOI] [PubMed] [Google Scholar]
- 51. Kelly MJ, Qiu J, Rønnekleiv OK. Estrogen modulation of G-protein-coupled receptor activation of potassium channels in the central nervous system. Ann NY Acad Sci. 2003;1007:6–16 [DOI] [PubMed] [Google Scholar]
- 52. Roepke TA, Malyala A, Bosch MA, Kelly MJ, Rønnekleiv OK. Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology. 2007;148:4937–4951 [DOI] [PubMed] [Google Scholar]
- 53. Rønnekleiv OK, Bosch MA, Zhang C. 17β-oestradiol regulation of gonadotrophin-releasing hormone neuronal excitability. J Neuroendocrinol. 2012;24:122–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res. 2010;1364:116–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Okamura H, Tsukamura H, Ohkura S, Uenoyama Y, Wakabayashi Y, Maeda K. Kisspeptin and GnRH pulse generation. Adv Exp Med Biol. 2013;784:297–323 [DOI] [PubMed] [Google Scholar]
- 56. Knobil E. Patterns of hypophysiotropic signals and gonadotropin secretion in the rhesus monkey. Biol Reprod. 1981;24:44–49 [DOI] [PubMed] [Google Scholar]
- 57. Kimura F, Nishihara M, Hiruma H, Funabashi T. Naloxone increases the frequency of the electrical activity of luteinizing hormone-releasing hormone pulse generator in long-term ovariectomized rats. Neuroendocrinology. 1991;53:97–102 [DOI] [PubMed] [Google Scholar]
- 58. Kinsey-Jones JS, Li XF, Luckman SM, O'Byrne KT. Effects of kisspeptin-10 on the electrophysiological manifestation of gonadotropin-releasing hormone pulse generator activity in the female rat. Endocrinology. 2008;149:1004–1008 [DOI] [PubMed] [Google Scholar]
- 59. Foradori CD, Amstalden M, Goodman RL, Lehman MN. Colocalisation of dynorphin A and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol. 2006;18:534–541 [DOI] [PubMed] [Google Scholar]
- 60. Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166:680–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wakabayashi Y, Yamamura T, Sakamoto K, Mori Y, Okamura H. Electrophysiological and morphological evidence for synchronized GnRH pulse generator activity among kisspeptin/neurokinin B/dynorphin A (KNDy) neurons in goats. J Reprod Dev. 2013;59:40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kesner JS, Wilson RC, Kaufman JM, et al. Unexpected responses of the hypothalamic gonadotropin-releasing hormone “pulse generator” to physiological estradiol inputs in the absence of the ovary. Proc Natl Acad Sci USA. 1987;84:8745–8749 [DOI] [PMC free article] [PubMed] [Google Scholar]