Abstract
Transgenic animal models are valuable for studying gene function in various tissue compartments. Mice with conditional deletion of genes in the uterus using the Cre-loxP system serve as powerful tools to study uterine biology. The uterus is comprised of 3 major tissue types: myometrium, stroma, and epithelium. Proliferation and differentiation in each uterine cell type are differentially regulated by ovarian hormones, resulting in spatiotemporal control of gene expression. Therefore, examining gene function in each uterine tissue type will provide more meaningful information regarding uterine biology during pregnancy and disease states. Although currently available Cre mouse lines have been very useful in exploring functions of specific genes in uterine biology, overlapping expression of these Cre lines in more than 1 tissue type and in other reproductive organs sometimes makes interpretation of results difficult. In this article, we report the generation of a new iCre knock-in mouse line, in which iCre is expressed from endogenous lactoferrin (Ltf) promoter. Ltf-iCre mice primarily direct recombination in the uterine epithelium in adult females and in immature females after estrogen treatment. These mice will allow for specific interrogation of gene function in the mature uterine epithelium, providing a helpful tool to uncover important aspects of uterine biology.
The uterus is a complex organ and has 3 major tissue compartments: myometrium, stroma, and epithelium. Coordinated actions of ovarian progesterone and estrogen regulate proliferation and differentiation of these uterine cell types in a spatiotemporal manner (1, 2). Disruption of this regulation leads to infertility and can initiate gynecological diseases, such as endometriosis, endometrial cancer, and many other conditions. More importantly, the uterine epithelium plays a critical role in embryo-uterine interactions for successful implantation. Currently, 4 Cre mouse lines are available for conditional inactivation of genes of interest in the uterus. Each of these Cre lines has its own unique uses, and they have been widely implemented to study gene function in the female reproductive tract (3–7).
Mice expressing Cre under the control of progesterone receptor (PR) (PR-Cre) have been widely used and have generated a wealth of information about gene function in uterine biology during pregnancy and in disease states (2). However, PR is expressed in all major uterine cell types, and thus, PR-Cre can delete floxed genes in all major uterine compartments (myometrium, stroma, and epithelium) (3, 6). Therefore, it is difficult to distinguish cell type-specific function of a gene if it is deleted in more than 1 cell type (8). In addition, PR is expressed in the oviduct, ovary, mammary gland, and pituitary. In this context, both oviductal and uterine defects were observed when a floxed Tsc1 gene was deleted by PR-Cre (5). Females with conditional deletion of Lgr5 by PR-Cre were also infertile, but the origin of defects was an ovarian failure to maintain progesterone secretion during pregnancy (9). Along the same line, anti-Müllerian hormone receptor type 2 (Amhr2)-Cre mice are commonly used to delete target genes in the stroma and myometrial compartments as well as in the ovary and oviduct (4–6). For deletion of genes of interest in the uterine epithelium, Wnt7a-Cre and Sprr2f-Cre mouse lines have been used. However, Cre activity under the control of the Sprr2f promoter is also observed in the cerebellum and kidney, and loxP recombination by Sprr2f-Cre in adult uterine epithelia is not uniform (7). Wnt7a-Cre deletes genes in the uterine epithelium and in epithelia of other organs, such as the hair follicular epithelium (see Supplemental Figure 2). Another unmet challenge in using PR-, Amhr2-, and Wnt7a-Cre mouse lines is that recombination occurs before the uterus is fully mature and in some cases affects uterine development (10–12), limiting the use of these lines in studying gene function in the adult uterus.
We sought to create a new mouse line for uterine epithelium-specific Cre recombination using a lactoferrin (Ltf) promoter (also called lactotransferrin). Ltf is a nonheme iron-binding glycoprotein, which is highly responsive to estrogen in the mouse uterus (13–17). Ltf is not expressed in immature mouse uteri, but it is robustly expressed in the uterine epithelium of adult mice (13, 15, 16). Furthermore, mice with constitutive deletion of Ltf are viable and fertile, albeit with minor alterations in iron homeostasis under normal conditions (18). In this study, we generated Ltf-iCre knock-in mice and show that iCre efficiently recombines floxed genes primarily in the uterine epithelium in adult females, and in immature females after estrogen treatment.
Materials and Methods
Targeting constructs
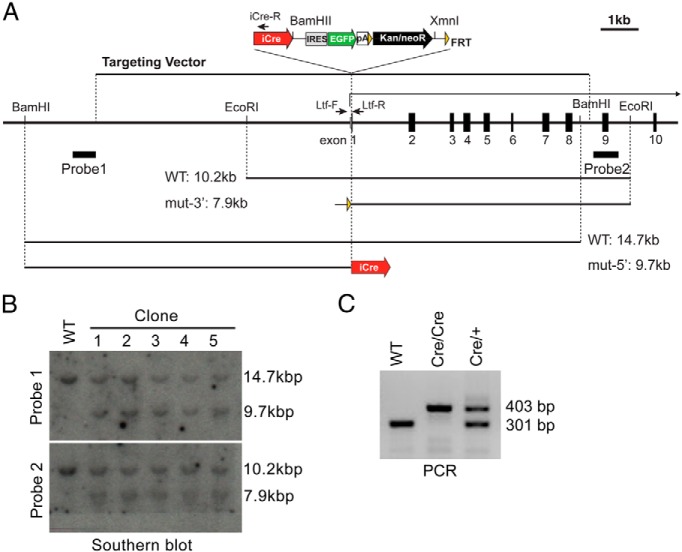
To create the Ltf-iCre knock-in targeting vector, the WI1–1277C21 fosmid that contains Ltf was acquired from the CHORI WIBR-1 mouse fosmid library and modified by a BAC recombineering method (19). A 25.5-kb region was removed from the 5′-end of the insert and replaced by a unique MluI site. Then, a 4.8-kb region at the 3′-end of the insert and a loxP site in the vector backbone were replaced by a diphtheria toxin fragment A expression cassette (20). As a result, the modified Ltf fosmid contains a 13.1-kb insert from the Ltf promoter to exon 8 of chr9: 111,012,552- 111,025,711 according to the December 2011 genome assembly (GRCm38/mm10) in the UCSC genome browser. Finally, an iCre-internal ribosomal entry site (Ires)-enhanced green fluorescent protein (EGFP)-flippase recognition target (FRT)-Neo/Kan-FRT cassette of pXL119 (a kind gift from Dr Gonzalo Alvarez-Bolado, Max Planck Institute, Munich, Germany) (21) with Kozak sequence replaced a part of exon 1 and intron 1 of Ltf at position chr9: 111,019,326- 111,019,369 in the GRCm38/mm10 genome assembly. The Ltf-iCre knock-in targeting vector was linearized by MluI for transfection.
Embryonic stem cell (ES cell) culture and gene targeting
The Ltf-iCre targeting construct was transfected into 129 and C57BL/6 hybrid G4 male ES cells (22) using a Bio-Rad Genepulser set at 500 μF and 0.24 kV. Neomycin-resistant cells were selected with 200 μg/mL G418, and ES colonies were picked 9–10 days after transfection. To confirm the targeted knock-in of iCre at the Ltf locus, Southern blot analyses were carried out. BamHI- and EcoRI/XmnI-digested genomic DNA were probed with probe 1 and probe 2, respectively, as shown in Figure 1A. Probes for Southern blotting were amplified from WI1–1277C21 by the following primers: probe1, Ltf5′P-F, GACATTCCTACTGCTCCTTGG and Ltf5′P-R, CCTGCTGTCCAGATGAGG; probe2, Ltf3′P-F, CACCAAGGACTGATGGATGA and Ltf3′P-R, TTCCATATTTTCCAAATGAACC. Thirteen homologously targeted ES clones were isolated out of 35 colonies picked (Figure 1B). Two Ltf-iCre-positive ES cell lines were microinjected into albino B6 mouse blastocysts to create mice (stock number 000058; The Jackson Laboratory). ES cell injection into blastocysts was performed at the Stem Cell and Transgenics Core at Cornell University (Ithaca, New York).
Figure 1.
Generation of Ltf-iCre knock-in mice. A, Map of the Ltf genomic region. The Ltf-iCre gene targeting vector is shown above the map. B, Southern blot analysis of BamHI-digested or EcoRI/XmnI-digested gDNA with probe 1 or 2, respectively. C, Genomic PCR for genotyping using Ltf-F, Ltf-R, and iCre-R primers. Ltf-F and iCre-R primer set amplified a 403-bp fragment from the targeted Ltf-iCre locus, whereas a 301-bp fragment is amplified from wild-type Ltf by Ltf-F and Ltf-R primers. WT, wild type.
Mice
The Ltf-iCre mouse line was generated and kept in a 129, C57BL/6 and albino B6 mixed background. To determine iCre localization and the efficiency of gene deletion, we mated them with B6.129S4-Gt(ROSA)26Sortm1Sor/J (R26Rf/f) mice. Genotyping LtfiCre/+ mice was done by PCR using tail gDNA with primers: 5′-GTTTCCTCCTTCTGGGCTCC-3′ (Ltf forward), 5′-TTTAGTGCCCAGCTTCCCAG-3′ (Ltf reverse), and 5′-CCTGTTGTTCAGCTTGCACC-3′ (iCre reverse). The primers Ltf forward and Ltf reverse amplify a wild-type band (301 bp), and the primers Ltf forward and iCre reverse amplify a Ltf-iCre band (403 bp) (Figure 1C). Estradiol-17β (100 ng/mouse) was sc injected into R26Rf/f/LtfiCre/+ female mice 2 times (once daily at the ages of 21 and 22 d), which were killed at the age of 30 days to collect tissue for further analysis. All procedures for the present study were reviewed and approved by the Cincinnati Children's Research Foundation's Institutional Animal Care and Use Committee, in accordance with NIH guidelines.
LacZ (gene encoding β-galactosidase) staining
LacZ staining was performed as previously described (6, 23). In brief, tissues were embedded in optimum cutting temperature (OCT) compound after fixation in 0.2% paraformaldehyde and infusion in 30% sucrose at 4°C. Frozen sections were stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside overnight at 37°C. The sections were counterstained with eosin.
Results
Generation of a new uterine epithelium-specific Cre mouse line
To create a uterine epithelium-specific Cre mouse line, we inserted iCre, which more efficiently deletes floxed genes under some experimental conditions as compared with standard Cre (24), under the control of the endogenous Ltf promoter by gene targeting (Figure 1). The iCre was followed by an Ires-EGFP to visualize Ltf promoter activity. Two Ltf-iCre targeted clones were used for blastocyst injection, and 8 chimeric male mice with 90%–100% contribution of Ltf-iCre ES clones as marked by coat color were selected for further analysis. Chimeras were bred with albino B6 mice twice to confirm germ line transmission. Male and female LtfiCre/+ mice were then mated to generate LtfiCre/iCre mice. Breeding of male and female LtfiCre/+ mice produced normal litter sizes (7.1 ± 0.8, n = 12). Furthermore, breeding of LtfiCre/iCre males and females also successfully produced pups (8.1 ± 1.0, n = 7). These results show that both LtfiCre/+ and LtfiCre/iCre mice are viable and fertile. We also examined pregnancy timing with Ltf+/+, LtfiCre/+, and LtfiCre/iCre females mated with wild-type males. Parturition timing, litter size, and weight of pups of Ltf+/+, LtfiCre/+, and LtfiCre/iCre females were comparable (Supplemental Table 1). These results provide evidence that iCre expression under the Ltf promoter does not affect pregnancy outcome.
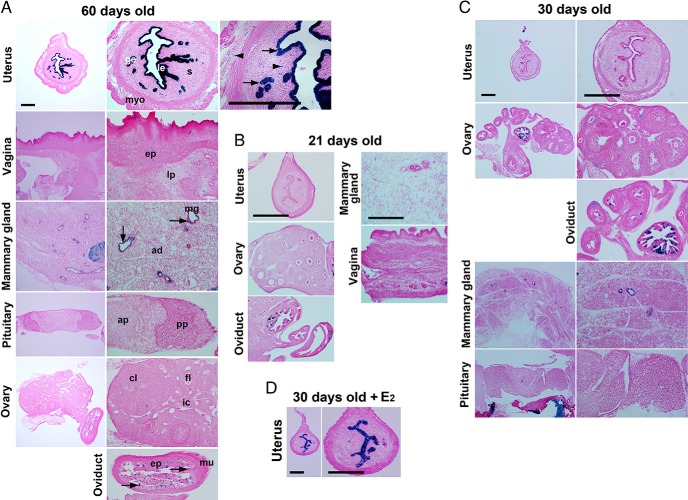
We next examined the efficiency of iCre recombination by crossing B6.129S4-Gt(ROSA)26Sortm1Sor/J (R26Rf/f) mice with LtfiCre/+mice. R26Rf/f mice induce β-galactosidase expression at the ROSA26 locus after Cre deletes a polyA signal flanked by loxP sites. As shown in Figure 2A, Ltf-iCre efficiently induced β-galactosidase expression in the uterine epithelium with nearly 100% efficiency as opposed to undetectable expression in the ovary and pituitary in mature R26Rf/f/LtfiCre/+ females. However, a limited population of cells in the oviduct, vagina, and mammary gland showed weak lacZ staining, and staining was also present in scattered neutrophils in the stroma and myometrium. The expression of Ltf in neutrophils in the uterine stromal bed was previously detected by in situ hybridization (16).
Figure 2.
LtfiCre/+ efficiently recombines loxP sites in the uterine epithelium. Conditional gene recombination induced by Ltf-iCre was visualized by lacZ staining (A, 60 d; B, 21 d; C, 30 d; and D, 30 d old with estradiol-17β [E2] treatment). Images in the left panels represent lower magnification, and those in the right panels are of higher magnification (A, C, and D). Arrows and arrowheads indicate lacZ-positive staining. Arrowheads depict lacZ-positive neutrophils in A. Scale bar, 400 μm. le, luminal epithelium; ge, glandular epithelium; myo, myometrium; s, stroma; ep, epithelium; lp, lamina propria; mg, mammary gland; ad, adipocyte; ap, anterior lobe of pituitary; pp, posterior pituitary; cl, corpus luteum; fl, follicle; ic, interstitial cell; mu, muscularis.
Ltf-iCre activity in immature mice
We next examined whether Ltf-iCre mediates floxed gene recombination in the uterus of immature mice (Figure 2, B and C). As expected, the number of lacZ-positive cells was very low to undetectable in the uterus at 21 and 30 days of age. LacZ staining was also detected in a subset of cells in the oviduct at the age of 21 days and in mammary glands at 30 days of age. To assess Ltf-iCre expression in the uterine precursor tissue in the fetal mesonephros, we examined Ltf-iCre activity in the Müllerian ducts on embryonic day 16 (vaginal plug = day 1). We crossed Ltf-iCre mice to B6;129S6-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J mice, which show tdTomato expression only after Cre-mediated recombination (due to Ltf-iCre-mediated excision of the polyA signal flanked by loxP sites) (Supplemental Figure 1). tdTomato expression was not detected in Müllerian ducts (marked by E-cadherin immunofluorescence), whereas it was detected in immune cells in the mesonephros; these are likely neutrophils, given that previous reports have demonstrated Ltf expression within neutrophils (16). These data suggest that Ltf-iCre does not induce loxP recombination in the fetal uterine precursor or in immature mice.
In contrast to Ltf-iCre, PR-Cre deleted floxed genes in all major uterine tissue compartments as early as 10 days of age (Supplemental Figure 2). PR-Cre also deleted floxed genes in the oviduct as early as 21 days old. In a similar manner, Amhr2-Cre deleted floxed genes in the stroma and myometrium in 10-day-old uteri, and also in the ovary and oviduct. Wnt7a-Cre deleted genes not only in uterine epithelia but also in the ovary and skin in 10-day-old mice. These results show that iCre expression under the regulation of the Ltf promoter generates more efficient Cre-loxP deletion primarily in the uterine epithelia of adult mice. Epithelial cell specificity of Ltf-iCre expression was also confirmed by examining EGFP expression from the iCre-Ires-EGFP cassette. Although EGFP expression was very low, but still detectable, without amplification of the signal by anti-GFP antibody staining, expression was observed in the uterine epithelium of mature mice (Supplemental Figure 3).
Because estrogen treatment can induce uterine epithelial Ltf expression in immature and mature mice (13, 14, 17), we examined whether Ltf-iCre can be induced by estrogen in immature mice. Indeed, we observed that estrogen treatment was effective in inducing Ltf-iCre activity in immature mice (Figure 2D). These results suggest that the estrogen-induced Ltf activation pathway is already present in immature mice, and Cre-loxP recombination can be induced by estrogen in immature Ltf-iCre mice before endogenous Ltf activation occurs.
Ltf-iCre activity in male reproductive tissues and other organs
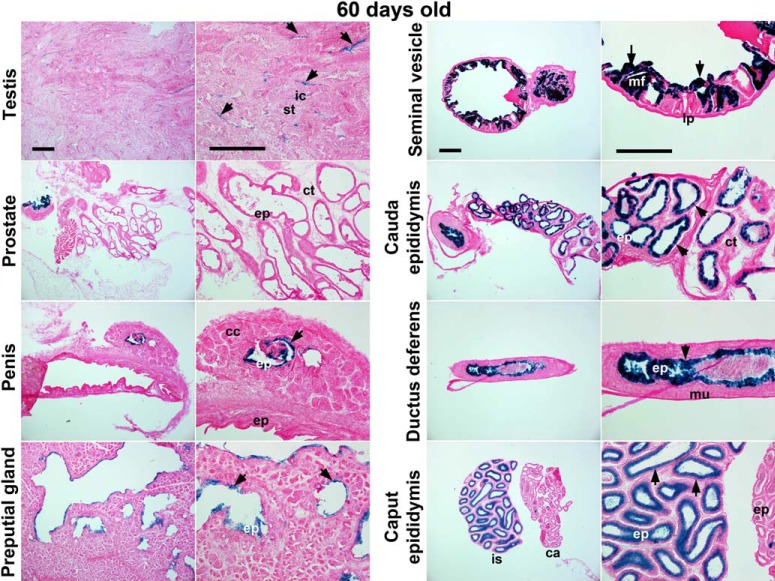
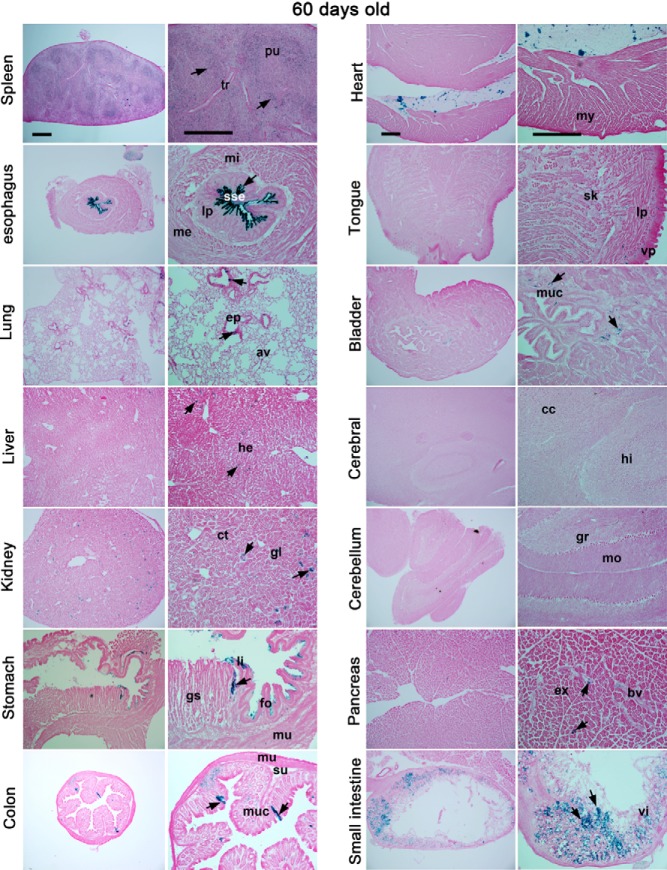
We also examined Cre-loxP recombination in other tissues in R26Rf/f/LtfiCre/+ mice. Among male reproductive organs, some interstitial cells in the testis and epithelial cells in nontestis organs were lacZ positive (Figure 3). As shown in Figure 4, most tissues were negative or only a subset of cells was positive for lacZ expression except the esophageal epithelium and spleen in R26Rf/f/LtfiCre/+ mice. Interestingly, lacZ-positive cells were also detected in the esophagus of R26Rf/f/PRCre/+ mice (Supplemental Figure 4). Because Ltf was reported to be expressed in preimplantation embryos (25), we examined Cre-loxP recombination in blastocysts using a double reporter mouse line (STOCK Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J) (Supplemental Figure 5). This mouse line expresses tdTomato in all tissues, but tdTomato expression is switched to EGFP expression when Cre-loxP recombination occurs. Because EGFP expressed from Ltf-iCre-Ires-EGFP was undetectable (Supplemental Figure 5, bottom panels), we expected that any detectable EGFP signal would originate from conversion of tdTomato to EGFP expression mediated by iCre. However, we failed to detect EGFP fluorescence in blastocysts obtained from these mice. This suggests that iCre driven by the Ltf promoter is not active in blastocysts.
Figure 3.
LtfiCre/+ induces recombination of loxP sites in male reproductive tissues. Conditional gene recombination induced by Ltf-iCre was visualized by lacZ staining. All samples are from 60-day-old males. Images in the left panels represent lower magnification, and those in the right panels are of higher magnification. Arrows indicate lacZ-positive staining. Scale bar, 400 μm. ic, interstitial cell; st, seminiferous tubule; ep, epithelium; ct, connective tissue; cc, corpus cavernosum; mf, mucosal fold; lp, lamina propia; mu, muscularis; is, intial segment of epididymis; ca, caput of epididymis.
Figure 4.
LtfiCre/+ shows limited recombination of loxP sites in other tissues. Conditional gene recombination induced by Ltf-iCre was visualized by lacZ staining. All samples are from 60-day-old females. Images in the left panels represent lower magnification, and those in the right panels are of higher magnification. Arrows indicate lacZ-positive staining. Scale bar, 400 μm. pu, pulp; tr, trabeculae; mi, muscularis interina; sse, stratified squamous epithelium; lp, lamina propria; me, muscularis externa; ep, epithelium; av, alveoli; he, hepatocyte; ct, convoluted tubule; gl, glomerulus; li, limiting ridge; gs, glandular stomach; fo, forestomach; mu, muscularis; su, submucosa; muc, mucosa, my, myocardium; sk, skeletal muscle; vp, ventral epithelium; cc, cerebral cortex; hi, hippocampus; gr, granular layer; mo, molecular layer; ex, exocrine pancreas; bv, blood vessel; vi, villus.
Discussion
We present here the generation of Ltf-iCre mice that show efficient and specific deletion of floxed genes in the uterine epithelium, which could be of great value in studying uterine epithelial gene function during pregnancy and in immature mice after hormonal stimulation. A variable, but generally low, level of Ltf-iCre activity was also observed in other tissues, such as male reproductive organs, neutrophils, spleen, liver, kidney, and epithelia of esophagus, lung, intestine, and bladder. Although the activity of Ltf-iCre was strongest and most consistent in the uterine epithelium, its expression was higher in neutrophils, spleen, and esophagus than the other nonuterine tissues, in which subsets of cells were lacZ positive. Thus, as with most Cre lines, some caution is raised as to the deletion of a target gene that may occur in other undesired tissues, potentially resulting in unexpected phenotypes. However, Ltf-iCre showed nearly 100% efficiency of recombination in the uterine epithelium, starting only in adult stages between 1 and 2 months of age. Additionally, we found very low to undetectable activity in the pituitary and ovary. Therefore, this Cre line will provide a new way to assess gene function specifically in the adult uterine epithelium, with perhaps relatively fewer concerns about confounding effects of gene deletion in other components of the reproductive tract or hormonal machinery.
It may also be worthwhile to examine whether Ltf deletion in the Ltf-iCre mice produces any phenotype, because Ltf has roles in defense against oxidative stress and infection and has immunomodulatory function under certain conditions (26, 27). However, Ltf knockout mice show a mild phenotype, with only minor alterations in iron homeostasis under normal conditions (18). We believe that using Ltf-iCre mice to induce conditional targeting of floxed genes in the uterine epithelium should not pose any appreciable problem, because the recombination of the target gene can be efficiently achieved with heterozygous Ltf-iCre alleles; 1 allele of Ltf remains intact to allow for normal immunomodulatory function. This new Ltf-iCre mouse line is likely to be a powerful tool to define the contribution of specific genes in uterine epithelial cell function under normal and abnormal uterine environments.
Acknowledgments
We thank Serenity Curtis for editing the manuscript. The iCre-Ires-EGFP-FRT-PGK-neo-FRT construct was kindly provided by Gonzalo Alvarez-Bolado (University of Heidelberg, Heidelberg, Germany). The G4 ES cell line was provided by Andras Nagy and Marina Gertsenstein (Mount Sinai Hospital, Canada). ES cell injection into blastocysts was performed by Christian Abratte and Robert Munroe (Stem Cell and Transgenics Core, Cornell University, Ithaca, NY). PR-Cre mice were initially provided by Francesco DeMayo and John B. Lydon (Baylor College of Medicine, Houston, TX). Amhr2-Cre and Wnt7a-Cre mice were provided by Richard Behringer (MD Anderson Cancer Center, Houston, TX).
This work was supported in part by National Institutes of Health Grants HD068524, DA06668, and PO1CA7783 and by March of Dimes (S.K.D.). J.T. is supported by a Japan Society for the Promotion of Science postdoctoral fellowship sponsored by Kyoto University (Kyoto, Japan) and Cincinnati Children's Hospital. Stem Cell and Transgenics Core, Cornell University is supported by New York State Department of Health Contract C024174.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Amhr2
- anti-Müllerian hormone receptor type 2
- EGFP
- enhanced green fluorescent protein
- ES cell
- embryonic stem cell
- FRT
- flippase recognition target
- Ires
- internal ribosomal entry site
- lacZ
- gene encoding β-galactosidase
- Ltf
- lactoferrin
- PR
- progesterone receptor
- PR-Cre
- Cre under the control of PR.
References
- 1. Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7(3):185–199 [DOI] [PubMed] [Google Scholar]
- 2. Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18(12):1754–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soyal SM, Mukherjee A, Lee KY, et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41(2):58–66 [DOI] [PubMed] [Google Scholar]
- 4. Arango NA, Kobayashi A, Wang Y, et al. A mesenchymal perspective of Müllerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev. 2008;75(7):1154–1162 [DOI] [PubMed] [Google Scholar]
- 5. Daikoku T, Yoshie M, Xie H, et al. Conditional deletion of Tsc1 in the female reproductive tract impedes normal oviductal and uterine function by enhancing mTORC1 signaling in mice. Mol Hum Reprod. 2013;19(7):463–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Daikoku T, Jackson L, Besnard V, Whitsett J, Ellenson LH, Dey SK. Cell-specific conditional deletion of Pten in the uterus results in differential phenotypes. Gynecol Oncol. 2011;122(2):424–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Contreras CM, Akbay EA, Gallardo TD, et al. Lkb1 inactivation is sufficient to drive endometrial cancers that are aggressive yet highly responsive to mTOR inhibitor monotherapy. Dis Model Mech. 2010;3(3–4):181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun X, Zhang L, Xie H, et al. Kruppel-like factor 5 (KLF5) is critical for conferring uterine receptivity to implantation. Proc Natl Acad Sci USA. 2012;109(4):1145–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun X, Terakawa J, Clevers H, Barker N, Daikoku T, Dey SK. Ovarian LGR5 is critical for successful pregnancy. FASEB J. 2014;28(5):2380–2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dunlap KA, Filant J, Hayashi K, et al. Postnatal deletion of Wnt7a inhibits uterine gland morphogenesis and compromises adult fertility in mice. Biol Reprod. 2011;85(2):386–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stewart CA, Wang Y, Bonilla-Claudio M, et al. CTNNB1 in mesenchyme regulates epithelial cell differentiation during Müllerian duct and postnatal uterine development. Mol Endocrinol. 2013;27(9):1442–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Franco HL, Dai D, Lee KY, et al. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 2011;25(4):1176–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Teng CT, Beard C, Gladwell W. Differential expression and estrogen response of lactoferrin gene in the female reproductive tract of mouse, rat, and hamster. Biol Reprod. 2002;67(5):1439–1449 [DOI] [PubMed] [Google Scholar]
- 14. Das SK, Tan J, Johnson DC, Dey SK. Differential spatiotemporal regulation of lactoferrin and progesterone receptor genes in the mouse uterus by primary estrogen, catechol estrogen, and xenoestrogen. Endocrinology. 1998;139(6):2905–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Newbold RR, Teng CT, Beckman WC, et al. Fluctuations of lactoferrin protein and messenger-ribonucleic-acid in the reproductive-tract of the mouse during the estrous cycle. Biol Reprod. 1992;47(5):903–915 [DOI] [PubMed] [Google Scholar]
- 16. McMaster MT, Teng CT, Dey SK, Andrews GK. Lactoferrin in the mouse uterus: analyses of the preimplantation period and regulation by ovarian steroids. Mol Endocrinol. 1992;6(1):101–111 [DOI] [PubMed] [Google Scholar]
- 17. Das SK, Flanders KC, Andrews GK, Dey SK. Expression of transforming growth factor-β isoforms (β 2 and β 3) in the mouse uterus: analysis of the periimplantation period and effects of ovarian steroids. Endocrinology. 1992;130(6):3459–3466 [DOI] [PubMed] [Google Scholar]
- 18. Ward PP, Mendoza-Meneses M, Cunningham GA, Conneely OM. Iron status in mice carrying a targeted disruption of lactoferrin. Mol Cell Biol. 2003;23(1):178–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee EC, Yu D, Martinez de Velasco J, et al. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73(1):56–65 [DOI] [PubMed] [Google Scholar]
- 20. Ogawa Y, Sun BK, Lee JT. Intersection of the RNA interference and X-inactivation pathways. Science. 2008;320(5881):1336–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao T, Zhou X, Szabó N, Leitges M, Alvarez-Bolado G. Foxb1-driven Cre expression in somites and the neuroepithelium of diencephalon, brainstem, and spinal cord. Genesis. 2007;45(12):781–787 [DOI] [PubMed] [Google Scholar]
- 22. George SH, Gertsenstein M, Vintersten K, et al. Developmental and adult phenotyping directly from mutant embryonic stem cells. Proc Natl Acad Sci USA. 2007;104(11):4455–4460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma W, Tan J, Matsumoto H, et al. Adult tissue angiogenesis: evidence for negative regulation by estrogen in the uterus. Mol Endocrinol. 2001;15(11):1983–1992 [DOI] [PubMed] [Google Scholar]
- 24. Shimshek DR, Kim J, Hübner MR, et al. Codon-improved Cre recombinase (iCre) expression in the mouse. Genesis. 2002;32(1):19–26 [DOI] [PubMed] [Google Scholar]
- 25. Ward PP, Mendoza-Meneses M, et al. Restricted spatiotemporal expression of lactoferrin during murine embryonic development. Endocrinology. 1999;140(4):1852–1860 [DOI] [PubMed] [Google Scholar]
- 26. Ward PP, Mendoza-Meneses M, Park PW, Conneely OM. Stimulus-dependent impairment of the neutrophil oxidative burst response in lactoferrin-deficient mice. Am J Pathol. 2008;172(4):1019–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. González-Chávez SA, Arévalo-Gallegos S, Rascón-Cruz Q. Lactoferrin: structure, function and applications. Int J Antimicrob Agents. 2009;33(4):301.e1–e8 [DOI] [PubMed] [Google Scholar]